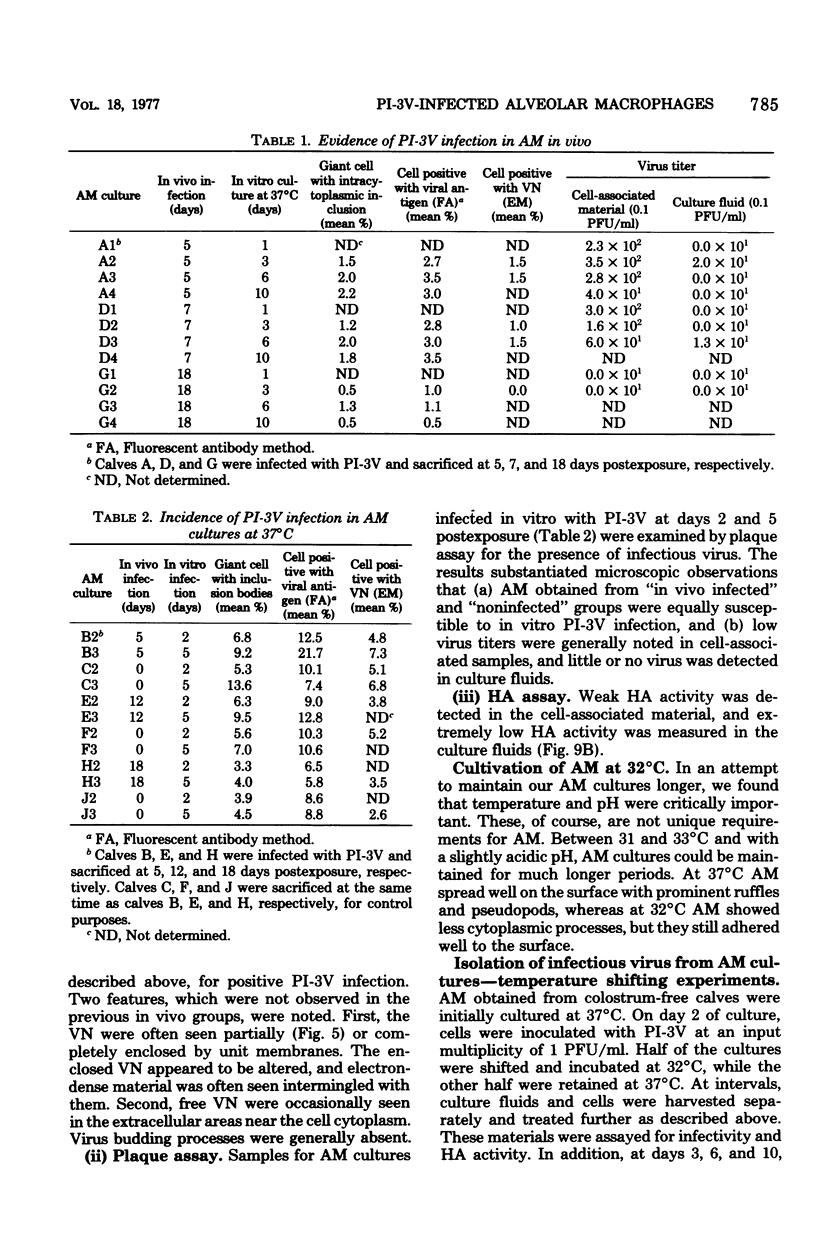
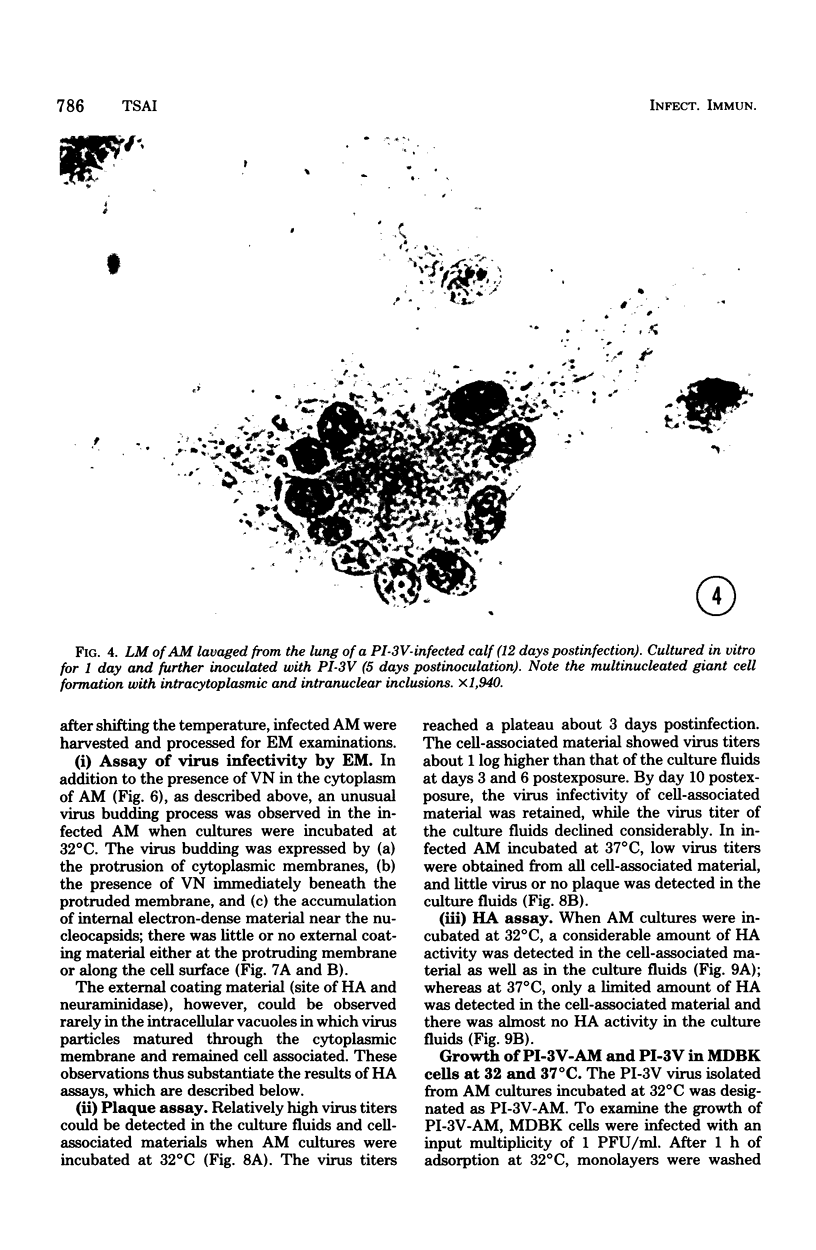
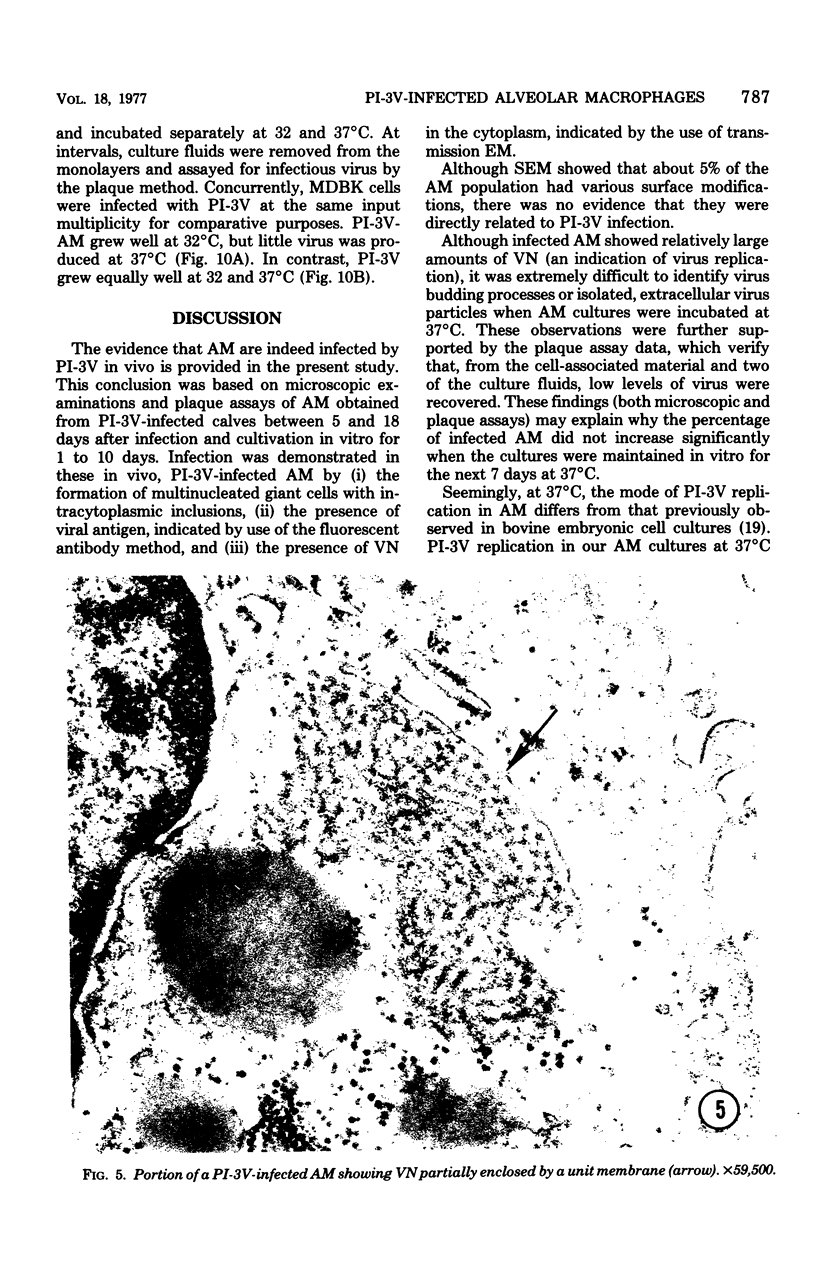
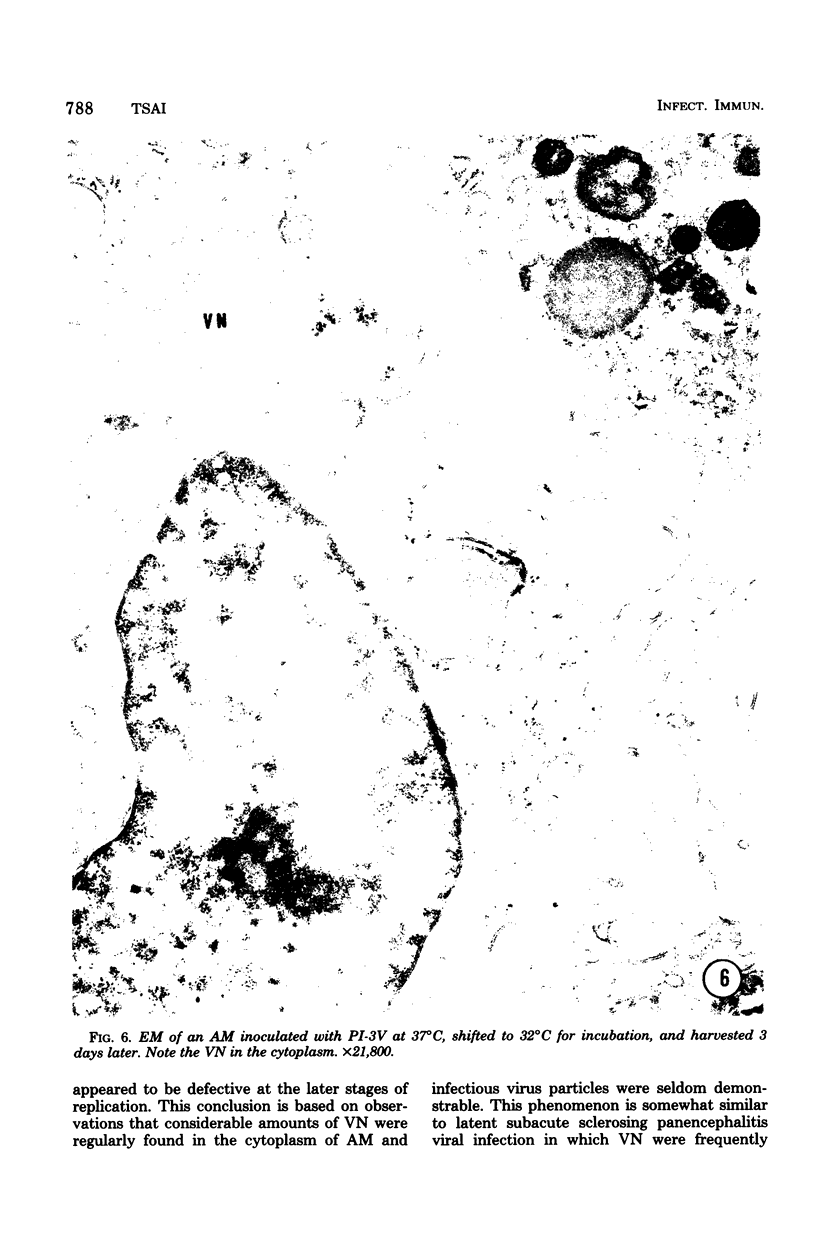
Abstract
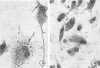
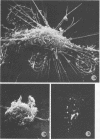
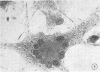
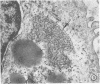
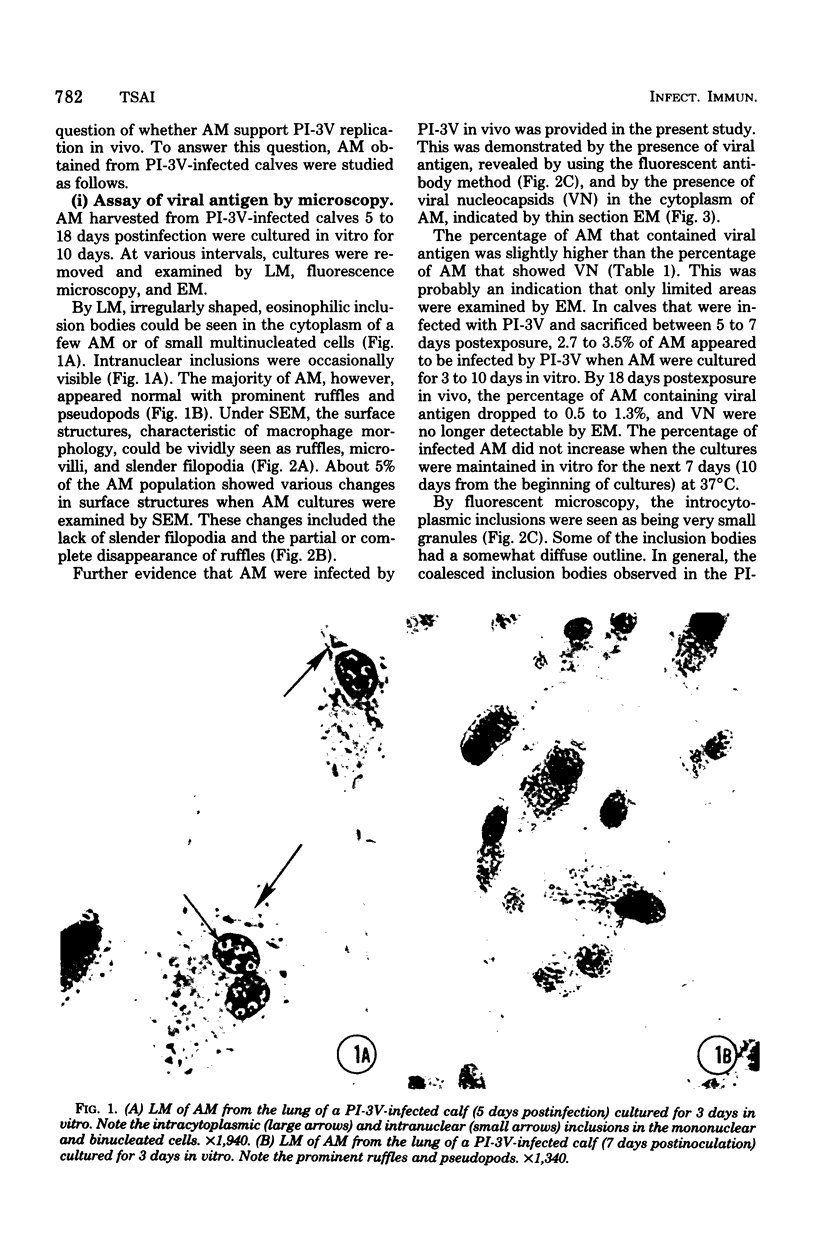
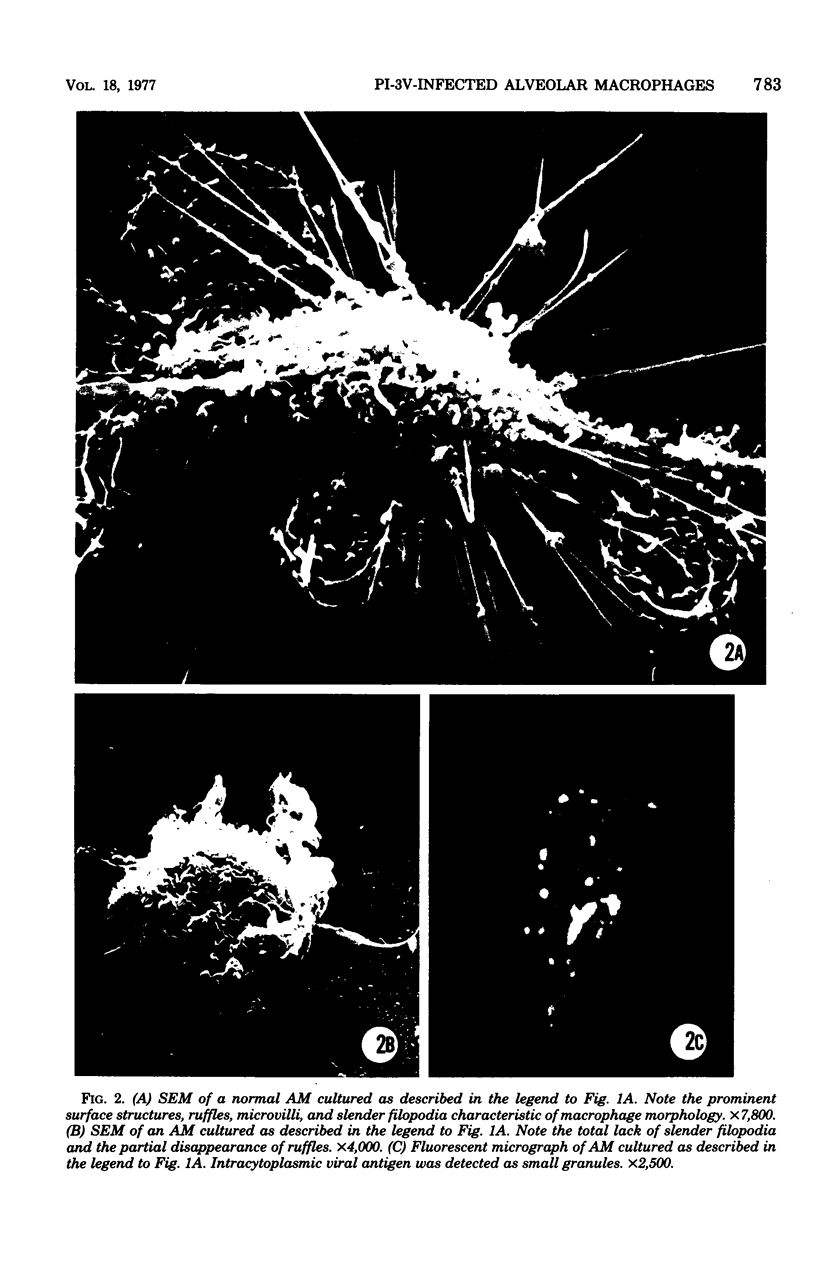
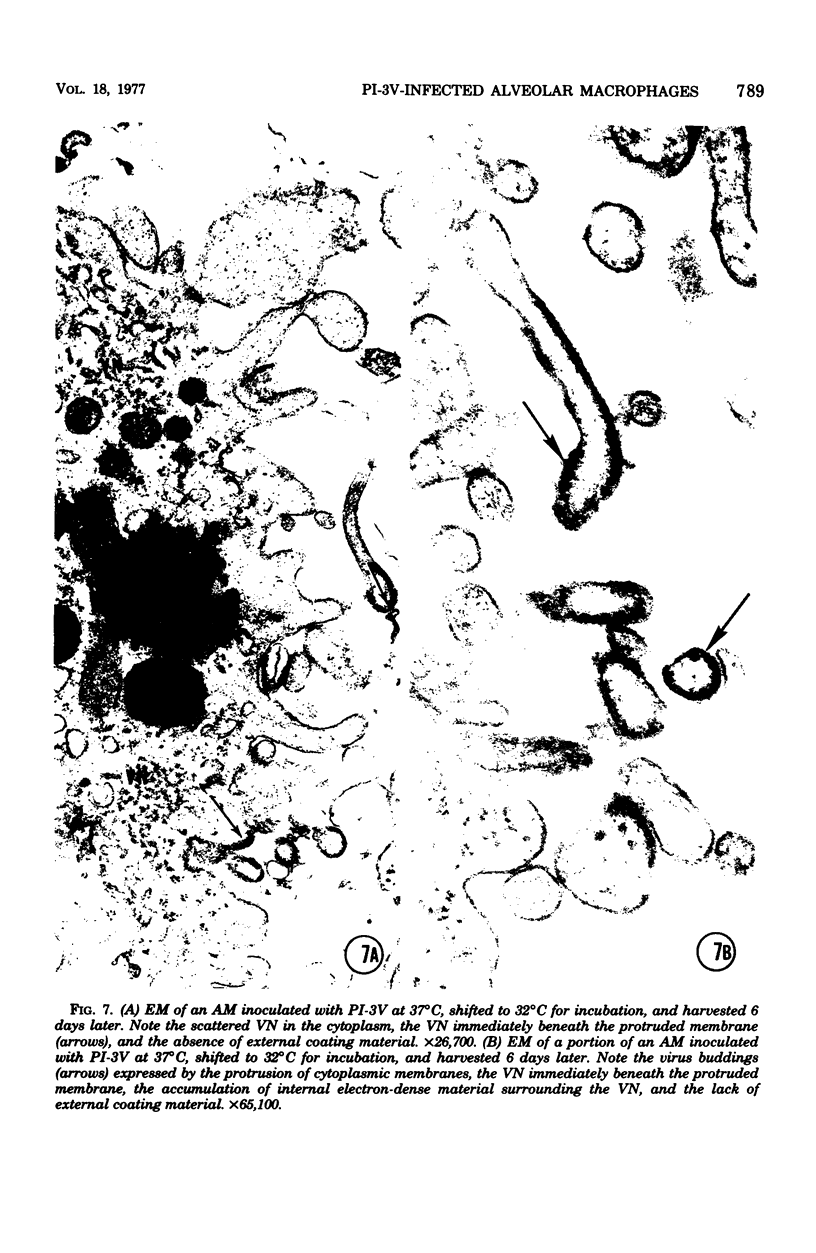
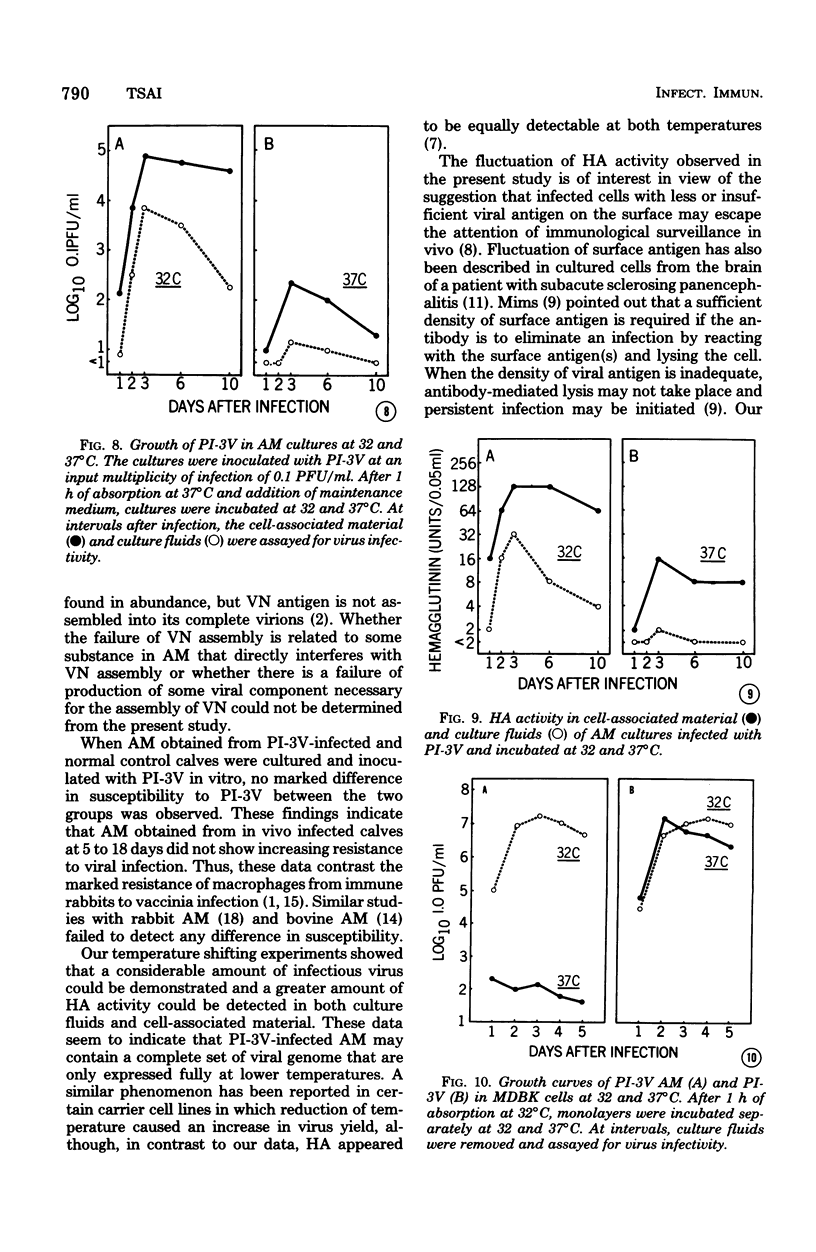
Approximately 2% of cultured alveolar macrophages (AM), originally lavaged from the lungs of parainfluenza type 3 virus (PI-3V)-infected calves, were observed to contain viral antigen (by fluorescent antibody method) or viral nucleocapsids (by electron microscopy). Plaque assays, however, indicated that virus titers were generally low when cultures were incubated at 37 degrees C for 10 days. AM, obtained from "in vivo infected" and "noninfected" calves, were found to be equally susceptible to further in vitro PI-3V infection when cultures were incubated at 37 degrees C. AM that were obtained from the lungs of normal calves, cultured at 37 degrees C, and inoculated with PI-3V were observed to produce relatively high virus titers when the incubation temperature was shifted down to 32 degrees C. Results from hemagglutinin assays showed that considerable amounts of hemagglutinin were detected when AM cultures were incubated at 32 degrees C, but only limited amounts were detected at 37 degrees C. Results from electron microscopic examinations at both temperatures substantiated the results of plaque and hemagglutinin assays. The PI-3V, isolated from AM cultures incubated at 32 degrees C, grew well in Madin-Darby bovine kidney cells at 32 degrees C, but little virus was produced at 37 degrees C. In contrast, parent PI-3V grew equally well at both temperatures. The results are discussed in terms of host susceptibility, temperature-sensitivity and virus maturation, and surface viral antigens and persistent viral infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila F. R., Schultz R. M., Tompkins W. A. Specific macrophage immunity to vaccinia virus: macrophage-virus interaction. Infect Immun. 1972 Jul;6(1):9–16. doi: 10.1128/iai.6.1.9-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Barbosa L. H., Hamilton R., Sever J. L. Comparison between productive and latent subacute sclerosing panencephalitis viral infection in vitro. An electron microscopic and immunoperoxidase study. Lab Invest. 1974 Mar;30(3):241–250. [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M. Pulmonary clearance of infectious agents. Annu Rev Med. 1968;19:315–336. doi: 10.1146/annurev.me.19.020168.001531. [DOI] [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Ito Y., Shimokata K., Nishiyama Y., Nagata I. Temperature-sensitive virus derived from BHK cells persistently infected with HVJ (Sendai virus). J Virol. 1975 Jan;15(1):55–63. doi: 10.1128/jvi.15.1.55-63.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Mims C. A. Factors in the mechanism of persistence of viral infections. Prog Med Virol. 1974;18(0):1–14. [PubMed] [Google Scholar]
- Oldstone M. B., Bokisch V. A., Dixon F. J., Barbosa L. H., Fuccillo D., Sever J. L. Subacute sclerosing panencephalitis: destruction of human brain cells by antibody and complement in an autologous system. Clin Immunol Immunopathol. 1975 May;4(1):52–52. doi: 10.1016/0090-1229(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1972 Feb;9(2):200–206. doi: 10.1128/jvi.9.2.200-206.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Probert M., Stott E. J., Thomas L. H. Interactions between calf alveolar macrophages and parainfluenza-3 virus. Infect Immun. 1977 Feb;15(2):576–585. doi: 10.1128/iai.15.2.576-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Woan M. C., Tompkins W. A. Macrophage immunity to vaccina virus: factors affecting macrophage immunity in vitro. J Reticuloendothel Soc. 1974 Jul;16(1):37–47. [PubMed] [Google Scholar]
- Shenk T. E., Koshelnyk K. A., Stollar V. Temperature-sensitive virus from Aedes albopictus cells chronically infected with Sindbis virus. J Virol. 1974 Feb;13(2):439–447. doi: 10.1128/jvi.13.2.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. Macrophages and viral immunity. Semin Hematol. 1970 Apr;7(2):185–214. [PubMed] [Google Scholar]
- Tompkins W. A., Zarling J. M., Rawls W. E. In vitro assessment of cellular immunity to vaccinia virus: contribution of lymphocytes and macrophages. Infect Immun. 1970 Dec;2(6):783–790. doi: 10.1128/iai.2.6.783-790.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K. S., Thomson R. G. Bovine parainfluenza type 3 virus infection: virus replication in bovine embryonic cell cultures and virion separation by rate-zonal centrifugation. Infect Immun. 1975 Apr;11(4):770–782. doi: 10.1128/iai.11.4.770-782.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]