Abundant root cortical aerenchyma improves plant growth under nitrogen-limiting conditions by decreasing root metabolic costs, enhancing soil exploration in deep soil strata, thereby increasing nitrogen acquisition at greater depths.
Abstract
Suboptimal nitrogen (N) availability is a primary constraint for crop production in developing nations, while in rich nations, intensive N fertilization carries substantial environmental and economic costs. Therefore, understanding root phenes that enhance N acquisition is of considerable importance. Structural-functional modeling predicts that root cortical aerenchyma (RCA) could improve N acquisition in maize (Zea mays). We evaluated the utility of RCA for N acquisition by physiological comparison of maize recombinant inbred lines contrasting in RCA grown under suboptimal and adequate N availability in greenhouse mesocosms and in the field in the United States and South Africa. N stress increased RCA formation by 200% in mesocosms and by 90% to 100% in the field. RCA formation substantially reduced root respiration and root N content. Under low-N conditions, RCA formation increased rooting depth by 15% to 31%, increased leaf N content by 28% to 81%, increased leaf chlorophyll content by 22%, increased leaf CO2 assimilation by 22%, increased vegetative biomass by 31% to 66%, and increased grain yield by 58%. Our results are consistent with the hypothesis that RCA improves plant growth under N-limiting conditions by decreasing root metabolic costs, thereby enhancing soil exploration and N acquisition in deep soil strata. Although potential fitness tradeoffs of RCA formation are poorly understood, increased RCA formation appears be a promising breeding target for enhancing crop N acquisition.
Nitrogen (N) deficiency is one of the most limiting factors in maize (Zea mays) production worldwide (Ladha et al., 2005). In developing countries such as those in sub-Saharan Africa, less than 20 kg N ha−1 is applied to fields of smallholder farmers due to high fertilizer cost (Azeez et al., 2006; Worku et al., 2007). In developed countries, intensive N fertilization is used to maintain satisfactory yield (Tilman et al., 2002). In the United States, N fertilizers are the greatest economic and energy cost for maize production (Ribaudo et al., 2011). However, less than half of the N applied to crops is actually acquired, and most of the remaining N becomes a source of environmental pollution (Raun and Johnson, 1999; Smil, 1999; Tilman et al., 2002). For example, N and phosphorus (P) effluents into marine systems from agriculture cause eutrophication and hypoxic zones (Diaz and Rosenberg, 2008; Robertson and Vitousek, 2009). Nitrate contamination in surface water and groundwater systems poses serious health risks, such as methemoglobinemia and N-nitroso-induced cancers (UNEP and WHRC, 2007). Emission of nitrous oxides from agricultural activities contributes to ozone damage and global warming (Kulkarni et al., 2008; Sutton et al., 2011). Furthermore, the production of N fertilizers requires considerable energy from fossil fuels, and since energy costs have risen in recent years, farmers face economic pressure from increasing N fertilizer costs, which are linked to higher food prices. It is estimated that a 1% increase in crop N efficiency could save more than $1 billion (U.S.) annually worldwide (Kant et al., 2011). Therefore, even a small improvement in N efficiency would have significant positive impacts on the environment and the economy.
Soil N is heterogenous and dynamic. The bioavailability of soil N depends on the balance between the rates of mineralization, nitrification, and denitrification. These processes are determined by several factors, including soil composition, microbial activity, soil temperature, and soil water status (Miller and Cramer, 2004). The predominant form of soil N available to plants in most agricultural systems is nitrate, which is highly soluble in water and thus mobile in the soil (Barber, 1995; Marschner, 1995). Mineralization of organic matter and/or the application of N fertilizer at the beginning of the growing season followed by precipitation and irrigation create a pulse of nitrate that may exceed the N acquisition capacity of seedlings and leach below the root zone. Therefore, it has been proposed that increasing the speed of root exploration of deep soil strata could benefit N acquisition (Lynch, 2013). However, the structural investments and metabolic expenditures of root systems are substantial and can exceed half of daily photosynthesis (Lambers et al., 2002). Therefore, full consideration of the costs and benefits of root systems is crucial for identifying root traits to improve crop production, especially in water- and nutrient-deficient environments (Lynch, 2007). Taking rhizoeconomics and the spatiotemporal availability of soil N into account, Lynch (2013) proposed a root ideotype for enhanced N acquisition in maize called Steep, Cheap, and Deep, in which Steep refers to architectural phenes and Cheap refers to phenes that reduce the metabolic cost of soil exploration. One element of this ideotype is abundant root cortical aerenchyma (RCA).
RCA consists of enlarged air spaces in the root cortex (Esau, 1977). RCA is known to form in response to hypoxia, and the role of RCA in improving oxygen transport to roots of many plant species under hypoxic conditions has been well researched (Vartapetian and Jackson, 1997; Jackson and Armstrong, 1999; Mano and Omori, 2007, 2013). Interestingly, RCA can also form in response to drought and edaphic stresses such as N, P, and sulfur deficiencies (Drew et al., 1989; Bouranis et al., 2003; Fan et al., 2003; Zhu et al., 2010a), which suggests that the benefit of RCA extends beyond facilitating oxygen transport. Several lines of evidence suggest that RCA enhances root metabolic efficiency under stress. Fan et al. (2003) found that RCA formation significantly reduced root segment respiration and P content of root tissue, which allowed greater shoot growth in soils with low P availability. Under drought, maize genotypes with high RCA formation had greater root length, deeper rooting, better leaf water status, and 8 times greater yield than closely related genotypes with low RCA (Zhu et al., 2010a). Effects of RCA on root respiration were more pronounced for large-diameter roots compared with small-diameter roots (Jaramillo et al., 2013). Results from the functional-structural plant model SimRoot showed that RCA formation could be an adaptive response to deficiency of N, P, and potassium by decreasing the metabolic cost of soil exploration. By reducing root respiration, RCA decreases the carbon cost of soil exploration, and by decreasing the N and P content of root tissue, RCA permits internal reallocation of nutrients to growing root tissue, which is particularly beneficial under conditions of low N and P availability (Postma and Lynch, 2011a). Under suboptimal P availability, RCA increased the growth of a simulated 40-d-old maize plant by 70% (Postma and Lynch, 2011b). In the case of N, RCA increased the growth of simulated maize plants up to 55% in low-N conditions, and plants benefit from RCA more in high-N-leaching environments than in low-N-leaching environments (Postma and Lynch, 2011a). In addition, the formation of RCA decreases critical soil nutrient levels, defined as the soil fertility below which growth is reduced, suggesting that cultivars with high RCA may require less fertilizer under nonstressed conditions. These in silico results suggest that RCA has potential utility for improving crop nutrient acquisition in both high- and low-input agroecosystems.
The overall objective of this research was to assess the utility of RCA for N acquisition in maize under N-limiting conditions. Maize near-isophenic recombinant inbred lines (RILs) sharing a common genetic background (i.e. descending from the same parents) with common root phenotypes but contrasting in RCA formation were grown under N stress to test the hypothesis that RCA formation is associated with reduced root respiration, reduced tissue nutrient content, greater rooting depth, enhanced N acquisition, and therefore greater plant growth and yield under N limitation.
RESULTS
RCA Formation and N Stress
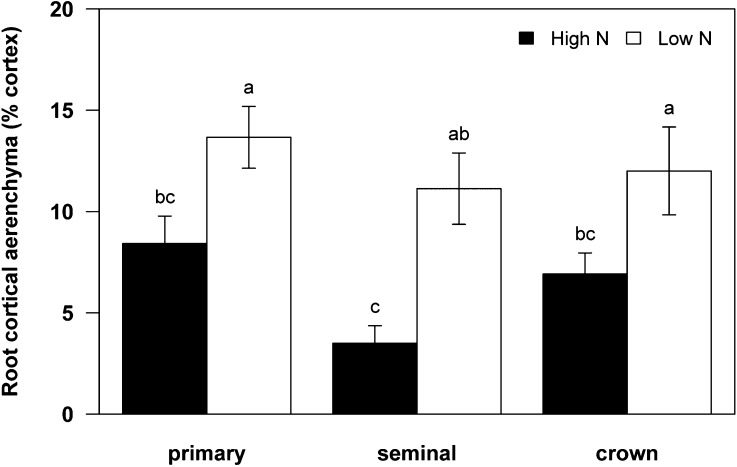
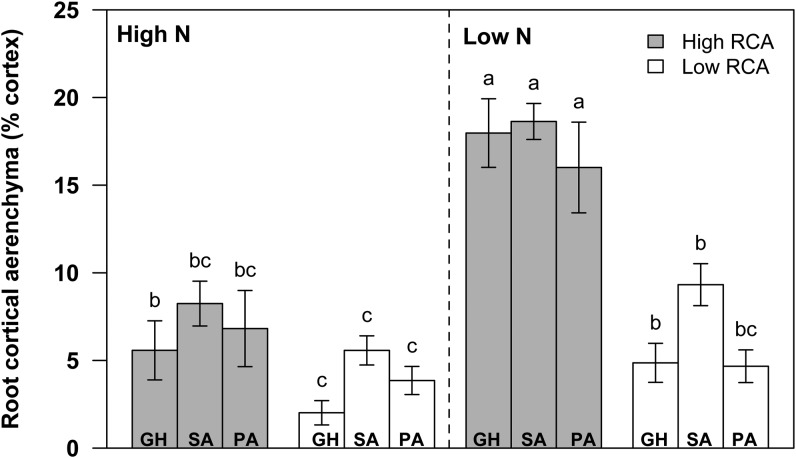
N stress substantially increased RCA of plants grown in mesocosms (GH2010 [see “Materials and Methods”]) by an average of 200% at 35 d after planting (DAP). The increase in RCA was significant in all root classes: primary roots (62%; P = 0.015), seminal roots (218%; P < 0.001), and second whorl crown roots (74%; P = 0.0454; Fig. 1). N stress did not affect root diameter, cortical cell file number, and xylem diameter of the root segments collected 20 to 24 cm from the base of the primary, seminal, and second whorl crown roots (Table I). The genotypes were grouped based on RCA phenotypes in the second whorl crown roots, which has been shown to be the representative position of RCA distribution in the maize root system (Burton et al., 2013b). Low-RCA RILs consisted of 133, 177, and 337, and high-RCA RILs consisted of 196, 199, and 345. We found that the differences among RCA phenotypes were accentuated by low-N treatment. Low-RCA RILs averaged 5% of the root cortical cross-sectional area as RCA, while high-RCA RILs averaged 18% RCA under low-N conditions (Fig. 2).
Figure 1.
Production of RCA as a percentage of cortical area in three root classes of maize harvested at 35 DAP under high-N and low-N conditions in soil mesocosms (GH2010). Data shown are means of four replicates ± se. Different letters represent significant differences (P < 0.05).
Table I. Root anatomical traits of different root classes at 35 DAP in the mesocosms.
Root segments were collected 20 to 24 cm from the base of the primary, seminal, and second whorl crown roots. Data shown are means of four replicates of six RILs grown under high- and low-N conditions. ns, N treatment had no significant effect at P = 0.05.
| Root Class | Treatment | Root Anatomical Traits |
Metaxylem Diameter | ||
|---|---|---|---|---|---|
| RCA | Root Diameter | Cortical Cell File No. | |||
| % | mm | mm | |||
| Primary | High N | 8.42 | 0.77 | 6.42 | 0.070 |
| Low N | 13.67 | 0.72 | 6.32 | 0.070 | |
| P | 0.02 | ns | ns | ns | |
| Seminal | High N | 3.49 | 0.63 | 6.40 | 0.063 |
| Low N | 11.13 | 0.63 | 6.12 | 0.067 | |
| P | 0 | ns | ns | ns | |
| Crown | High N | 6.91 | 0.77 | 7.20 | 0.078 |
| Low N | 12.01 | 0.72 | 7.00 | 0.072 | |
| P | 0.02 | ns | ns | ns | |
Figure 2.
Production of RCA between high-RCA and low-RCA maize RILs grown under high-N and low-N conditions and harvested at 35 DAP in soil mesocosms (GH) in 2010 and at 63 DAP in the field in South Africa (SA) and Pennsylvania (PA). The data shown are means of four replicates ± se. Different letters represent significant differences (P < 0.05) compared within each location.
At the field site in South Africa, N stress increased RCA of the plants by an average of 102% at flowering. Low-RCA RILs (1, 157, and 177) averaged 9% RCA, while high-RCA RILs (31, 34, and 338) averaged 19% RCA under N stress (Fig. 2). At the field site in Pennsylvania, N stress increased RCA of the plants by an average of 94% at flowering. Low-RCA RILs (1, 85, 97, 157, and 165) averaged 5% RCA, while high-RCA RILs (56, 82, 224, 284, and 353) averaged 16% RCA under N stress. RCA of high-RCA RILs was significantly greater than that of low-RCA RILs under low-N conditions in all environments (P < 0.05; Fig. 2).
RCA, Root Respiration, and Root Tissue N Content
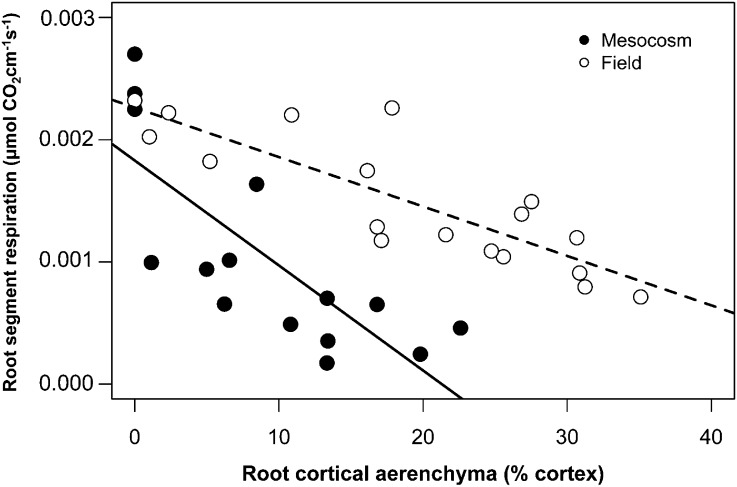
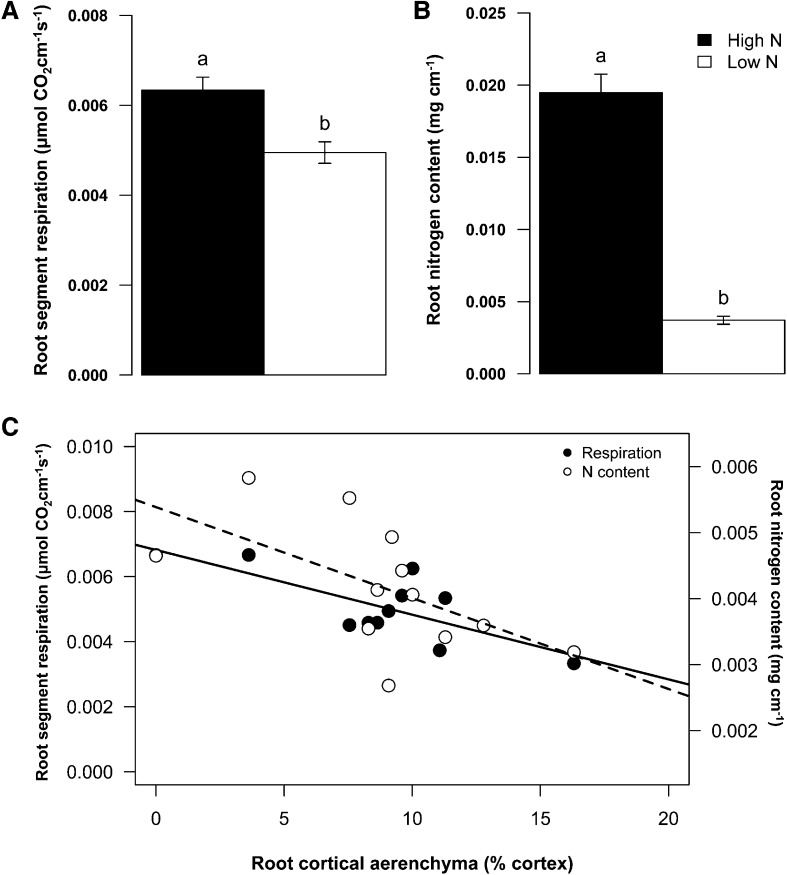
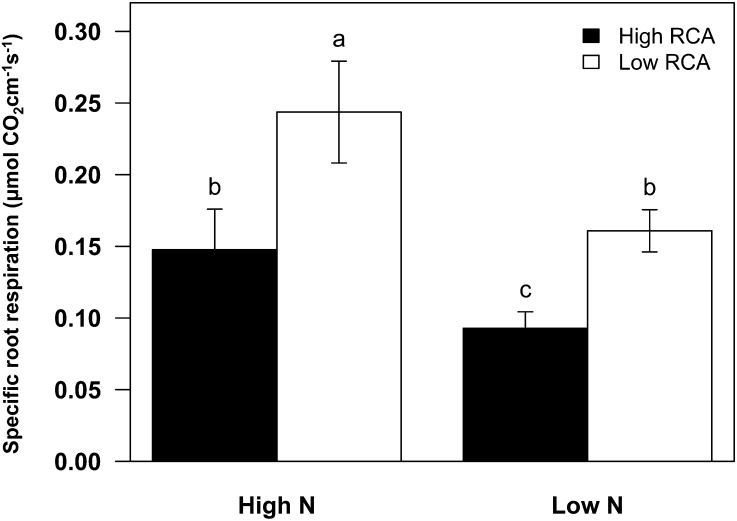
RCA reduced root respiration in both mesocosm studies (GH2010 and GH2013) and in the field (Figs. 3–5). High-RCA RILs had less specific root respiration than low-RCA RILs by 39% under high-N conditions and by 42% under low-N conditions in GH2010 (Fig. 4). In GH2013, N stress reduced root segment respiration of the second whorl crown roots by 1.3-fold and the N content by 5.25-fold (Fig. 5, A and B; P < 0.001). Under low-N conditions RCA was negatively correlated with root segment respiration (r = −0.75, P < 0.05) and root tissue N content (r = −0.60, P < 0.05). The regression equation between root segment respiration and RCA indicated that the conversion of 10% and 11% of cortical area to RCA reduced root segment respiration and N content by 50% (Fig. 5C).
Figure 3.
Negative correlation of root segment respiration with RCA in soil mesocosms (GH2010; r = −0.78, P < 0.001) and in the field (r = −0.85, P < 0.001).
Figure 5.
N stress reduced root segment respiration (A) and root N content (B) in second whorl crown roots in soil mesocosms (GH2013). RCA was negatively correlated with root respiration (r = −0.75, P < 0.05) and N content (r = −0.60, P < 0.05) under low-N conditions (C).
Figure 4.
Specific root respiration (i.e. root respiration per unit of root length derived from the respiration of whole intact root systems) in high- and low-RCA genotypes at 35 DAP in both high- and low-N conditions in the mesocosms in 2010. Data shown are means of four replicates ± se. Different letters represent significant differences (P < 0.05).
RCA and Root Growth
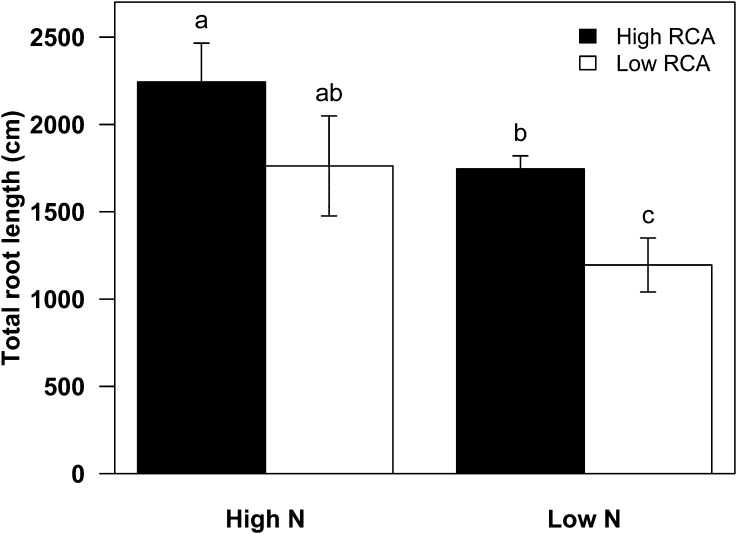
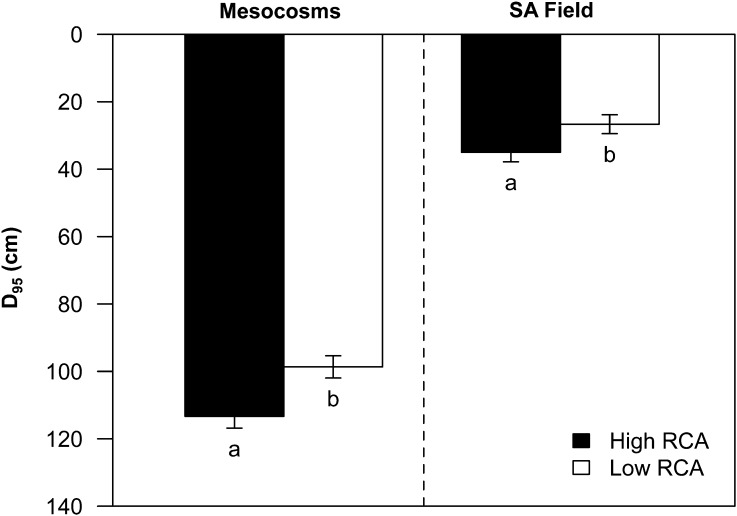
In GH2010, N stress reduced the average total root length of all genotypes by 42%. High-RCA RILs had 35% greater total root length than the low-RCA RILs under low-N conditions (P < 0.05; Fig. 6). N stress increased rooting depth (the depth attained by the 95th percentile of root length [D95]) of all genotypes by 29%. D95 of high-RCA RILs was 15% greater than that of low-RCA RILs under low-N conditions (Fig. 7; Supplemental Fig. S1). In South Africa, the D95 of high-RCA RILs was 31% greater than that of low-RCA RILs at flowering under low-N conditions (Fig. 7).
Figure 6.
Total root length of high- and low-RCA RILs at 35 DAP under high- and low-N conditions in mesocosms (GH2010). Data shown are means of four replicates ± se. Different letters represent significant differences (P < 0.05).
Figure 7.
D95 of maize lines at 35 DAP in mesocosms (GH2010) and at 63 DAP in the field in South Africa (SA) under low-N conditions. Data shown are means of four replicates ± se. Different letters represent significant differences (P < 0.05) within the experiment.
Photosynthesis, N Acquisition, and Shoot Mass
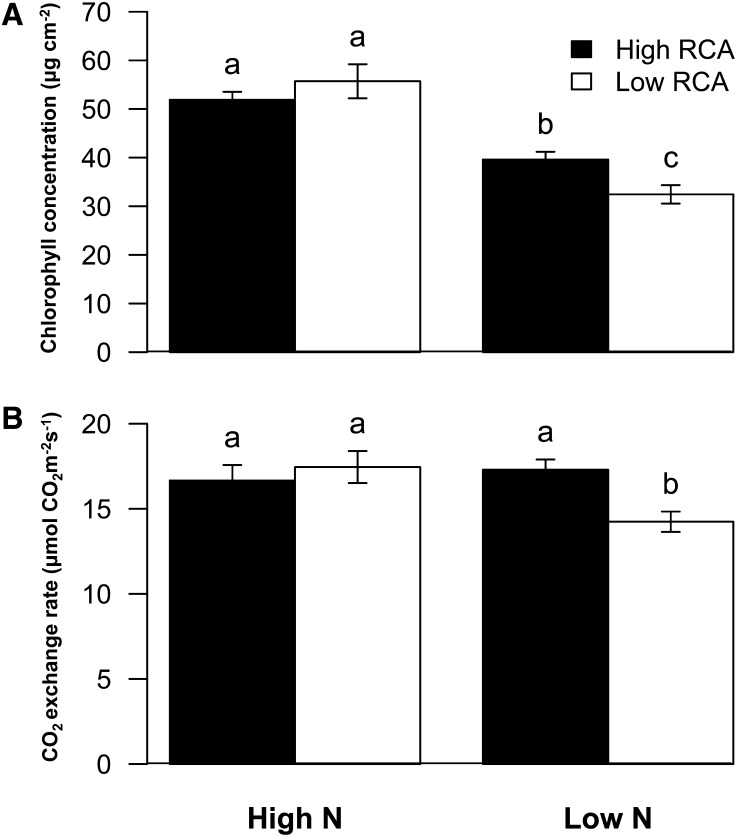
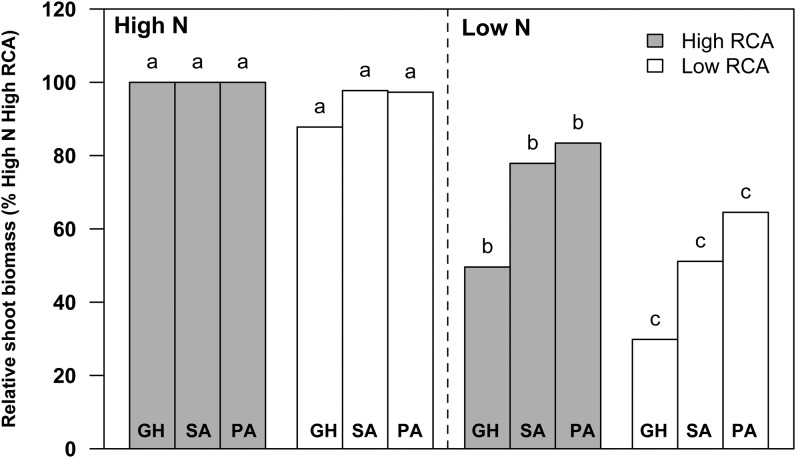
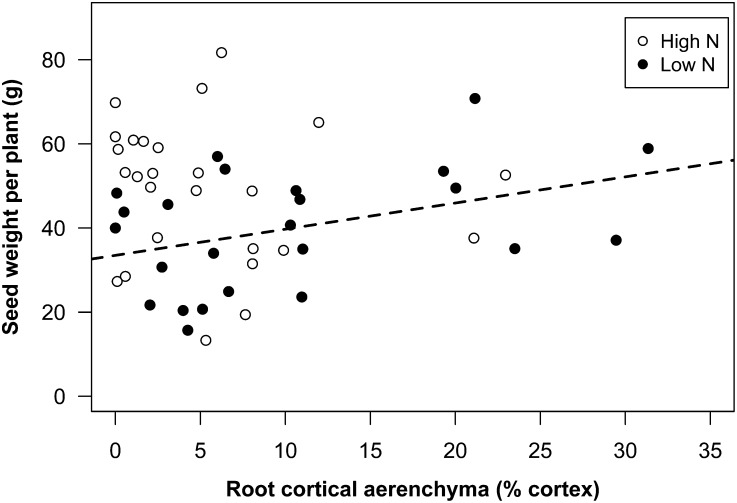
Under low-N conditions in mesocosms the chlorophyll content of high-RCA RILs was 22% greater than that of low-RCA RILs (Fig. 8A). N stress reduced leaf photosynthetic rates on average by 8%. The high-RCA RILs had 22% greater photosynthetic rates than the low-RCA RILs under low-N conditions (Fig. 8B). In GH2010, N stress reduced the shoot biomass of all genotypes by 58%. Under N stress, high-RCA RILs had 66% more shoot mass and 68% greater tissue N content at 35 DAP compared with low-RCA RILs (Fig. 9; Supplemental Fig. S2). In the field in South Africa, N stress reduced shoot mass by an average of 35% at flowering. The high-RCA RILs had 52% greater shoot mass and 81% greater tissue N content than low-RCA RILs at flowering under low-N conditions (Fig. 9; Supplemental Fig. S2). In the field in Pennsylvania, N stress reduced shoot mass by an average of 36% at flowering. The high-RCA RILs had 31% greater shoot mass and 28% greater tissue N content than low-RCA RILs under low-N conditions (Fig. 9; Supplemental Fig. S2). The regression equation between grain yield and RCA indicated that the grain yield of the highest RCA genotypes was 58% greater than that of genotypes with no RCA under low-N conditions (Fig. 10).
Figure 8.
Chlorophyll concentration (A) and photosynthesis rate (B) of high- and low-RCA RILs at 35 DAP in both high- and low-N conditions in mesocosms (GH2010). Data shown are means of four replicates ± se. Different letters represent significant differences (P < 0.05).
Figure 9.
Relative shoot biomass under high-N and low-N conditions at 35 DAP in soil mesocosms (GH) in 2010 and at flowering (63 DAP) in the field in South Africa (SA) and Pennsylvania (PA). The data shown are means of four replicates ± se. Different letters represent significant differences (P < 0.05) compared within each location. Baseline values for shoot mass are as follows: GH = 1.77 g, SA = 75.28 g, and PA = 159.08 g.
Figure 10.
Correlation between yield and percentage of RCA (% cortex) under high-N (not significant) and low-N (r = 0.40, P = 0.05) conditions in the field in Pennsylvania.
DISCUSSION
In this study, we show that N stress induces RCA expression in greenhouse and field conditions, which confirms earlier reports in solution culture (He et al., 1992). This effect was stronger in maize lines with high RCA formation under high N (Fig. 2). Experiments in mesocosms revealed that RCA substantially reduced root respiration and tissue N content (Figs. 3–5). Under suboptimal N availability, high-RCA RILs had greater rooting depth than low-RCA RILs in the field in South Africa (Fig. 7; Supplemental Fig. S1). High-RCA RILs had greater shoot biomass than low-RCA RILs under low-N conditions in all environments observed (Fig. 9). At the field site in Pennsylvania, RCA was associated with 58% increased grain yield under low-N conditions (Fig. 10). Our results are consistent with the hypothesis that RCA enhances N acquisition by reducing root metabolic costs, decreasing tissue N content, and permitting greater rooting depth, enhanced N acquisition, and greater plant growth under suboptimal N conditions.
In this study, we evaluated the utility of RCA in RILs segregating for RCA expression but sharing a common genetic background. In studies of the effects of individual alleles, it is desirable to compare isogenic lines varying for that allele. RCA is a typical quantitative trait controlled by many alleles in unknown ways (Saengwilai 2013). Analysis of three maize RIL populations (B73 × Mo17, OH43 × W64a, and NY821 × H99) identified five quantitative trait loci (QTLs) for aerenchyma area explaining from 4.7% to 9.4% of phenotypic variation and six QTLs for percentage aerenchyma explaining from 5.6% to 12.9% of phenotypic variation (Burton, 2010). Different QTLs were observed in the three populations, and QTLs observed in these maize RILs did not correspond with previously reported QTLs for aerenchyma induced by hypoxia in maize × teosinte (Zea nicaraguensis) crosses (Mano et al., 2007). Therefore, it is not possible to generate simple isogenic lines that vary for RCA formation across maize inbreds: many allele variants and combinations would need to be generated and compared for such a study. This study is focused on the phenome, and specifically on the physiological utility of RCA. For such a study, it is desirable to vary RCA while holding other aspects of the plant phenotype as constant as possible. RILs are ideal for this purpose, since each RIL represents a distinct genotype combining a shared set of alleles from common parents. In these experiments, our goal was to select near-isophenic RILs with common root phenotypes other than RCA, to minimize the potential effects of variation in nodal root number, root growth angles, lateral root branching, and crown root diameter (Supplemental Table S1) in root deployment and N acquisition. An alternative way to compare contrasting isophenic lines is in silico, where every feature of the plant phenotype can be controlled, as accomplished in SimRoot (Postma and Lynch, 2011a, 2011b). The combination of results from the field and from mesocosms is noteworthy, as the field includes variable environmental factors such as soil temperature, soil biota, and soil physical properties that may affect the results, while mesocosms are simplified soil environments that permit greater environmental control and more detailed measurement of root properties. The fact that our results with contrasting RILs in mesocosms and two field environments agree with each other as well as with previous in silico results is strong evidence that they are robust.
We found variation in RCA formation in maize RILs under unstressed conditions and greater RCA formation with suboptimal availability of N. These results are consistent with other studies (He et al., 1992; Zhu et al., 2010a). Interestingly, not all RILs increased RCA in response to N stress, particularly low-RCA RILs (Fig. 2). Genetic variation for the degree of RCA formation in response to N stress suggests that breeders could select for genotypes with consistently high, low, or plastic RCA. The utility of the phenotypic plasticity of RCA is currently unknown, but genetic control and the utility of plastic traits such as root hair length have been documented in maize (Zhu et al., 2010b).
RCA reduces root respiration (Fig. 3; Fan et al., 2003; Zhu et al., 2010a). Root respiration associated with growth, maintenance, and ion uptake are major components of root metabolic costs (Lambers et al., 1996; Lynch and Ho, 2005). Without root maintenance respiration, simulated maize plants had up to 72% greater growth under nutrient-limiting conditions (Postma and Lynch, 2011a, 2011b). An additional benefit of RCA is reallocation of nutrients from cortical tissue, which is predicted by simulation modeling to be an important function in N- and P-deficient plants (Postma and Lynch, 2011a). In this study, we found that high-RCA RILs had less root respiration than low-RCA RILs under both stressed and nonstressed conditions (Fig. 4). High RCA was also associated with reduced root tissue N content in low-N soils (Fig. 5). N in lysed root tissue of high-RCA plants could be reabsorbed and utilized to support plant growth, as evidenced by greater root and shoot growth of high-RCA RILs compared with low-RCA RILs in low-N soils. These results are consistent with responses found under suboptimal availability of P and water (Fan et al., 2003; Zhu et al., 2010a). The results support our hypothesis that reduced root maintenance costs allow high-RCA RILs to support a larger root system and have greater soil exploration than low-RCA RILs.
Fan et al. (2003) showed that 20% RCA reduced root respiration by 50% in seminal root segments of maize. In our study, we found that around 30% RCA is needed to reduce root respiration of crown root segments by half (Fig. 3). Crown and seminal root anatomy are fundamentally similar, but these root classes differ in size and number of cells; crown roots tend to have greater diameter, more cortical cell layers, and larger cortical area (Burton et al., 2013a). It has been shown that root respiration is substantially influenced by living portions of the root segments such as living cells in the cortex (Jaramillo et al., 2013). Since crown roots have a larger proportion of living tissue than seminal roots, we would expect that more RCA would be required in order to significantly affect root respiration in crown roots.
The distribution of roots in soil influences nutrient and water acquisition efficiency. For example, shallow rooting is beneficial for the acquisition of topsoil-available nutrients such as P and potassium (Lynch and Brown, 2001), while deeper rooting allows plants to acquire highly mobile resources such as water and nitrate before they are lost from the root zone (Kristensen and Thorup-Kristensen, 2004; Ho et al., 2005; Postma and Lynch, 2011a; Zhu et al., 2010a). Under low-N conditions, high-RCA RILs had greater D95 in the mesocosms and in the field (South Africa) than the low-RCA RILs (Fig. 7). Since the high-RCA RILs had reduced metabolic costs for root maintenance compared with the low-RCA RILs, the high-RCA RILs are able to support more root growth, resulting in greater rooting depth, which could enhance N acquisition in low-N soils. Enhanced N acquisition in the deep soil profile resulted in greater leaf N content, chlorophyll content, and photosynthesis, which benefitted overall plant growth and yield (Figs. 8–10).
In the field, we found that the utility of RCA was greater in the loamy sand of the South African field site than in the silt loam of Pennsylvania. Although the relative reduction in shoot mass caused by N stress was similar between sites, plants in South Africa were 2.5 times smaller than plants in Pennsylvania under low-N conditions (Fig. 9), which indicates that they suffered from greater stress. The temperature in South Africa was greater than that in Pennsylvania and may have been supraoptimal for these temperate maize lines. At flowering, the shoot biomass of high-RCA RILs in South Africa was 52% greater than that of low-RCA RILs, whereas the shoot biomass of high-RCA RILs in Pennsylvania was only 31% higher than that of low-RCA genotypes. In high-leaching environments such as the loamy sand in South Africa, the benefit of increased rooting depth could be more pronounced, since nitrate leaching is more rapid in coarser soils. These results are consistent with simulation results (Postma and Lynch, 2011a).
Selection for high RCA may indirectly select for greater ethylene sensitivity (He et al., 1992), which may affect other adaptive root traits. In this study, we carefully selected RILs and compared root phenes such as angle, number of crown roots, and root branching under high- and low-N conditions (Supplemental Table S1). We found no significant difference for other root anatomical phenes between high- and low-RCA RILs grown in mesocosms (Table I). We conclude that the results observed in this study are primarily due to contrasting RCA phenotypes.
Knowledge of interactions among phenes is essential in developing ideotypes for nutrient-efficient crops. Interactions among root phenes could result in synergistic or antagonistic effects on resource acquisition. As an example of an antagonistic interaction, increased adventitious rooting in common bean (Phaseolus vulgaris) reduces the growth of lateral roots arising from the tap and basal roots, which results in reduced P acquisition in low-P soils (Walk et al., 2006). As an example of a synergistic interaction, under low-P conditions, common bean gains more benefit from having long root hair length combined with shallow root angle than would be predicted from the additive benefits of each phene in isolation (Miguel, 2011). As for RCA, simulation modeling predicts synergism between RCA and lateral root branching density in maize under low-P conditions (Postma and Lynch, 2011a). Under low-N conditions, RCA benefits metabolically costly root phenes such as a greater number of crown roots, because higher crown root number allows greater volume of soil exploration at the expense of root growth and maintenance (York et al., 2013). Since RCA reduces metabolic costs for root growth in general, we propose that RCA may also be synergistic with root phenes that enhance soil exploration in different soil domains, such as root angle.
Substantial genetic variation for RCA occurs in maize and its relatives in the genus Zea (Burton et al., 2013a). This suggests that there may be costs associated with RCA. It has been shown that RCA contributes to reduced root hydraulic conductivity in maize roots under low-P conditions (Fan et al., 2007). RCA formation also inhibits radial transportation of nutrients such as phosphate and calcium (Hu et al., 2014), although the importance of these small effects in older root segments for nutrient uptake of entire root systems is unclear. In addition, RCA may affect the colonization and spread of microbes within roots. For example, in wheat (Triticum aestivum), cultivars with high root cortical cell death are more susceptible to common root rot (Deacon et al., 1982). RCA may have less effect on disease susceptibility than does cortical cell death, since after RCA formation the epidermis remains intact. RCA formation may reduce mycorrhizal symbiosis, which requires living cortical tissue. RCA may also affect the mechanical strength of roots, especially in plant species that lack a structural support in the outer part of the cortex, although maize was not in that category in a study of resistance to radial compression (Striker et al., 2007). The cost/benefit of RCA and its interactions with other root traits are likely to be complex and may differ in different environments. This merits research.
There is increasing evidence that RCA enhances water and nutrient capture under drought and edaphic stress (Fan et al., 2003; Zhu et al., 2010a; Postma and Lynch, 2011a, 2011b). This report empirically demonstrates the benefit of RCA for N acquisition from low-N soils. Genetic variation of RCA is present in several important agronomic species, including wheat, barley (Hordeum vulgare), sorghum (Sorghum bicolor), rice (Oryza sativa), common bean, and maize (Liljeroth, 1995; Colmer, 2003; Fan et al., 2003; Haque et al., 2010, 2012; Zhu et al., 2010a; Promkhambut et al., 2011), making RCA amenable to plant breeding. We suggest that increased RCA formation may be a promising breeding target for enhancing N acquisition from low-N soils and for reducing the N requirement of high-input agriculture.
MATERIALS AND METHODS
Greenhouse Mesocosm Study
Plant Materials
Seeds of maize (Zea mays) RILs from the Intermated B73 and Mo17 (IBM) population were obtained from Dr. Shawn Kaeppler (University of Wisconsin, Madison; Senior et al., 1996; Kaeppler et al., 2000). Previous screening indicated that RILs 337, 133, and 177 had low RCA and RILs 196, 199, and 345 had high RCA under low-N conditions. These RILs were planted in greenhouse mesocosms in 2010 (GH2010). A set of six IBM RILs (14, 111, 106, 43, 101, and 199) was planted in greenhouse mesocosms in 2013 (GH2013) to examine the effect of RCA on root tissue N content.
Experimental Design
The experiments were arranged in a randomized complete block design. The factors were two N regimes (high- and low-N conditions), six RILs, and four replicates over four blocks. Planting was staggered 1 d between replicates with time of planting as a block effect.
Growth Conditions
Plants were grown from October 4 to November 24, 2010, for GH2010 and from September 23 to October 29, 2013, for GH2013. The greenhouse is located on the campus of Pennsylvania State University in University Park (40°48′N, 77°51′W), with a photoperiod of 14/10 h at 28°C/24°C. Seeds were soaked for 1 h in a fungicide solution consisting of benomyl (Benlate; E.I. DuPont) and 1.3 m metalaxyl (Allegiance; Bayer CropScience) and then were surface sterilized in 10% (v/v) NaOCl for 1 min. The seeds were pregerminated in rolled germination paper (Anchor Paper) soaked with 0.5 mm CaSO4 and placed in darkness at 28°C in a germination chamber for 2 d. At planting, the plants were transferred to mesocosms consisting of polyvinyl chloride cylinders 15.7 cm in diameter and 160 cm in height. The mesocosms were lined with transparent high-density polyethylene film to facilitate root sampling at harvest. The growth medium consisted of a mixture (volume based) of 50% medium-size (0.5–0.3 mm) commercial-grade sand (Quikrete), 35% horticultural vermiculite, 5% Perlite (Whittemore), and 10% topsoil. The topsoil was collected from the Russell E. Larson Agricultural Research Center in Rock Springs, PA (fine, mixed, semiactive, mesic Typic Hapludalf, pH 6.7, silt loam). Thirty-three liters of the mixture was used in each mesocosm to ensure the same bulk density of the media. One day before planting, the mesocosms were saturated with 5 L of a nutrient solution adjusted to pH 6. In GH2010, the nutrient solution for the high-N treatment consisted of (in μm): NO3 (7,000), NH4 (1,000), P (1,000), potassium (3,000), calcium (2,000), SO4 (500), magnesium (500), chlorine (25), boron (12.5), manganese (1), zinc (1), copper (0.25), molybdenum (0.25), and Fe-diethylene triamine pentaacetic acid (100). For the low-N treatment, NO3 and NH4 were reduced to 70 and 10 μm, respectively. In GH2013, nitrate was used as the only N source for both high- and low-N treatments. Two germinated seeds were sown per mesocosm and were thinned after 4 d to one plant per mesocosm. Plants were watered every other day with 100 mL of deionized water. Environmental data were collected hourly in the greenhouse using a HOBO U10-003 data logger (Onset). Soil solutions were collected at 20-cm depth intervals weekly using a microsampler 2.5 mm in diameter and 9 cm in length (Soilmoisture Equipment). The solutions were stored at −80°C until processing. The concentrations of nitrate in the solutions were determined using the vanadium (III) chloride protocol according to Doane and Horwáth (2003).
Root Sampling, Root Segment Respiration, and Root Distribution in Mesocosms
Shoots and roots were harvested at 35 DAP. At harvest, the polyethylene liners were removed from the mesocosms and laid on a root washing station. Root segments were collected 20 to 24 cm from the base of the primary, seminal, and second whorl crown roots. The samples were stored in 75% (v/v) ethanol at 4°C until processing and analysis. For root distribution studies, the liners were divided into 20-cm segments starting from the base of the shoot. Roots were cut and separated from each segment by carefully washing with tap water. The roots were preserved in 75% (v/v) ethanol. Total root lengths were obtained by scanning and analyzing preserved root samples using WinRHIZO Pro (Régent Instruments). Whole-root respiration was measured 1 d before harvest in GH2010 according to Jaramillo et al. (2013). In short, an acrylic plate was placed around a single plant on the top of the mesocosm and carefully sealed with modeling clay around the stem of the plant. The plate was connected to an LI-6200 infrared gas analyzer (IRGA; LI-COR) with polyethylene tubing to measure the respiration of the whole-root system. Carbon dioxide concentration was monitored for 2 min for each plant. Root respiration per unit of length was calculated by dividing the rate of whole-root respiration by the total root length obtained by WinRHIZO Pro as described above. Root segment respiration was measured on three 4-cm root segments of second-whorl crown roots in GH2010 and on three 8-cm root segments in GH2013. The segments were excised 20 cm from the base of the root, and lateral roots were removed with a Teflon-coated blade. Twenty minutes after excision, the samples were placed in a chamber connected to an LI-6200 IRGA (LI-COR) in GH2010 and to an LI-6400 IRGA (LI-COR) in GH2013. For both experiments, the temperature of the chamber was maintained at 27°C using a water bath. Carbon dioxide evolution from the root segments was recorded every 5 s for 180 s. After the respiration measurements, the root segments were stored in 75% (v/v) ethanol for anatomical analysis.
RCA Measurement
In GH2010, root cross sections were obtained by hand sectioning with Teflon-coated double-edged stainless steel blades (Electron Microscopy Sciences). The root sections were examined on a Diaphot inverted light microscope (Nikon) at 2.8× magnification. Three sections were selected as subsamples for image capture. The microscope was fitted with a black-and-white XC-77 CCD Video Camera Module (Hamamatsu). ImageMaster 5.0 software (Photon Technology International) was used to capture and save images. Analysis of images was performed in MatLab 7.6 2008a (The MathWorks) using RootScan, which is a program for semiautomated image analysis of anatomical traits in root cross sections (Burton et al., 2012). RCA was expressed as a percentage of the root cortical area. In GH2013, the roots were ablated using laser ablation tomography (Saengwilai, 2013). In brief, laser ablation tomography is a semiautomated system that uses a laser beam to vaporize or sublimate the root at the camera focal plane ahead of an imaging stage. The sample is incremented, vaporized or sublimated, and imaged simultaneously. The cross-section images were taken using a Canon T3i camera with a 5× microlens (MP-E 65 mm) on the laser-illuminated surface.
Shoot Dry Weight and Plant N Status
For both GH2010 and GH2013, 1 d prior to harvest, leaf gas exchange of the second youngest fully expanded leaves was measured with an LI-6400 IRGA (LI-COR) using a red-blue light at photosynthetically active radiation intensity of 1,200 μmol photons m−2 s−1 and constant CO2 concentration of 400 µL L−1. At harvest, 6-mm-diameter leaf discs were collected from the second youngest fully expanded leaves for chlorophyll measurement. Chlorophyll was extracted in 80% (v/v) acetone. The concentrations of chlorophyll a and b in the extracts were determined at wavelengths of 663.2 and 646.8 nm with a spectrophotometer (Lichtenthaler and Buschmann, 2001). Shoots and root segments were dried at 60°C for 72 h prior to dry weight determination. The shoots were ground, and 2 to 3 mg of ground tissues was used for tissue N analysis using an elemental analyzer (Series II CHNS/O Analyzer 2400; PerkinElmer).
Field Studies
Field Conditions, Experimental Design, and Plant Materials
Experiments were carried out from February to April in 2010 at Alma, Limpopo Province, Republic of South Africa (24°33′00.12S, 28°07′25.84E, 1235 meters above sea level) and from June to August in 2011 at the Russell Larson Research and Education Center of Pennsylvania State University in Rock Springs (40°42′37′′0.52N, 77°57′07′′0.54W, 366 meters above sea level). The soils at the experimental sites were a Clovelly loamy sand (Typic Ustipsamment) in Alma and Hagerstown silt loam (fine, mixed, semiactive, mesic Typic Hapludalf) in Rock Springs. Based on soil analysis at the beginning of the growing season, N fertilizers were applied at the rate of 30 kg N ha−1 five times until flowering, resulting in 150 kg N ha−1 in total for high-N plots at Alma. Low-N plots received 30 kg N ha−1 only at the beginning of the growing season. At Rock Springs, fields were amended with 915 g m−2 sawdust to immobilize soil N. High-N plots were fertilized with 150 kg N ha−1 urea, while low-N plots did not receive any N fertilizer. In both environments, soil nutrient levels of other macronutrients and micronutrients were adjusted to meet the requirements for maize production as determined by soil tests. Pest control and irrigation were carried out as needed. Based on previous experiments conducted in the field (P. Saengwilai, K.M. Brown, and J.P. Lynch, unpublished data), six IBM RILs consisting of low-RCA RILs (1, 157, and 177) and high-RCA RILs (31, 34, and 338) were planted at Alma and 10 IBM RILs consisting of low-RCA RILs (1, 85, 97, 157, and 165) and high-RCA RILs (56, 82, 224, 284, and 353) were planted at Rock Springs. The experiments were arranged in a split-plot design, with the two N treatments as the whole-plot factor and genotype as the split-plot factor. Five-row plots of each genotype (6 m long) were randomly assigned within each whole plot. Row width was 75 cm, and distance within a row was 23 cm, resulting in a planting density of 5.8 plants m−2. The plants were harvested at 9 weeks after planting (flowering stage) at the South African and Pennsylvania fields.
Root Sampling, Root Segment Respiration, and Root Distribution in the Field
At harvest, three 4-cm root segments of second whorl crown roots were excised from 8 to 12 cm away from the base of the root, and lateral roots were removed with a Teflon-coated blade. The three root segments were placed in a tube chamber connected to a LI-6400 IRGA (LI-COR). The temperature of the chamber was maintained at 27°C using a water bath. Carbon dioxide evolution from the root segments was recorded every 5 s for 180 s. After the respiration measurements, the root segments were stored in 75% (v/v) ethanol for anatomical analysis.
For root distribution, soil cores were taken within a planting row midway between two plants with soil-coring equipment (Giddings Machine). The cores were divided into 10-cm segments, and roots were extracted from each soil segment.
Root length was obtained as described previously for mesocosm samples. Percentages of root length at each depth were calculated in each soil core. D95 was calculated by linear interpolation between the cumulative root lengths (Trachsel et al., 2013).
Shoot Dry Weight, Chlorophyll Measurements, Tissue N Content, and Yield
One day prior to harvest, leaf gas exchange of the ear leaves was measured with a LI-6400 IRGA (LI-COR) using a red-blue light at photosynthetically active radiation intensity of 1,800 μmol photons m−2 s−1 and constant CO2 concentration of 360 µL L−1. At Rock Springs, 6-mm-diameter leaf discs were collected from the ear leaves for chlorophyll measurement. Chlorophyll was extracted in 80% (v/v) acetone. The concentrations of chlorophyll a and b in the extracts were determined at wavelengths of 663.2 and 646.8 nm with a spectrophotometer (Lichtenthaler and Buschmann, 2001). Shoots were dried at 60°C for 72 h prior to dry weight determination. The leaves and stems were ground, and 2 to 3 mg of ground tissues was taken for tissue N analysis using an elemental analyzer (Series II CHNS/O Analyzer 2400; PerkinElmer). Yield was collected at physiological maturity in the field study in Pennsylvania.
Statistical Analysis
Statistical analyses were performed using R version 2.15.1 (R Development Core Team). Linear mixed-effect models were fit using the function lme from the package nlme (Pinheiro et al., 2012), and a two-way ANOVA was used for comparisons between high- and low-RCA groups (or individual RILs), N levels, and the interaction between these main effects. A protected lsd posthoc test (α = 0.05) and Tukey’s honestly significant difference method (α = 0.05) were used for multiple comparisons. Correlations and linear regressions were carried out between shoot and root traits with RCA and root respiration and between RCA and yield.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Root length density at different soil depths in mesocosms and in the field in South Africa.
Supplemental Figure S2. Relative tissue nitrogen content at 35 DAP in soil mesocosms and at flowering (63 DAP) in the field in South Africa and Pennsylvania.
Supplemental Table S1. Root phenotypes of maize lines.
Supplementary Material
Acknowledgments
We thank Bob Snyder, Curtis Frederick, and Johan Prinsloo for the management of the experiments in greenhouse mesocosms and in the field in the United States and South Africa; Francis Harriman, Gina Riggio, and Michael Williams for assistance with sectioning and image analysis; and Larry M. York and Johannes Postma for review of the article.
Glossary
- N
nitrogen
- P
phosphorus
- RCA
root cortical aerenchyma
- RIL
recombinant inbred line
- DAP
days after planting
- D95
the depth attained by the 95th percentile of root length
- QTLs
quantitative trait loci
- IBM
Intermated B73 and Mo17
- IRGA
infrared gas analyzer
Footnotes
This work was supported by the National Science Foundation/Basic Research to Enhance Agricultural Development (grant no. 4184–UM–NSF–5380) and the Howard G. Buffett Foundation.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Azeez J, Adetunji M, Lagoke S. (2006) Response of low-nitrogen tolerant maize genotypes to nitrogen application in a tropical Alfisol in northern Nigeria. Soil Tillage Res 91: 181–185 [Google Scholar]
- Barber SA (1995) Soil Nutrient Bioavailability: A Mechanistic Approach. John Wiley & Sons, New York [Google Scholar]
- Bouranis DL, Chorianopoulou SN, Siyiannis VF, Protonotarios VE, Hawkesford MJ. (2003) Aerenchyma formation in roots of maize during sulphate starvation. Planta 217: 382–391 [DOI] [PubMed] [Google Scholar]
- Burton AL (2010) Phenotypic evaluation and genetic basis of anatomical and architectural traits in the genus Zea PhD thesis. Pennsylvania State University, University Park [Google Scholar]
- Burton AL, Brown KM, Lynch JP. (2013a) Phenotypic diversity of root anatomical and architectural traits in Zea species. Crop Sci 53: 1042–1055 [Google Scholar]
- Burton AL, Lynch JP, Brown KM. (2013b) Spatial distribution and phenotypic variation in root cortical aerenchyma of maize (Zea mays L.). Plant Soil 367: 263–274 [Google Scholar]
- Burton AL, Williams M, Lynch JP, Brown KM. (2012) RootScan: software for high-throughput analysis of root anatomical traits. Plant Soil 357: 189–203 [Google Scholar]
- Colmer TD. (2003) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann Bot (Lond) 91: 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon JW, Lewis SJ, Road WM. (1982) Natural senescense of the root cortex of spring wheat in relation to susceptibility to common root rot (Cochliobolus sativus) and growth of a free-living nitrogen-fixing bacterium. Plant Soil 66: 13–20 [Google Scholar]
- Diaz RJ, Rosenberg R. (2008) Spreading dead zones and consequences for marine ecosystems. Science 321: 926–929 [DOI] [PubMed] [Google Scholar]
- Doane TA, Horwáth WR. (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36: 2713–2722 [Google Scholar]
- Drew MC, He CJ, Morgan PW. (1989) Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiol 91: 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K (1977) Anatomy of Seed Plants, Ed 2. John Wiley & Sons, New York [Google Scholar]
- Fan M, Bai R, Zhao X, Zhang J. (2007) Aerenchyma formed under phosphorus deficiency contributes to the reduced root hydraulic conductivity in maize roots. J Integr Plant Biol 49: 598–604 [Google Scholar]
- Fan M, Zhu J, Richards C, Brown KM, Lynch JP. (2003) Physiological roles for aerenchyma in phosphorus-stressed roots. Funct Plant Biol 30: 493–506 [DOI] [PubMed] [Google Scholar]
- Haque E, Oyanagi A, Kawaguchi K. (2012) Aerenchyma formation in the seminal roots of Japanese wheat cultivars in relation to growth under waterlogged conditions. Plant Prod Sci 15: 164–173 [Google Scholar]
- Haque ME, Abe F, Kawaguchi K. (2010) Formation and extension of lysigenous aerenchyma in seminal root cortex of spring wheat (Triticum aestivum cv. Bobwhite line SH 98 26) seedlings under different strengths of waterlogging. Plant Root 4: 31–39 [Google Scholar]
- He CJ, Morgan PW, Drew MC. (1992) Enhanced sensitivity to ethylene in nitrogen- or phosphate-starved roots of Zea mays L. during aerenchyma formation. Plant Physiol 98: 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. (2005) Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol 32: 737–748 [DOI] [PubMed] [Google Scholar]
- Hu B, Henry A, Brown KM, Lynch JP. (2014) Root cortical aerenchyma inhibits radial nutrient transport in maize (Zea mays). Ann Bot (Lond) 113: 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Armstrong W. (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1: 274–287 [Google Scholar]
- Jaramillo RE, Nord EA, Chimungu JG, Brown KM, Lynch JP. (2013) Root cortical burden influences drought tolerance in maize. Ann Bot (Lond) 112: 429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF. (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low P and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40: 358–364 [Google Scholar]
- Kant S, Bi YM, Rothstein SJ. (2011) Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J Exp Bot 62: 1499–1509 [DOI] [PubMed] [Google Scholar]
- Kristensen HL, Thorup-Kristensen K. (2004) Root growth and nitrate uptake of three different catch crops in deep soil layers. Soil Sci Soc Am J 68: 529–537 [Google Scholar]
- Kulkarni MV, Groffman PM, Yavitt JB. (2008) Solving the global nitrogen problem: it’s a gas! Front Ecol Environ 6: 199–206 [Google Scholar]
- Ladha JK, Pathak HP, Krupnik TJ, Six J, van Kessel C. (2005) Efficiency of fertilizer nitrogen in cereal production: retrospect and prospect. Adv Agron 87: 86–156 [Google Scholar]
- Lambers H, Atkin O, Millenaar F (2002) Respiratory patterns in roots in relation to their functioning. In Y Waisel, A Eshel, T Beeckman, U Kafkafi, eds, Plant Roots: The Hidden Half, Ed. 3. Marcel Dekker, New York, pp 521–552 [Google Scholar]
- Lambers H, Atkin O, Scheurwater I (1996) Respiratory patterns in roots in relation to their functioning. In Y Waisel, A Eshel, T Beeckman, U Kafkaki, eds, Plant Roots: The Hidden Half, Ed. 2. Marcel Dekker, New York, pp 323–362 [Google Scholar]
- Lichtenthaler HK, Buschmann C. (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 431–438 [Google Scholar]
- Liljeroth E. (1995) Comparisons of early root cortical senescence between barley cultivars, Triticum species and other cereals. New Phytol 130: 495–501 [DOI] [PubMed] [Google Scholar]
- Lynch JP. (2007) Rhizoeconomics: the roots of shoot growth limitations. HortScience 42: 1107–1109 [Google Scholar]
- Lynch JP. (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot (Lond) 112: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. (2001) Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant Soil 237: 225–237 [Google Scholar]
- Lynch JP, Ho MD. (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269: 45–56 [Google Scholar]
- Mano Y, Omori F. (2007) Breeding for flooding tolerant maize using “teosinte” as a germplasm resource. Plant Root 1: 17–21 [Google Scholar]
- Mano Y, Omori F. (2013) Relationship between constitutive root aerenchyma formation and flooding tolerance in Zea nicaraguensis. Plant Soil 370: 447–460 [Google Scholar]
- Mano Y, Omori F, Takamizo T, Kindiger B, Bird RM, Loaisiga CH, Takahashi H. (2007) QTL mapping of root aerenchyma formation in seedlings of a maize × rare teosinte “Zea nicaraguensis” cross. Plant Soil 295: 103–113 [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants, Ed 2. Academic Press, San Diego [Google Scholar]
- Miguel M (2011) Functional role and synergistic effect of root traits for phosphorus acquisition efficiency and their genetic basis in common bean (Phaseolus vulgaris L.). PhD thesis. Pennsylvania State University, University Park [Google Scholar]
- Miller AJ, Cramer MD. (2004) Root nitrogen acquisition and assimilation. Plant Soil 274: 1–36 [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team.(2012) nlme: linear and nonlinear mixed effects models, R package version 3.1-103 ed. http://CRAN.Rproject.org/package=nlme [Google Scholar]
- Postma JA, Lynch JP. (2011a) Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol 156: 1190–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Lynch JP. (2011b) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Ann Bot (Lond) 107: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promkhambut A, Polthanee A, Akkasaeng C, Younger A. (2011) Growth, yield and aerenchyma formation of sweet and multipurpose sorghum (Sorghum bicolor L. Moench) as affected by flooding at different growth stages. Aust J Crop Sci 5: 954–965 [Google Scholar]
- Raun WR, Johnson GV. (1999) Improving nitrogen use efficiency for cereal production. Agron J 91: 357–363 [Google Scholar]
- Ribaudo M, Delgado J, Hansen L, Livingston M, Mosheim R, Williamson J (2011) Nitrogen in Agricultural Systems: Implications for Conservation Policy. US Department of Agriculture, Washington, DC
- Robertson GP, Vitousek PM. (2009) Nitrogen in agriculture: balancing the cost of an essential resource. Annu Rev Environ Resour 34: 97–125 [Google Scholar]
- Saengwilai P (2013) Root traits for efficient nitrogen acquisition and genome-wide association mapping of root anatomical traits in maize (Zea mays L.). PhD thesis. Pennsylvania State University, University Park [Google Scholar]
- Senior ML, Chin ECL, Lee M, Smith JSC, Stuber C. (1996) Simple sequence repeat markers developed from maize sequences found in the GENBANK database: map construction. Crop Sci 36: 1676–1683 [Google Scholar]
- Smil V. (1999) Nitrogen in crop production: an account of global flows. Global Biogeochem Cycles 13: 647–662 [Google Scholar]
- Striker GG, Insausti P, Grimoldi AA, Vega AS. (2007) Trade-off between root porosity and mechanical strength in species with different types of aerenchyma. Plant Cell Environ 30: 580–589 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Oenema O, Erisman JW, Leip A, van Grinsven H, Winiwarter W. (2011) Too much of a good thing. Nature 472: 159–161 [DOI] [PubMed] [Google Scholar]
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. (2002) Agricultural sustainability and intensive production practices. Nature 418: 671–677 [DOI] [PubMed] [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP. (2013) Maize root growth angles become steeper under low N conditions. Field Crops Res 140: 18–31 [Google Scholar]
- UNEP and WHRC (2007) Reactive Nitrogen in the Environment: Too Much or Too Little of a Good Thing. United Nations Environment Programme, Paris [Google Scholar]
- Vartapetian BB, Jackson MB. (1997) Plant adaptations to anaerobic stress. Ann Bot (Lond) 79: 3–20 [Google Scholar]
- Walk TC, Jaramillo R, Lynch JP. (2006) Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 279: 347–366 [Google Scholar]
- Worku M, Bänziger M, Schulte auf’m Erley G, Friesen D, Diallo AO, Horst WJ. (2007) Nitrogen uptake and utilization in contrasting nitrogen efficient tropical maize hybrids. Crop Sci 47: 519–528 [Google Scholar]
- York LM, Nord EA, Lynch JP. (2013) Integration of root phenes for soil resource acquisition. Front Plant Sci 4: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Brown KM, Lynch JP. (2010a) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33: 740–749 [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang C, Lynch JP. (2010b) The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Funct Plant Biol 37: 313–322 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.