Exceptionally well-preserved fossils shed light on the earliest roots and their interactions with the environment.
Abstract
Geological sites of exceptional fossil preservation are becoming a focus of research on root evolution because they retain edaphic and ecological context, and the remains of plant soft tissues are preserved in some. New information is emerging on the origins of rooting systems, their interactions with fungi, and their nature and diversity in the earliest forest ecosystems. Remarkably well-preserved fossils prove that mycorrhizal symbionts were diverse in simple rhizoid-based systems. Roots evolved in a piecemeal fashion and independently in several major clades through the Devonian Period (416 to 360 million years ago), rapidly extending functionality and complexity. Evidence from extinct arborescent clades indicates that polar auxin transport was recruited independently in several to regulate wood and root development. The broader impact of root evolution on the geochemical carbon cycle is a developing area and one in which the interests of the plant physiologist intersect with those of the geochemist.
Roots were an early development in plant life, evolving on land during the Devonian Period, 416 to 360 million years ago (Gensel et al., 2001; Raven and Edwards, 2001; Boyce, 2005; Kenrick, 2013). Here, we use the term root to denote a multicellular organ characterized by special features including gravitropic response, endogenous branching, root hairs, and a protective root cap. The Devonian Period was a time of enormous change, which witnessed the evolution of forest ecosystems from an earlier diminutive herbaceous vegetation of small leafless plants with simpler rhizoid-based rooting systems (RBRSs). Roots combined with a fully integrated vascular system were essential to the evolution of large plants, enabling them to meet the requirements of anchorage and the acquisition of water and nutrients (Boyce, 2005). Plants in the earliest forests (approximately 398 million years ago) already displayed an astonishing diversity of roots encompassing extinct forms and others that are comparable in many ways to those of modern gymnosperms (Stein et al., 2007; Meyer-Berthaud et al., 2010; Giesen and Berry, 2013). From the outset, symbiotic associations with fungi were important (Taylor et al., 2004; Strullu-Derrien and Strullu, 2007; Bonfante and Genre, 2008), and it is clear that mycorrhizae and plant roots have coevolved in many different ways (Brundrett, 2002; Wang and Qiu, 2006; Taylor et al., 2009b; Strullu-Derrien et al., 2014). Roots and RBRSs can be observed in many geological contexts, but much recent research has focused on a handful of exceptional fossil sites in which plants were preserved in their growth positions (Stein et al., 2012) and in some in which this was also accompanied by complete soft-tissue preservation to the cellular level (Trewin and Rice, 2004). These sites are providing a rich source of new data on the nature of early roots and RBRSs and on their interactions with fungi, especially the origins of mycorrhizal symbioses (Taylor et al., 2004; Strullu-Derrien et al., 2014). Increasingly, paleontologists are turning to the discoveries of developmental biology to interpret features of fossils and to advance a more synthetic view of the evolution of key tissues and organ systems (Rothwell et al., 2014). Aspects of a plant’s physiology can leave fingerprints in fossils, providing insights into the nature and prevalence of developmental regulators such as auxin (Rothwell et al., 2008; Sanders et al., 2011). The combined weight of evidence demonstrates that once plants made the transition to the land, roots evolved in a piecemeal fashion independently in several different clades, rapidly acquiring and extending functionality and complexity. As roots evolved, they influenced the development of soils and the weathering of land surfaces, which had major consequences for the geochemical carbon cycle (Field et al., 2012; Lenton et al., 2012; Taylor et al., 2012).
ROOTS AND RBRSs
Roots and RBRSs are preserved as fossils in a variety of sedimentary contexts of varying quality (Retallack, 2001). The best and most complete earliest evidence comes from the Rhynie Chert (including the nearby Windyfield Chert), which is a 407-million-year-old site in Scotland that captures a period when plant life on land was at an early stage of development (Trewin and Rice, 2004). Here, plants grew on sandy substrates in and around the margins of ephemeral ponds and lakes on an alluvial plain (Trewin, 1994; Fayers and Trewin, 2004). The cherts formed as siliceous sinters that were deposited during multiple episodes of hot spring activity (Rice et al., 2002). This resulted in inundation and preservation of whole plants sometimes in their growth positions as well as the underlying soil. Petrographic thin sections are the method most widely employed to investigate and to reconstruct the plants, and they reveal amazing details ranging from the overall form to subcellular structures (Taylor et al., 2009b). The plants were small and herbaceous, with simple vascular tissues and typically leafless bifurcating axes, some of which functioned as upright stems and others as RBRSs (Fig. 1). Here, the term axis (plural: axes) is preferred over stem, rhizome, and root because in the first land plants, these organ systems differed in important aspects of structure and function to their equivalents in living plants (Tomescu et al., 2014). Another key difference from modern bryophytes or vascular plants is that life cycles showed a much greater degree of similarity between gametophytes (haploid sexual phase) and sporophytes (diploid phase; Kerp et al., 2004; Taylor et al., 2005). Similar organ and tissues systems were expressed in both phases of the life cycle. The Rhynie Chert thus provides a system in which one can investigate the nature and potentially the behavior of rooting structures at an early stage of plant evolution.
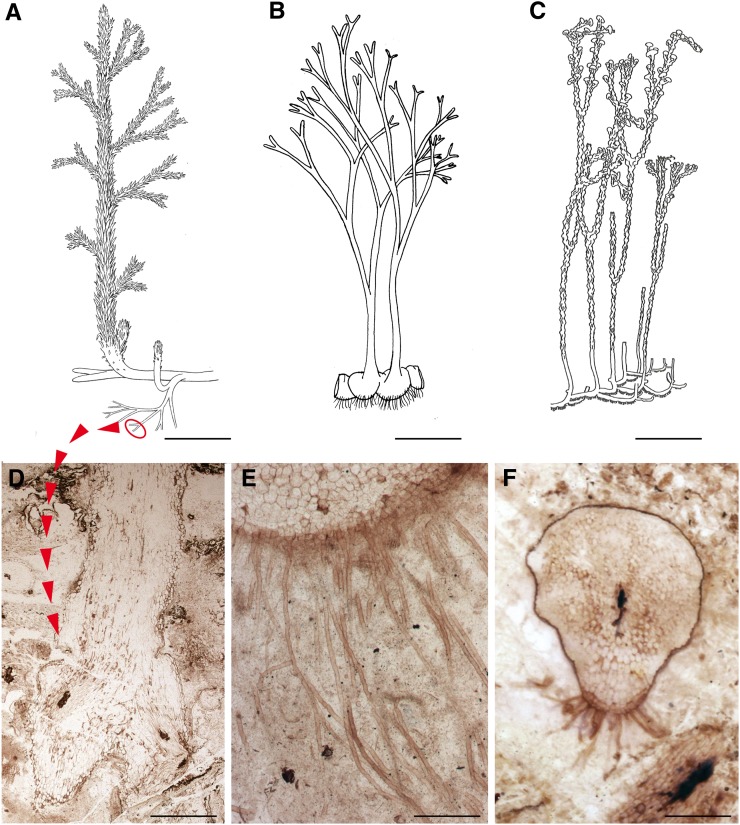
Figure 1.
Early land plants from the Lower Devonian Rhynie Chert and RBRSs. A and B, Reconstructions of the plants. A, A. mackiei (reprinted with permission from Kidston and Lang, 1921). B, H. lignieri (reprinted with permission from Eggert, 1974). C, N. aphylla (reprinted with permission from Kerp et al., 2001). D to F, Rooting structures. D, Longitudinal section of the rooting system of A. mackiei showing dichotomized branching (slide PB V67913 from the Natural History Museum, London). E, Transverse section of a corm bearing rhizoids of H. lignieri (slide PB SC 3137 from the Natural History Museum, London). F, Transverse section of a rhizome of N. aphylla showing a ridge on the ventral surface that bears the rhizoids (slide P 2808 from the University of Munster; photograph courtesy of H. Kerp). Bar = 4 cm in A, 3 cm in B and C, 1 mm in D, 0.45 mm in E, and 1.5 mm in F.
Tip-Growing Cells
Almost all land plants develop tip-growing filamentous cells at the interface between plant and soil. These take the form of root hairs in vascular plant sporophytes and rhizoids in bryophytes and in the free-living gametophytes of some vascular plants (e.g. clubmosses, ferns, and horsetails). RBRSs develop in very small plants where rhizoids both anchor the thallus to the substrate, and are thought to play a key role in the scavenging and uptake of relatively immobile ions such as potassium and phosphate (Jones and Dolan, 2012). Most of the Rhynie Chert plants bore unicellular, smooth-walled rhizoids (e.g. Horneophyton lignieri and Nothia aphylla; Fig. 1, E and F); unlike their living relatives, these were expressed in both the sporophyte and, where known, the gametophyte phases of the life cycle (Kerp et al., 2004), underlining their autonomous nature. The development of rhizoids on rhizomatous axes exhibited variation and flexibility. They were quite short and present on all surfaces in some species (Lyon and Edwards, 1991), whereas they were longer and confined to ridges of tissues or perhaps further localized in patches in others (Kerp et al., 2001; Edwards, 2004; Fig. 1F). In some species, the presence of rhizoids was associated with underlying tissue differentiation and the development of apparent transfusion tissues and vascular parenchyma linking them to the vascular system (Kerp et al., 2001). Furthermore, rhizoids were capable of developing from different types of epidermal cells and in different positions, including from stomatal subsidiary cells and from the epidermis of rhizomatous axis-borne structures such as multicellular spines (Edwards, 2004; Kenrick, 2013). These observations indicate that among early vascular plants, rhizoids were capable of being induced generally on rhizomatous axes and probably also on aerial axes and associated appendages when in proximity to the soil. Furthermore, their induction was associated with some additional differentiation in underlying tissue systems, indicating that they played a role in water transport and the absorption of minerals. Curiously, rhizoids appear to be absent from the Rhynie Chert clubmoss Asteroxylon mackiei (Edwards, 2004), indicating that tip-growing cells were not essential to all early vascular plants (Fig. 1D). The widespread occurrence of simple RBRSs in land plants (bryophytes and early fossil vascular plants) indicates that this form of rooting system preceded the evolution of true roots and may well have been a legacy inherited from green algal ancestors (Jones and Dolan, 2012). Striking confirmation of the shared evolutionary history of tip-growing cells recently came from a comparison of rhizoid development in the moss Physcomitrella patens and root hair formation in the flowering plant Arabidposis (Arabidopsis thaliana), demonstrating that a highly conserved molecular pathway controls the development of both cell types across land plants (Menand et al., 2007).
Rhizoid-Bearing Organs
In RBRSs of early vascular plants, the rhizoid-bearing axes were barely distinguishable from aerial axes. Both exhibited great morphological equivalence as well as developmental interchangeability to some extent (Kenrick, 2002, 2013). Most Rhynie Chert plants had prostrate rhizomatous axes in which the basic patterning of tissues resembled that of the aerial axes. In transverse section, one can recognize the epidermis, stomates in some, hypodermis, cortex, and a vascular cylinder (Kerp et al., 2001; Edwards, 2004). There is no endodermis. The principal indicator of rooting function is the presence of rhizoids. The rhizomatous axes are thought to have been surficial in some species, whereas they may have been subterranean in others (Fig. 1D), but shallowly so, to depths of a few millimeters (Kerp et al., 2001). One species is thought to have been a geophyte, with short-lived probably seasonal aerial parts that developed from a persistent rhizomatous axis; in another, the aerial axes terminated in lobed rhizoid-bearing bases rather than an extended rhizome (Kerp et al., 2001). Rhizomatous axes, however, appear to have been the norm both in the Rhynie Chert and at other early sites (Gensel et al., 2001; Raven and Edwards, 2001; Edwards, 2004; Kenrick, 2013). The phylogenetic placement of these early fossils implies that rhizomatous growth was characteristic of the early vascular plants. It has been documented in protracheophytes (Rhyniophytes) and within the vascular plants in basal members of lycophyte and the euphyllophyte clades (Gensel et al., 2001; Kenrick, 2013; Fig. 2A). The earliest rooting structures in vascular plants were therefore broadly equivalent to aerial axes that were modified by the presence of rhizoids.
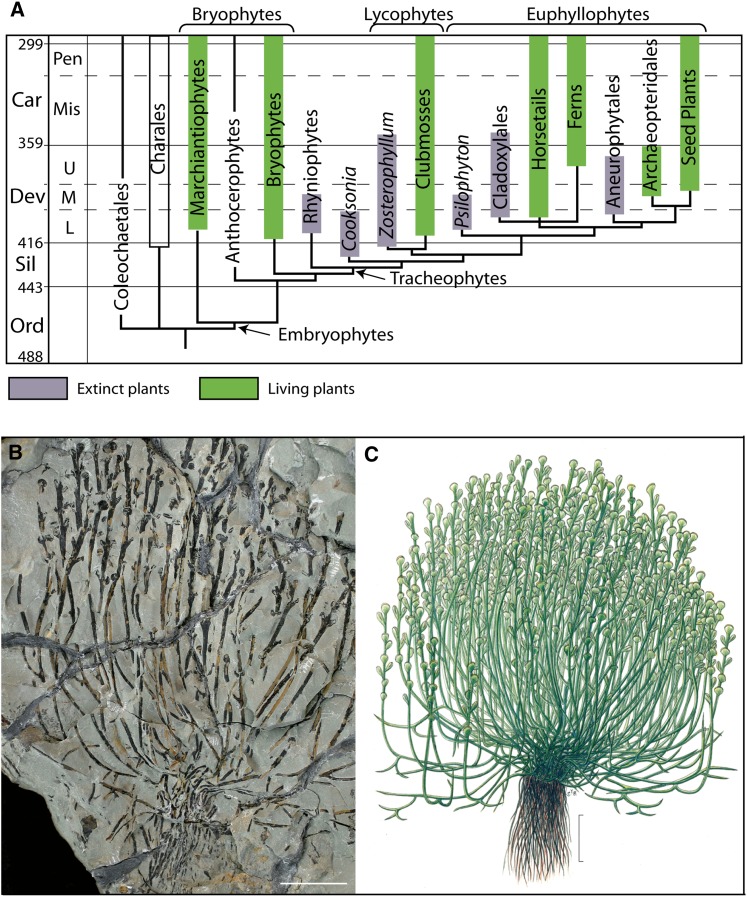
Figure 2.
A, Simplified phylogenetic tree showing the minimum stratigraphic ranges of selected groups based on fossils (thick bars) and their minimum implied range extensions (thin lines). Extinct and living plant groups are shown. Adapted from Kenrick and Crane (1997b). B, Early rooting system in Zosterophyllum shengfengense from the Lower Devonian of Qujing City, Yunnan Province, south China. The plant shows a tufted habit of numerous aerial axes, which are supported by a rhizome and shallow rooting system. C, Reconstruction of Z. shengfengense (photograph in B and reconstruction in C courtesy of Hao Shougang). Car, Carboniferous; Dev, Devonian; L, lower; M, middle; Mis, Mississippian; Ord, Ordovician; Pen, Pennsylvanian; Sil, Silurian; U, upper. Bars = 10 mm in B and 20 mm in C.
Roots
Some of the earliest evidence of roots comes from 419- to 408-million-year-old clubmosses (e.g. Drepanophycus spinaeformis) and their close relatives in the extinct zosterophylls (e.g. Bathurstia denticulate; Gensel et al., 2001). These plants bore distinctive branches that developed from aerial axes but were typically narrower and shorter, irregularly bifurcating, often with a sinuous appearance, and without leaves. A rooting function is further suggested by their orientation. Evidence from several fossils shows root-like structures growing downward from points along prostrate axes (Gensel et al., 2001). In other species, aerial axes are all upright and root-like structures emerged from the base in a dense tuft (Hao et al., 2010; Fig. 2, B and C). Because these fossils preserve overall form but not anatomical detail, it is not known whether root development was endogenous. Similarly, the presence or absence of root caps and root hairs cannot be determined. Where there is anatomical preservation, as in the Rhynie Chert, the rooting axes appear to be exogenous and root hairs are absent (Raven and Edwards, 2001). Organs such as these possessed some of the attributes of roots, and they provide the earliest evidence of a gravitropic response in plants.
Dormant meristematic regions that had the potential to develop into new aerial axes and perhaps rooting systems were common to many early vascular plants, indicating that apical regulation of lateral meristem development was active. These dormant meristems were most frequent on rhizomatous axes, but they were also present on aerial axes where they could be abundant (Kenrick and Crane, 1997a; Gensel et al., 2001). They were vascular, exogenous, and they are known to have developed into new aerial axes in some species, whereas it is possible that they developed into rooting structures in others (Edwards and Kenrick, 1986; Remy et al., 1986; Li, 1992). It has been suggested that the function of dormant meristems in the life cycle of the plant was to facilitate vegetative growth or reproduction. Sedimentological evidence shows that many such plants inhabited disturbed marginal aquatic settings where they were prone to catastrophic burial by flood (Edwards and Kenrick, 1986). The combination of numerous dormant meristems and rhizomatous growth may have been an adaptation to occasional burial in sediment and would have led to the formation of extensive thickets of genetically identical individuals.
ROOTS OF TREES
Roots became increasingly elaborate and diverse as forest ecosystems evolved, reflecting both the independent evolution of trees in several major clades of plants and the co-option of roots to a variety of additional functions. Such diversity is already evident in the earliest known fossil forest, which is preserved in 385-million-year-old sediments near Gilboa, New York (Fig. 3A). Here the fossils occur as sediment-filled casts of tree stumps (Stein et al., 2007), and they are also found within the soils from which the stumps have been removed, providing remarkable evidence of forest structure and the nature of the plant community (Mintz et al., 2010; Stein et al., 2012). The ecological setting was a tropical-subtropical, coastal wetland that was subject to periodic disturbance through flooding. At Gilboa, trees could exceed 8 m in height, and the dominant form belonged to an extinct group (Cladoxylales; Fig. 2A) thought to be intermediate between early vascular plants and living ferns. In overall habit, they bore a resemblance to tree ferns, but the trunks were anchored at the bases by many narrow (approximately 1–2 cm), unbranched, overlapping roots, rather like modern palms (Stein et al., 2012; Fig. 4A). This general growth form is corroborated by remarkably complete fossils at other sites (Giesen and Berry, 2013). It is probable that the trunks of these plants were hollow, reed like, and fast growing. The trees may have been capable of rapid establishment, and probably contributed significantly to substrate stability because of the nature of the root mass.
Figure 3.
The rooting systems from the Gilboa Forest (Riverside Quarry, NY). A, Eospermatopteris (Cladoxylales) root mound. Circular central depression, raised rim, and radiating system of attached roots. B, Aneurophytalean rhizome (photographs in A and B courtesy of C. Berry). C, Probable aneurophytalean rhizome, with numerous apparently unbranched appendages interpreted as roots (specimen collected by Goldring, NYSM 6575; photograph courtesy of W. Stein). Bar = 11 cm.
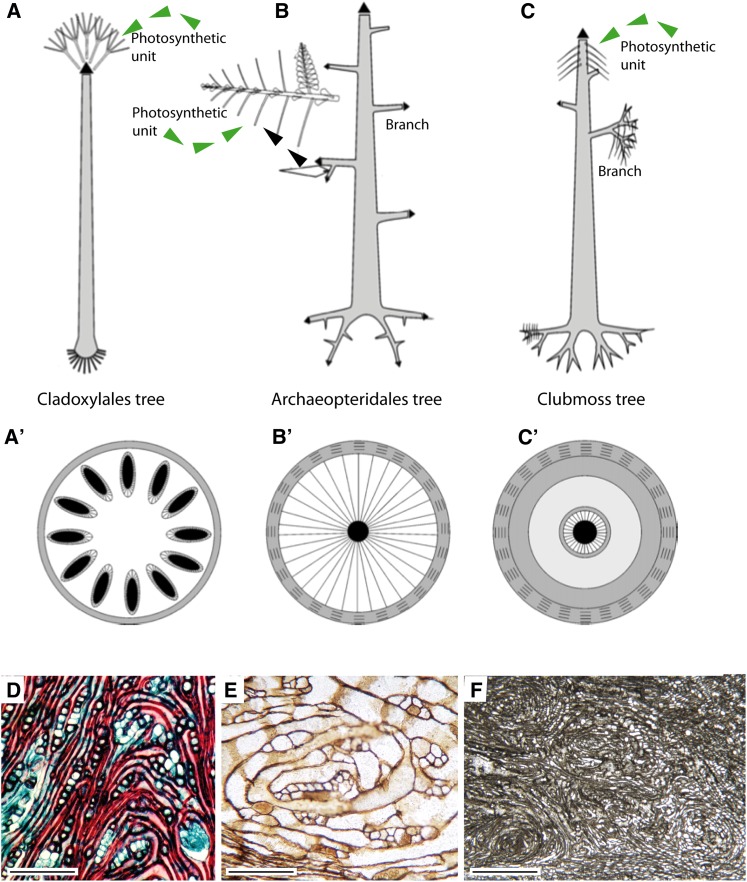
Figure 4.
A to C, Comparative architecture (aerial part and rooting system) of three principal arborescent strategies of the Middle-Upper Devonian and transverse section of the corresponding trunks (Cladoxylales [A and A′], Archaeopteridales [B and B′], and arborescent clubmoss [C and C′]). The color scheme is as follows: black, primary vascular tissue; gray, cortex; and striped, secondary tissue (on white background, wood; on gray background, secondary cortex; scheme courtesy of B. Meyer-Berthaud). D to F, Tangential sections of living and fossil wood showing spiral patterns reflecting the deviation in the axial flow of auxin. D, Spiral patterns of wood distal to diverging branches in the extant Pinus halepensis (photograph courtesy of G. Rothwell). E, Circular tracheids and interspersed parenchyma from area distal to branch stele in the fossil Callixylon whiteanum (Archeopteridales) from the Upper Devonian of Oklahoma (photograph courtesy of G. Rothwell). F, Callixylon roots (species unidentified) from the Upper Devonian of Morocco. Spiral wood pattern in a longitudinal section of a main root (slide MD600/3/2-Cl2 from the collections of the Museum of Geology and Paleontology, University of Tuebingen; photograph courtesy of B. Meyer-Berthaud). Length of tissue = 1.04 mm in D and 0.27 mm in E. Scale bar = 0.7 mm.
Small clubmoss trees were also present at Gilboa. Although the root systems of the clubmosses were not preserved at this site, evidence from younger sediments shows that these trees possessed large bifurcating root systems that extended at shallow depths horizontally forming a stable platform for the pole-like trunk (Fig. 4C). Furthermore, it is likely that in early growth stages, development of the root system preceded that of the shoot system (Phillips and DiMichele, 1992). A third growth form at Gilboa is interpreted as a liana. Narrow woody stems with adventitious roots ran between the trees and probably climbed into them (Fig. 3, B and C). These belonged to plants in the extinct progymnosperms (Aneurophytales; Fig. 2A), which were a grade of free-sporing plants that were closely related to seed plants (Taylor et al., 2009b). Fossil evidence from early sites such as Gilboa shows that the evolution of large plants and in particular trees placed increasing demands on root systems, and that these were solved in a variety of ways. There was, however, no straightforward correlation between increasing plant stature aboveground and the size of root systems (Stein et al., 2012; Meyer-Berthaud et al., 2013). There is still much to learn about the roots of the Gilboa plants. In particular, the details of internal anatomical structure remain frustratingly elusive.
Much of the evidence of root systems in early forests comes from the casts of stumps and roots and their traces preserved in paleosols. But where fossilization of roots is also associated with cellular permineralization, one can obtain additional insights into tissue systems and their development. In the earliest forest ecosystems, the progymnosperms were among the most abundant and cosmopolitan forms of early shrubs, lianas, and trees. These were relatives of modern gymnosperms, but they differed in key features. Progymnosperms resembled ferns in their mode of reproduction (free sporing) and in the general form of their leaves; however, unlike ferns, they developed gymnospermous-type wood in stems and branches (Rothwell and Lev-Yadun, 2005). The similarities with modern arborescent gymnosperms are now also known to extend to their roots. The extinct Archaeopteridales were trees that grew to substantial sizes (Fig. 4B), and they dominated riparian habitats 383 to 359 million years ago. Anatomy of internal tissue systems when it is preserved shows that in many respects these root systems resembled those of arborescent conifers (Meyer-Berthaud et al., 2013). Their roots were extensive and woody, and they developed several orders of branching. Primary and secondary order branches were large and basically indeterminate. These features, together with the observation that the wood developed growth rings, confirm that the roots of Archaeopteridales were capable of continuous perennial growth and long-term survival in the soil (Algeo et al., 2001). Evidence from petrified trunks shows that in addition to a well-developed root system, Archaeopteridales possessed adventitious latent primordia similar to those produced by some living trees, which eventually develop into roots on stem cuttings (Meyer-Berthaud et al., 1999). The roots also bore numerous narrow adventitious rootlets that had determinate growth and developed endogenously (Meyer-Berthaud et al., 2013). The capacity for indeterminate growth of main root branches combined with an ability to develop adventitious rootlets provided these early trees with a flexible root system facilitating the exploitation of soil volume and enabling a dynamic response to changing soil conditions.
POLAR AUXIN TRANSPORT
As well as providing direct evidence on the structure and diversity of early rooting systems, fossils might also contribute to our understanding of the mechanisms regulating plant development. Experimentation and molecular developmental methods are not available to the paleontologist, but the fingerprints of regulatory mechanisms are sometimes discernable in fossils, providing insights into when and in what context they might have evolved (Sanders et al., 2007; Rothwell et al., 2014). Thus, features of roots such as the gravitropic response and the endogenous development of lateral roots, which are regulated by auxin (Fukaki and Tasaka, 2009; Overvoorde et al., 2010), when observed in fossils indicate that similar mechanisms were operating in ancient plants. Rothwell and Lev-Yadun (2005) showed that the patterning of wood development in early trees belonging to the progymnosperms was under polar auxin transport (PAT) regulation. In modern woods, the obstruction of basipetal auxin flow by branches leads to the development of a distinctive circular pattern of xylem cells in the wood above the branch (Sachs and Cohen, 1982). Similar patterns were observed in permineralized fossil woods of Archaeopteridales in both the trunk (Rothwell and Lev-Yadun, 2005) and the root (Meyer-Berthaud et al., 2013), signifying that the same developmental processes were at work (Fig. 4, D–F). Circular patterns of xylem cells were also observed in tree clubmosses (Rothwell et al., 2008). Again, these features are positioned above root traces providing evidence that as in modern seed plants, PAT proceeded from the shoot apex to root apex (Sanders et al., 2011). Because wood had not yet evolved in the most recent common ancestor of progymnosperms, horsetails, and clubmosses, it is likely that the secondary growth of these trees evolved separately but in parallel, with each clade independently recruiting a common PAT regulatory pathway (Rothwell et al., 2008).
FUNGAL PARTNERSHIPS
Fungal symbioses involving mycorrhizae characterize over 90% of living plant species (Wang and Qiu, 2006), where they play an important role in the uptake of phosphate (Parniske, 2008). Arbuscular mycorrhizal (AM) fungi (Glomeromycota; Schüßler et al., 2001) form associations widely in the roots of vascular plants and also in thalli and gametophytes of liverworts, hornworts, and lycophytes (Read et al., 2000; Smith and Read, 2008). They are characterized by intracellular fungal structures (i.e. arbuscules and hyphal coils) and often storage organs termed vesicles. Recent inferences from phylogenetic studies and direct observations of early fossils demonstrate that AM fungi are ancient (Brundrett, 2002; Taylor et al., 2004; Taylor and Krings, 2005; Berbee and Taylor, 2007; Krings et al., 2007a; Strullu-Derrien and Strullu, 2007). Much of the early fossil evidence comes from the Rhynie Chert. Arbuscule-like structures developed in the cortex of one stem group vascular plant (Remy et al., 1994; Fig. 5, A and B). Furthermore, there is evidence that infection occurred very early in the plant’s ontogeny, while the gametophyte was still attached to its spore. In this early fossil, the mycorrhizal infection was also clearly more extensive than that in living vascular plants. Arbuscule-like structures developed in the cortical tissues of both rhizomatous axes and aerial axes, with infection reported to extend to the tips in both regions (Taylor et al., 2004). This is a key point of difference between early stem group vascular plants and their modern relatives in which AM fungi are confined to roots (Taylor et al., 2004; Strullu-Derrien and Strullu, 2007). The colonization of aerial photosynthetic axes in early fossils is more similar to the type of infection seen in the thalli of living liverworts and hornworts and in the gametophytes of clubmosses. Because these plants do not possess roots, the associations are best referred to as mycorrhizal like (Smith and Read, 2008) or paramycorrhizae, a term introduced to denote colonization of photosynthetic thalli and aerial axes, as opposed to eumycorrhizae, which means colonization of root systems only (Strullu-Derrien and Strullu, 2007). Paramycorrhizae seem to typify plants with RBRSs.
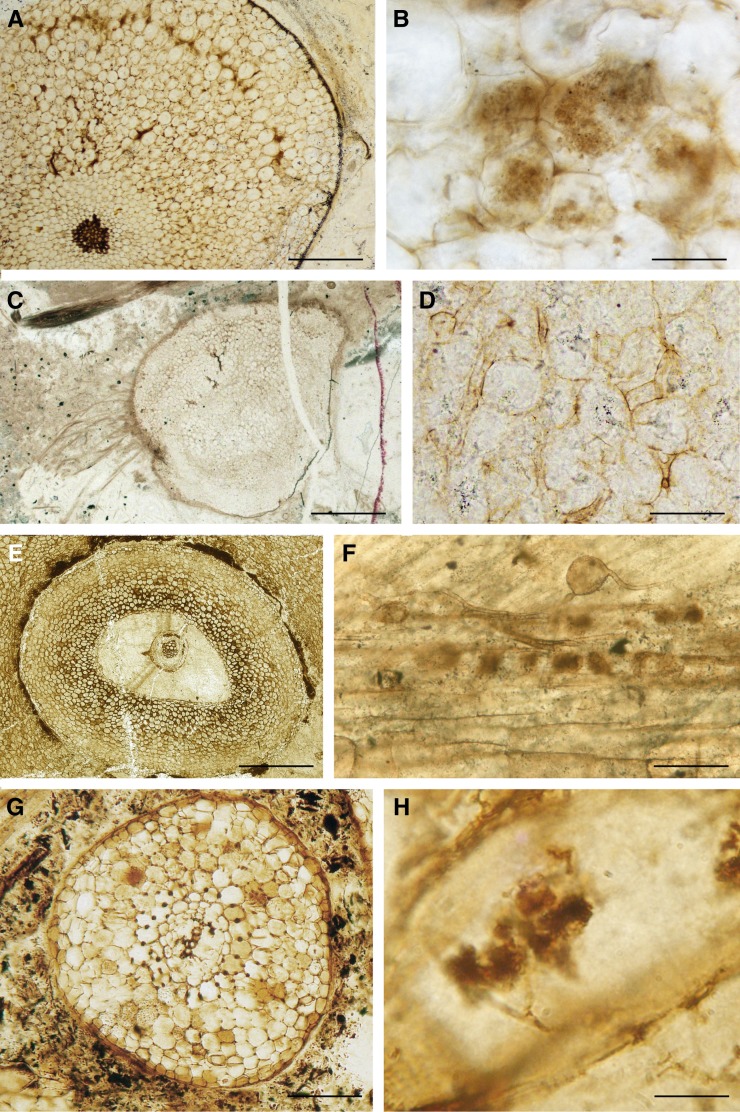
Figure 5.
Fungal partnerships in Devonian and Carboniferous plants. A and B, Fungal endophyte of the glomeromycotan type in A. major from the Devonian Rhynie Chert. A, Transverse section of an aerial axis showing the well-defined colonized zone in the outer cortex (slide PB V15637 from the Natural History Museum, London). B, Arbuscule-like structures in an aerial axis (slide from the University of Munster; photograph courtesy of H. Kerp). C and D, Colonization of the mucoromycotean type in H. lignieri from the Devonian Rhynie Chert. C, Transverse section of a corm; a zonation of fungal colonization is visible within the corm. D, Intercellular branched thin-walled and intercellular thick-walled hyphae are present. E, Arborescent clubmoss rootlet from the Upper Carboniferous of Great Britain (slide PB V11472 from the Natural History Museum, London). F, AM-like fungi in stigmarian appendage. Trunk hyphae, intercalary vesicle (left), and putative arbuscule-like structures (right) are visible (slide BSPG 1964X from the Bavarian State Collection for Paleontology and Geology; photograph courtesy of M. Krings). G, Cordaites rootlet from the Upper Carboniferous of Grand’Croix, France, colonized by AM fungus. The cortex comprises a reticulum of phi thickenings that are prominent in cells located close to the vascular cylinder (slide Lignier Collection no. 194 from the University of Caen). H, Detail of an arbuscule-like structure. The hyphal trunk of the arbuscule-like structure branches repeatedly forming a bush-like tuft within the cell (slide Lignier Collection no. 194 from the University of Caen). Bars = 0.55 mm in A, 30 µm in B, 1.1 mm in C, 120 µm in D, 1.5 mm in E, 70 µm in F, 1.25 mm in G, and 18 µm in H.
Whereas some paramycorrhizae in the Rhynie Chert plants developed arbuscule-like structures, others apparently did not, or at least their development has not been observed. In some species, a clear zone of fungal infection was present in the stem cortex, but only vesicles have been described (Karatygin et al., 2006). In addition, some individual axes show little sign of infection, whereas others are more heavily infected, raising the possibility that the fungal associations were transient or perhaps different phases of infection were preserved. Much still remains to be learned about the infection pathway of paramycorrhizae, especially how they developed within the aerial axes. In modern vascular plants, infection by AM fungi commences with the formation of an appressorium, which is an inconspicuous flattened hyphal organ that develops at the root epidermis facilitating the penetration of cells and tissues (Parniske, 2008). The occurrence of appressoria needs further investigation in the Rhynie Chert plants. In one species (Aglaophyton major), it is thought that hyphae entered the axes in the region of the rhizoids (Taylor et al., 1995). A different mode of colonization probably occurred in another plant (N. aphylla), in which fungal infection was initially intracellular in rhizoids and tissues of the rhizoidal ridge and later became intercellular in the cortex of the RBRSs and the base of aerial axes (Krings et al., 2007b). In a third plant (H. lignieri), it has been inferred that colonization of the upright axes occurred through the epidermis because of the proximity of hyphae to the base of the aerial axis (Strullu-Derrien et al., 2014).
All putative paramycorrhizal associations in the Rhynie Chert plants have been attributed to Glomeromycota, but the recent discovery of two new endophytes in one plant species demonstrates greater diversity (Strullu-Derrien et al., 2014). The fungus colonizing the cortical tissues of the aerial axes possessed intercellular hyphae with arbuscule-like structures, vesicles, and spores, resembling Glomeromycota. The parenchymatous tissues of the RBRS were colonized by a different form with intercellular hyphae and intracellular hyphal coils, resembling the infection by Mucoromycotina observed in some modern liverworts, hornworts, and lycophytes (Bidartondo et al., 2011; Fig. 5, C and D). The mode of infection by Mucoromycotina remains unknown; however, because hyphae were absent from the rhizoids, a route through the epidermis seems plausible. The growing body of data emerging from the Rhynie Chert shows that fungal associations in early land plants were more diverse than previously assumed, both in terms of their development within plants and in their affinities to major fungal clades, challenging the long-held paradigm that the early endophytes were exclusively Glomeromycota.
In contrast with the wealth of information emerging from the Rhynie Chert, our knowledge of fungal symbioses associated with roots in the earliest forest ecosystems is sparse, reflecting in part a research focus on the plants themselves and in part a lack of suitably preserved petrified material, especially during the early evolution of forests in the middle and latter parts of the Devonian Period (398–359 million years ago). Krings et al. (2011) reported an AM-like fungus in the root system of an arborescent clubmoss from the latter part of the Carboniferous Period (315 million years ago). These plants bore an extensive root system with a unique evolutionary history. The roots were a modified shoot and the rootlets were modified leaves (Rothwell, 1995). This type of root system therefore had a different evolutionary origin to that in any other major plant group. The AM-like fungus developed near the tip of the rootlets. Large vesicles or spores were observed attached to hyphal threads (Fig. 5, E and F). Other hyphae penetrated cortical cells to form multibranched structures interpreted as arbuscule like.
One developing source of information on the coevolution of plants and fungi is historic slide collections of petrified plants made during the 20th century and earlier. The original purpose of these collections was to document the anatomy of stems, reproductive organs, and roots of long extinct plants, but it is now realized that they are an important source of information on plant endobionts. Historical collections were used to document AM-like fungi in early relatives of the conifers also found in deposits of the Carboniferous Period (Strullu-Derrien et al., 2009; Fig. 5, G and H). Here, fungal colonization occurred in rootlets that were undergoing primary growth, and it was characterized by the absence of an intercellular phase and by the development of intraradical hyphae. Vesicles were not observed, but small arbuscule-like structures developed in some of the cortical cells (Fig. 5H). A well-developed endodermis was observed in these rootlets, probably creating a barrier to fungal colonization of the vascular system. The systematic examination of historic slide collections of petrified plants promises to yield a large and valuable new source of information on associated endobiotic fungi and the plants’ responses to infection.
EARTH SYSTEMS
The evolution of root systems in plants had influence that extended far beyond the microenvironment of the soil and the local effects on individual plants. Roots had an enormous impact on the evolution of other aspects of plant morphology and on the development of soil ecosystems. Their effect on the evolution of Earth’s atmosphere and climate through the Phanerozoic is an exciting and developing area of research (Bergman et al., 2004; Beerling and Berner, 2005; Berner et al., 2007; Beerling, 2012; Kenrick et al., 2012). Living organisms are part of the biogeochemical carbon cycle, in which CO2 is drawn down from the atmosphere through two main processes. One involves photosynthesis followed by burial of organic matter in sediments. The second depends on root-mediated weathering of calcium and magnesium silicates in surface rocks and soils (Berner and Kothavala, 2001). This weathering process incorporates many steps, including the synthesis of plant- and atmosphere-derived carbonic or organic acids, the conversion of CO2 to HCO3− in soil and groundwater, as well as transport of HCO3− through rivers to the sea and its eventual precipitation as a component of limestone or dolomite on the ocean floor. A vegetation of large plants with extensive root systems and associated mycorrhizal fungi greatly enhances the weathering effect (Berner and Kothavala, 2001; Taylor et al., 2009a, 2012). Carbon sequestered in these ways is released back into the atmosphere slowly over tens or hundreds of millions of years through oxidative weathering of buried carbon and through the thermal breakdown of marine carbonates, resulting in degassing to the Earth’s surface. Considered over geological timescales, the net effect of the presence of plants with roots on land is to draw CO2 out of the atmosphere, and this is thought to have had a major effect on climate, contributing to the glaciations of the latter part of the Paleozoic Era. The root-mediated weathering of silicates is therefore an important variable in biogeochemical models (Berner and Kothavala, 2001; Bergman et al., 2004; Berner et al., 2007), but the effects of different types of vegetation are still poorly understood. The root systems of large vascular plants (i.e. trees) are thought to exert the most significant effect, but recent laboratory-based research indicates that the RBRSs of mosses also considerably enhanced silicate weathering over background abiotic levels (Lenton et al., 2012). Further research on modeling the effects of simple root systems is needed, but early results indicate that even modest RBRSs in land plants may have had major effects on the chemistry of the atmosphere and on climate during the early colonization of the land.
Deeper soils and larger plants go hand in hand, but the evolution of both may have been more intimately connected than is generally supposed. One of the most remarkable phenomena of the Devonian Period was the independent and more or less concurrent evolution of trees in several plant groups (Kenrick and Davis, 2004). Mosbrugger (1990) argued that this phenomenon may in fact have been caused by positive feedback between soil and plant, mediated by the roots. In essence, the idea is based on the observation that larger plants generally require more stable and comparatively deeper soils, and that plants themselves contribute to soil formation. Under circumstances such as these, increments in plant size are expected to lead to increments in the quality and depth of the soil profile, which in turn would provide soils suitable for even larger plants. Therefore, it is thought that plant size and soil depth increased in parallel until other limiting factors came into play. Feedbacks such as this between plants and aspects of their environment are increasingly seen as important and pervasive in early terrestrial ecosystems. For example, there is evidence that the initial evolution of leaves was influenced by falling concentrations of atmospheric CO2 during the Devonian Period, which itself was driven by root induced surficial weathering of rocks and the sequestration of carbon in sediments and soils (Osborne et al., 2004). Plant, soil, and atmosphere are therefore linked in an intricate network of geophysiological feedbacks (Beerling and Berner, 2005).
CONCLUSION
Exceptional sites of fossil preservation such as the 407-million-year-old Rhynie Chert provide direct evidence on the nature and function of roots and RBRSs in early terrestrial ecosystems and on their interactions with fungi. RBRSs predate the evolution of roots, and they were widespread in both the sporophyte and gametophyte generations of early vascular plants.
Roots evolved in a piecemeal fashion and independently in several major clades of plants, rapidly acquiring and extending functionality and complexity. Specific aspects of root evolution that are still poorly understood but amenable to investigation include cell patterning in root meristems, the development of lateral roots, and the evolution of root caps and root hairs.
Even though regulatory mechanisms in plants are molecular and dynamic, they leave their marks in tissues and organs, which can be preserved and observed in fossils. Recent research indicates that PAT was recruited independently in several major plant clades to regulate the development of wood and roots.
A growing body of data emerging from the Rhynie Chert demonstrate that fungal associations in early land plants were more diverse than previously assumed, both in terms of their development within plants and in their affinities to major fungal clades, challenging the long-held paradigm that the early endophytes were exclusively Glomeromycota. Mucoromycotina were also present as mycorrhizal endophytes of RBRSs in the earliest plants.
Large historic slide collections of petrified plants made during the 20th century and earlier with the original purpose of documenting the anatomy of stems, reproductive organs, and roots are an important and developing source of new information on the plant eukaryote endophytes of roots and RBRSs.
The evolution of RBRSs and roots had a major impact on the geochemical carbon cycle, leading to drawdown of atmospheric CO2 and influencing climate change on a global scale. Further quantification of the rates of weathering by different types of root and RBRS is needed to improve carbon cycle models.
Acknowledgments
We thank Hao Shougang (Beijing), Bill Stein (Binghamton), Gar Rothwell (Ohio), Hans Kerp (Munster), Chris Berry (Cardiff), Brigitte Meyer-Berthaud (Montpellier), and Michael Krings (Munich) as well as the New Phytologist, Comptes-Rendus Palevol, American Journal of Botany, and International Journal of Plant Sciences for permission to reuse figures.
Glossary
- RBRS
rhizoid-based rooting system
- PAT
polar auxin transport
- AM
arbuscular mycorrhizal
Footnotes
This work was supported by a European Commission Marie Curie Intra-European Fellowship for Career Development (grant no. SYMBIONTS GA–2011–298735 to C.S.-D.) and FP6 SYNTHESYS (grant no. SE-TAF 3444 to C.S.-D.).
References
- Algeo TJ, Scheckler SE, Maynard JB. (2001) Effects of the Middle to Late Devonian spread of vascular land plants on weathering regimes, marine biotas, and global climate. In Gensel PG, Edwards D, eds, Plants Invade the Land: Evolutionary and Environmental Consequences. Columbia University Press, New York, pp 213–236 [Google Scholar]
- Beerling DJ. (2012) Atmospheric carbon dioxide: a driver of photosynthetic eukaryote evolution for over a billion years? Philos Trans R Soc Lond B Biol Sci 367: 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerling DJ, Berner RA. (2005) Feedbacks and the coevolution of plants and atmospheric CO2. Proc Natl Acad Sci USA 102: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbee ML, Taylor JW. (2007) Rhynie chert: a window into a lost world of complex plant-fungus interactions. New Phytol 174: 475–479 [DOI] [PubMed] [Google Scholar]
- Bergman NM, Lenton TM, Watson AJ. (2004) COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am J Sci 304: 397–437 [Google Scholar]
- Berner RA, Kothavala Z. (2001) GEOCARB III; a revised model of atmospheric CO2 over Phanerozoic time. Am J Sci 301: 182–204 [Google Scholar]
- Berner RA, Vandenbrooks JM, Ward PD. (2007) Evolution. Oxygen and evolution. Science 316: 557–558 [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Read DJ, Trappe JM, Merckx V, Ligrone R, Duckett JG. (2011) The dawn of symbiosis between plants and fungi. Biol Lett 7: 574–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P, Genre A. (2008) Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends Plant Sci 13: 492–498 [DOI] [PubMed] [Google Scholar]
- Boyce KC. (2005) The evolutionary history of roots and leaves. In Holbrook NM, Zwieniecki MA, eds, Vascular Transport in Plants. Elsevier, San Diego, pp 479–500 [Google Scholar]
- Brundrett MC. (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154: 275–304 [DOI] [PubMed] [Google Scholar]
- Edwards D. (2004) Embryophytic sporophytes in the Rhynie and Windyfield cherts. Trans R Soc Edinb Earth Sci 94: 397–410 [Google Scholar]
- Edwards D, Kenrick P. (1986) A new zosterophyll from the Lower Devonian of Wales. Bot J Linn Soc 92: 269–283 [Google Scholar]
- Eggert DA. (1974) The sporangium of Horneophyton lignieri (Rhyniophytina). Am J Bot 61: 405–413 [Google Scholar]
- Fayers SR, Trewin NH. (2004) A review of the palaeoenvironments and biota of the Windyfield Chert. Trans R Soc Edinb Earth Sci 94: 325–339 [Google Scholar]
- Field KJ, Cameron DD, Leake JR, Tille S, Bidartondo MI, Beerling DJ. (2012) Contrasting arbuscular mycorrhizal responses of vascular and non-vascular plants to a simulated Palaeozoic CO2 decline. Nat Commun 3: 835. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69: 437–449 [DOI] [PubMed] [Google Scholar]
- Gensel PG, Kotyk ME, Basinger JF. (2001) Morphology of above- and below-ground structures in Early Devonian (Pragian-Emsian) plants. In Gensel PG, Edwards D, eds, Plants Invade the Land: Evolutionary and Environmental Perspectives. Columbia University Press, New York, pp 83–102 [Google Scholar]
- Giesen P, Berry CM. (2013) Reconstruction and growth of the early tree Calamophyton (Pseudosporochnales, Cladoxylopsida) based on exceptionally complete specimens from Lindlar, Germany (mid-Devonian): organic connection of Calamophyton branches and Duisbergia trunks. Int J Plant Sci 174: 665–686 [Google Scholar]
- Hao S, Xue J, Guo D, Wang D. (2010) Earliest rooting system and root:shoot ratio from a new Zosterophyllum plant. New Phytol 185: 217–225 [DOI] [PubMed] [Google Scholar]
- Jones VA, Dolan L. (2012) The evolution of root hairs and rhizoids. Ann Bot (Lond) 110: 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatygin IV, Snigirevskaya NS, Demchenko KN. (2006) Species of the genus Glomites as plant mycobionts in Early Devonian ecosystems. Paleontol J 40: 572–579 [Google Scholar]
- Kenrick P. (2002) The telome theory. In Cronk QCB, Bateman RM, Hawkins JA, eds, Developmental Genetics and Plant Evolution. Taylor & Francis, London, pp 365–387 [Google Scholar]
- Kenrick P. (2013) The origin of roots. In Eshel A, Beeckman T, eds, Plant Roots: The Hidden Half, Ed 4. Taylor & Francis, London, pp 1–13 [Google Scholar]
- Kenrick P, Crane PR. (1997a) The Origin and Early Diversification of Land Plants: A Cladistic Study. Smithsonian Institution Press, Washington, DC [Google Scholar]
- Kenrick P, Crane PR. (1997b) The origin and early evolution of plants on land. Nature 389: 33–39 [Google Scholar]
- Kenrick P, Davis PG. (2004) Fossil Plants. Natural History Museum, London [Google Scholar]
- Kenrick P, Wellman CH, Schneider H, Edgecombe GD. (2012) A timeline for terrestrialization: consequences for the carbon cycle in the Palaeozoic. Philos Trans R Soc Lond B Biol Sci 367: 519–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerp H, Hass H, Mosbrugger V. (2001) New data on Nothia aphylla Lyon 1964 ex El-Saadawy et Lacey 1979, a poorly known plant from the Lower Devonian Rhynie Chert. In Gensel PG, Edward D, eds, Plants Invade the Land: Evolutionary and Environmental Perspectives. Columbia University Press, New York, pp 52–82 [Google Scholar]
- Kerp H, Trewin NH, Hass H. (2004) New gametophytes from the Early Devonian Rhynie Chert. Trans R Soc Edinb Earth Sci 94: 411–428 [Google Scholar]
- Kidston R, Lang WH. (1921) On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part IV. Restorations of the vascular cryptogams, and discussion on their bearing on the general morphology of the pteridophyta and the origin of the organization of land-plants. Trans R Soc Edinb 52: 831–854 [Google Scholar]
- Krings M, Taylor TN, Hass H, Kerp H, Dotzler N, Hermsen EJ. (2007a) An alternative mode of early land plant colonization by putative endomycorrhizal fungi. Plant Signal Behav 2: 125–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings M, Taylor TN, Hass H, Kerp H, Dotzler N, Hermsen EJ. (2007b) Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. New Phytol 174: 648–657 [DOI] [PubMed] [Google Scholar]
- Krings M, Taylor TN, Taylor EL, Dotzler N, Walker C. (2011) Arbuscular mycorrhizal-like fungi in Carboniferous arborescent lycopsids. New Phytol 191: 311–314 [DOI] [PubMed] [Google Scholar]
- Lenton TM, Crouch M, Johnson M, Pires N, Dolan L. (2012) First plants cooled the Ordovician. Nat Geosci 5: 86–89 [Google Scholar]
- Li CS. (1992) Hsua robusta, an Early Devonian plant from Yunnan Province, China and its bearing on some structures of early land plants. Rev Palaeobot Palynol 71: 121–147 [Google Scholar]
- Lyon AG, Edwards D. (1991) The first zosterophyll from the Lower Devonian Rhynie Chert, Aberdeenshire. Trans R Soc Edinb Earth Sci 82: 324–332 [Google Scholar]
- Menand B, Yi K, Jouannic S, Hoffmann L, Ryan E, Linstead P, Schaefer DG, Dolan L. (2007) An ancient mechanism controls the development of cells with a rooting function in land plants. Science 316: 1477–1480 [DOI] [PubMed] [Google Scholar]
- Meyer-Berthaud B, Decombeix AL, Ermacora X. (2013) Archaeopterid root anatomy and architecture: new information from permineralized specimens of Famennian age from Anti-Atlas (Morocco). Int J Plant Sci 174: 364–381 [Google Scholar]
- Meyer-Berthaud B, Scheckler SE, Wendt J. (1999) Archaeopteris is the earliest known modern tree. Nature 398: 700–701 [Google Scholar]
- Meyer-Berthaud B, Soria A, Decombeix AL. (2010) The land plant cover in the Devonian: a reassessment of the evolution of the tree habit. In Vecoli M, Clément G, Meyer-Berthaud B, eds, The Terrestrialization Process: Modelling Complex Interactions at the Biosphere-Geosphere Interface. Geological Society, London, pp 59–70 [Google Scholar]
- Mintz JS, Driese SG, White JD. (2010) Environmental and ecological variability of Middle Devonian (Givetian) forests in Appalachian Basin paleosols, New York, United States. Palaios 25: 85–96 [Google Scholar]
- Mosbrugger V. (1990) The tree habit in land plants: A functional comparison of trunk constructions with a brief introduction into the biomechanics of trees. Lect Notes in Earth Sci 28: 1–161 [Google Scholar]
- Osborne CP, Beerling DJ, Lomax BH, Chaloner WG. (2004) Biophysical constraints on the origin of leaves inferred from the fossil record. Proc Natl Acad Sci USA 101: 10360–10362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T. (2010) Auxin control of root development. Cold Spring Harb Perspect Biol 2: a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M. (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6: 763–775 [DOI] [PubMed] [Google Scholar]
- Phillips TL, DiMichele WA. (1992) Comparative ecology and life-history biology of arborescent lycopsids in late Carboniferous swamps of Euramerica. Ann Mo Bot Gard 79: 560–588 [Google Scholar]
- Raven JA, Edwards D. (2001) Roots: evolutionary origins and biogeochemical significance. J Exp Bot 52: 381–401 [DOI] [PubMed] [Google Scholar]
- Read DJ, Ducket JG, Francis R, Ligron R, Russell A. (2000) Symbiotic fungal associations in ‘lower’ land plants. Philos Trans R Soc Lond B Biol Sci 355: 815–830, discussion 830–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy W, Hass H, Schultka S. (1986) Anisophyton potoniei nov. spec. aus den Kühlbacher Schichten (Emsian) vom Steinbruch Ufersmühle, Wiehltalsperre. Argumenta Palaeobotanica 7: 123–138 [Google Scholar]
- Remy W, Taylor TN, Hass H, Kerp H. (1994) Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci USA 91: 11841–11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack GJ. (2001) Soils of the Past: An Introduction to Paleopedology, Ed 2. Blackwell Science, Oxford [Google Scholar]
- Rice CM, Trewin NH, Anderson LI. (2002) Geological setting of the Early Devonian Rhynie cherts, Aberdeenshire, Scotland; an early terrestrial hot spring system. J Geol Soc 159: 203–214 [Google Scholar]
- Rothwell GW. (1995) The fossil history of branching: implications for the phylogeny of land plants. In Hoch PC, Stephenson AG, eds, Experimental and Molecular Approaches to Plant Biosystematics. Missouri Botanical Garden, St. Louis, pp 71–86 [Google Scholar]
- Rothwell GW, Lev-Yadun S. (2005) Evidence of polar auxin flow in 375 million-year-old fossil wood. Am J Bot 92: 903–906 [DOI] [PubMed] [Google Scholar]
- Rothwell GW, Sanders H, Wyatt SE, Lev-Yadun S. (2008) A fossil record for growth regulation: the role of auxin in wood evolution. Ann Mo Bot Gard 95: 121–134 [Google Scholar]
- Rothwell GW, Wyatt SE, Tomescu AMF. (2014) Plant evolution at the interface of paleontology and developmental biology: An organism-centered paradigm. Am J Bot 101: 899–913 [DOI] [PubMed] [Google Scholar]
- Sachs T, Cohen D. (1982) Circular vessels and the control of vascular differentiation in plants. Differentiation 21: 22–26 [Google Scholar]
- Sanders H, Rothwell GW, Wyatt S. (2007) Paleontological context for the developmental mechanisms of evolution. Int J Plant Sci 168: 719–728 [Google Scholar]
- Sanders H, Rothwell GW, Wyatt SE. (2011) Parallel evolution of auxin regulation in rooting systems. Plant Syst Evol 291: 221–225 [Google Scholar]
- Schüßler A, Schwarzott D, Walker C. (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105: 1413–1421 [Google Scholar]
- Smith SE, Read DJ. (2008) Mycorrhizal Symbiosis. Academic Press, Cambridge [Google Scholar]
- Stein WE, Berry CM, Hernick LV, Mannolini F. (2012) Surprisingly complex community discovered in the mid-Devonian fossil forest at Gilboa. Nature 483: 78–81 [DOI] [PubMed] [Google Scholar]
- Stein WE, Mannolini F, Hernick LV, Landing E, Berry CM. (2007) Giant cladoxylopsid trees resolve the enigma of the Earth’s earliest forest stumps at Gilboa. Nature 446: 904–907 [DOI] [PubMed] [Google Scholar]
- Strullu-Derrien C, Kenrick P, Pressel S, Duckett JG, Rioult JP, Strullu DG. (2014) Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: novel insights into ancestral plant-fungus symbioses. New Phytol 203: 964–979 [DOI] [PubMed] [Google Scholar]
- Strullu-Derrien C, Rioult JP, Strullu DG. (2009) Mycorrhizas in upper carboniferous Radiculites-type cordaitalean rootlets. New Phytol 182: 561–564 [DOI] [PubMed] [Google Scholar]
- Strullu-Derrien C, Strullu DG. (2007) Mycorrhization of fossil and living plants. C R Palevol 6: 483–494 [Google Scholar]
- Taylor LL, Banwart SA, Valdes PJ, Leake JR, Beerling DJ. (2012) Evaluating the effects of terrestrial ecosystems, climate and carbon dioxide on weathering over geological time: a global-scale process-based approach. Philos Trans R Soc Lond B Biol Sci 367: 565–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LL, Leake JR, Quirk J, Hardy K, Banwart SA, Beerling DJ. (2009a) Biological weathering and the long-term carbon cycle: integrating mycorrhizal evolution and function into the current paradigm. Geobiology 7: 171–191 [DOI] [PubMed] [Google Scholar]
- Taylor TN, Kerp H, Hass H. (2005) Life history biology of early land plants: deciphering the gametophyte phase. Proc Natl Acad Sci USA 102: 5892–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Klavins SD, Krings M, Taylor EL, Kerp H, Hass H. (2004) Fungi from the Rhynie Chert: a view from the dark side. Trans R Soc Edinb Earth Sci 94: 457–473 [Google Scholar]
- Taylor TN, Krings M. (2005) Fossil microorganisms and land plants: associations and interactions. Symbiosis 40: 119–135 [Google Scholar]
- Taylor TN, Remy W, Hass H, Kerp H. (1995) Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia 87: 560–573 [Google Scholar]
- Taylor TN, Taylor EL, Krings M. (2009b) Paleobotany: The Biology and Evolution of Fossil Plants, Ed 2. Academic Press, Amsterdam [Google Scholar]
- Tomescu AMF, Wyatt SE, Hasebe M, Rothwell GW. (2014) Early evolution of the vascular plant body plan - the missing mechanisms. Curr Opin Plant Biol 17: 126–136 [DOI] [PubMed] [Google Scholar]
- Trewin NH. (1994) Depositional environment and preservation of biota in the Lower Devonian hot-springs of Rhynie, Aberdeenshire, Scotland. Trans R Soc Edinb Earth Sci 84: 433–442 [Google Scholar]
- Trewin NH, Rice CM, editors (2004) The Rhynie Chert Host-Spring System: Geology, Biota and Mineralization, Vol 94, Part 4. Royal Society of Edinburgh, Edinburgh [Google Scholar]
- Wang B, Qiu YL. (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16: 299–363 [DOI] [PubMed] [Google Scholar]