Abstract
Objectives
This study sought to test 2 hypotheses: 1) fibroblast growth factor (FGF)-23 identifies patients with stable ischemic heart disease (SIHD) at high risk of cardiovascular events independent of clinical factors, renal function, and established cardiovascular biomarkers; and 2) FGF-23 identifies patients who derive greater clinical benefit from angiotensin-converting enzyme inhibitor therapy.
Background
FGF-23 is an endocrine regulator of mineral metabolism and markedly elevated levels are associated with cardiovascular events in patients with chronic kidney disease. Data in patients with SIHD are more sparse.
Methods
FGF-23 levels were measured in 3,627 patients with SIHD randomly assigned to trandolapril or placebo within the PEACE (Prevention of Events With Angiotensin-Converting Enzyme) trial and followed up for a median of 5.1 years.
Results
After adjustment for clinical risk predictors, left ventricular ejection fraction, markers of renal function, and established cardiovascular biomarkers, FGF-23 concentration was independently associated with an increased risk of cardiovascular death or heart failure among patients allocated to placebo (quartile 4 hazard ratio: 1.73; 95% confidence interval, 1.09 to 2.74; p = 0.02) and significantly improved metrics of discrimination. Furthermore, among patients in the top quartile of FGF-23 levels, trandolapril significantly reduced cardiovascular death or incident heart failure (hazard ratio: 0.45; 95% confidence interval: 0.28 to 0.72), whereas there was no clinical benefit in the remaining patients (hazard ratio: 1.07; 95% confidence interval: 0.75 to 1.52; p interaction = 0.0039). This interaction was independent of and additive to stratification based on renal function.
Conclusions
Elevated levels of FGF-23 are associated with cardiovascular death and incident heart failure in patients with SIHD and identify patients who derive significant clinical benefit from angiotensin-converting enzyme inhibitor therapy regardless of renal function.
Keywords: angiotensin-converting enzyme inhibitors, biomarkers, coronary artery disease, fibroblast growth factor-23, kidney
Fibroblast growth factor (FGF)-23 is a phosphatonin, a circulating endocrine regulator of mineral metabolism that rises in the earliest stages of renal impairment (1–3). Markedly elevated levels of FGF-23, observed in patients with moderate-to-severe chronic kidney disease (CKD), are associated with an increased risk of mortality (4,5). Data also suggest that FGF-23 levels at the higher end of the range seen within the general population may be associated with an increased risk of cardiovascular events in patients at risk for or with stable ischemic heart disease (SIHD) (6–10). However, the extent to which FGF-23 is a significant predictor of cardiovascular events independent of clinical comorbidities, conventional markers of renal function, and established cardiovascular biomarkers is unknown. Furthermore, whereas biomarkers of reduced renal function identify patients with SIHD who derive greater benefit from angiotensin-converting enzyme (ACE) inhibitor therapy (11,12), whether levels of FGF-23 can do the same or better remains untested. Therefore, we tested the hypotheses that in patients with SIHD, higher levels of FGF-23 were associated with an increased risk of cardiovascular events and identified patients who derived greater clinical benefit from ACE inhibition.
Methods
Study design and participants
The PEACE (Prevention of Events with Angiotensin-Converting Enzyme) Trial was a randomized trial of trandolapril versus placebo in 8,290 participants age ≥50 years with SIHD, left ventricular ejection fraction >40%, and serum creatinine ≤2.0 mg/dl that enrolled patients from November 1996 through June 2000 (13,14). All participants from the United States and Canada were eligible for biospecimen sampling at the discretion of each clinical center, and approximately half agreed to participate. All participants provided written informed consent, and this study was approved by the relevant institutional review boards. The current analysis included all patients who had an enrollment blood sample available for measurement of FGF-23 (n = 3,627). There were no clinically relevant differences between patients included in the substudy and the overall trial population (Online Table 1).
Biomarkers
FGF-23 levels were measured with a well-established C-terminal human enzyme-linked immunoabsorbent assay (Immunotopics, San Clemente, California) (15) in the Thrombolysis in Myocardial Infarction (TIMI) Clinical Trials Laboratory (Boston, Massachusetts) as detailed in the supplemental Methods section in the Online Appendix. In adults with preserved renal function, normal values for this assay are 55 ± 50 reference units (RU)/ml (15). Baseline levels of the N-amino terminal fragment of the prohormone B-type natriuretic peptide (NT-proBNP), cardiac troponin T measured with the high-sensitivity assay (hs-cTnT), C-reactive protein measured with a highly sensitive assay (hs-CRP), midregional pro-atrial natriuretic peptide, midregional pro-adrenomedullin, C-terminal proendothelin-1, estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease equation (16), cystatin C, and urinary albumin to creatinine ratio (ACR) have been determined in this population previously (12,17–21). All biochemical testing was performed by study personnel who were unaware of the clinical outcome and treatment assignment.
Endpoints
On the basis of prior data regarding the predictive ability of FGF-23 (6,7), the primary outcome of this analysis was the composite of cardiovascular death or hospitalization for heart failure. Additionally, we explored other major adverse cardiovascular events that had been part of the primary endpoint for the parent PEACE trial, including myocardial infarction (MI), stroke, and coronary revascularization. All clinical events were documented and adjudicated before this biomarker was measured (13).
Statistical analyses
Participants were separately divided into quartiles according to their baseline FGF-23 levels and descriptive analyses of baseline characteristics were performed (see supplemental Methods section in the Online Appendix). Cumulative event rates were calculated across quartiles of FGF-23 with the Kaplan-Meier method and compared by use of a trend test. Cumulative event rates were also calculated stratifying patients on the basis of FGF-23 levels and established biomarkers of renal function, specifically, eGFR, cystatin C (using a cut point of the top quartile, ≥0.91 mg/l, which also approximates the top 2.5 percentile of the normal reference range [22]), and urinary ACR (using sex-specific cut points of ≥25 µg/mg in women and ≥17 µg/mg in men to define microalbuminuria as previously described [18]).
The association between FGF-23 levels and outcomes was estimated among placebo-assigned patients using Cox proportional-hazards models to derive hazard ratios (HR) and 95% confidence intervals (CI) for elevation in FGF-23 levels. Models were adjusted for the following clinical risk factors: age, sex, weight, history of hypertension, history of diabetes mellitus, current tobacco use, prior MI, prior coronary revascularization, systolic blood pressure, eGFR, and left ventricular ejection fraction. Models containing the aforementioned clinical variables were then also adjusted for 2 additional biomarkers of renal function: cystatin C and urinary ACR. Further adjustment to the described clinical and renal models was also performed by adding the established and novel biomarkers delineated in the preceding text under Biomarkers. The incremental performance of FGF-23 was evaluated by calculating changes in the C-statistic, integrated discrimination improvement, and category-free net reclassification improvement metrics (23).
To determine whether FGF-23 levels could be used to identify patients in whom ACE inhibition resulted in greater clinical benefit, hazard ratios for the effect of trandolapril on the risk of cardiovascular death or heart failure were estimated in patients stratified by FGF-23 level. To test for statistically significant effect modification, a Cox proportional-hazards model was created that included a term for trandolapril, a term for FGF-23 risk category, and an interaction term. Further subcategorization was done by additional stratification using eGFR (≥60 or <60 ml/min/1.73 m2) according to standard criteria for defining advanced CKD (24), and a recently developed multimarker score (21). All p values were 2-sided and values of p < 0.05 were considered to be statistically significant. STATA/EC (version 12.1, STATA Corp, College Station, Texas) and R (version 2.12.1, R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses.
Results
Baseline characteristics
Among the 3,627 participants with a baseline measurement, the median level of FGF-23 was 50.6 RU/ml (interquartile range, 38.7 to 69.9). The distribution of FGF-23 levels was similar to that in a healthy population (adults, 55 ± 50 RU/ml [15]), but lower than in patients with stages 2 through 4 CKD (median, 145.5 RU/ml; interquartile range, 95.8 to 239.1) (5). The baseline characteristics of placebo patients according to quartile of FGF-23 are shown in Table 1. In general, higher baseline levels of FGF-23 were associated with older age, female sex, hypertension, diabetes mellitus, current tobacco use, and reduced eGFR, but with a lower rate of prior MI. The correlation at baseline between FGF-23 and established or experimental markers of renal function and cardiovascular risk was moderate to weak, with the strongest correlations being with cystatin C and midregional pro-adrenomedullin (rho 0.36 and 0.39, respectively, p < 0.001 for both) (Online Table 2).
Table 1.
Baseline Characteristics by FGF-23 Quartiles in the Placebo Arm
| Quartiles of FGF-23 (RU/ml) | p Value for Trend* |
||||
|---|---|---|---|---|---|
| 1 (<38.81; N = 454) |
2 (38.81–50.05; N = 454) |
3 (50.06–70.20; N = 454) |
4 (>70.20; N = 453) |
||
| Age (yrs) | 62.0 ± 7.7 | 63.8 ± 8.1 | 64.9 ± 8.3 | 65.7 ± 8.4 | <0.0001 |
| Female | 48 (10.6) | 53 (11.7) | 87 (19.2) | 132 (29.1) | <0.0001 |
| Weight (kg) | 82.5 ± 14.2 | 84.6 ± 15.2 | 83.5 ± 16.1 | 84.5 ± 17.0 | 0.47 |
| Hypertension | 184 (40.5) | 198 (43.6) | 206 (45.4) | 226 (49.9) | 0.004 |
| Current tobacco use | 65 (14.3) | 48 (10.6) | 68 (15.0) | 99 (21.9) | 0.0003 |
| Diabetes mellitus | 58 (12.8) | 62 (13.7) | 78 (17.2) | 89 (19.7) | 0.002 |
| Prior MI | 276 (60.8) | 270 (59.5) | 261 (57.5) | 240 (53.0) | 0.014 |
| Prior PCI or CABG | 331 (72.9) | 306 (67.4) | 340 (74.9) | 347 (76.6) | 0.047 |
| SBP (mm Hg) | 131.8 ± 16.4 | 131.9 ± 15.9 | 135.3 ± 17.4 | 134.8 ± 17.5 | 0.001 |
| DBP (mm Hg) | 78.4 ± 9.7 | 78.6 ± 9.9 | 78.9 ± 10.6 | 77.3 ± 10.5 | 0.18 |
| eGFR (mL/min/1.73 m2) | 81.3 ± 18.5 | 80.7 ± 19.7 | 76.8 ± 17.9 | 74.4 ± 20.8 | <0.0001 |
| Apo B (mg/dl) | 106.0 ± 23.6 | 106.3 ± 22.2 | 107.0 ± 21.4 | 108.5 ± 25.3 | 0.13 |
| Apo A1 (mg/dl) | 139.6 ± 23.7 | 137.7 ± 25.5 | 140.3 ± 25.8 | 137.9 ± 25.4 | 0.57 |
| LVEF (%) | 58.3 ± 9.4 | 58.6 ± 9.9 | 59.0 ± 9.5 | 58.9 ± 9.7 | 0.31 |
Values are mean ± SD or n (%). Plasma FGF-23 concentrations are reported in reference units (RU)/ml.
The trend test refers to a 1-degree of freedom test for linear trend across quartiles.
Apo = apolipoprotein; CABG = coronary artery bypass grafting; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; FGF = fibroblast growth factor; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; SBP = systolic blood pressure; RU = reference units.
FGF-23 levels and clinical endpoints
Among 1,815 placebo-assigned patients, 114 experienced cardiovascular death or incident heart failure over a median 5.1 years of follow-up. Higher baseline levels of FGF-23 were strongly associated with the subsequent risk of cardiovascular death or heart failure, with a 35% increased risk per 1-SD increase in log-transformed FGF-23 levels (HR: 1.35; 95% CI: 1.20 to 1.53; p < 0.0001). Risk increased across quartiles of FGF-23, particularly among patients who were in the highest quartile of FGF-23 (HR: 3.32; 95% CI: 1.95 to 5.66; p < 0.0001) (Fig. 1). A similar pattern was seen for the individual endpoints of cardiovascular death and heart failure (HR: 3.16; 95% CI: 1.54 to 6.49; p = 0.0009, and HR: 4.44; 95% CI: 2.04 to 9.63; p < 0.0001, respectively) (Online Table 3). As expected on the basis of prior work (6,7), FGF-23 was not associated with the incidence of MI, stroke, unstable angina, or coronary revascularization (Online Table 3).
Figure 1. Cumulative Incidence Curves for the Composite of Cardiovascular Death or Heart Failure Among Patients in the Placebo Arm (n = 1,815).
Patients are categorized by quartiles of fibroblast growth factor (FGF)-23. The p value is for log-rank test for trend across quartiles.
After adjustment for traditional clinical risk factors (age, sex, weight, hypertension, diabetes mellitus, current tobacco use, prior MI, prior coronary revascularization, and systolic blood pressure), eGFR, and left ventricular ejection fraction, elevated concentrations of FGF-23 remained independently associated with an increased risk of cardiovascular death or heart failure per 1-SD increase (adjusted HR: 1.31; 95% CI: 1.13 to 1.51; p = 0.0004). Quartile analysis demonstrated that the independent association between higher levels of FGF-23 and the incidence of cardiovascular death or heart failure was evident in those patients in the top quartile of FGF-23 levels (adjusted HR: 2.31; 95% CI: 1.32 to 4.05; p = 0.003) as was the risk of cardiovascular death or heart failure individually (Table 2). The addition of FGF-23 to the clinical model significantly improved metrics of discrimination, including an improvement in the C-statistic from 0.764 (95% CI: 0.718 to 0.810) to 0.784 (95% CI: 0.740 to 0.829), an integrated discrimination improvement of 1.65%, and a net reclassification improvement of 0.43 (all p < 0.05).
Table 2.
Association of FGF-23 Levels and Clinical Outcomes in the Placebo Arm Adjusted for Clinical Findings at Baseline*
| Outcome | HR (95% CI) per 1-SD Increase in Log-Transformed FGF-23 |
p Value | HR (95% CI) Across Quartiles (RU/ml Range)† | p Value for Trend |
|||
|---|---|---|---|---|---|---|---|
| 1 (<38.81) |
2 (38.81–50.05) |
3 (50.06–70.20) |
4 (>70.20) |
||||
| CV death, HF | 1.31 (1.13–1.51) | 0.0004 | Referent | 0.75 (0.38–1.50) | 1.07 (0.58–1.98) | 2.31 (1.32–4.05) | 0.002 |
| CV death | 1.35 (1.11–1.63) | 0.002 | Referent | 0.92 (0.39–2.19) | 1.20 (0.53–2.70) | 2.14 (1.003–4.57) | 0.038 |
| HF | 1.36 (1.12–1.66) | 0.002 | Referent | 0.64 (0.21–1.97) | 1.07 (0.43–2.69) | 3.28 (1.46–7.38) | 0.0003 |
Covariates in the model include conventional clinical factors: age, sex, weight, history of hypertension, history of diabetes mellitus, current tobacco use, prior myocardial infarction, prior percutaneous coronary intervention or coronary artery bypass graft surgery, systolic blood pressure, estimated glomerular filtration rate, and left ventricular ejection fraction. In quartile analyses, a trend test refers to a 1-degree of freedom test for linear trend across quartiles.
Plasma FGF-23 concentrations are reported in reference units (RU)/ml.
CI = confidence interval; CV = cardiovascular; FGF = fibroblast growth factor; HF = heart failure; HR = hazard ratio; RU = reference units.
In addition to eGFR, the risk associated with elevated FGF-23 levels was additive to and independent of other biomarkers of renal function, including cystatin C and urinary ACR, with comparable elevated risk of cardiovascular death or heart failure in patients with levels in the highest category of either FGF-23 or another renal biomarker, and markedly elevated risk among patients presenting in the highest risk category of both biomarkers simultaneously (Fig. 2). Moreover, in multivariable analyses adjusting for clinical characteristics, eGFR, cystatin C, and urinary ACR, patients with an FGF-23 level in the fourth quartile still had a 2-fold elevation in risk for cardiovascular death or heart failure (adjusted HR: 2.00; 95% CI: 1.28 to 3.14; p = 0.003) compared to those in quartiles 1 through 3. Furthermore, FGF-23 in the top quartile was an independent risk factor for cardiovascular death or heart failure even when evaluated in patients with normal renal function as defined by GFR ≥60 ml/min/1.73 m2 (HR: 2.54; 95% CI: 1.59 to 4.07; p < 0.0001), cystatin C <0.91 mg/l (HR: 2.26; 95% CI: 1.20 to 4.24; p = 0.011), or absence of microalbuminuria (HR: 2.20; 95% CI: 1.10 to 4.38; p = 0.025).
Figure 2. 6-Year Incidence Rates for the Composite of Cardiovascular Mortality or Heart Failure in Placebo Patients Stratified by FGF-23, and Either eGFR, Cystatin C, or Microalbuminuria.
Patients are categorized dichotomously according to whether their level of FGF-23 was in the top quartile (high) or not (low) and their eGFR was <60 ml/min/1.73 m2 or not (A) and their cystatin C level was in the top quartile (≥0.91 mg/l: high) or not (<0.91 mg/l: low) (B), and by the presence or absence of microalbuminuria (urinary albumin to creatinine ratio of ≥25 µg/mg in women and ≥17 µg/mg in men) (C). P values in figure represent global p value for differences in rates. Furthermore, for all pairwise comparisons p < 0.05. eGFR = estimated glomerular filtration rate; FGF = fibroblast growth factor.
We next assessed whether FGF-23 remained a significant predictor of risk after adjusting for well-established cardiovascular biomarkers, specifically NT-proBNP, hs-cTnT, and hs-CRP. Even after adding all 3 of these biomarkers to a model adjusted for the aforementioned clinical covariates and biomarkers of renal function, FGF-23 levels in the top quartile remained a significant independent predictor of cardiovascular death or heart failure (adjusted HR: 1.73; 95% CI: 1.09 to 2.74; p = 0.02). Midregional pro-atrial natriuretic peptide and midregional pro-adrenomedullin are alternative biomarkers of cardiovascular stress (21). Starting with a model that contained the aforementioned clinical covariates, biomarkers of renal function, and established cardiovascular biomarkers, applying a forward selection algorithm to FGF-23, midregional pro-atrial natriuretic peptide and midregional pro-adrenomedullin resulted in only FGF-23 achieving significance and entering and staying in the model (p = 0.02).
Interaction with trandolapril therapy
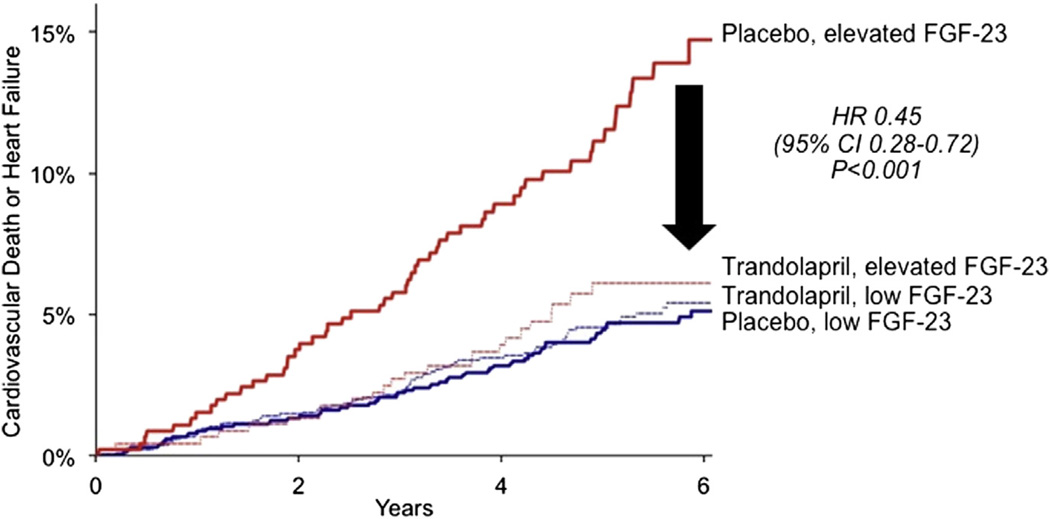
We observed a significant interaction between FGF-23 levels and the effect of trandolapril with respect to cardiovascular mortality or heart failure (p interaction = 0.0039). Among patients treated with trandolapril, there was not a gradient of risk with increasing FGF-23 levels, and consequently, among patients in the top quartile of FGF-23, trandolapril significantly reduced the risk of cardiovascular death or heart failure by 55% (HR: 0.45; 95% CI: 0.28 to 0.72) (Fig. 3), whereas no benefit was observed in patients with lower levels of FGF-23 (HR: 1.07; 95% CI: 0.75 to 1.52). Similar trends were observed for the individual risk of cardiovascular death and heart failure events (Online Table 4). The absolute risk reduction with ACE inhibitor therapy over 6 years among patients with SIHD in the highest risk category of FGF-23 was 8.62%, representing a number-needed-to-treat of 12 patients to prevent one additional cardiovascular death or incident heart failure. Furthermore, the gradient of clinical benefit with trandolapril defined by an elevated FGF-23 level was additive to that seen using renal function (Online Fig. 1) or biomarkers of cardiovascular stress (Online Fig. 2). There was no interaction among FGF-23 levels, trandolapril therapy, and atherothrombotic events (Online Table 5).
Figure 3. Cumulative Incidence Curves for the Composite of Cardiovascular Death or Heart Failure in Patients Categorized by FGF-23 Level and Treatment With Trandolapril.
Red lines indicate patients with high FGF-23 levels (>70.20 RU/ml): the solid line indicates patients treated with placebo (n = 455); and the dashed line indicates patients treated with trandolapril (n = 451). Blue lines indicate patients with low FGF-23 levels (≤70.20 RU/ml): the solid line indicates patients treated with placebo (n = 1,360); and the dashed line indicates patients treated with trandolapril (n = 1,361). CI = confidence interval; FGF = fibroblast growth factor; HR = hazard ratio.
Discussion
The results of this investigation support the hypothesis that higher levels of FGF-23 are associated with cardiovascular mortality and incident heart failure in patients with SIHD. Of note, akin to what has been observed for hs-CRP (25), risk was seen with plasma FGF-23 levels well within the observed range in the general population without known cardiovascular disease or renal impairment (7,15). We also show that FGF-23 provides incremental prognostic information even after adjusting for clinical risk factors, renal function, and cardiovascular biomarkers. Lastly, leveraging data from a randomized controlled trial, we demonstrate that patients with higher levels of FGF-23 received clinical benefit from ACE inhibitor therapy, independent of renal function.
FGF-23 is a phosphatonin that is synthesized and secreted by osteoblasts into the circulation. At normal levels, FGF-23 acts primarily in the kidney to maintain phosphate homeostasis by inducing urinary phosphate excretion (26,27). Elevated FGF-23 levels have previously been associated with progression of renal dysfunction and mortality in patients with CKD (4,5,28). However, when examining the association of FGF-23 levels with cardiovascular outcomes in patients without CKD, the results have been less clear. One study in 833 patients with SIHD did show an association between FGF-23 levels and mortality, but there was no adjustment for established cardiovascular biomarkers such as B-type natriuretic peptide and cardiac troponin (6). In studies of community-dwelling individuals with a low prevalence of or no known coronary disease, associations between FGF-23 levels and major adverse cardiovascular events were either absent (7) or severely attenuated after adjustment for renal function (8,9). To our knowledge, this is the first report to demonstrate that in patients with stable coronary disease, the adverse cardiovascular risk associated with higher levels of FGF-23 is independent of renal function, whether defined using eGFR, cystatin C, or microalbuminuria, and significant even in those with normal renal function as defined using the previously described markers. Moreover, we show that FGF-23 remains an independent prognostic factor even after adjusting for established cardiovascular biomarkers including NT-proBNP, hs-cTnT, and hs-CRP.
In addition to defining a population at high risk for adverse cardiovascular prognosis, the results of this study also support the hypothesis that elevated FGF-23 levels identify a population in whom ACE inhibitor therapy was effective at lowering this risk. These findings build on our previous work demonstrating that ACE inhibition yielded greater clinical benefits among patients with SIHD who have evidence of renal dysfunction defined as a low eGFR (11,12). Importantly, FGF-23 and eGFR offered additive stratification in terms of the clinical benefit of ACE inhibition, suggesting they provide complementary value.
There are several possible pathobiological explanations for the association between elevated circulating levels of FGF-23 and the risk of cardiovascular death and incident heart failure as opposed to atherothrombotic events. Elevated levels of FGF-23 may be an early marker of subclinical renal disease (1–3,28), and/or disrupted mineral homeostasis, each of which might lead to cardiovascular toxicity. However, given the observed prognostic significance independent of multiple established renal biomarkers, one must consider that FGF-23 may have direct links to the cardiovascular system. To that end, there are FGF receptors in the heart (29). The near absence of Klotho (a necessary coreceptor for FGF-23 in the distal renal tubule) (30,31) in the heart had previously led to the assumption that FGF-23 could not mediate any direct cardiac effects. Challenging that notion, however, investigators have shown that, via calcineurin/nuclear factor of activated T-cells pathways independent of Klotho, FGF-23 can induce myocyte hypertrophy and left ventricular hypertrophy in animal models (32,33). Furthermore, clinical studies have demonstrated that FGF-23 is independently associated with adverse left ventricular remodeling and is an independent predictor of survival in patients with established heart failure (32,34–38). Investigators have recently demonstrated the presence of Klotho in the human vasculature where it, and as a result, FGF-23 indirectly, appears to exert anticalcific effects (39,40). However, in our study there were only nonsignificant trends for an association between FGF-23 levels in the highest quartile and the risk of MI and stroke, consistent with weak or absent associations seen in other studies (6–8,10).
Data are also emerging for the link between FGF-23, Klotho, and the renin-angiotensin system. Specifically, angiotensin II negatively regulates Klotho expression (41), which, in turn, would result in a compensatory increase in FGF-23 levels. Furthermore, FGF-23 directly suppresses angiotensin-converting enzyme 2 (ACE2) expression (42). Unlike ACE, ACE2 is a negative regulator of the renin-angiotensin system, promoting vasodilation and natriuresis (43). Thus, one can speculate that patients with higher levels of angiotensin II would have relative suppression of Klotho and increased circulating FGF-23 levels, with resultant left ventricular hypertrophy, vascular stiffness, and ACE2-induced activation of the renin-angiotensin system, all of which would contribute to an increased risk of cardiovascular death or heart failure rather than contributing to progressive or unstable atherosclerotic plaque. ACE inhibition would then be particularly beneficial in this setting.
Study limitations
This analysis was performed in a selection of patients participating in a clinical trial rather than from the general population; however, the demographics and clinical characteristics of the cohort are typical for patients with SIHD. It should be noted that cardiovascular death and heart failure were not the primary endpoint of the parent clinical trial; however, they were the outcomes most strongly associated with FGF-23 levels in prior studies, have been shown to be reduced by ACE inhibition, and thus were the logical choice to examine for the prognostic value of FGF-23 and for a treatment interaction. Although we did not have data on parathyroid hormone, phosphate, calcium, or vitamin D levels, we controlled for eGFR, cystatin C, and urinary ACR, renal biomarkers strongly associated with these substances. Moreover, there is no clear evidence supporting an independent association between mineral levels and cardiovascular events, especially in patients without CKD (44). We do not have data on renin and angiotensin II levels, which are optimally assessed on fresh samples, and have no data on the modifiability of FGF-23 levels over time in this study. Major strengths of our study include its robust sample size and number of observed clinical events; detailed collection of subjects’ clinical and laboratory data; risk estimation with consideration of clinical risk factors and conventional markers of cardiac and renal function; and randomization of medical therapy allowing for an unbiased measure of clinical effectiveness stratified by baseline risk. Nevertheless, studies in other populations are welcome to confirm our findings.
Conclusions
Elevated levels of the phosphatonin FGF-23 were independently associated with cardiovascular death and incident heart failure in patients with SIHD. Trandolapril therapy significantly attenuated this relationship. These observations suggest this novel biomarker may be helpful in estimating future cardiovascular risk and help to predict the response to ACE inhibitor therapy in patients with SIHD.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the efforts of the PEACE patients, investigators, research coordinators, and committee members.
The PEACE trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI; N01HC65149) with support from Knoll Pharmaceuticals and Abbott Laboratories, which also provided the study medication. Dr. Udell was supported in part by a Postdoctoral Research Fellowship from the Canadian Institutes for Health Research (CIHR; Ottawa, Canada) and Canadian Foundation for Women’s Health (Ottawa, Canada). Dr. Sabatine was supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL094390. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Reagent for measurement of high-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide were provided by Roche Diagnostics; midregional pro-atrial natriuretic peptide, midregional proadrenomedullin, and C-terminal pro-endothelin-1 were provided by B.R.A.H.M.S. GmbH. Drs. Morrow, Hoffman, Braunwald, and Sabatine, and S. Sloan are members of the TIMI Study Group, which has received grant support from Amgen, AstraZeneca, Athera, Beckman Coulter, BG Medicine, Bristol-Myers Squibb, Buhlmann Laboratories, Daiichi Sankyo Co Ltd, Eli Lilly and Co, Esai, GlaxoSmithKline, Johnson & Johnson, Merck and Co., Nanosphere, Novartis Pharmaceuticals, Ortho-Clinical Diagnostics, Pfizer, Randox, Roche Diagnostics, Sanofi-Aventis, Siemens, and Singulex. Dr. Morrow has received grant support from Abbott Diagnostics, Beckman-Coulter, Nanosphere, Ortho-Clinical Diagnostics, Randox, Singulex, Amgen, Astra-Zeneca, Bristol-Myers-Squibb, Daiichi Sankyo, Esai, GlaxoSmithKline, Pfizer, Sanofi-Aventis, and Takeda; grants and personal fees from BG Medicine, Eli Lilly, Gilead, Johnson & Johnson, Merck & Company, Novartis, and Roche Diagnostics; and personal fees from Critical Diagnostics, Genentech, Instrumentation Laboratory, Konica-Minolta, and Servier outside the submitted work. Dr. Omland has received grants, personal fees, and nonfinancial support from Roche Diagnostics during the conduct of the study; grants, personal fees, and nonfinancial support from Abbott Laboratories; personal fees from Siemens Healthcare; and grants and nonfinancial support from AstraZeneca outside the submitted work. Dr. Pfeffer has received grant support from Amgen, Celladon, Novartis, and Sanofi-Aventis; consulting fees from Aastrom, Amgen, Bristol-Myers Squibb, Cerenis, Concert, Genzyme, Hamilton Health Sciences, Keryx, Medtronic, Merck and Co, Novartis, Roche Diagnostics, Servier, Teva, the University of Oxford, and Xoma; and is listed as a co-inventor on patents awarded to Brigham and Women’s Hospital regarding the use of inhibition of the renin-angiotensin system that are licensed to Boehringer Ingelheim and Novartis and are irrevocably transferred to charity. Dr. Braunwald has received grant support from Knoll Pharmaceuticals and Abbott Laboratories (as a supplement to the PEACE trial). Dr. Sabatine has received grant support from Abbott Laboratories, Accumetrics, Amgen, AstraZeneca, AstraZeneca/Bristol-Myers Squibb Alliance, BRAHMS GmbH, Bristol-Myers Squibb/Sanofi-Aventis Joint Venture, Critical Diagnostics, Daiichi-Sankyo, Eisai, Genzyme, GlaxoSmithKline, Intarcia, Merck, Nanosphere, Roche Diagnostics, Sanofi-Aventis, Takeda; and personal fees from Aegerion, Amgen, AstraZeneca/Bristol-Myers Squibb Alliance, Bristol-Myers Squibb/Sanofi-Aventis Joint Venture, Daiichi-Sankyo/Lilly, diaDexus, GlaxoSmithKline, Intarcia, Merck, Ortho-Clinical Diagnostics, Pfizer, Sanofi-Aventis, Vertex, and Zeus outside the submitted work.
Abbreviations and Acronyms
- ACE
angiotensin-converting enzyme
- ACR
albumin to creatinine ratio
- CI
confidence interval
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- FGF
fibroblast growth factor
- HR
hazard ratio
- hs-CRP
high-sensitivity C-reactive protein
- hs-cTnT
high-sensitivity cardiac troponin T
- MI
myocardial infarction
- NT-proBNP
N-amino terminal fragment of the prohormone B-type natriuretic peptide
- RU
reference units
- SIHD
stable ischemic heart disease
Footnotes
APPENDIX
For an expanded Methods section, and supplemental tables and figures, please see the online version of this article.
All other authors have reported they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 2.Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant. 2010;25:993–997. doi: 10.1093/ndt/gfp699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isakova T, Xie H, Yang W, et al. for the Chronic Renal Insufficiency Cohort (CRIC) Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor EN, Rimm EB, Stampfer MJ, Curhan GC. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. 2011;161:956–962. doi: 10.1016/j.ahj.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60:200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ärnlöv J, Carlsson AC, Sundstrom J, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. 2013;83:160–166. doi: 10.1038/ki.2012.327. [DOI] [PubMed] [Google Scholar]
- 10.Scialla JJ, Xie H, Rahman M, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokmakova MP, Skali H, Kenchaiah S, et al. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) study. Circulation. 2004;110:3667–3673. doi: 10.1161/01.CIR.0000149806.01354.BF. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, Rice MM, K AJ, et al. Prevention of Events with ACE inhibition (PEACE) Investigators. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation. 2006;114:26–31. doi: 10.1161/CIRCULATIONAHA.105.592733. [DOI] [PubMed] [Google Scholar]
- 13.Braunwald E, Domanski MJ, Fowler SE, et al. for the PEACE Trial Investigators. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Domanski M, Rosenberg Y, et al. Prevention of events with angiotensin-converting enzyme inhibition (the PEACE study design). Prevention of Events with Angiotensin-Converting Enzyme Inhibition. Am J Cardiol. 1998;82:25H–30H. doi: 10.1016/s0002-9149(98)00488-3. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Omland T, Sabatine MS, Jablonski KA, et al. Prognostic value of B-type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol. 2007;50:205–214. doi: 10.1016/j.jacc.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, Lin J, Solomon CG, et al. Prevention of Events With ACE Inhibition (PEACE) Investigators. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation. 2007;116:2687–2693. doi: 10.1161/CIRCULATIONAHA.107.723270. [DOI] [PubMed] [Google Scholar]
- 19.Sabatine MS, Morrow DA, Jablonski KA, et al. for the PEACE Investigators. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 20.Omland T, de Lemos JA, Sabatine MS, et al. for the Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabatine MS, Morrow DA, de Lemos JA, et al. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation. 2012;125:233–240. doi: 10.1161/CIRCULATIONAHA.111.063842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlmann EJ, Hock KG, Issitt C, et al. Reference intervals for plasma cystatin C in healthy volunteers and renal patients, as measured by the Dade Behring BN II System, and correlation with creatinine. Clin Chem. 2001;47:2031–2033. [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Pencina KM, Janssens ACJW, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–481. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Balk E, et al. for the National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 26.Larsson TE. The role of FGF-23 in CKD-MBD and cardiovascular disease: friend or foe? Nephrol Dial Transplant. 2010;25:1376–1381. doi: 10.1093/ndt/gfp784. [DOI] [PubMed] [Google Scholar]
- 27.Zisman AL, Wolf M. Recent advances in the rapidly evolving field of fibroblast growth factor 23 in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:335–342. doi: 10.1097/mnh.0b013e328338f536. [DOI] [PubMed] [Google Scholar]
- 28.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Pasumarthi KB, Padua RR, et al. Adult cardiomyocytes express functional high-affinity receptors for basic fibroblast growth factor. Am J Physiol. 1995;268:H1927–H1938. doi: 10.1152/ajpheart.1995.268.5.H1927. [DOI] [PubMed] [Google Scholar]
- 30.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 31.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 32.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Touchberry CD, Green TM, Tchikrizov V, et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304:E863–E873. doi: 10.1152/ajpendo.00596.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Seiler S, Cremers B, Rebling NM, et al. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J. 2011;32:2688–2696. doi: 10.1093/eurheartj/ehr215. [DOI] [PubMed] [Google Scholar]
- 37.Plischke M, Neuhold S, Adlbrecht C, et al. Inorganic phosphate and FGF-23 predict outcome in stable systolic heart failure. Eur J Clin Invest. 2012;42:649–656. doi: 10.1111/j.1365-2362.2011.02631.x. [DOI] [PubMed] [Google Scholar]
- 38.Poss J, Mahfoud F, Seiler S, et al. FGF-23 is associated with increased disease severity and early mortality in cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2013;2:211–218. doi: 10.1177/2048872613494025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim K, Lu TS, Molostvov G, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 40.Moe SM. Klotho: a master regulator of cardiovascular disease? Circulation. 2012;125:2181–2183. doi: 10.1161/CIRCULATIONAHA.112.104828. [DOI] [PubMed] [Google Scholar]
- 41.de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603–1609. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai B, David V, Martin A, et al. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One. 2012;7:e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boehm M, Nabel EG. Angiotensin-converting enzyme 2—a new cardiac regulator. N Engl J Med. 2002;347:1795–1797. doi: 10.1056/NEJMcibr022472. [DOI] [PubMed] [Google Scholar]
- 44.Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.