Roots play a key role in determining long life in perennials.
Abstract
Maximum lifespan greatly varies among species, and it is not strictly determined; it can change with species evolution. Clonal growth is a major factor governing maximum lifespan. In the plant kingdom, the maximum lifespans described for clonal and nonclonal plants vary by an order of magnitude, with 43,600 and 5,062 years for Lomatia tasmanica and Pinus longaeva, respectively. Nonclonal perennial plants (those plants exclusively using sexual reproduction) also present a huge diversity in maximum lifespans (from a few to thousands of years) and even more interestingly, contrasting differences in aging patterns. Some plants show a clear physiological deterioration with aging, whereas others do not. Indeed, some plants can even improve their physiological performance as they age (a phenomenon called negative senescence). This diversity in aging patterns responds to species-specific life history traits and mechanisms evolved by each species to adapt to its habitat. Particularities of roots in perennial plants, such as meristem indeterminacy, modular growth, stress resistance, and patterns of senescence, are crucial in establishing perenniality and understanding adaptation of perennial plants to their habitats. Here, the key role of roots for perennial plant longevity will be discussed, taking into account current knowledge and highlighting additional aspects that still require investigation.
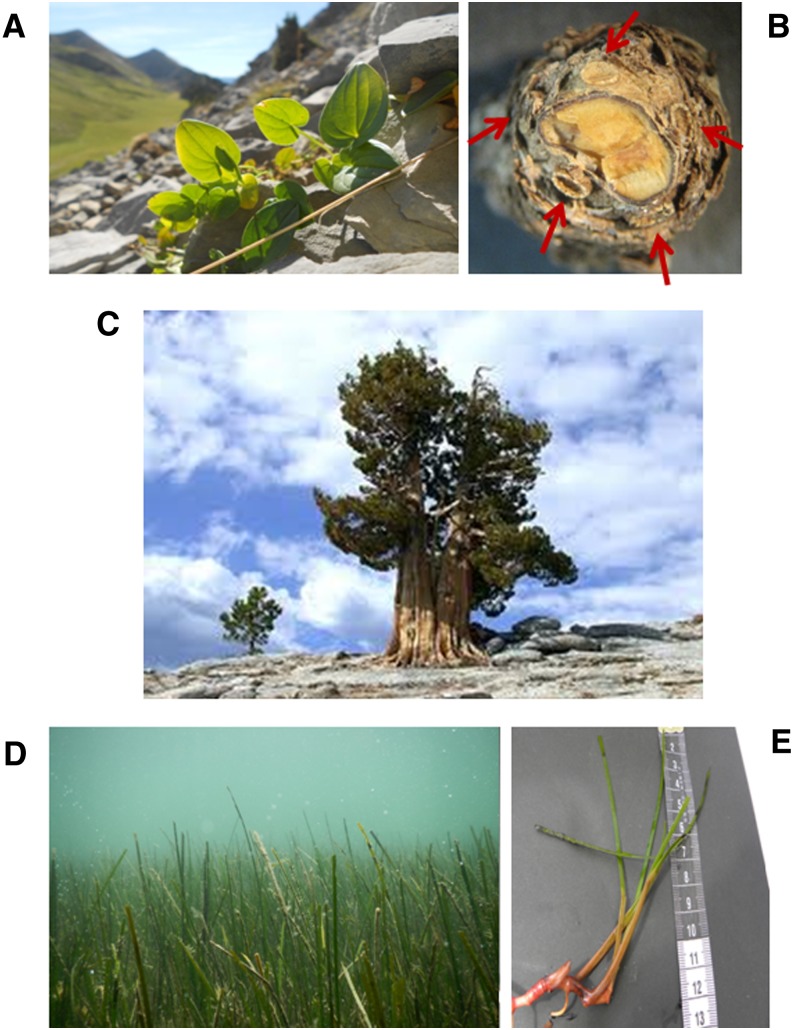
There is enormous diversity among the types of perennial plants and among their patterns of aging (Jones et al., 2014). Perennial plants can be divided into herbaceous (or perennial herbs) and woody perennials (trees and shrubs), and therefore, they represent very diverse organisms in size and complexity from some herbs that weigh a few grams to huge trees like sequoias (Sequoia sempervirens). Among perennial herbs, the slowest growing species described thus far, Borderea pyrenaica (a small geophyte growing in the Central Pyrenees of northeastern Spain), is also the one with the longest maximum lifespan (350 years; Fig. 1). Interestingly, fecundity of this species increases with aging, representing a case of negative senescence (Garcia et al., 2011; Morales et al., 2013). If mortality falls as size increases and if size increases with age, then mortality will fall with age, and negative senescence occurs (Vaupel et al., 2004). Negative senescence is not common in the tree of life, but it seems to occur in not only some perennial herbs, such as B. pyrenaica (Garcia et al., 2011) and Plantago lanceolata (Roach and Gampe, 2004), but also, other phylogenetically distant organisms, such as turtles (Jones et al., 2014). Other perennial herbs with higher biomass production rates and consequently, larger sizes, such as stinging nettle (Urtica dioica), are much shorter-lived (a few years only). In this case, however, perenniality is achieved by allocating an important part of their energy to asexual reproduction (production of stolons; i.e. clonal propagation), giving rise to new entire clonal plants (Koskela, 2002). Indeed, this process happens in several other plant species with rapid growth that we commonly find in gardens, such as strawberries (Fragaria × ananassa) or raspberries (Rubus idaeus). Stolons can be produced aboveground or underground (in the latter case, forming rhizomes). Van Dijk (2009) elegantly reviewed the direct and indirect methods currently used to estimate plant age in clonal and nonclonal plants, showing several examples of plant species using clonal propagation with maximum lifespans of thousands of years, with the most notable example, King’s Lomatia (Lomatia tasmanica), being dated at 43,600 years (Lynch et al., 1998). Only one wild-living clone of this species is known. Clonal propagation is the only means for propagation, because it is a sterile ancient clone. When a branch falls, that branch produces new roots, establishing a new plant that is genetically identical to its parent (Lynch et al., 1998). Here, the production of new roots becomes essential for achieving potential immortality. Another example of extreme longevity is the bristlecone pine (Pinus longaeva), with a maximum lifespan of 5,062 years. It holds the record of longevity of a single individual within the plant kingdom, which was observed by Tom Harlan during 2012 in a living individual of this species in the White Mountains (the location has not been reported; Earle, 2013).
Figure 1.
Examples of extreme longevity in perennial plants. A, B. pyrenaica, the perennial herb with the longest lifespan described to date. B, A cross section of the tuber of B. pyrenaica showing the scars left by the five meristematic points in the spiral. C, P. longaeva, the species with the individual with the longest lifespan ever recorded (not using clonal propagation). D, C. nodosa meadow, with a detail of the rhizomes (E) that allow clonal propagation and potential immortality in this species. [See online article for color version of this figure.]
The enormous diversity in lifespans within a species responds to specific life history traits and mechanisms evolved by each individual to adapt to its habitat. Particularities of roots in perennial plants, such as meristem indeterminacy, modular growth, stress resistance, and patterns of senescence, are crucial in understanding adaptation of perennial plants to their habitats, explaining differences in longevity. Here, the key role of roots in providing long lifespans in perennial plants will be discussed, taking into account current knowledge and highlighting additional aspects that still require investigation.
ROOTS OF PERENNIALITY
Like in chess, in which all pieces have to protect the king, the condition for a long life in a perennial plant is to protect the roots (or at least, the capability to regenerate roots quickly, such as in the example provided above). Roots are essential for nutrient and water uptake, hormone production, shoot growth and reproduction, and many other functions. Most importantly, they bear the central core of the plant and the basis for plant growth and development from germination to senescence: the root meristems. In a simplistic (reduced) view of the biology of a plant, the root and shoot apical meristems, together with the vascular tissues, form the essential core of life. A perennial plant cannot be considered dead until all of its aboveground or underground meristems have died. For instance, a tree will still be alive if just one of the apical shoot meristems and one of the root meristems are alive and if they are connected by a functional vascular system. Therefore, during plant development, all tissues will play an altruistic role to serve the meristems (the kings in the chess metaphor), which in turn, will form as many chess boards (growth modules) as possible, with their own respective kings (meristems) and functional pieces (vascular tissues, leaves, flowers, etc.).
This kind of development is already marked at the embryonic stage, during which a shoot and a root meristem start a new chess game. In an annual plant, the game is short, because at a given stage, all kings produced aboveground decide to finish the game and give rise to reproductive structures, thus allowing the next generation to play chess very quickly. In a perennial plant, chess boards are also provided to the following generation (one time per flowering season), but not all shoot apical meristems enter a determinate flowering stage, so that several chess boards (thousands in a large tree) are distributed and several kings and the corresponding pieces play at the same time and place. In this way, the plant can live much longer, although also at a higher cost and complexity.
Root systems are populations of exploring units that sustain all perennial aboveground development. Root apical meristems allow the plants to explore the soil vertically, whereas lateral meristems develop new roots from the pericycle and provide the possibility to obtain water and nutrients laterally. An endogenous developmental program imposes an ordered arrangement of the position of new lateral roots, but environmental stimuli, such as nutrient levels, or mechanical stimuli also affect the patterning of lateral root production (Richter et al., 2009). The lifespans of cohorts of roots in perennial plants can be very variable, ranging from a few weeks (strawberries and apple trees [Malus × domestica]) to over 35 weeks (sugar maple [Acer saccharum]; Eissenstat and Yanai, 1997). Aside from long lifespans, roots of perennials can sustain long lives thanks to efficient turnover. In conifers, 30% to 86% of the fine roots turn over annually, similar to the values reported for deciduous trees (for review, see Fogel, 1983). Root system development is unique to each species, not only structurally but also temporally. In woody perennials, completion of the vertical expansion of the structural root system may require many years (for instance, 70 years in Scots pine [Pinus sylvestris]; Fogel, 1983).
Tubers are modified roots with a role in storage. They are generally present in geophytes, in which the perennial organ (the tuber) is kept underground for the entire plant lifespan, while it gives rise to aboveground parts seasonally. Examples of plants with true root tubers (not those plants derived from aerial parts, such as potatoes [Solanum tuberosum]) include plants of the genera Mirabilis spp., Smallanthus spp., or a special case of extreme longevity, Borderea spp. (Garcia et al., 2011). B. pyrenaica and Borderea chouardii, which belong to the Dioscoreaceae family, are relict species from the Tertiary that can currently be found in isolated populations in the Central Pyrenees (Garcia et al., 2011). In both cases, the root (a tuber with spiral growth; Fig. 1) houses the secret for long life, which can reach more than 300 years based on scars left in the tuber after each growing season (Garcia et al., 2011). Forced by extreme conditions at 2,100 meters above sea level, the tuber produces aboveground organs during the spring (stem, leaves, and flowers); all aboveground parts enter senescence after reproduction during the summer, but the tuber remains alive. By using one single meristematic point each year from the five possible points, the plant reduces potential damage caused by accumulated mutations in the meristems by 80%. The alternative use of meristematic points in the tuber, dormancy during most of the year, and reduced growth rates can explain long life in this species, in which the tuber plays a crucial role (Morales et al., 2013).
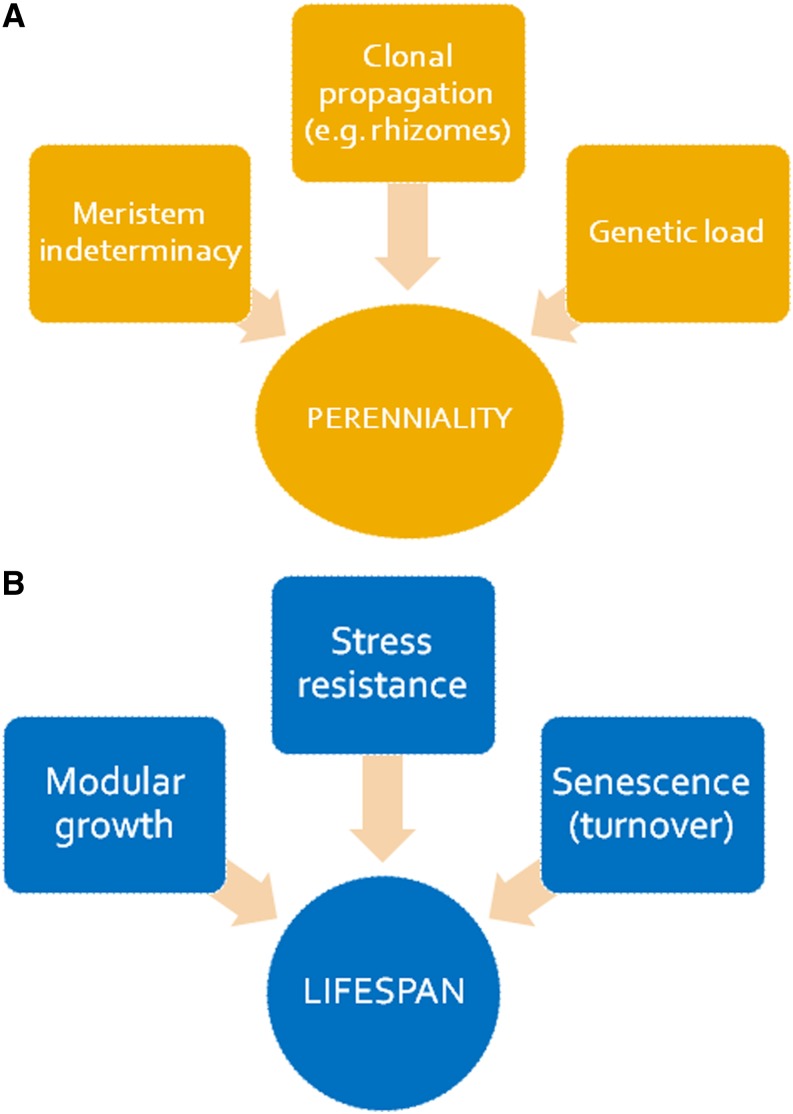
Perennial plants have evolved several times from their annual ancestors during evolution (Soltis et al., 2013). Indeed, Melzer et al. (2008) showed that the silencing of two genes is sufficient for converting Arabidopsis (Arabidopsis thaliana) to a perennial growth habit. Silencing of the genes encoding the MADS box proteins SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 and FRUITFULL, which were previously identified to control flowering time and affect determinacy of all meristems, established phenotypes common to the lifestyle of perennial plants, such as indeterminate growth and the development of shrub-like structures, thus indicating the importance of meristem indeterminacy to establish perenniality in an evolutionary context (Fig. 2). The establishment of perenniality is, however, not always under control of aboveground tissues. Another means to establish perenniality in a given species is through the formation of a rhizome. If a rhizome is separated into pieces, each piece may be able to give rise to a new plant, which is indicative of the rhizome’s potential for propagation. Therefore, rhizomes not only serve a role in reserve (nutrient) storage, but they are also a means for lateral propagation in many species. For instance, in the aquatic little Neptune grass (Cymodocea nodosa), the formation of rhizomes can allow propagation of the plant over hundreds of square kilometers, forming sea grass meadows (Fig. 1; Terrados, 1993). In the genus Oryza spp., the capacity to form rhizomes has evolved in some rice congeners, such as Oryza longistaminata, that show a perennial growth habit. This species is a perennial wild rice with strong rhizomes. Propagation through rhizomes makes the plant (in this case, the clone) potentially immortal. Hu et al. (2011) have shown that the initiation and development of the rhizomatous trait in O. longistaminata are controlled by very complex gene networks involving several plant hormones and regulatory genes. This study has identified several quantitative trait loci, paving the way to the characterization of new genes responsible for this important trait. Quantitative genetic traits also seem to control perenniality in temperate forage grasses. Evidence for this finding comes from studies of crosses between annuals and perennials of the genus Lolium spp. (Thomas, 1995). Similarly, a single chromosome addition from the perennial plant Thinopyrum elongatum is sufficient to confer a perennial growth habit to annual wheat (Lammer et al., 2004). These findings open an exciting area for future research, because they can potentially be used to develop perennial cereal crops for sustainable farming.
Figure 2.
A, Traits that determine perenniality. B, Root traits that determine a long lifespan in perennial plants. [See online article for color version of this figure.]
STRESS RESISTANCE
Evidence obtained thus far indicates that perennial plants are generally more resistant to environmental stresses than annual plants, with traits common to roots of perennial plants conferring stress resistance. Modular growth allows temporal adjustments to the phenotype as new parts are added during development. This way is one way to change the allocation of resources to different functions, such as growth or reproduction, thus providing enormous plant plasticity and potential for adaptation to the environment. Root modularity forms the basis for adaptation of roots of perennial plants to biotic and abiotic stresses. The ability to make new modules at the root level adds plasticity to the response of the plant root as a whole and improves resistance to environmental stresses. Ryser and Eek (2000) evaluated the tradeoffs between acquisition capacities for aboveground and belowground resources in response to different irradiance levels and nitrogen supply in the perennial grasses Dactylis glomerata and Dactylis polygama, the latter being more shade tolerant and exhibiting a longer leaf lifespan. Results showed that phenotypic plasticity tends to maximize resource acquisition and growth rate in the short term, whereas the higher tissue mass density and the longer leaf lifespan of shade-tolerant species indicate reduced loss rates as a more advantageous adaptation to shade in the long term.
Boot et al. (1986) examined the differences in drought response, in terms of mortality, flower production, and water potential, in potted U. dioica (perennial) and Urtica urens (annual) plants exposed to gradual water deficit. U. dioica was more resistant to drought stress than U. urens in terms of mortality. In this species, reduction in mortality rates was associated with drought hardening, a reduction in growth, and a delay in flower production. In contrast, growth and reproduction were maintained at increased risk of drought mortality in U. urens. In this species, water potentials decreased sharply after exposure to drought, which correlated with high mortality rates. Consistent with predictions for adaptive stress resistance, perennial plants allocate proportionately more biomass to roots and rhizomes and produce smaller, thicker, and longer-lived leaves than those plants grown near optimal conditions (Moriuchi and Winn, 2005). Increased resource allocation to roots, particularly in rhizomes, is a stress resistance trait, because as discussed before, roots (and particularly, tubers and rhizomes as perennial storage organs) provide the safety to resume growth when environmental constraints, such as water or nutrient availability, have passed.
In another study, Wahl and Ryser (2000) evaluated the root characteristics of 19 perennial grass species from different habitats and related these parameters to the ecological behavior of the species. Relative growth rate of roots correlated with anatomical characteristics that contribute to root robustness, whereas plant height correlated with characteristics associated with axile root hydraulic conductance. It was found that, for a given root diameter, slow-growing species had smaller, albeit more numerous, xylem vessels, indicating a higher resistance to cavitation and protection against embolisms. Plant height correlated positively with root and xylem cross-sectional area, indicating a need for a high transport capacity in roots of species that attain a large size at maturity.
Dormancy of both aboveground and belowground meristems is another important trait for stress resistance. The positive role played by summer dormancy and to a lesser extent, root depth in enhancing the survival of temperate perennial grasses in Australia, such as orchardgrass (D. glomerata) and tall fescue (Lolium arundinaceum), has been shown (Nie and Norton, 2005). In these studies, the performance of cultivars and populations with these traits has been compared with germplasm without them, which makes this work priceless, unequivocally indicating that dormancy is an important trait conferring stress tolerance in perennial plants.
Sectoriality is another important trait that affects plant responses to biotic and abiotic stressors. Aboveground and belowground growth and development require the bidirectional transport of resources within the plant. However, transport is generally restricted by vascular architecture to specific subunits, known as integrated physiological units (Watson and Casper, 1984). This restricted transport within integrated physiological units is referred to as sectoriality. Split-root experiments with tomato (Lycopersicon esculentum) have shown that fertilization to isolated lateral roots generates heterogeneity in leaf morphology, side-shoot growth, and accumulation of phenolics, thus affecting plant–herbivore interactions. Specifically, leaflets with direct connections to these lateral roots were larger than leaflets in other sectors lacking direct vascular connections. Moreover, side-shoot production was greater in the connected sectors (Orians et al., 2002). This is only one example of how different modules compete for resources and how modularity and sectoriality affect the physiology of perennial plants and their interaction with other organisms and the environment.
SENESCENCE
Senescence can be studied at different levels of organization from cells to entire plants. We will illustrate here as an example the contrasting results that can be obtained in studies with a similar aim but in which different plant parts were analyzed. In the first study, Lanner and Connor (2001) compared the accumulation of somatic mutations in vegetative meristems of bristlecone pines of various ages. No evidence of accumulation of mutations in the meristems was found, regardless of whether the analyzed tree was 23 or up to 4,713 years old. In the second study, Flanary and Kletetschka (2005) found that telomere length and telomerase activity correlate with aging in this species, with samples dated up to 3,500 years, thus suggesting that lifespan is limited by the number of cell divisions. This study, however, used somatic (including both foliar and root) tissues to perform analyses and not meristems. Therefore, this study indicates that the number of cell divisions in somatic tissues of an older individual is more limited than in those tissues from a younger individual. This finding suggests that aging reduces the potential maximum lifespan of modules but not the lifespan of the tree. As long as the tree keeps the capacity to make new modules and maintains viable shoot and root meristems, it will keep the capacity for growth, although obviously at reduced rates. Indeed, this result is what has been shown in leaves as trees age. Photosynthetic and leaf growth rates decline with aging (Mencuccini et al., 2005; Vanderklein et al., 2007), but this age-related decline does not explain increased mortality with aging. Environmental stresses, such as heat waves and extreme drought, are the main cause of mortality in pine trees (van Mantgem et al., 2009).
Root biomass accumulation and depth are key determinants of hydraulic conductance, which together with elevated temperatures, respiration, and biotic factors, strongly influence plant response to extreme drought and mortality in pines and other conifers (McDowell et al., 2008; Brodribb and Cochard, 2009). Consequently, root turnover (including growth and senescence) will be one of the major factors controlling mortality rates in these trees and many other species of temperate climates. Indeed, senescence of fine roots in conifers has been attributed to stresses imposed on the root system by drought and extreme soil temperatures among other factors (Lyr and Hoffman, 1967).
Bingham (2012) investigated the effects of mechanical root damage, shading of the shoot before defoliation, soil temperature, microbial inoculation, and nature of the growth substrate on root longevity in Trifolium pratense. Root longevity, which was defined as the time span until more than 80% of root cells lost viability after shoot excision, was mostly affected by the light environment and the soil temperature, and in general, root senescence was strongly negatively correlated with sugar contents. Webb et al. (2010) also studied root senescence and nitrogen release in the same species after temporary or prolonged abiotic stress. Results showed a strong nitrogen release from roots under prolonged stress, which indicated the start of cellular breakdown (i.e. loss of membrane integrity) of the root system, coinciding with the failure of plants to recover. Interestingly, Webb et al. (2010) characterized a cysteine protease gene (Tp-cp8) that may be of particular importance for root senescence in this species. In a comparative study of root senescence across four species, including Populus tremuloides, Acer rubrum, A. saccharum, and Betula alleghaniensis, Kunkle et al. (2009) evaluated the changes in fine root biomass and nitrogen contents from live to dead roots. Overall, root mass decreased 28% to 40%, nitrogen uncorrected for mass loss increased 10% to 35%, nitrogen per root length decreased 5% to 16%, nitrogen/calcium ratio declined 14% to 48%, and, most importantly, nitrogen corrected for mass declined 12% to 28%. Kunkle et al. (2009) suggest that, given the magnitude of senescence-related root mass loss and uncertainties about calcium dynamics in senescing roots, nitrogen loss corrected for mass loss is likely the most reliable estimate of nitrogen loss and hence, root senescence. Sugar and nitrogen contents seem, therefore, to be determining in the dynamics of root senescence in perennial plants and need to be used together with analyses of hydraulic conductance at the whole-plant level to unravel the causes of mortality in trees and other perennial plants.
CONCLUSION AND PERSPECTIVES
It is concluded that roots play a crucial role in determining the lifespan of perennial species. Tubers are the secret for a long life in several geophytes, such as B. pyrenaica. Rhizomes provide the means for clonal propagation and therefore, potential immortality of the clone, which has been shown in C. nodosa. Meristem indeterminacy, modular growth, stress resistance, and patterns of root turnover are crucial for understanding adaptation to their habitats and long life of trees and other perennial plants.
Aging is a part of life, something that will affect each individual in a given species differently. It should be noted that the maximum lifespan for a given species is not determined, but it can change with evolution. The most notable example that has been studied is humans, for whom maximum lifespan has enormously increased from hunter-gatherers to today (Burger et al., 2012). Unfortunately, there is no information about how the maximum lifespan has been shaped by evolution in any plant species, but it is very likely that the maximum lifespan of a huge diversity of plant species has also changed during evolutionary times. Indeed, it is very likely that we will discover additional examples of extreme longevity within the plant kingdom in the near future. These examples will increase our understanding of how roots contribute to long lifespans in perennial plants and could probably also be used to illustrate our roots to immortality.
Certainly, perennial plants show that regenerative biomedicine is the key for long life in humans, because clonal growth is the crucial step to potential immortality in perennial plants. However, this potential immortality does not mean that the individual will be immortal; it just means that the mentioned individual will not suffer the wear and tear of aging. Clonal perennial plants pay the price of losing individuality (where does a meadow start or stop?). What price will humans pay for replacing their organs thanks to regenerative biomedicine?
Acknowledgments
Melanie Morales and Laura Siles (University of Barcelona) provided pictures for inclusion in Figure 1. I thank Andreas M. Fischer (Montana State University, Bozeman, MT) and two anonymous reviewers for critical reading of the manuscript.
Footnotes
This work was supported by the Spanish Government (project no. BFU2012–32057).
Some figures in this article are displayed in color online but in black and white in the print edition.
References
- Bingham IJ. (2012) Factors affecting the longevity of clover roots following shoot excision and its implications for managing N cycling in arable cropping systems. Soil Biol Biochem 50: 199–207 [Google Scholar]
- Boot R, Raynal DJ, Grime JP. (1986) A comparative study of the influence of drought stress on flowering in Urtical dioica and U. urens. J Ecol 74: 485–495 [Google Scholar]
- Brodribb TJ, Cochard H. (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149: 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger O, Baudisch A, Vaupel JW. (2012) Human mortality improvement in evolutionary context. Proc Natl Acad Sci USA 109: 18210–18214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle CJ (2013) Pinus longaeva. The Gymnosperm Database. http://www.conifers.org/pi/Pinus_longaeva.php (January 15, 2014)
- Eissenstat DM, Yanai RD. (1997) The ecology of root lifespan. Adv Ecol Res 27: 1–60 [Google Scholar]
- Flanary BE, Kletetschka G. (2005) Analysis of telomere length and telomerase activity in tree species of various life-spans, and with age in the bristlecone pine Pinus longaeva. Biogerontology 6: 101–111 [DOI] [PubMed] [Google Scholar]
- Fogel R. (1983) Root turnover and productivity of coniferous forests. Plant Soil 71: 75–85 [Google Scholar]
- Garcia MG, Dahlgren JP, Ehrlén J. (2011) No evidence of senescence in a 300-year-old mountain herb. J Ecol 99: 1424–1430 [Google Scholar]
- Hu F, Wang D, Zhao X, Zhang T, Sun H, Zhu L, Zhang F, Li L, Li Q, Tao D, et al. (2011) Identification of rhizome-specific genes by genome-wide differential expression analysis in Oryza longistaminata. BMC Plant Biol 11: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OR, Scheuerlein A, Salguero-Gómez R, Camarda CG, Schaible R, Casper BB, Dahlgren JP, Ehrlén J, García MB, Menges ES, et al. (2014) Diversity of ageing across the tree of life. Nature 505: 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela T. (2002) Variation in life-history traits among Urtica dioica populations with different history in parasitism by the holoparasitic plant Cuscuta europaea. Evol Ecol 16: 433–454 [Google Scholar]
- Kunkle KE, Palecki M, Ensor L, Hubbard KG, Robinson D, Redmond K, Easterling D. (2009) Trends in twentieth-century U.S. snowfall using a quality-controlled dataset. J Atmos Ocean Tech 26: 33–44 [Google Scholar]
- Lammer D, Cai X, Arterburn M, Chatelain J, Murray T, Jones S. (2004) A single chromosome addition from Thinopyrum elongatum confers a polycarpic, perennial habit to annual wheat. J Exp Bot 55: 1715–1720 [DOI] [PubMed] [Google Scholar]
- Lanner RM, Connor KF. (2001) Does bristlecone pine senesce? Exp Gerontol 36: 675–685 [DOI] [PubMed] [Google Scholar]
- Lynch AJJ, Barnes RW, Cambecèdes J, Vaillancourt RE. (1998) Genetic evidence that Lomatia tasmanica (Proteaceae) is an ancient clone. Aust J Bot 46: 25–33 [Google Scholar]
- Lyr H, Hoffman G. (1967) Growth rates and growth periodicity of tree roots. In Romberger JA, Mikola O, eds, International Review of Forestry Research, Vol 2. Academic Press, New York, pp 181–236 [Google Scholar]
- McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, et al. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178: 719–739 [DOI] [PubMed] [Google Scholar]
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T. (2008) Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat Genet 40: 1489–1492 [DOI] [PubMed] [Google Scholar]
- Mencuccini M, Martínez-Vilalta J, Vanderklein D, Hamid HA, Korakaki E, Lee S, Michiels B. (2005) Size-mediated ageing reduces vigour in trees. Ecol Lett 8: 1183–1190 [DOI] [PubMed] [Google Scholar]
- Morales M, Oñate M, Garcia MG, Munné-Bosch S. (2013) Photo-oxidative stress markers reveal absence of physiological deterioration with ageing in Borderea pyrenaica, an extraordinarily long-lived herb. J Ecol 101: 555–565 [Google Scholar]
- Moriuchi KS, Winn AA. (2005) Relationships among growth, development and plastic response to environment quality in a perennial plant. New Phytol 166: 149–158 [DOI] [PubMed] [Google Scholar]
- Nie Z, Norton MR. (2005) Stress tolerance and persistence of perennial grasses: the role of the summer dormancy trait in temperate Australia. Crop Sci 49: 2405–2411 [Google Scholar]
- Orians CM, Ardón M, Mohammad BA. (2002) Vascular architecture and patchy nutrient availability generate within-plant heterogeneity in plant traits important to herbivores. Am J Bot 89: 270–278 [DOI] [PubMed] [Google Scholar]
- Richter GL, Monshausen GB, Krol A, Gilroy S. (2009) Mechanical stimuli modulate lateral root organogenesis. Plant Physiol 151: 1855–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach DA, Gampe J. (2004) Age-specific demography in Plantago: uncovering age-dependent mortality in a natural population. Am Nat 164: 60–69 [DOI] [PubMed] [Google Scholar]
- Ryser P, Eek L. (2000) Consequences of phenotypic plasticity vs. interspecific differences in leaf and root traits for acquisition of aboveground and belowground resources. Am J Bot 87: 402–411 [PubMed] [Google Scholar]
- Soltis DE, Mort ME, Latvis M, Mavrodiev EV, O’Meara BC, Soltis PS, Burleigh JG, Rubio de Casas R. (2013) Phylogenetic relationships and character evolution analysis of Saxifragales using a supermatrix approach. Am J Bot 100: 916–929 [DOI] [PubMed] [Google Scholar]
- Terrados J. (1993) Sexual reproduction and seed banks of Cymodocea nodosa (Ucria) Ascherson meadows on the southeast Mediterranean coast of Spain. Aquat Bot 46: 293–299 [Google Scholar]
- Thomas HM. (1995) Meiosis of triploid Lolium. II. Discrepancies between the analyses of chromosome configurations at metaphase I in inverse autoallotriploid combinations. Heredity (Edinb) 75: 446–452 [Google Scholar]
- Vanderklein D, Martínez-Vilalta J, Lee S, Mencuccini M. (2007) Plant size, not age, regulates growth and gas exchange in grafted Scots pine trees. Tree Physiol 27: 71–79 [DOI] [PubMed] [Google Scholar]
- Van Dijk H. (2009) Ageing effects in an iteroparous plant species with a variable life span. Ann Bot (Lond) 104: 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mantgem PJ, Stephenson NL, Byrne JC, Daniels LD, Franklin JF, Fulé PZ, Harmon ME, Larson AJ, Smith JM, Taylor AH, et al. (2009) Widespread increase of tree mortality rates in the western United States. Science 323: 521–524 [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Baudisch A, Dölling M, Roach DA, Gampe J. (2004) The case for negative senescence. Theor Popul Biol 65: 339–351 [DOI] [PubMed] [Google Scholar]
- Wahl S, Ryser P. (2000) Root tissue structure is linked to ecological strategies in grasses. New Phytol 148: 459–471 [DOI] [PubMed] [Google Scholar]
- Watson MA, Casper BB. (1984) Morphogenetic constraints on patterns of carbon distribution in plants. Annu Rev Ecol Syst 15: 233–258 [Google Scholar]
- Webb KJ, Jensen EF, Heywood S, Morris SM, Linton PE, Hooker JE. (2010) Gene expression and nitrogen loss in senescing root systems of red clover (Trifolium pratense). J Agric Sci 148: 579–591 [Google Scholar]