The endodermis acts as a barrier to solute and water movement but also has important functions in signaling and morphogenesis.
Abstract
The root endodermis is characterized by the Casparian strip and by the suberin lamellae, two hydrophobic barriers that restrict the free diffusion of molecules between the inner cell layers of the root and the outer environment. The presence of these barriers and the position of the endodermis between the inner and outer parts of the root require that communication between these two domains acts through the endodermis. Recent work on hormone signaling, propagation of calcium waves, and plant-fungal symbiosis has provided evidence in support of the hypothesis that the endodermis acts as a signaling center. The endodermis is also a unique mechanical barrier to organogenesis, which must be overcome through chemical and mechanical cross talk between cell layers to allow for development of new lateral organs while maintaining its barrier functions. In this review, we discuss recent findings regarding these two important aspects of the endodermis.
Soil contains water and dissolved nutrients needed for plant growth, but also holds pathogens and toxic compounds that can be detrimental to the plant. The root system, which is directly in contact with soil particles, can integrate environmental cues to adjust its development in order to optimize nutrient (Péret et al., 2011; Lynch, 2013) and water uptake (Cassab et al., 2013; Lynch, 2013; Bao et al., 2014) or avoid regions of high salinity (Galvan-Ampudia et al., 2013). Once anchored in the soil, roots must deal with the constraints of their local environment and develop specific barriers to balance uptake of nutrients, water, and interactions with symbionts with protection against detrimental biotic and abiotic factors.
In young roots, these barriers are mainly formed by the deposition of hydrophobic polymers such as lignin and suberin within the primary cell wall of the endodermis, which separates the pericycle from the cortex (Fig. 1), and of the exodermis, which lies between the cortex and the epidermis (Nawrath et al., 2013). Although formation of an exodermis is species dependent, the endodermis is a distinguishing figure of extant vascular plants (Raven and Edwards, 2001). Within this layer, two barriers (i.e. the Casparian strip and the suberin lamellae) are sequentially deposited and regulate water and nutrient movements between the inner and outer parts of the root. In this review, we discuss how the presence of these two major endodermal barriers affects communication between the different cell layers of the root. We focus on recent articles highlighting the importance of the endodermis in this communication during various biological and developmental processes.
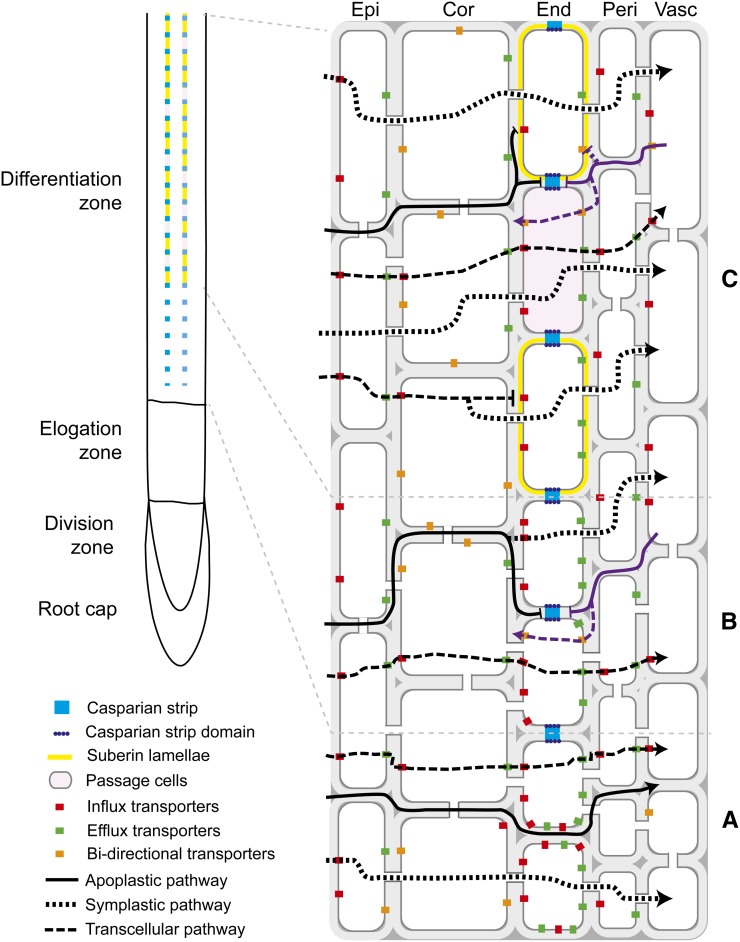
Figure 1.
Endodermal barriers affect radial movement of water and solutes through the root. A, At the root tip, to move from the soil to the outer tissues of the root and then into the stele, water and solute molecules can use either the apoplastic (black lines), symplastic (dotted lines), or transcellular (dashed lines) pathways. B, The deposition of the Casparian strip in the endodermis prevents the free apoplastic diffusion of molecules between the outer part and the inner part of the root forcing molecules to pass through the symplast of endodermal cells. C, The deposition of suberin lamellae prevents the uptake of molecules from the apoplast directly into the endodermis forcing molecules to enter the symplast from more outer tissue layers. Suberin deposition is also likely to prevent the backflow of water and ions out of the stele. Passage cells are unsuberized and may facilitate the uptake of water and nutrients in older parts of the root. Cor, Cortex; End, endodermis; Epi, epidermis; Peri, pericycle; Vasc, vasculature. Figure redrawn and modified from Geldner et al. (2013).
ENDODERMAL BARRIERS AS REGULATORS OF WATER AND NUTRIENT UPTAKE
Water and solutes move between the cell layers of the root using either the apoplast or the symplast (Fig. 1). The apoplast consists of the cell walls, extracellular spaces, and the lumen of tracheary elements, whereas the symplast is the continuum of cytoplasm between cells united through plasmodesmata (Steudle and Peterson, 1998). Solutes and water can also repeatedly cross plasma membranes and tonoplasts going from cell to cell to reach the stele, a transport route usually referred to as the directional transcellular pathway (Steudle and Peterson, 1998; Geldner, 2013). The symplastic and transcellular modes are difficult to distinguish experimentally and are usually referred to as the cell-to-cell pathway. Because molecules have to cross the plasma membrane at least once in this pathway, their flow is largely controlled by the presence, number, and gating of transporters localized at the plasma membranes. For example, the expression levels, localization, and gating of aquaporin water channels have been shown to play a major role in controlling water flow within the root (Chaumont and Tyerman, 2014). However, the plasma membrane is not a perfect barrier against entry of undesirable compounds into the symplast; passive diffusion of various molecules through phospholipid bilayers can occur and movement through membrane transporters is not always specific to a single molecular species. If harmful compounds enter the symplast through these pathways, they can later be transported out of the cells or sequestered in vacuoles, preventing them from being further transported to the stele (Schaaf et al., 2006; Miwa et al., 2007). Such control does not exist in the apoplast, where molecules likely diffuse freely depending on concentration gradients or are carried by bulk water flow driven by transpiration (Enstone et al., 2002). The mobility of some ions, such as calcium, can be affected by the composition of the extracellular pectocellulosic matrix (Gilliham et al., 2011).
As the root differentiates, the free apoplastic diffusion of solutes between the outer cell layers and the vasculature is prevented by the formation of an apoplastic barrier in the extracellular matrix of the endodermis called the Casparian strip. The Casparian strip is a highly localized lignin impregnation (Naseer et al., 2012) of the primary cell wall that forms a longitudinal belt-like structure in the middle of the anticlinal walls of endodermal cells (Fig. 1). This deposition is coordinated within the whole layer to form a supracellular network that completely isolates the apoplast of the stele from the apoplast of the outer tissue layers. The role of the Casparian strip as a barrier for solutes and ions has been suggested by the absence of diffusion of fluorescent dyes beyond the Casparian strip into the stele (Alassimone et al., 2010), by the accumulation of salts at the cortical side of the Casparian strip (Nagahashi et al., 1974; Alassimone et al., 2012), and by the drop in root pressure observed after puncturing the endodermis (Peterson et al., 1993; Steudle and Peterson, 1998). However, it is still unclear how well water and small dissolved solutes can move through the Casparian strip (Steudle and Peterson, 1998; Steudle, 2000; Geldner, 2013). In absence of apoplastic water flow directly through the Casparian strip, aquaporins could provide a route for water uptake, and their transcriptional and posttranslational regulation could allow for rapid control of water flow across the endodermis into the stele (Bramley et al., 2007). This model is supported by the high expression levels of some aquaporin isoforms in the endodermis (Chaumont and Tyerman, 2014).
The molecular actors involved in the local deposition of the Casparian strip in endodermal cells have just begun to be discovered (Roppolo et al., 2011; Hosmani et al., 2013; Lee et al., 2013). The Casparian strip membrane domain proteins accumulate in the subdomain of the plasma membrane where the Casparian strip will form and likely guide the formation of a protein-rich membrane domain, termed the Casparian strip domain (CSD; Roppolo et al., 2011). The CSD anchors the plasma membrane to the cell wall (Alassimone et al., 2010) and recruits the enzymes necessary for lignin biosynthesis (Hosmani et al., 2013; Lee et al., 2013). Interestingly, it has been shown that membranous proteins cannot diffuse freely within this region (Alassimone et al., 2010). As a result, the CSD creates two distinct and exclusive plasma membrane domains, possibly reinforcing the polarized localization of several ion transporters observed at earlier stages of endodermal differentiation (Ma et al., 2006, 2007; Alassimone et al., 2010). The asymmetric distribution of influx and efflux carriers at endodermal plasma membranes associated with the apoplastic barrier provided by the Casparian strip ensures control of solute movement from the outer tissue layers to the vasculature and prevents the backflow of ions from the stele to the apoplast of the cortex when transpiration is low (Steudle and Peterson, 1998). However, because of the bidirectionality of water transport through aquaporins, backflow of water to the outer part of the root could still occur under unfavorable conditions such as in dry soils (Bramley et al., 2007). Tight regulation of aquaporin expression levels, localization, and gating is likely essential to balance uptake and loss of water in such conditions (Bramley et al., 2007).
After the formation and maturation of the Casparian strip, the endodermis can undergo a second stage of differentiation, which consists of the deposition of a hydrophobic secondary cell wall lying between the primary cell wall and the plasma membrane that completely covers the cell surface (Fig. 1). These lamellae are made of suberin, an aliphatic polyester highly resistant to degradation, but they likely do not function as efficient apoplastic barriers (Geldner, 2013). Indeed, in the enhanced suberin1 (esb1) mutant, which is defective in Casparian strip formation, the free diffusion of propidium iodide into the stele is not blocked as early as in wild-type plants. However, this defect cannot be compensated by the strong ectopic deposition of suberin observed in the endodermis relatively close to the root tip in these genetic backgrounds (Hosmani et al., 2013). This suggests that the Casparian strip and suberin lamellae do not have interchangeable functions. Instead, suberin lamellae might prevent the uptake of solutes and water from the apoplast directly into endodermal cells, forcing solutes to use the symplastic pathway from more outer tissue layers. Geldner (2013) proposed that the outer tissues essentially increase the absorptive surface area of the root and funnel solutes to the endodermis through the symplast. Thus, restricting the uptake of solutes from the apoplast by the endodermis may have a low impact on nutrient uptake if entry into the symplast initially occurs at outer tissue layers. Conversely, water movement should be strongly affected by the deposition of suberin lamellae, provided that entry of water into the symplast occurs primarily at the endodermis, and that flow through plasmodesmata is slower than flow through endodermal plasma membranes. Suberin deposition may also be important for preventing bulk movement of water out of the stele. This role is consistent with observations that suberin deposition can be enhanced in more mature regions of the root (Naseer et al., 2012) and in response to environmental stress (Chen et al., 2011). However, experimental results regarding the importance of the suberin lamellae in controlling the radial hydraulic conductivity of roots have been variable, with both positive and negative effects observed (Steudle and Peterson, 1998; Baxter et al., 2009; Ranathunge and Schreiber, 2011). Overall, the effect of the suberin lamellae on solutes and water movement is likely dependent on relative transport capabilities of the endodermis compared with more outer tissue layers.
It has been suggested that the participation of the endodermis in nutrient uptake can be particularly important in the regions of the root where Casparian bands have formed but suberin lamellae are not yet established (Geldner, 2013). However, it is worth noting that secondary cell walls are not deposited in every endodermal cell (Naseer et al., 2012). Patchy patterns observed with fluorol yellow staining or with expression of GUS reporters of suberin biosynthesis genes point to the existence of passage cells that lack secondary cell wall deposition in the proximity of xylem poles (Wu et al., 2011; Naseer et al., 2012). These passage cells may be hubs of endodermal nutrient uptake (Geldner, 2013). For example, PHOSPHATE1 (PHO1) phosphate transporters are expressed in individual endodermal cells that might correspond to passage cells adjacent to protoxylem (Hamburger et al., 2002). The concomitant use of PHO1:GUS and suberin staining would be necessary to clarify the importance of passage cells in phosphate transport to the stele. The expression of aquaporins in passage cells has not yet been analyzed to our knowledge, so it is still unclear whether passage cells are important contributors to water uptake in the more mature region of the root.
Once the Casparian strip and suberin lamellae are deposited, molecules such as nutrients, ions, and hormones have to pass through the endodermis cytoplasm to move between the inner and outer parts of the root, making this cell layer a potential hub for receiving and transferring signals between cell layers. This function of the endodermis was recently reviewed (Dinneny, 2014) and is particularly important during biotic and abiotic stresses.
ENDODERMIS AS A COMMUNICATION CENTER DURING ROOT RESPONSES TO THE EXTERNAL ENVIRONMENT
The function of diffusion barriers in the endodermis is especially important when considering the uptake of toxic solutes into the root. In high-saline environments, the endodermis limits free apoplastic diffusion of sodium ions into the vascular stream. This leads to the accumulation of sodium ions in tissues peripheral to the endodermis (Møller et al., 2009). Under drought conditions, suberization in the endodermis can be accelerated and may prevent the desiccation of inner tissue layers (Enstone et al., 2002; Henry et al., 2012). Thus, the Casparian strip and suberin lamellae not only prevent uptake of toxic compounds but may also prevent the loss of water under unfavorable environments (Soukup et al., 2007; Baxter et al., 2009; Chen et al., 2011).
Because of its role in solute and water transport, the endodermis may act as a signaling center during the perception of stressful environmental conditions and subsequent regulation of growth and development. Recent studies have highlighted the importance of the endodermis in regulating root growth under salt stress through abscisic acid (ABA) signaling. Duan et al. (2013) reveal that the postemergence growth of lateral roots is strongly suppressed during salt stress in Arabidopsis (Arabidopsis thaliana). ABA signaling is specifically induced in these quiescent lateral roots and is necessary for growth inhibition. A GALACTOSE4/Virion Protein 16-Upstream Activation Sequence transactivation system (Haseloff and Hodge, 2001) was used to express the mutant protein abi1-1 in different tissue types to interrupt ABA signaling in a spatially restricted manner. Endodermal ABA signaling was found to be most important to inhibit lateral root growth during salt stress. Surprisingly, although suppression of ABA signaling allowed lateral roots to bypass growth quiescence, growth was not prolonged indefinitely and eventually these roots senesced, suggesting that other aspects of salinity tolerance were not properly regulated (Duan et al., 2013). Primary roots enter a similar, but more short-lived quiescent phase when seedlings are treated with high concentrations of salt; however, in this case, endodermal ABA signaling promotes growth recovery, perhaps as a result of interactions with the ethylene-signaling pathway (Sharp, 2002; Geng et al., 2013). ABA-responsive genes are up-regulated in all tissue layers of the root under salt stress (Dinneny et al., 2008); thus, the downstream targets of ABA must somehow be unique in the endodermis in order to regulate growth.
Calcium is a versatile signaling component involved in regulating multiple physiological and developmental responses such as stomatal opening (Allen et al., 2001) and root hair elongation (Ehrhardt et al., 1996; Wymer et al., 1997). Through the induction of rapid changes in cytosolic calcium concentration, cells use calcium to signal environmental stresses such as cold (Knight et al., 1996; Carpaneto et al., 2007), drought, salt stress (Knight et al., 1997), and biotic stress (Blume et al., 2000; Navazio et al., 2007). To quantify changes in cytosolic calcium concentration, calcium-binding proteins have been identified and used to develop Förster resonance energy transfer-based sensors (Horikawa et al., 2010). Choi et al. (2014) recently utilized a high-affinity calcium sensor in Arabidopsis to characterize a calcium-signaling wave, which propagates rapidly from a point of local salt treatment to the rest of the root system. Intriguingly, upon localized salt treatment at a lateral root tip, the signal travels to the rest of the root system through the cortex and endodermis at speeds of up to 400 μm/s, eventually reaching the shoot (Choi et al., 2014). Activation of stress-responsive genes was detected within 1 to 2 min in the tissue directly treated with salt, and was detected within 10 min in the aerial part of the plant. The transcriptional regulation of some of these genes can be affected by applying lanthanum (III) chloride, which inhibits calcium channels. This suggests that the calcium wave plays an important role in systemic responses under salt stress. Among these stress-induced genes, a vacuole channel protein, TWO-PORE CHANNEL1 (TPC1), shows very rapid and transient induction in the shoot within 1 min of treatment and is also involved in determining the speed with which the calcium wave propagates (Choi et al., 2014). This study provides an interesting mechanism for rapid and long-distance signaling after localized salt exposure. With the rapid propagation of calcium signals from a localized stressed region to the rest of the root system and further to the shoot, plants can trigger systemic stress-responsive programs as a preventive mechanism against further damage from stressful environments.
These observations highlight a potentially important role of the endodermis in calcium signaling and downstream responses under salt stress, which is supported by the work done by Kiegle et al. (2000). By expressing a calcium reporter, aequorin, in specific cell types, Kiegle et al. (2000) discovered that prolonged cytosolic calcium oscillation occurs in the endodermis and pericycle upon osmotic or salt-stress treatment. It will be interesting to examine tissue-specific functions of TPC1 to determine whether the roles of cortical and endodermal calcium signaling can be differentiated. Whether suberin deposition in the endodermis affects the rate of signal propagation by affecting the flow of calcium from the apoplast to endodermal cells would also be intriguing to determine. In addition, calcium signaling in guard cells has been shown to enhance ABA regulation of stomatal movements (Allen et al., 1999; Webb et al., 2001). Experiments to determine whether similar cross talk occurs between these pathways during regulation of root growth may be fruitful.
Aside from abiotic stresses, the plant root system also faces challenges and opportunities from microorganisms in soil. The Casparian strip and suberin lamellae can function as physical barriers to exclude pathogenic microbes (Bernards, 2002; Schreiber and Franke, 2011), but the root is also capable of forging symbioses with beneficial microbes to assist in nutrient acquisition. One of the most common of such symbioses occurs between plants and arbuscular mycorrhizae (AM). Penetrating fungal hyphae differentiate and form a branch-like structure with a large membrane interface in the root cortex (Genre et al., 2008). This network of hyphae indirectly increases the contact area between the root system and the soil, which facilitates the uptake of water and nutrients by the plant. Reciprocally, the fungi obtain carbon resources from the phloem of the root (Parniske, 2008). Here, the endodermis may function to monitor selective nutrient exchange processes between the cortex and vascular tissue; therefore, signaling events in the endodermis may be important to facilitate communication between the root and fungal symbionts.
AM development is greatly dependent on plant-derived signals. For example, an endogenous plant hormone, strigolactone, has been identified as an important branching factor for fungal hyphae (Akiyama et al., 2005; Besserer et al., 2006). In petunia (Petunia hybrida), the identification of a strigolactone transporter, PLEIOTROPIC DISEASE RESISTANCE-LIKE1, provides a mechanism to guide the entry of arbuscular hyphae through hypodermal passage cells (Kretzschmar et al., 2012). Recent work suggested that strigolactone signaling may have endodermis-specific functions during the regulation of root branching (Koren et al., 2013). It will be interesting for future studies to determine whether such endodermal-specific strigolactone signaling is also necessary for AM infection.
Gibberellins (GAs), another class of plant hormones, may also have endodermis-specific roles in controlling root development and AM colonization. In Arabidopsis, fluorescently labeled GA derivatives predominantly accumulate in elongating endodermal cells in the root (Shani et al., 2013) and DELLA proteins, which antagonize GA signaling, function in the endodermis to suppress primary root elongation and meristem size (Ubeda-Tomás et al., 2008, 2009). DELLA proteins may also interact with ABA signaling in the endodermis to suppress the postemergence growth of lateral roots under salt stress (Duan et al., 2013). GA was recently found to inhibit AM colonization, and DELLA proteins are important in this process (Foo et al., 2013; Yu et al., 2014). Indeed, Floss et al. (2013) recently reported that AM formation in the root cortex is strongly suppressed in the Medicago truncatula double mutant della1/della2. Surprisingly, fungal hyphae are still able to absorb nutrients from the mutant plant, but fail to produce spores and complete their life cycle (Floss et al., 2013). By expressing the dominant mutant protein della1-Δ18 under both the native promoter of MtDELLA1 and Mt PHOSPHATE TRANSPORTER9, a phosphate transporter, the AM defect in the della1/della2 double mutant can be rescued. In both situations, the mutant protein is mainly localized in the nuclei of cells of the endodermis and stele, indicating a possible role of the endodermis in regulating AM formation in the cortex (Floss et al., 2013).
The above examples illustrate the importance of the endodermis in coordinating signals to regulate root growth and responses to biotic and abiotic stimuli. This tissue layer also plays a central role in mediating cross talk between different cell layers during development of new lateral organs.
ENDODERMIS AS A BARRIER AND FACILITATOR OF LATERAL ORGAN MORPHOGENESIS
The root system of higher plants is a highly branched network, and new lateral branches are initiated deep within the inner tissue layers of the parent root. In many dicot species, lateral root primordia are derived from the pericycle, a layer of cells that lies immediately internal to the endodermis. As primordia develop and mature, they must pass through overlying tissue layers in order to emerge from the parent root and enter the soil environment. This process must be highly regulated in order to allow the primordium to emerge without damaging the parent root and significantly compromising barriers provided by the endodermis.
During lateral root emergence in Arabidopsis, enzymatic wall loosening takes place in the epidermis and cortex to allow developing primordia to push aside overlying cells as they expand and elongate. However, recent work suggests that the endodermis behaves differently during this process. Simple separation of endodermal cells would likely compromise the diffusion barrier provided by this layer, so it is likely that alternative mechanisms are employed to allow lateral root primordia to emerge through it. A common theme for all overlying tissue layers is a role of auxin signaling in setting cellular modifications in motion. The auxin influx carrier LIKE AUX13 (LAX3) was shown to be critical for proper emergence of lateral root primordia based on the decreased number of emerged lateral roots in lax3-null mutants (Swarup et al., 2008). This transporter is expressed in cortical and epidermal cells immediately overlying developing primordia and is required for expression of wall-remodeling enzymes by these cells (Fig. 2).
Figure 2.
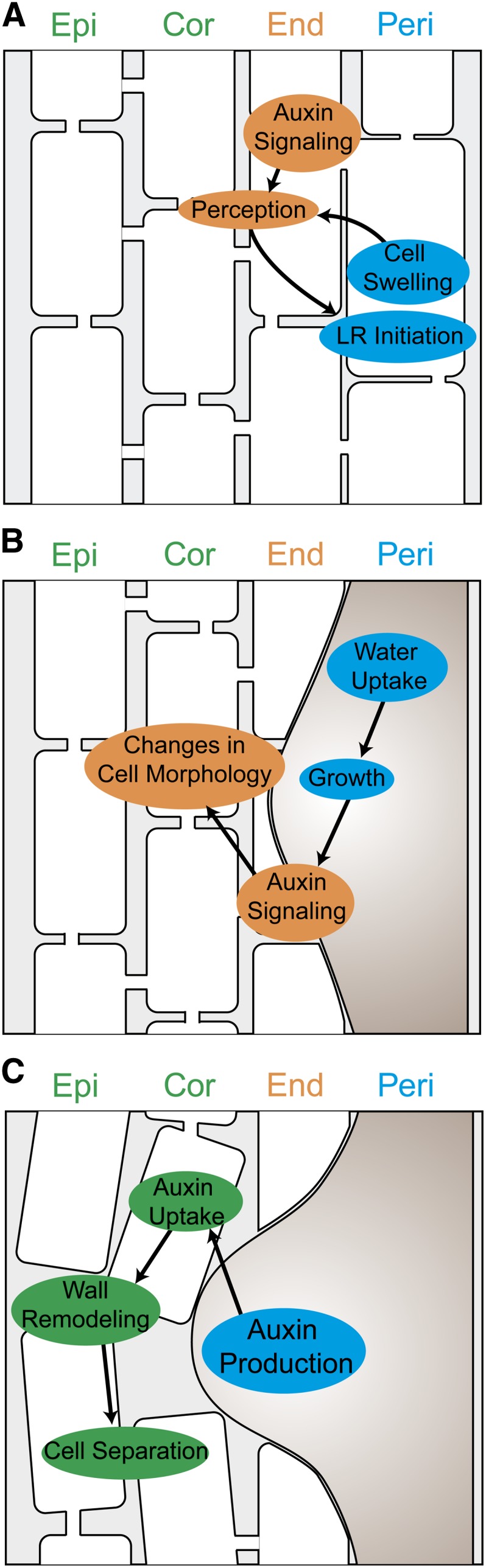
Mechanical and auxin signals mediate cell-to-cell communication during lateral root development. A, Cell swelling in the pericycle sends a mechanical signal to the endodermis. The endodermis responds in an auxin-dependent manner to allow the pericycle cell to undergo the first asymmetric division marking lateral root initiation. B, The endodermis responds to turgor-driven growth of the developing lateral root primordium by undergoing auxin-dependent cell morphological changes. These changes allow the primordium to pass through the endodermis without creating significant apoplastic gaps between the primordium and neighboring endodermal cells. C, Auxin produced by the developing primordium is taken up by overlying cells in the cortex and endodermis through the transporter LAX3. Auxin signaling in these cells then activates expression of cell wall-remodeling enzymes that allow the cells to separate from one another as the primordium pushes past them. Events are color coded according to the tissue layer they occur in. Cor, Cortex; End, endodermis; Epi, epidermis; LR, lateral root; Peri, pericycle.
Auxin signaling is also activated in endodermal cells overlying developing primordia, and has been linked to induction of wall-remodeling enzymes such as PECTIN LYASE2 and GLYCOSYL HYDROLASE17 (Swarup et al., 2008). However, these enzymes lack the ability to degrade the lignified Casparian strip. Vermeer et al. (2014) show that cells of the endodermis instead undergo dramatic morphological changes to accommodate growth of new lateral roots (Fig. 2). The cells become thinner and flatter in regions in contact with developing primordia, accompanied by small, localized breaks in the Casparian strip. These modifications cause the endodermal cells to press tightly around the base of the developing primordium, allowing for emergence without creating significant gaps in the diffusion barrier that would occur if the cells completely separated from one another.
Auxin signaling in the endodermis is necessary for the morphological changes that occur to accommodate developing primordia. This was demonstrated through endodermis-specific expression of short hypocotyl2-2 (shy2-2), which encodes a dominant-negative allele of an AUXIN RESISTANT/INDOLE-3-ACETIC ACID INDUCIBLE (Aux/IAA) protein normally expressed in the endodermis, hypocotyl, and other tissues (Vermeer et al., 2014). Lateral root primordia and emerged lateral roots are completely absent from plants expressing this transgene, implying that the block occurs very early in development. Although exogenous auxin treatment partially rescues this phenotype, developing primordia appear to be flatter and more elongated, and emerge at a much lower frequency than those of wild-type plants. These morphological defects could be attributable to an imbalance of mechanical forces between the primordium and overlying tissue layers during development.
The primordium must generate a force to push past the cells of the outer tissue layers, and these layers must make modifications that allow them to yield to this force (Fig. 2). Accordingly, altering either side of this coordinated interaction leads to developmental defects. The force that allows primordia to push past overlying cells arises as a result of water uptake by the cells of the primordia, which causes them to expand and maintain turgor pressure. Thus, it was proposed that water movement would be a critical factor in mediating the outgrowth of new lateral roots. Péret et al. (2012) tested this hypothesis through a combination of mathematical modeling and reverse genetic approaches. Predictions made using the model were tested empirically through misexpression of the aquaporin PLASMA MEMBRANE INTRINSIC PROTEIN2;1. Both overexpression and null mutations of this gene lead to delays in lateral root emergence and flattening of developing primordia. Similar defects are observed when the ability of overlying tissues to yield to the force from primordia is disrupted. This was demonstrated by blocking auxin perception in tissues outside of developing primordia, which prevents these cells from undergoing auxin-dependent changes to their morphology and/or cell wall structure (Lucas et al., 2013). An inducible enhancer-trap system was used to express auxin resistant3-1 in all root cells outside of developing primordia. This gene encodes a dominant-negative Aux/IAA protein that leads to constitutive suppression of the auxin-signaling pathway. Expression of this transgene leads to delayed emergence coupled with flattening of primordia, similar to what is observed with misexpression of aquaporins during lateral root development. Interestingly, the expression of aquaporin genes is tightly regulated by auxin during lateral root emergence (Péret et al., 2012), implicating a role for this hormone both in the generation of force by the primordium and the yielding to this force by outer tissue layers.
How can signaling from one hormone lead to different outcomes in different tissue layers? In the way that ABA plays tissue-specific roles during salt stress (Duan et al., 2013), auxin apparently acts in multiple tissue layers during lateral root development, serving to generate a mechanical force in some layers while decreasing resistance to mechanical force elsewhere. It is likely that other conditions or factors that are unique to each cell type give rise to these tissue-specific responses. For instance, cell wall loosening is activated by auxin to allow the cells in the outer tissue layers to separate as lateral root primordia grow. Cell wall loosening may also play a role in primordia themselves, since wall relaxation is limiting in turgor-mediated cellular expansion (Cosgrove, 1993; Schopfer, 2006). Perhaps it is the water status of these cells and the rate they can take up water that distinguish developing primordia from overlying tissues during lateral root development.
It appears that mechanical interactions between lateral root primordia and surrounding tissue layers are important for proper morphogenesis, and the endodermis may play a central role in this mechanical cross talk. Blocking auxin signaling in the endodermis through misexpression of shy2-2 has effects on development preceding lateral root initiation (Vermeer et al., 2014). Cells of the pericycle increase in volume immediately preceding the first asymmetric cell division that marks lateral root initiation, a process that requires endodermal auxin signaling (Vermeer et al., 2014). It was proposed that resistance of the endodermis to pericycle cell swelling might be perceived by the pericycle cell and used to gauge the level of mechanical resistance provided by the endodermis. The endodermis would then respond to this force by undergoing the morphological adjustments necessary to accommodate the developing primordium (Fig. 2). The molecular mechanisms by which plant cells sense such mechanical forces remain to be elucidated, although numerous efforts have been made to address this issue (Hamant, 2013).
Although considerable progress has been made on the role of the endodermis in lateral root emergence in Arabidopsis, relatively few studies have been done beyond this species. The endodermis plays a more active role in the generation of new lateral roots in many species. In maize (Zea mays) and other cereal crops, the lateral root cap and epidermis of new lateral roots are of endodermal origin (Orman-Ligeza et al., 2013). Histology of developing lateral root primordia in maize also suggests that the lignin in the walls of the endodermal cells that contribute to the primordium is degraded (Karas and McCully, 1973). However, it is unclear how this delignification occurs and whether there are any mechanisms employed by the plant to compensate for this localized breakdown of the apoplastic barrier. Suberin deposition has been observed in endodermal cells surrounding lateral root primordia in Arabidopsis (Martinka et al., 2012); such a modification could serve to provide some level of protection in species in which endodermal delignification takes place. Many species also employ programmed cell death in outer tissue layers to clear a path for emergence of developing primordia. The effects of such processes on maintaining endodermal diffusion barriers, if any, remain unexplored.
CONCLUSION
It is generally accepted that the endodermis protects the inner part of the root from the external environment by developing both an apoplastic diffusion barrier (the Casparian strip) and later a barrier to the movement of molecules directly from the apoplast into the endodermis (the suberin lamellae). Nevertheless, many mysteries remain concerning the precise effects that these barriers have on the flow of water and solutes. Despite the fact that these barriers differentially affect the movement of molecules through the endodermis, the Casparian strip and the suberin lamellae are not always well distinguished when studies have analyzed specific biological functions of the endodermis. A more rigorous and systematic characterization of the developmental progression of the endodermis should be made for this purpose. Mutants specifically affecting one barrier would be useful, but as highlighted with the esb1 mutant (Hosmani et al., 2013), cross talk between lignin and suberin deposition might make this kind of approach difficult. Regarding solute movement, it is likely that different molecules will preferentially use different paths and their movement is also likely dependent on the age of the root, the species under study, and environmental conditions. All of these parameters should be brought together to synthesize a more holistic understanding of solute flow in roots.
The endodermis is also a useful signaling center, mediating interactions between the root and its environment as well as between different cell types. An important question in this regard is how the endodermis perceives various inputs that must be transduced to affect biological processes. Regarding high salinity, the activation and rapid propagation of calcium signaling could serve as a mechanism. Hormone intermediates, such as auxin and ABA, also provide a means of communication during development and environmental stress. In addition, recent work points to an important role for perception of mechanical cues in mediating cross talk between neighboring cell layers. The responses of the endodermis to this wide array of signals appear to be central to a number of biological processes, including root-microbe interactions and the morphogenesis of new lateral organs. However, many of these signals are activated and perceived in multiple tissue layers. What molecular properties of the endodermis allow it to generate unique responses compared with other cell types? In addition, many of these signaling processes, such as long-distance propagation of calcium waves and stress-induced calcium oscillations, appear to involve the endodermis as well as tissue layers that neighbor it. Is this simply a consequence of symplastic connections between these cells and the endodermis, or do the actions of these cells have functional importance as well? The utilization of tissue-specific genetic tools will be of great utility in addressing these questions.
The role of the endodermis as a center for signaling during development and environmental stress has become increasingly apparent. Future work into the basic biology of this protective barrier layer and its functions in signaling will provide a greater understanding of the mechanisms underlying how plants perceive and respond to their external environment to regulate internal physiology and development.
Acknowledgments
We thank members of the Dinneny laboratory for helpful comments and suggestions on the article.
Glossary
- CSD
Casparian strip domain
- ABA
abscisic acid
- AM
arbuscular mycorrhizae
Footnotes
This work was supported by the Carnegie Institution for Science Endowment (funding to J.R.D.) and a National Science Foundation Graduate Research Fellowship (grant no. DGE–1147470 to N.E.R.).
References
- Akiyama K, Matsuzaki K, Hayashi H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Alassimone J, Naseer S, Geldner N. (2010) A developmental framework for endodermal differentiation and polarity. Proc Natl Acad Sci USA 107: 5214–5219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alassimone J, Roppolo D, Geldner N, Vermeer JE. (2012) The endodermis: development and differentiation of the plant’s inner skin. Protoplasma 249: 433–443 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. (1999) Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11: 1785–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Aggarwal P, Robbins NE, 2nd, Sturrock CJ, Thompson MC, Tan HQ, Tham C, Duan L, Rodriguez PL, Vernoux T, et al. (2014) Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc Natl Acad Sci USA 111: 9319–9324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE. (2009) Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5: e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards MA. (2002) Demystifying suberin. Can J Bot 80: 227–240 [Google Scholar]
- Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Bécard G, Séjalon-Delmas N. (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol 4: e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume B, Nürnberger T, Nass N, Scheel D. (2000) Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12: 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley H, Turner DW, Tyerman SD, Turner NC. (2007) Water flow in the roots of crop species: the influence of root structure, aquaporin activity, and waterlogging. Adv Agron 96: 133–196 [Google Scholar]
- Carpaneto A, Ivashikina N, Levchenko V, Krol E, Jeworutzki E, Zhu JK, Hedrich R. (2007) Cold transiently activates calcium-permeable channels in Arabidopsis mesophyll cells. Plant Physiol 143: 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassab GI, Eapen D, Campos ME. (2013) Root hydrotropism: an update. Am J Bot 100: 14–24 [DOI] [PubMed] [Google Scholar]
- Chaumont F, Tyerman SD. (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164: 1600–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Cai X, Wu X, Karahara I, Schreiber L, Lin J. (2011) Casparian strip development and its potential function in salt tolerance. Plant Signal Behav 6: 1499–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S. (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111: 6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (1993) Wall extensibility: its nature, measurement and relationship to plant cell growth. New Phytol 124: 1–23 [DOI] [PubMed] [Google Scholar]
- Dinneny JR. (2014) A gateway with a guard: how the endodermis regulates growth through hormone signaling. Plant Sci 214: 14–19 [DOI] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. (2008) Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320: 942–945 [DOI] [PubMed] [Google Scholar]
- Duan L, Dietrich D, Ng CH, Chan PMY, Bhalerao R, Bennett MJ, Dinneny JR. (2013) Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 25: 324–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. (1996) Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85: 673–681 [DOI] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA, Ma F. (2002) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21: 335–351 [Google Scholar]
- Floss DS, Levy JG, Lévesque-Tremblay V, Pumplin N, Harrison MJ. (2013) DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 110: E5025–E5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Ross JJ, Jones WT, Reid JB. (2013) Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Ann Bot (Lond) 111: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, Julkowska MM, Darwish E, Gandullo J, Korver RA, Brunoud G, Haring MA, Munnik T, Vernoux T, Testerink C. (2013) Halotropism is a response of plant roots to avoid a saline environment. Curr Biol 23: 2044–2050 [DOI] [PubMed] [Google Scholar]
- Geldner N. (2013) The endodermis. Annu Rev Plant Biol 64: 531–558 [DOI] [PubMed] [Google Scholar]
- Geng Y, Wu R, Wee CW, Xie F, Wei X, Chan PMY, Tham C, Duan L, Dinneny JR. (2013) A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 25: 2132–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Faccio A, Barker DG, Bonfante P. (2008) Prepenetration apparatus assembly precedes and predicts the colonization patterns of arbuscular mycorrhizal fungi within the root cortex of both Medicago truncatula and Daucus carota. Plant Cell 20: 1407–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliham M, Dayod M, Hocking BJ, Xu B, Conn SJ, Kaiser BN, Leigh RA, Tyerman SD. (2011) Calcium delivery and storage in plant leaves: exploring the link with water flow. J Exp Bot 62: 2233–2250 [DOI] [PubMed] [Google Scholar]
- Hamant O. (2013) Widespread mechanosensing controls the structure behind the architecture in plants. Curr Opin Plant Biol 16: 654–660 [DOI] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y. (2002) Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14: 889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff JP, Hodge S (2001) Gene expression. In Google Patents [Google Scholar]
- Henry A, Cal AJ, Batoto TC, Torres RO, Serraj R. (2012) Root attributes affecting water uptake of rice (Oryza sativa) under drought. J Exp Bot 63: 4751–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K, Yamada Y, Matsuda T, Kobayashi K, Hashimoto M, Matsu-ura T, Miyawaki A, Michikawa T, Mikoshiba K, Nagai T. (2010) Spontaneous network activity visualized by ultrasensitive Ca(2+) indicators, yellow Cameleon-Nano. Nat Methods 7: 729–732 [DOI] [PubMed] [Google Scholar]
- Hosmani PS, Kamiya T, Danku J, Naseer S, Geldner N, Guerinot ML, Salt DE. (2013) Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root. Proc Natl Acad Sci USA 110: 14498–14503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas I, McCully ME. (1973) Further studies of the histology of lateral root development in Zea mays. Protoplasma 77: 243–269 [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR. (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23: 267–278 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Koren D, Resnick N, Mayzlish Gati E, Belausov E, Weininger S, Kapulnik Y, Koltai H. (2013) Strigolactone signaling in the endodermis is sufficient to restore root responses and involves SHORT HYPOCOTYL 2 (SHY2) activity. New Phytol 198: 866–874 [DOI] [PubMed] [Google Scholar]
- Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E. (2012) A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483: 341–344 [DOI] [PubMed] [Google Scholar]
- Lee Y, Rubio MC, Alassimone J, Geldner N. (2013) A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412 [DOI] [PubMed] [Google Scholar]
- Lucas M, Kenobi K, Von Wangenheim D, Voß U, Swarup K, De Smet I, Van Damme D, Lawrence T, Péret B, Moscardi E. (2013) Lateral root morphogenesis is dependent on the mechanical properties of the overlaying tissues. Proc Natl Acad Sci USA 110: 5229–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot (Lond) 112: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. (2006) A silicon transporter in rice. Nature 440: 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. (2007) An efflux transporter of silicon in rice. Nature 448: 209–212 [DOI] [PubMed] [Google Scholar]
- Martinka M, Dolan L, Pernas M, Abe J, Lux A. (2012) Endodermal cell-cell contact is required for the spatial control of Casparian band development in Arabidopsis thaliana. Ann Bot (Lond) 110: 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. (2007) Plants tolerant of high boron levels. Science 318: 1417. [DOI] [PubMed] [Google Scholar]
- Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M. (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 21: 2163–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi G, Thomson WW, Leonard RT. (1974) The casparian strip as a barrier to the movement of lanthanum in corn roots. Science 183: 670–671 [DOI] [PubMed] [Google Scholar]
- Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N. (2012) Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci USA 109: 10101–10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Moscatiello R, Genre A, Novero M, Baldan B, Bonfante P, Mariani P. (2007) A diffusible signal from arbuscular mycorrhizal fungi elicits a transient cytosolic calcium elevation in host plant cells. Plant Physiol 144: 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Schreiber L, Franke RB, Geldner N, Reina-Pinto JJ, Kunst L. (2013) Apoplastic diffusion barriers in Arabidopsis. Arabidopsis Book 11: e0167, /10.1199/tab.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman-Ligeza B, Parizot B, Gantet PP, Beeckman T, Bennett MJ, Draye X. (2013) Post-embryonic root organogenesis in cereals: branching out from model plants. Trends Plant Sci 18: 459–467 [DOI] [PubMed] [Google Scholar]
- Parniske M. (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6: 763–775 [DOI] [PubMed] [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. (2011) Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci 16: 442–450 [DOI] [PubMed] [Google Scholar]
- Péret B, Li G, Zhao J, Band LR, Voß U, Postaire O, Luu DT, Da Ines O, Casimiro I, Lucas M, et al. (2012) Auxin regulates aquaporin function to facilitate lateral root emergence. Nat Cell Biol 14: 991–998 [DOI] [PubMed] [Google Scholar]
- Peterson CA, Murrmann M, Steudle E. (1993) Location of the major barriers to water and ion movement in young roots of Zea mays L. Planta 190: 127–136 [Google Scholar]
- Ranathunge K, Schreiber L. (2011) Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. J Exp Bot 62: 1961–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Edwards D. (2001) Roots: evolutionary origins and biogeochemical significance. J Exp Bot 52: 381–401 [DOI] [PubMed] [Google Scholar]
- Roppolo D, De Rybel B, Tendon VD, Pfister A, Alassimone J, Vermeer JE, Yamazaki M, Stierhof YD, Beeckman T, Geldner N. (2011) A novel protein family mediates Casparian strip formation in the endodermis. Nature 473: 380–383 [DOI] [PubMed] [Google Scholar]
- Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, von Wirén N. (2006) AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Biol Chem 281: 25532–25540 [DOI] [PubMed] [Google Scholar]
- Schopfer P. (2006) Biomechanics of plant growth. Am J Bot 93: 1415–1425 [DOI] [PubMed] [Google Scholar]
- Schreiber L, Franke RB. (2011) Endodermis and exodermis in roots. In eLS. John Wiley & Sons, Chichester, UK, /10.1002/9780470015902.a0002086.pub2 [Google Scholar]
- Shani E, Weinstain R, Zhang Y, Castillejo C, Kaiserli E, Chory J, Tsien RY, Estelle M. (2013) Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proc Natl Acad Sci USA 110: 4834–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE. (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25: 211–222 [DOI] [PubMed] [Google Scholar]
- Soukup A, Armstrong W, Schreiber L, Franke R, Votrubová O. (2007) Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytol 173: 264–278 [DOI] [PubMed] [Google Scholar]
- Steudle E. (2000) Water uptake by roots: effects of water deficit. J Exp Bot 51: 1531–1542 [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. (1998) How does water get through roots? J Exp Bot 49: 775–788 [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Ubeda-Tomás S, Federici F, Casimiro I, Beemster GT, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett MJ. (2009) Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr Biol 19: 1194–1199 [DOI] [PubMed] [Google Scholar]
- Ubeda-Tomás S, Swarup R, Coates J, Swarup K, Laplaze L, Beemster GT, Hedden P, Bhalerao R, Bennett MJ. (2008) Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nat Cell Biol 10: 625–628 [DOI] [PubMed] [Google Scholar]
- Vermeer JE, von Wangenheim D, Barberon M, Lee Y, Stelzer EH, Maizel A, Geldner N. (2014) A spatial accommodation by neighboring cells is required for organ initiation in Arabidopsis. Science 343: 178–183 [DOI] [PubMed] [Google Scholar]
- Webb AA, Larman MG, Montgomery LT, Taylor JE, Hetherington AM. (2001) The role of calcium in ABA-induced gene expression and stomatal movements. Plant J 26: 351–362 [DOI] [PubMed] [Google Scholar]
- Wu H, Jaeger M, Wang M, Li B, Zhang BG. (2011) Three-dimensional distribution of vessels, passage cells and lateral roots along the root axis of winter wheat (Triticum aestivum). Ann Bot (Lond) 107: 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S. (1997) Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J 12: 427–439 [DOI] [PubMed] [Google Scholar]
- Yu N, Luo D, Zhang X, Liu J, Wang W, Jin Y, Dong W, Liu J, Liu H, Yang W, et al. (2014) A DELLA protein complex controls the arbuscular mycorrhizal symbiosis in plants. Cell Res 24: 130–133 [DOI] [PMC free article] [PubMed] [Google Scholar]