Extended branching order classification describes morphological variability in root systems and respiration better than traditional root diameter classes or root orders alone.
Abstract
Within branched root systems, a distinct heterogeneity of traits exists. Knowledge about the ecophysiology of different root types is critical to understand root system functioning. Classification schemes have to match functional root types as closely as possible to be used for sampling and modeling. Among ecophysiological root traits, respiration is of particular importance, consuming a great amount of carbon allocated. Root architecture differs between the four deciduous tree seedlings. However, two types of terminal root segments (i.e. first and second orders), white colored and brown colored, can be distinguished in all four species but vary in frequency, their morphology differing widely from each other and higher coarse root orders. Root respiration is related to diameter and tissue density. The use of extended root ordering (i.e. order and color) explains the variance of respiration two times as well as root diameter or root order classes alone. White terminal roots respire significantly more than brown ones; both possess respiration rates that are greater than those of higher orders in regard to dry weight and lower in regard to surface area. The correlation of root tissue density to respiration will allow us to use this continuous parameter (or easier to determine dry matter content) to model the respiration within woody root systems without having to determine nitrogen contents. In addition, this study evidenced that extended root orders are better suited than root diameter classes to picture the differences between root functional types. Together with information on root order class frequencies, these data allow us to calculate realistic, species-specific respiration rates of root branches.
In response to environmental parameters, whole-root systems exhibit high plasticity at different hierarchical scales, such as physiology, anatomy, morphology, and/or biomass (Deak and Malamy, 2005; Gruber et al., 2011). However, within branched systems of single-root units, a distinct heterogeneity of root traits is also present, especially in but not limited to woody root systems (Rewald et al., 2011). Thus, a key to the understanding of the functioning of root systems is knowledge of the traits of individual root segments, especially those related to water and nutrient uptake as well as carbon invested. Currently, several systems are used to classify roots within a branching root system. It is important that classes, when used as a basis for root sampling, are not arbitrary but matched as closely as possible to functional categories (Pregitzer et al., 1998); thus, classification schemes that are able to represent different types of root segments best have to be identified.
The most commonly used classification system in woody plants is based on root diameters (Böhm, 1979), usually distinguishing rather ephemeral fine roots (diameter = 0–2 mm) from woody coarse roots (diameter ≥ 2 mm). Root diameter is one of the most important input parameters for root and rhizosphere modeling (Himmelbauer et al., 2004), likely because of the convenient determination by image analyses programs or during hand sorting. In contrast, two principal ways to number segments are used: centrifugal (i.e. basal to distal) or centripetal (i.e. distal to basal) ordering (Uylings et al., 1975). Today, the centrifugal ordering scheme is most commonly used (Fitter, 1996), especially by researchers describing root system development and root phenology of crop species (Chen et al., 2011; Clark et al., 2013; Leitner et al., 2014), but it has also been applied on tree roots (Pregitzer et al., 1997; Majdi et al., 2001). In contrast, ordering in centripetal systems (Strahler, 1957) is initiated at most distal segments, and order number is increased when two root segments of equal order meet (Pregitzer, 2002). Although the dynamics of centripetal systems are opposite to those of root system development, they group functionally similar terminal (most distal) tree segments, such as root tips, into the same order. In addition to those approaches, several researchers have successfully used individual classification parameters to distinguish root segments according to color (Goldfarb et al., 1990; Bouma et al., 2000) or characteristic branching patterns (e.g. cluster roots; Neumann and Martinoia, 2002). With respect to color, tree roots are predominantly regarded to be white when first produced, turning brown with age (Wells and Eissenstat, 2003).
The respiration of CO2 from fine roots is an important component of the terrestrial carbon cycle. Root respiration (RR) accounts for 25% to 60% of total soil respiration (Pregitzer et al., 1997; Epron et al., 1999; Dannoura et al., 2006a, 2006b) and consumes up to 75% of carbon allocated to roots (Majdi et al., 2007). Predictions on whole-plant carbon budget estimate that total plant respiration is about one-half of gross primary production (Chapin et al., 2012). In temperate forests, belowground net primary production is about 40% of total net primary production. However, large uncertainties remain in quantifying the allocation of carbon to tree root systems in general and the amount of RR of the whole-tree carbon budget in particular. Therefore, factors that describe the metabolic activity of roots and associated microbes are an important component of determining plant carbon budgets and allocation pattern more precisely. Physiologically, fine RR is critical for root maintenance and growth and one important variable determining the uptake efficiency of roots (George et al., 2003) alongside construction costs (Poorter, 1994). Ion transport across membranes may account for 25% to 50% of RR (Lambers et al., 2008). Previous measurements emphasized that fine RR rates can be highly variable (Pregitzer et al., 1998; Makita et al., 2009). This variability is probably partially because of the arbitrary classification of fine roots based on diameter rather than an anatomical or physiological basis (Bouma et al., 2000; Makita et al., 2009). Although positive correlations between fine RR and nitrogen contents have been established, with young roots having greater nitrogen contents, much of the variation in RR rate within a diameter class must be caused by other factors (Pregitzer et al., 1998). In the last decade, evidence increased that root traits often vary according to the position of individual roots segments among the root branching hierarchy (Pagès and Kervella, 1990; Pregitzer et al., 2002; Wang et al., 2006; Guo et al., 2008a, 2008b; Valenzuela-Estrada et al., 2008; Beyer et al., 2013) and that analysis by root order is one powerful approach to understand complex woody root systems under stress (Rewald et al., 2012). Thus, it is surprising that few attempts (Jia et al., 2013) have been made to test if root order-based classification is covering root system heterogeneity in respiration in a meaningful way and morphological parameters on which it may be based.
The aim of this study is to describe the relationship between RR of four deciduous European tree species and continuous root morphological traits and root classes. Our hypotheses are that: (1) root architectural and morphological traits of woody tree species can be best described by extended centripetal root order classification and (2) RR is highly reflected by morphological traits. (3) Additionally, if sampling classes are used, extended root order classification has a significantly greater explanatory value than root diameter classes. Furthermore, we evaluate if upscaling of respiration rates per root order class can be used to compare species-specific RR rates—information that is scarce for European tree species.
RESULTS
Plant Allometry
The seedlings of the four species differ in total biomass, ranging from 30 (Acer platanoides) to 64 g (Quercus robur). Biomass is distributed differently between aboveground organs and the root system (Table I), whereas the root mass fractions (RMFs) of Q. robur and Tilia cordata seedlings are 0.34 and 0.36, respectively, and A. platanoides and Carpinus betulus have RMFs of 0.47 and 0.42, respectively. Significant differences of biomass distribution exist between root order classes. In A. platanoides, root orders ≥ 3 hold significantly more biomass than terminal root orders; the same tendency is found in Q. robur and T. cordata root systems (Table I). In contrast, the root system of C. betulus is composed of a significantly greater amount of brown 1 + 2 roots, indicating a more terminally branched root system. All four species possess relatively small amounts of white terminal roots. However, the relative biomass of root order class 1 + 2 white on the total root system is significantly greater in A. platanoides seedlings (6.2%) compared with the three other species (0.1%–2.8%).
Table I. Absolute plant biomass per organ (grams dry weight) and relative biomass distribution between the three extended root order classes ≥3, 1 + 2 brown, and 1 + 2 white (percentage).
Different small letters indicate significant biomass differences within each species, and different capital letters indicate significant differences of the relative root biomass between species within the same root order class (mean ± se, n = 5; Tukey test, P < 0.05).
| Organ |
A. platanoides |
C. betulus |
Q. robur |
T. cordata |
||||
|---|---|---|---|---|---|---|---|---|
| Biomass | Relative Root Biomass | Biomass | Relative Root Biomass | Biomass | Relative Root Biomass | Biomass | Relative Root Biomass | |
| g | % | g | % | g | % | g | % | |
| Leaves | 3.8 ± 0.4 b | — | 8.5 ± 0.7 b | — | 3.8 ± 0.6 c | — | 2.8 ± 0.6 cd | — |
| Stem | 12.1 ± 1.3 a | — | 16.3 ± 0.9 a | — | 37.8 ± 4.1 a | — | 20.6 ± 1.1 a | — |
| Root order ≥ 3 | 9.6 ± 0.9 a | 70.1 ± 4.4 A | 7.4 ± 0.4 a | 41.1 ± 3.1 B | 15.6 ± 2.0 b | 71.8 ± 1.9 A | 7.9 ± 0.8 b | 59.9 ± 3.5 A |
| Root order 1 + 2, brown | 3.4 ± 0.7 b | 23.7 ± 4.5 B | 10.3 ± 1.0 b | 56.0 ± 3.3 A | 6.5 ± 1.3 bc | 28.1 ± 1.9 B | 5.0 ± 0.6 bc | 38.0 ± 3.4 B |
| Root order 1 + 2, white | 0.8 ± 0.1 b | 6.2 ± 0.9 A | 0.5 ± 0.1 c | 2.8 ± 0.7 B | 0.03 ± 0.02 c | 0.1 ± 0.1 B | 0.3 ± 0.1 d | 2.2 ± 0.8 B |
Root Morphology
Significant differences in morphological parameters were found between the three distinguished root order classes and/or species (Table II). In general, root order classes ≥ 3 of all four species have a significantly larger root diameter, smaller specific root area (SRA), and lower root tissue density (RTD) than terminal root orders 1 + 2. No significant differences were found between species in regard to root diameter and SRA of root orders ≥ 3. However, T. cordata root segments of the order class ≥ 3 have a significantly lower RTD (approximately 25%–34%) than C. betulus and Q. robur roots, with A. platanoides roots’ tissue density being midway between them. In a comparison of classification approaches (root order or diameter based), SRA and RTD of root order class ≥ 3 and diameter class ≥ 2 mm (coarse roots) are similar, because most root orders ≥ 3 had a diameter above 2 mm (data not shown).
Table II. Root diameter, SRA, and RTD of the species A. platanoides, C. betulus, Q. robur, and T. cordata arranged by root order classes ≥3, 1 + 2 brown, and 1 + 2 white.
Different small letters indicate significant differences of parameters between root order classes and within species, and different capital letters indicate significant difference of parameters between species within the same root order class (mean ± se, n = 5–18; Tukey-Kramer test, P < 0.05).
| Root Order Class | Root Diameter |
SRA |
RTD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acer sp. | Carpinus sp. | Quercus sp. | Tilia sp. | Acer sp. | Carpinus sp. | Quercus sp. | Tilia sp. | Acer sp. | Carpinus sp. | Quercus sp. | Tilia sp. | |
| mm | cm2 g−1 | g cm−3 | ||||||||||
| Root order ≥ 3 | 2.60 ± 0.16 aA | 2.36 ± 0.20 aA | 2.40 ± 0.13 aA | 2.66 ± 0.10 aA | 44 ± 4 cA | 38 ± 4 bA | 40 ± 2 bA | 45 ± 2 bA | 0.40 ± 0.02 aBC | 0.47 ± 0.02 aA | 0.44 ± 0.02 aAB | 0.35 ± 0.02 aC |
| Root order 1 + 2, brown | 0.42 ± 0.02 bA | 0.38 ± 0.02 bAB | 0.39 ± 0.02 cAB | 0.32 ± 0.01 cB | 712 ± 114 bA | 665 ± 45 aA | 441 ± 29 aB | 667 ± 60 aA | 0.13 ± 0.01 bB | 0.18 ± 0.01 bAB | 0.25 ± 0.01 bA | 0.25 ± 0.04 bA |
| Root order 1 + 2, white | 0.59 ± 0.02 bC | 0.67 ± 0.04 bBC | 1.30 ± 0.16 bA | 0.81 ± 0.06 bB | 877 ± 61 aA | 654 ± 46 aA | 540 ± 162 aA | 750 ± 122 aA | 0.08 ± 0.00 cA | 0.09 ± 0.00 cA | 0.08 ± 0.01 cA | 0.08 ± 0.01 cA |
Differences in root morphology between the brown and white root order classes 1 + 2 are evidenced most strongly by RTD (Table II); white terminal roots of all four species have significant one-third to two-third lower RTD compared with brown root order class 1 + 2. Across species, white terminal roots are thicker than brown terminal roots; however, the differences were found to be significant in T. cordata and Q. robur seedlings only, with approximately 2.5 to 3 times larger average root diameters of white terminal roots. In regard to SRA, white root order class 1 + 2 tended to possess a larger SRA then brown terminal roots in A. platanoides, Q. robur, and T. cordata, although this difference was only significant between A. platanoides terminal root classes. Significant species-specific differences in terminal root morphology exist (e.g. A. platanoides seedlings have the significantly largest diameter of brown terminal roots and the significantly smallest diameter of white terminal root orders; Table II). No significant differences of white root orders 1 + 2 were found between species in regard to SRA and RTD. The root morphological parameters are not shown for root diameter class 0 to 2 mm (fine roots) because of the overrepresentation of white terminal roots in the applied sampling scheme; in general, however, all values of brown root orders 1 + 2 change slightly toward the values of white root order class 1 + 2 if expressed as diameter class 0 to 2 mm, because only eight roots (i.e. A. platanoides, 3; C. betulus, 2; Q. robur, 3) of the root order class ≥ 3 were thinner than 2 mm in diameter.
To test for a possible relation between RTD and root dry matter content (RDMC) in roots of woody seedlings, a nonlinear correlation analysis was performed per species (Supplemental Fig. S1). The R2 results indicate that that RDMC is exponentially related to RTD by 83% to 96%. A transspecies R2 of 0.90 was calculated [RTD(RDMC) = 0.03e(6.07 × (RDMC − 0.58)].
RR
RR was calculated based on root segment dry weight (RRDWT), surface area (RRSA), and tissue volume (RRVOL). RRDWT values were related to either the continuous variables root diameter and RTD (Figs. 1 and 2) or the root order and diameter classes that were decided (Fig. 3). RRSA and RRVOL values are only displayed according to root order classes (Supplemental Fig. S2).
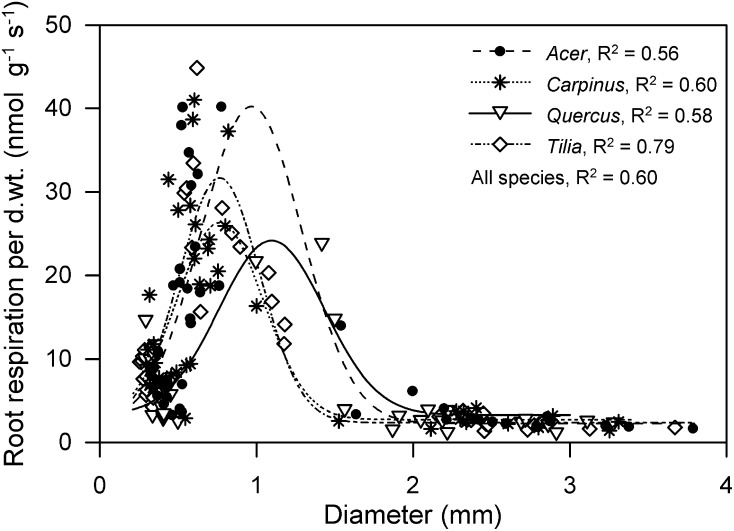
Figure 1.
RRDWT (nanomoles gram−1 second−1) of the species A. platanoides, C. betulus, Q. robur, and T. cordata in relation to root diameter. Gauss curves were fitted per species and all data points (line not drawn). The R2 results (P < 0.001) indicate that between 56% and 79% of the between root segments variance in respiration is accounted for by the diameter (per species, n = 33–45; all species, n = 164). d.wt., Dry weight.
Figure 2.
RRDWT (nanomoles gram−1 second−1) of the species A. platanoides, C. betulus, Q. robur, and T. cordata in relation to RTD. Exponential decay curves were fitted per species and all data points (line not drawn). The R2 results (P < 0.001) indicate that between 67% and 78% of the between root segments variance in respiration is accounted for by the tissue density (per species, n = 33–45; all species, n = 164). d.wt., Dry weight.
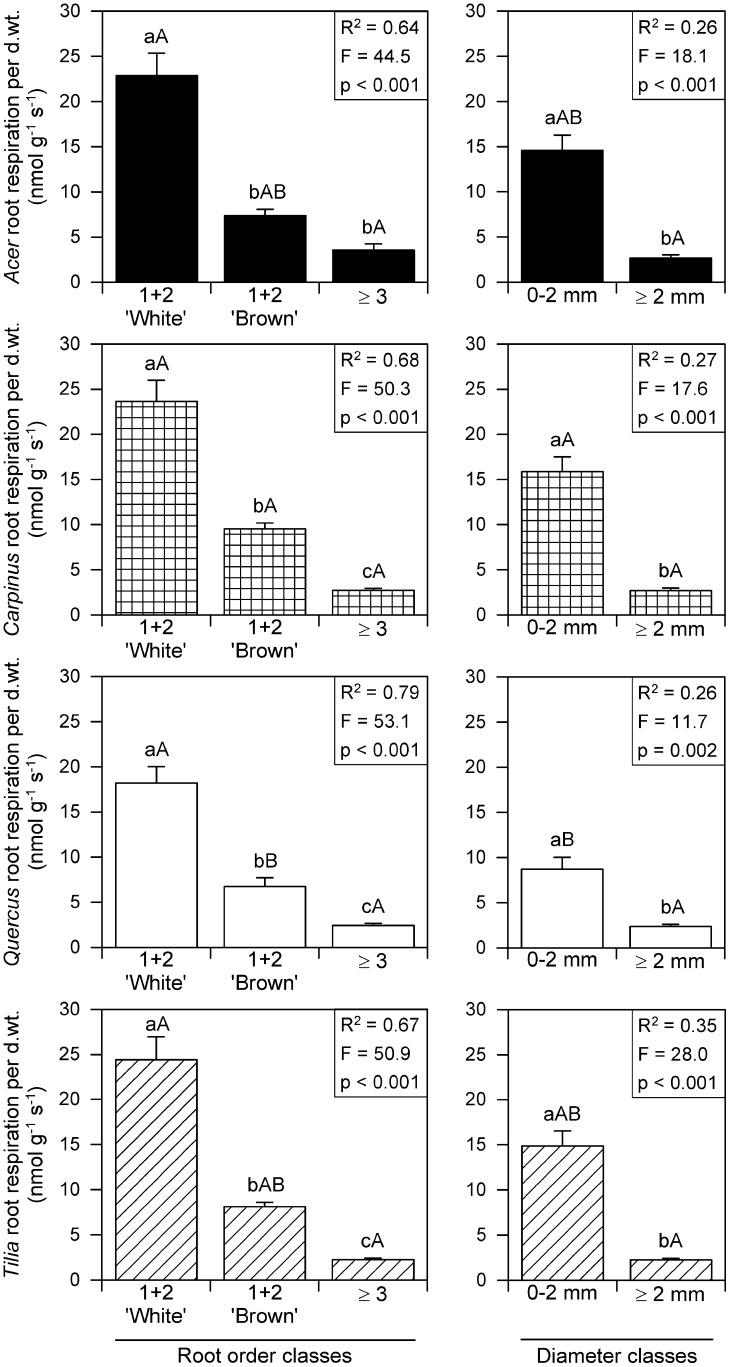
Figure 3.
RRDWT (nanomoles gram−1 second−1) of the species A. platanoides, C. betulus, Q. robur, and T. cordata arranged by root order classes 1 + 2 white, 1 + 2 brown, and ≥3 (left) or root diameter classes 0 to 2 mm (fine roots) and ≥2 mm (coarse roots; right). Different small letters indicate significant differences between classes within species, and different capital letters indicate significant difference between species within the same class (mean + se; Tukey-Kramer test, P < 0.05; root order classes, n = 5–18; diameter classes, n = 11–39). Key results from a one-way ANOVA (proc general linear model) with the class variables order classes (left) or diameter classes (right) and the dependent variable RRDWT are given in insets. The R2 results indicate that between 64% and 69% of the between root segments variance in respiration is accounted for by root order class sorting and between 26% and 35% of the between root segments variance in respiration is accounted for by root diameter class. d.wt., Dry weight.
RRDWT of the four species varies between 1.0 and 44.9 nmol of CO2 g−1 s−1 under the given experimental conditions. The RRDWT of all four species showed a unimodal distribution in regard to root diameter (0.2–3.8 mm); all species possess distinct maxima of RRDWT and the largest variability around 1 mm of root diameter: the white terminal roots (Fig. 1). Thus, RRDWT < 12 nmol g−1 s−1 is common for roots <0.5 and >2 mm in diameter. Interpreting the R2 results as percentages of variance indicates that between 56% and 79% of the between root segments variance in respiration is accounted for by the diameter if fitted with a unimodal (Gauss) curve (Fig. 1). The equation (R2 = 0.60, P < 0.0001) describing the RR rates per dry weight in relation to root diameter for the four species combined is RRDWT(diameter) = 3.09 + 24.78 × exp(−((diameter − 0.81)/0.38)2). RRDWT of all four species showed an exponential decay distribution in regard to RTD (0.02–0.75 g cm−3); all species possess maxima of RRDWT in the root segments with the lowest RTD (Fig. 2). The R2 values indicate that between 67% and 78% of the between root segments variance in respiration is accounted for by the RTD, which is a greater percentage compared with the relation of RRDWT to root diameter. The transspecies equation (R2 = 0.67, P < 0.0001) describing RRDWT relation to RTD is RRDWT(RTD) = 3.28 + exp(−15.49 × (RTD − 0.27)).
Arranged by either root order or diameter classes, the lower classes (i.e. terminal roots) possess significantly greater RRDWT than root segments of orders ≥ 3 (Fig. 3, left) or diameters ≥ 2 mm (Fig. 3, right). In all four species, white root orders 1 + 2 have a significant, up to 3-fold greater RRDWT than brown terminal root orders; average RRDWT of white terminal root segments varies between 18.2 nmol g−1 s−1 (Q. robur) and 23.6 nmol g−1 s−1 (C. betulus). Significant differences were found between the RRDWT of the brown root order class 1 + 2; C. betulus roots (9.5 nmol g−1 s−1) respired significantly (approximately 40%) more per dry weight. than Q. robur roots (6.7 nmol g−1 s−1), with A. platanoides and T. cordata brown terminal roots RRDWT values in between them. No significant differences in RRDWT were found between species’ more mature roots (i.e. root order class ≥ 3 or ≥2 mm in diameter). A comparison of the coefficient of determinations (R2; Fig. 3, insets) shows that the root order-based classification of root segments RRDWT provides better fits than the classical root diameter approach in all four species.
If RR is not expressed related to segment dry weight (Fig. 3) but to surface area (RRSA) or tissue volume (RRVOL), different patterns appear (Supplemental Fig. S2). In A. platanoides, C. betulus, and T. cordata, RRSA of root order class ≥ 3 is significantly greater than RRSA of both terminal root order classes; in Q. robur, it is greater than the 1 + 2 brown root order class (because of low sample nos. of 1 + 2 white). In all four species, the RRSA of the root order class 1 + 2 brown is significantly lowest—with means around 0.01 nmol cm−2 s−1. Significant species-specific differences were found between the RRSA of A. platanoides 1 + 2 white root segments and Q. robur and T. cordata roots of the same class, with Acer spp. RRSA being significantly lower. T. cordata RRSA of root order class ≥ 3 is significantly lower than the RRSA values of A. platanoides and C. betulus. Interestingly, RRVOL shows the most distinct species-specific pattern. RRVOL of 1 + 2 brown and ≥3 root order classes is not significantly different in A. platanoides and C. betulus, whereas RRVOL white and brown terminal roots do not differ significantly in both Q. robur and T. cordata (Supplemental Fig. S2).
Two-way ANOVA results, testing the influence of the class variables species, root order class (1 + 2 white, 1 + 2 brown, and ≥3), diameter class (0–2 mm and ≥2 mm), and species × class effects on the dependent variables RRDWT, RRSA, and RRVOL, can be found in Table III. The results highlight that species and root order classes significantly (P < 0.001) explain the variance in RRDWT, whereas diameter class does not. RRSA is significantly influenced by both root order class and diameter as well as their cross effects; only a trend regarding species influence on RRSA exists. RRVOL is significantly influenced by species, root order, and diameter classes (Table III).
Table III. Two-way ANOVA (proc general linear model) results testing the influence of the class variables species (A. platanoides, C. betulus, Q. robur, and T. cordata), root order class (≥3, 1 + 2 brown, and 1 + 2 white), diameter class (0–2 and ≥2 mm), and species × class effects on the dependent variables RRDWT, RRSA, and RRVOL.
DF, Degree of freedom; SS I, sum of squares Type 1.
| Source | DF |
RRDWT |
RRSA |
RRVOL |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SS I | F | P | SS I | F | P | SS I | F | P | ||
| Species | 3 | 863 | 8.56 | <0.001 | 0.001 | 2.43 | 0.067 | 5.07 | 4.07 | 0.008 |
| Root order class | 2 | 11,800 | 175.63 | <0.001 | 0.088 | 295.74 | <0.001 | 17.16 | 20.65 | <0.001 |
| Diameter class | 1 | 28 | 0.86 | 0.355 | 0.001 | 7.77 | 0.006 | 4.26 | 10.26 | 0.002 |
| Species × root order class | 6 | 99 | 0.49 | 0.814 | 0.007 | 8.10 | <0.001 | 1.56 | 3.75 | 0.002 |
| Species × diameter class | 2 | 38 | 0.57 | 0.568 | 0.004 | 14.51 | <0.001 | 2.42 | 2.91 | 0.057 |
| Model | 14 | 12,829 | 27.28 | <0.001 | 0.102 | 48.87 | <0.001 | 38.25 | 6.58 | <0.001 |
| Error | 177 | 5,912 | 0.026 | 73.52 | ||||||
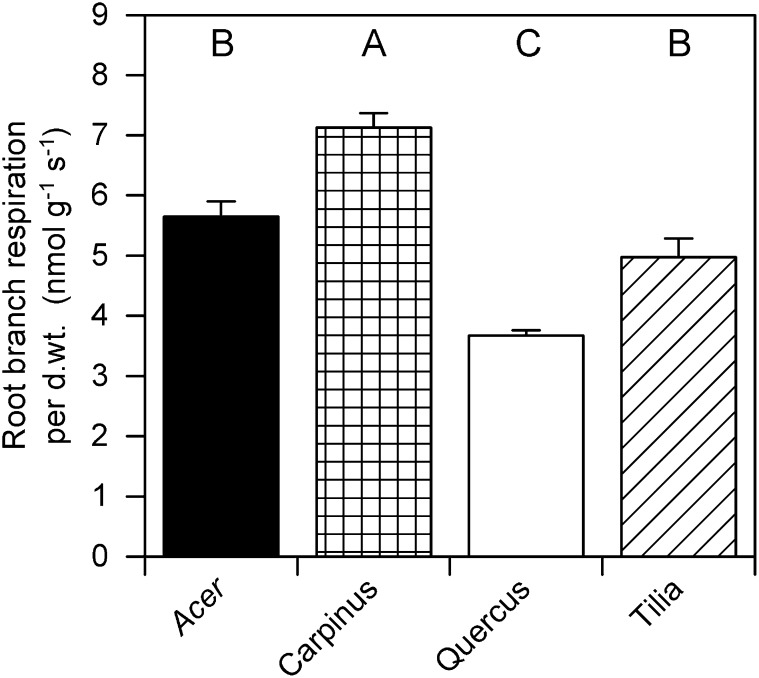
The calculated root respiration of root branches (RRBranch DWT; i.e. the respiration of root order classes set in proportion to their respective biomass frequency) varied significantly between species (Fig. 4). C. betulus root branches have a significantly greater RRBranch DWT (7.1 ± 0.2 nmol g−1 s−1) and Q. robur root branches have a significantly lower respiration (3.7 ± 0.1 nmol g−1 s−1) than the two other species. RRBranch DWT values of A. platanoides and T. cordata root systems are both approximately 5 nmol g−1 s−1 and not significantly different from each other (Fig. 4).
Figure 4.
RRBranch DWT (nanomoles gram−1 second−1) of the species A. platanoides, C. betulus, Q. robur, and T. cordata taking biomass frequencies of root order classes 1 + 2 white, 1 + 2 brown, and ≥3 and their respiration rates (RRDWT) into account. Different capital letters indicate significant difference between species (mean + se; Tukey-Kramer test, P < 0.05; n = 5). d.wt., Dry weight.
DISCUSSION
Root Morphological Traits of Four Deciduous European Tree Species
More persistent root segments in seedlings of the tree species A. platanoides, C. betulus, T. cordata, and Q. robur occur at root order 3 and higher. Roots of these orders possess secondary growth, evidenced by the large root diameter and the highest tissue density (RTD), and hold the lowest SRA. The ephemeral part of the seedlings’ root systems, therefore, mainly consists of the two terminal root orders. Similarly, root orders 1 and 2 of other woody species have been found previously to possess a shorter longevity than root segments of the order 3 (Valenzuela-Estrada et al., 2008; Huang et al., 2010).
Recently, Beyer et al. (2013) reported that “[morphological] analyses based on root orders produce a precise picture of species differences”; the authors studied seedlings of the Central European tree species Fraxinus excelsior and Fagus sylvatica (Beyer et al., 2013). We assume that root systems are not strictly organized in terms of root orders but in terms of different root types; types may often but not always coincide with root orders. In this study, the terminal root order class 1 + 2 had to be divided into two types by extended centripetal root order classification: white or brown terminal roots. Other than color, the two terminal root types are clearly distinguished from each other in terms of morphology (Table II). In general, white terminal root segments are thicker than brown terminal roots and possess very low RTD, resulting in significantly greater SRA in A. platanoides and Q. robur seedlings. A qualitative visual examination of cross sections led to the impression that white root segments possess a thicker, less dense cortex compared with brown terminal roots (data not shown). The different RTD of terminal roots order classes may indicate a greater longevity of brown terminal roots (Ryser, 1996). In all four species, brown root orders 1 + 2 were more frequent in terms of biomass and abundance than white terminal roots, although clear species-specific branching patterns exist (Table I). Similarly, Lipp and Andersen (2003) found that white roots of Pinus ponderosa trees comprise a relatively small portion of total root biomass in the field. Classification by root color was frequently used in previous studies, often as proxy for root age, white being regarded as the color of young root segments (Goldfarb et al., 1990; Hendrick and Pregitzer, 1992; Hassan et al., 2008). In this study, however, brown root tips seemed as vital as white ones, and the relatively short duration of the experiment deems it unrealistic that many white root segments already matured to brown terminal roots. Because no spatial segregation between both terminal root types has been noticed (i.e. white root segments did not grow preferably at pot surfaces) and the potting substrate (quartz sand) as well as the irrigation and fertilization were very homogeneous, we conclude that white and brown terminal roots of the studies species represent functional different root types instead of age classes. This is further substantiated by the observation that swollen root tips, indicating the presence of ectomycorrhizal symbionts, were only found on brown terminal roots (data not shown). Thus, although a development of white to brown roots has been reported frequently, color is used in minirhizotron, and also, previous respiration studies (Lipp and Andersen, 2003) have used it to approximate root age, it needs to be concluded from our data that white terminal root segments are not (in all tree species) predecessors of brown roots but can be functionally different distal root types. Future studies should show if both types mature to brown root segments of higher order.
Our study is, thus, questioning if centripetal analyses by root order alone or diameter class are sufficiently detailed classification schemes to represent the morphological heterogeneity of woody root systems. All four species possess significant differences between root morphological parameters, especially within lower root order classes, which was previously shown for other tree species (Guo et al., 2008a, 2008b; Beyer et al., 2013). However, across species, very similar morphological differences can be recognized between the three root order classes, proving the suitability of the chosen classes to describe the morphological heterogeneity. Thus, we suggest expanding the centripetal root ordering scheme by visual parameters, such as color, in future studies, likely allowing for most precise descriptions of root systems’ morphological heterogeneity. Workload can be significantly reduced if similar root orders are grouped; for morphological parameters, this can be decided on by fast preassessments.
Explanatory Value of Root Diameter, Tissue Density, and Root Order Classes for Respiration
RR rates strongly depend on abiotic (e.g. temperature, moisture, and rooting medium; Zogg et al., 1996; Cheng et al., 2005; Jarvi and Burton, 2013) and biotic (e.g. competitors and symbionts; Trocha et al., 2010; Meier et al., 2013) factors. The measured respiration rates of brown terminal roots and root orders ≥ 3 of seedlings of four European deciduous tree species are comparable with RR rates of other mature tree species (Burton et al., 2002; Jia et al., 2013) and even respiration rates of some grass roots (Maček et al., 2005) reported earlier. Lambers et al. (1993) reported that root excision does not have a substantial effect on RR when assessed within 30 min of excision.
Respiration of aboveground and belowground tissues is often positively correlated with nitrogen concentrations for woody and herbaceous plants alike (Ryan et al., 1996; Reich et al., 1998; Tjoelker et al., 2005). Burton et al. (2002) reported that very fine root (diameter < 1 mm) nitrogen concentration explained up to 91% of the variability of RR among different tree species. However, Desrochers et al. (2002) did not find a correlation between fine root nitrogen and respiration in Populus tremuloides, and root nitrogen concentrations are not as easy to determine as root morphological parameters. Thus, one aim of this study was to find relationships between RR rates of four deciduous European tree species and root morphological traits. There has been much discussion of the best morphological trait that distinguishes species’ differences in comparative studies. Of particular interest is whether root diameter or RTD best captures species’ physiological differences (Comas and Eissenstat, 2004). Although root diameter was identified by Comas and Eissenstat (2004) to be the better indicator of differences in potential growth rate among woody species, our study shows that RR of the four species is highly related (R2 = 0.67–0.78) to RTD in an exponential decay fashion (Fig. 2). Least dense root tissues (i.e. white root segments) possess the highest respiration rates (RRDWT) across species. Root diameter can be useful for the approximation of RR as well but only after creating laborious calibration curves (R2 = 0.56–0.79); in this study, the best-fitting curve followed a unimodal distribution, with the highest RRDWT in (mostly white) fine root segments with diameter of approximately 0.8 mm (Fig. 1). Our results are in contrast to previous studies (e.g. studies by Pregitzer et al. [1998] and Desrochers et al. [2002]), which found that RR of mature Acer saccharum trees and P. tremuloides seedlings, respectively, declined with increasing root diameter; however, much information was lost in these studies by using root diameter classes (discussion below).
RTD is a fundamental trait in comparative root ecology, and it is being increasingly used as an indicator of plant species’ resource use strategy (Birouste et al., 2013). Because of its high ecological importance, tissue density is now measured routinely in many studies worldwide comparing species and growth plasticity under environmental conditions (Kembel and Cahill, 2011). Our study can show that RTD is a parameter outlining convergent patterns (Meinzer, 2003) of RR among different tree species much better than root diameter. It has similar explanatory value as nitrogen tissue concentrations (approximately 70%; Pregitzer et al., 1998) on respiration rates and thus, could be used for approximating respirational differences within and between root systems. Because RTD is highly correlated to RDMC (Supplemental Fig. S1), the ease of determining RDMC could replace RTD when root volume cannot be determined.
Root systems are often sampled by classes to enable researchers to process samples in a systematic and timely fashion. Previously, several studies found that respiration rates of tree roots decline with increasing root diameter classes (Pregitzer et al., 1998; Makita et al., 2009). In accordance, differences in RRDWT among root diameter classes in this study are congruent with hypothesized effects of variation in root function on metabolic activity; fine roots had the highest RRDWT rates, and coarse roots had low RRDWT rates, consistent with their structural and transport functions (Fig. 3). However, several previous studies did also report that broadly defined root classes, especially the lower diameter root segments, do not accurately reflect functional root categories (Pregitzer et al., 1998; Makita et al., 2009). The comparison of coefficients of determination between the two classification schemes used in this study shows that the extended root order classes have a greater explanatory value then the classical root diameter classes in describing RR and thus, are obviously better at representing functional root classes. R2 values of root order classes are between 0.64 and 0.79, whereas R2 values of root diameter classes are between 0.26 and 0.35. The explanatory value of standard centripetal root ordering would be rather similar to root diameter classes because of the fact that root orders 1 and 2 possess diameters < 2 mm, whereas most higher order segments of the tested species have a diameter ≥ 2 mm (Fig. 1; Table II). Furthermore, ANOVA showed that species and root order class significantly (P < 0.001) influence RRDWT, whereas diameter does not (P = 0.355; Table III). However, for RRSA and RRVOL (Supplemental Fig. S2), which are rather rarely calculated (Asplund and Curtis, 2001; Desrochers et al., 2002; Dannoura et al., 2006a, 2006b), significant influences of root diameter were found. RR is dependent on tissue porosity to gas diffusion (Asplund and Curtis, 2001); therefore, it can be anticipated that increasing diameter will increase diffusional limitations in tissues and alter respiration kinetics. Our finding that RRSA is higher in the ≥3 root order class than the distal classes, whereas the opposite is found when calculating RRDWT is likely based on the different functional anatomy of root orders (i.e. the ratio of respiring to structural mass is changing as roots age). Thus, RRDWT may overrate the heavy, structural, nonrespiring mass when calculated for woody roots, and RRSA may a more valuable expression of RR because of its direct interrelationship to water and nutrient uptake densities.
Species-Specific Differences in RR
Much comparative ecophysiological research has focused on contrasting species-specific behavior or ecological strategies with regard to regulation of basic physiological processes, such as transpiration, photosynthesis, and growth, leading to an emphasis on divergence rather than convergence in plant functioning (Meinzer, 2003). Our study shows that mass-based RR rates of root order classes are rather similar between the four tree species. No significant differences between species’ RRDWT values were found in the white and ≥3 root order class; brown terminal roots or roots of the diameter class of 0 to 2 mm of C. betulus had a greater RRDWT, and those of Q. robur had a smaller RRDWT (Fig. 3). Earlier results by Asplund and Curtis (2001) suggested that, under conditions where oxygen availability is not limiting, the specific respiration rates of three crop species’ root tips are rather similar. Our results emphasize, however, that tree root systems can also possess rather convergent functional units. If those units are measured, differences between tree species’ respiration rates seem minimal, leading often to nonsignificant differences between species of the same habitat (Burton et al., 2002). Jia et al. (2013) found recently that the RR rates of Larix gmelinii and Fraxinus mandshurica are rather similar when accessed by root order. However, because the frequency of root order classes is species specific (Table I), a clearer picture of species-specific RR rates can be archived if RR rates per class are weighed by their respective frequency. In our study, the respiration of representative root branches was significantly different for C. betulus (high) and Q. robur (low), and A. platanoides and T. cordata had values of RRBranch DWT in between them (Fig. 4). The lowest respiration rates found in Q. robur seem to fit the ecological traits of this species: slow growing and late successional. However, recently, Jacob (2013) showed that mature T. cordata trees have up to 5 times higher fine root growth rates than C. betulus in the Hainich National Park in central Germany; growth rates of a different Acer sp. (Acer pseudoplatanus) were in between them. In addition, Jacob (2013) found no significant differences in fine root turnover rates between the three tree species, which are all mid- to late-successional species. Thus, the calculated RRBranch DWT of seedlings seems at least not to be directly related to the root growth and turnover rates of mature trees; it is currently unknown if RR changes during ontogeny in perennials. In barley (Hordeum vulgare), the rates of both shoot and RR on a dry weight basis decrease throughout the lifecycle but with a more rapid decline in shoots than roots (Winzeler et al., 1976). Although a considerable part of RR is used for uptake and transport of ions (Veen, 1981), it remains currently unknown how nutrition influenced the respiration in our experiment and if the RR of the four species reacted differently to the supplied nutrient solution. However, the species-specific differences are strongly driven by the frequency of white terminal roots; although they comprise a relatively small portion of biomass, estimates of autotrophic respiration need to account for the substantially greater rates of white RR. If the branching pattern will change during the growing seasons or with ontogeny, species-specific RR rates will change independently from other factors, such as temperature and resource availability. However, at a given point in time, the calculated RRBranch DWT results identify species-specific difference more explicitly than comparing the more common brown terminal roots or diameter classes (Fig. 3). Thus, we propose that future studies should calculate species-specific respiration rates according to the outlined methodology to address both root physiological and architectural differences sufficiently. In addition, other root types, such as ectomycorrhizal root tips, should be separately considered as well if they compose a significant part of root systems. Future studies determining the influence of ontogeny on the respiration of woody root types are urgently needed.
MATERIALS AND METHODS
Species and Growth Conditions
Two-year-old seedlings of the Central European tree species Acer platanoides, Carpinus betulus, and Tilia cordata and 3-year-old seedlings of Quercus robur were obtained from a tree nursery (Murauer Forstpflanzen, Ort im Innkreis, Austria). Other than differing widely in their ecological and physiological niches (e.g. with regard to shade tolerance; Ellenberg and Leuschner, 2010), A. platanoides and T. cordata form symbioses predominantly with arbuscular mycorrhiza, and C. betulus and Q. robur trees have ectomycorrhizal symbionts. Plants were established from seeds under similar environmental conditions (with respect to climate, soil type, and fertigation). In early April of 2013, plants were dug up, and root systems were rinsed carefully. Ten dormant trees per species were planted in 7-L pots (diameter = 22 cm) with a perforated bottom; pots were filled with washed quartz sand (grain size distribution: 25%, 0.2–1 mm; and 75%, 1–2 mm in diameter), allowing for highly controlled water and nutrient supply and nondestructive root system rinsing. After planting, seedlings were grown for 6 weeks ex situ before being transferred to two growth chambers (HGC 1514; Weiss Gallenkamp); species were distributed equally between chambers. Growth chamber temperature was 24°C/20°C day-night for 10 d; after this period, plants were grown constantly at 20°C. The chosen temperature represents a compromise between species-specific soil temperature optima (i.e. 15°C–25°C) reported earlier (Lyr, 1996). Relative humidity was set at 50% during the day and 60% during the night; the length of the photoperiod was 14 h at 400 µmol m−2 s−1 (fluorescent). Plants were irrigated every 2nd day with an excess amount of nutrient solution (Supplemental Table S1) to prevent water scarcity and nutrient accumulation. After 3 weeks at 20°C, plants were randomly harvested for analyses.
Plant Allometry and Root System Architecture
Five plants per species were harvested to determine the biomass allocation between aboveground (leaves and stem) and belowground organs and within root systems. Three root order classes were distinguished per root system: root orders 1 + 2 of white color (visually also less branched and thicker in diameter), root orders 1 + 2 of brown color, and root orders ≥ 3. Root order class 1 + 2 represents the two terminal root orders (i.e. root tips and the next root segment; additional details on color and root order classification are in Goldfarb et al., 1990, and Rewald et al., 2011, respectively) and could be distinguished by color with certainty; we name our classification scheme “extended centripetal root order classification.” No spatial segregation between the two different terminal root order classes was noticed. Separation of root orders 1 and 2 was not performed because of their visual similarity and the associated excessive workload. Root order class ≥ 3 represents structural woody roots of the root order three and higher and was separated by removing all first and second order roots (if necessary) by manual clipping under an illuminated magnifying glass (10×). A maximum root system branching of five orders was noticed. The dry weight (48 h at 70°C) of leaves and stems and the root order classes were determined to a precision of 0.1 mg. RMF, calculated as the proportion of plant dry mass in roots, as well as the proportions of the three root order classes within the root system (percentage) were calculated. The proportion of root order classes was used to determine species-specific root branch respiration rates (see below).
RR and Morphology
RR rates (i.e. approximately |oxygen depletion|) were measured in a stirred (100%) cuvette filled with 2.5 mL of aerated nutrient solution (Supplemental Table S1) at 20.0°C ± 0.1°C (F12-ED; Julabo Labortechnik) using a Clark type oxygen sensor (S1 Oxygraph Electrode Disc) and a DW1 Electrode Chamber connected to a control box (Oxygraph Control Unit; Hansatech Instruments Ltd). The electrode was calibrated daily using demineralized, oxygen-saturated water followed by sodium dithionite to establish zero-oxygen conditions. The PC Program Oxygraph Plus (Hansatech) was used to records signals each 1 s and calculate the oxygen depletion rate over a 15-min time period; readings were taken starting 5 to 10 min after root segment insertion to allow for temperature equilibration. The cuvette was refilled with aerated nutrient solution (20°C; Supplemental Table S1) before each measurement. Because of that and the high stirrer speed, a depletion of the oxygen boundary layer at the root surface, restricting respiration, seems unlikely (compare with Asplund and Curtis, 2001).
In total, 194 RR measurements were conducted on nine to 10 plants per species. Three classes of root segments, root orders 1 + 2 of white or brown color and root orders ≥ 3 (predominantly root orders 3 and 4), were studied as described above. Replicate numbers were A. platanoides, C. betulus, and T. cordata root orders 1 + 2 white (n = 18), root orders 1 + 2 brown (n = 18), and root order 3 (n = 18) and Q. robur root orders 1 + 2 white (n = 5), root order 1 + 2 brown (n = 15), and root order 3 (n = 15). Lower replicate numbers in white Q. robur root orders 1 + 2 were caused by lack of sampling material. A maximum of two measurements per order class were performed per plant individual. Average diameter of root segments ranged from 0.25 to 3.79 mm. Ectomycorrhizal root tips were only found on brown root tips and excluded from the analysis because of the low rate of mycorrhization (1%–5%). Root sections were cut from the root systems immediately (5–10 min) before measurements to minimize the influence of excision on RR (Lambers et al., 1993; Fukuzawa et al., 2012), and they were rinsed carefully in nutrient solution (20°C) before being entered into the cuvette. The remaining root system was kept undisturbed in soil at room temperature covered by opaque, nutrient solution-soaked towels until the next segment was excised; leaves were illuminated during sampling, allowing photosynthesis to continue (Lipp and Andersen, 2003). After the measurements, 164 root segments were scanned with WinRhizo 2012 Pro (Regent Instruments Inc.) to determine surface area (centimeters2), volume (centimeters3), and average diameter (millimeters). The dry weight (48 h at 70°C) of all samples was determined to a precision of 0.1 mg. On approximately 50% of the samples, the fresh weight (after gently blotting the surface dry) was determined before drying and weighing. The weight and morphological data were used to calculate the SRA (centimeters2 gram−1), RTD (grams centimeter−3), and RDMC (grams dry weight grams fresh weight−1). RDMC was related to RTD to test its suitability to replace RTD in woody species (Birouste et al., 2013).
The measured oxygen depletion rate (nanomoles O2 millilter−1 minute−1) was set equal to RR by sign reversal; possible other O2-expending metabolic processes were considered negligible. Respiration rates were expressed per 1 s and related to RRSA (nanomoles centimeter−2 second−1), RRVOL (nanomoles centimeter−3 second−1), and RRDWT (nanomoles gram−1 second−1). Nonlinear correlation curves between average diameter/RTD and RRDWT were fitted for 33 to 45 measurements per species. In addition to root order classes, RRDWT was grouped in two root diameter classes (i.e. 0–2 mm [fine roots] and ≥2 mm [coarse roots]) to compare the coefficient of determination between the two classification approaches—diameter or extended root order class. The standard root order classes were not analyzed, because the datasets were nearly identical with the root diameter classes because of their diameter distributions. To determine species-specific RRBranch DWT, the average RR (RRDWT) per root order class was weighted by the respective biomass (Rewald et al. [2012] discuss a similar calculation with regard to branch water flux densities). This was done separately for the five dissected root systems per species, allowing us to use five true replicates per species for statistical analysis.
Statistics
Statistical calculations were conducted with the PC program SAS 9.2, 32 bit (SAS Institute). Datasets were tested for Gaussian distribution with the Shapiro-Wilk test and homogeneity of variances with the Levene test. A general linear model was used to test for significant influences of species and root morphological traits on RR. Parametric Tukey test was used for pairwise comparison of root morphological traits and respiration rates; critical α was set at 0.05. Curves in Figures 1 and 2 and Supplemental Figure S1 were fitted least square minimized with a maximum of 1,000 iterations; critical α was set at 0.001. Coefficient of determination value (R2) is reported in percentage to illustrate the proportion of total variation that is replicated by the respective models.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. RTD (grams centimeter−3) of the species A. platanoides, C. betulus, Q. robur, and T. cordata in relation to RDMC (grams dry weight grams fresh weight−1).
Supplemental Figure S2. RRSA (nanomoles centimeter−2 second−1) and RRVOL (nanomoles centimeter−3 second−1) of the species A. platanoides, C. betulus, Q. robur, and T. cordata arranged by root order classes 1 + 2 white (WT), 1 + 2 brown (BR), and ≥3.
Supplemental Table S1. Composition of the 100× stock solutions (macro, micro, and iron separated) for plant fertigation and as solution during the respiration measurements; pH values were adjusted to prevent iron precipitation.
Supplementary Material
Acknowledgments
We thank two anonymous reviewers and the editor for comments that improved an earlier draft of this article.
Glossary
- RDMC
root dry matter content
- RMF
root mass fraction
- RR
root respiration
- RRBranch DWT
root respiration of root branches
- RRDWT
root respiration root segment dry weight
- RRSA
root respiration surface area
- RRVOL
root respiration tissue volume
- RTD
root tissue density
- SRA
specific root area
Footnotes
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Asplund PT, Curtis WR. (2001) Intrinsic oxygen use kinetics of transformed plant root culture. Biotechnol Prog 17: 481–489 [DOI] [PubMed] [Google Scholar]
- Beyer F, Hertel D, Leuschner C. (2013) Fine root morphological and functional traits in Fagus sylvatica and Fraxinus excelsior saplings as dependent on species, root order and competition. Plant Soil 373: 143–156 [Google Scholar]
- Birouste M, Zamora-Ledezma E, Bossard C, Pérez-Ramos IM, Roumet C. (2013) Measurement of fine root tissue density: a comparison of three methods reveals the potential of root dry matter content. Plant Soil 374: 299–313 [Google Scholar]
- Böhm W. (1979) Methods of Studying Root Systems. Springer, Berlin [Google Scholar]
- Bouma TJ, Bryla D, Li Y, Eissenstat DM. (2000) Is maintainance respiration in roots a constant? In Stokes A, ed, The Supporting Roots of Trees and Woody Plants - Form, Function and Physiology. Kluwer Academic, Dordrecht, The Netherlands, pp 391–396 [Google Scholar]
- Burton AJ, Pregitzer KS, Ruess RW, Hendrik RL, Allen MF. (2002) Root respiration in North American forests: effects of nitrogen concentration and temperature across biomes. Oecologia 131: 559–568 [DOI] [PubMed] [Google Scholar]
- Chapin FS, III, Matson PA, Vitousek PM. (2012) Plant carbon budgets. In Chapin FS, III, Matson PA, Vitousek PM, eds, Principles of Terrestrial Ecosystem Ecology, Ed 2. Springer, New York, pp 157–181 [Google Scholar]
- Chen YL, Dunbabin VM, Diggle AJ, Siddique KHM, Rengel Z. (2011) Development of a novel semi-hydroponic phenotyping system for studying root architecture. Funct Plant Biol 38: 355–363 [DOI] [PubMed] [Google Scholar]
- Cheng W, Fu S, Susfalk RB, Mitchell RJ. (2005) Measuring tree root respiration using (13)C natural abundance: rooting medium matters. New Phytol 167: 297–307 [DOI] [PubMed] [Google Scholar]
- Clark RT, Famoso AN, Zhao K, Shaff JE, Craft EJ, Bustamante CD, McCouch SR, Aneshansley DJ, Kochian LV. (2013) High-throughput two-dimensional root system phenotyping platform facilitates genetic analysis of root growth and development. Plant Cell Environ 36: 454–466 [DOI] [PubMed] [Google Scholar]
- Comas LH, Eissenstat DM. (2004) Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Funct Ecol 18: 388–397 [Google Scholar]
- Dannoura M, Kominami Y, Tamai K, Goto Y, Jomura M, Kanazawa Y. (2006a) Short-term evaluation of the contribution of root respiration to soil respiration in a broad-leaved secondary forest in the southern part of Kyoto prefecture. J Agric Meteorol 62: 15–21 [Google Scholar]
- Dannoura M, Kominami Y, Tamai K, Jomura M, Miyama T, Goto Y, Kanazawa Y. (2006b) Development of an automatic chamber system for long-term measurements of CO2 flux from roots. Tellus B Chem Phys Meterol 58: 502–512 [Google Scholar]
- Deak KI, Malamy J. (2005) Osmotic regulation of root system architecture. Plant J 43: 17–28 [DOI] [PubMed] [Google Scholar]
- Desrochers A, Landhäusser SM, Lieffers VJ. (2002) Coarse and fine root respiration in aspen (Populus tremuloides). Tree Physiol 22: 725–732 [DOI] [PubMed] [Google Scholar]
- Ellenberg H, Leuschner C. (2010) Vegetation Mitteleuropas mit den Alpen, Ed 6. Ulmer, Stuttgart, Germany [Google Scholar]
- Epron D, Farque L, Lucot E, Badot PM. (1999) Soil CO2 efflux in a beech forest: the contribution of root respiration. Ann Sci 56: 289–295 [Google Scholar]
- Fitter A. (1996) Characteristics and functions of root systems. In Waisel Y, Eshel A, Kafkafi U, eds, Plant Roots: The Hidden Half, Ed 2. Marcel Decker, New York, pp 1–17 [Google Scholar]
- Fukuzawa K, Dannoura M, Shibata H. (2012) Fine root dynamics and root respiration. In Mancuso S, ed, Measuring Roots: An Updated Approach. Springer, Berlin, pp 291–302 [Google Scholar]
- George K, Norby RJ, Hamilton JG, DeLucia EH. (2003) Fine-root respiration in a loblolly pine and sweetgum forest growing in elevated CO2. New Phytol 160: 511–522 [DOI] [PubMed] [Google Scholar]
- Goldfarb D, Hendrick R, Pregitzer K. (1990) Seasonal nitrogen and carbon concentrations in white, brown and woody fine roots of sugar maple (Acer saccharum Marsh). Plant Soil 126: 144–148 [Google Scholar]
- Gruber V, Zahaf O, Diet A, de Zélicourt A, de Lorenzo L, Crespi M. (2011) Impact of the environment on root architecture in dicotyledoneous plants. In de Oliveira AC, Varshney RK, eds, Root Genomics. Springer, Heidelberg, pp 113–132 [Google Scholar]
- Guo D, Li H, Mitchell RJ, Han W, Hendricks JJ, Fahey TJ, Hendrick RL. (2008a) Fine root heterogeneity by branch order: exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol 177: 443–456 [DOI] [PubMed] [Google Scholar]
- Guo D, Xia M, Wei X, Chang W, Liu Y, Wang Z. (2008b) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol 180: 673–683 [DOI] [PubMed] [Google Scholar]
- Hassan S, Liu W, Ma JKC, Thomas CR, Keshavarz-Moore E. (2008) Characterization of mechanical properties of transgenic tobacco roots expressing a recombinant monoclonal antibody against tooth decay. Biotechnol Bioeng 100: 803–809 [DOI] [PubMed] [Google Scholar]
- Hendrick RL, Pregitzer KS. (1992) The demography of fine roots in a northern hardwood forest. Ecology 73: 1094–1104 [Google Scholar]
- Himmelbauer ML, Loiskandl W, Kastanek F. (2004) Estimating length, average diameter and surface area of roots using two different image analyses systems. Plant Soil 260: 111–120 [Google Scholar]
- Huang G, Zhao XY, Zhao HL, Huang YX, Zuo XA. (2010) Linking root morphology, longevity and function to root branch order: a case study in three shrubs. Plant Soil 336: 197–208 [Google Scholar]
- Jacob A (2013) Effects of tree species composition on fine root biomass and dynamics in the rhizosphere of deciduous tree stands in the Hainich National Park (Thuringia). PhD thesis. University of Göttingen, Gottingen, Germany [Google Scholar]
- Jarvi MP, Burton AJ. (2013) Acclimation and soil moisture constrain sugar maple root respiration in experimentally warmed soil. Tree Physiol 33: 949–959 [DOI] [PubMed] [Google Scholar]
- Jia S, McLaughlin NB, Gu J, Li X, Wang Z. (2013) Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol 33: 579–589 [DOI] [PubMed] [Google Scholar]
- Kembel SW, Cahill JF., Jr (2011) Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS ONE 6: e19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, III, Pons TL. (2008) Respiration. In Lambers H, Chapin FS, III, Pons TL, eds, Plant Physiological Ecology. Springer, New York, pp 101–150 [Google Scholar]
- Lambers H, Van der Werf A, Bergkotte M. (1993) Respiration: The alternative pathway. In Hendry GAF, Grime JP, eds, Methods of Comparitive Plant Ecology: A Laboratory Manual. Chapman and Hall, London, pp 140–144 [Google Scholar]
- Leitner D, Felderer B, Vontobel P, Schnepf A. (2014) Recovering root system traits using image analysis exemplified by two-dimensional neutron radiography images of lupine. Plant Physiol 164: 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp CC, Andersen CP. (2003) Role of carbohydrate supply in white and brown root respiration of ponderosa pine. New Phytol 160: 523–531 [DOI] [PubMed] [Google Scholar]
- Lyr H. (1996) Effect of the root temperature on growth parameters of various European tree species. Ann Sci For 53: 317–323 [Google Scholar]
- Maček I, Pfanz H, Francetič V, Batič F, Vodnik D. (2005) Root respiration response to high CO2 concentrations in plants from natural CO2 springs. Environ Exp Bot 54: 90–99 [Google Scholar]
- Majdi H, Damm E, Nylund JE. (2001) Longevity of mycorrhizal roots depends on branching order and nutrient availability. New Phytol 150: 195–202 [Google Scholar]
- Majdi H, Nylund JE, Agren GI. (2007) Root respiration data and minirhizotron observations conflict with root turnover estimates from sequential soil coring. Scand J For Res 22: 299–303 [Google Scholar]
- Makita N, Hirano Y, Dannoura M, Kominami Y, Mizoguchi T, Ishii H, Kanazawa Y. (2009) Fine root morphological traits determine variation in root respiration of Quercus serrata. Tree Physiol 29: 579–585 [DOI] [PubMed] [Google Scholar]
- Meier IC, Angert A, Falik O, Shelef O, Rachmilevitch S. (2013) Increased root oxygen uptake in pea plants responding to non-self neighbors. Planta 238: 577–586 [DOI] [PubMed] [Google Scholar]
- Meinzer FC. (2003) Functional convergence in plant responses to the environment. Oecologia 134: 1–11 [DOI] [PubMed] [Google Scholar]
- Neumann G, Martinoia E. (2002) Cluster roots: an underground adaptation for survival in extreme environments. Trends Plant Sci 7: 162–167 [DOI] [PubMed] [Google Scholar]
- Pagès L, Kervella J. (1990) Growth and development of root systems: geometrical and structural aspects. Acta Biotheor 38: 289–302 [Google Scholar]
- Poorter H. (1994) Construction costs and payback time of biomass: A whole plant perspective. In Roy J, Garnier E, eds, A whole plant perspective on carbon-nitrogen interactions. SPB Academic, The Hague, pp 111–127 [Google Scholar]
- Pregitzer KS. (2002) Fine roots of trees - a new perspective. New Phytol 154: 267–270 [DOI] [PubMed] [Google Scholar]
- Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL. (2002) Fine root architecture of nine North American trees. Ecol Monogr 72: 293–309 [Google Scholar]
- Pregitzer KS, Kubiske ME, Yu CK, Hendrick RL. (1997) Relationships among root branch order, carbon, and nitrogen in four temperate species. Oecologia 111: 302–308 [DOI] [PubMed] [Google Scholar]
- Pregitzer KS, Laskowski MJ, Burton AJ, Lessard VC, Zak DR. (1998) Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol 18: 665–670 [DOI] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS, Vose JM, Volin JC, Gresham C, Bowman WD. (1998) Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia 114: 471–482 [DOI] [PubMed] [Google Scholar]
- Rewald B, Ephrath JE, Rachmilevitch S. (2011) A root is a root is a root? Water uptake rates of Citrus root orders. Plant Cell Environ 34: 33–42 [DOI] [PubMed] [Google Scholar]
- Rewald B, Raveh E, Gendler T, Ephrath JE, Rachmilevitch S. (2012) Phenotypic plasticity and water flux rates of Citrus root orders under salinity. J Exp Bot 63: 2717–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MG, Hubbard RM, Pongracic S, Raison RJ, McMurtrie RE. (1996) Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol 16: 333–343 [DOI] [PubMed] [Google Scholar]
- Ryser P. (1996) The importance of tissue density for growth and life span of leaves and roots: a comparison of five ecologically contrasting grasses. Funct Ecol 10: 717–723 [Google Scholar]
- Strahler AN. (1957) Quantitative analysis of watershed geomorphology. Trans Am Geophys Union 38: 913–920 [Google Scholar]
- Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D. (2005) Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol 167: 493–508 [DOI] [PubMed] [Google Scholar]
- Trocha LK, Mucha J, Eissenstat DM, Reich PB, Oleksyn J. (2010) Ectomycorrhizal identity determines respiration and concentrations of nitrogen and non-structural carbohydrates in root tips: a test using Pinus sylvestris and Quercus robur saplings. Tree Physiol 30: 648–654 [DOI] [PubMed] [Google Scholar]
- Uylings HB, Smit GJ, Veltman WA. (1975) Ordering methods in quantitative analysis of branching structures of dendritic trees. Adv Neurol 12: 347–354 [PubMed] [Google Scholar]
- Valenzuela-Estrada LR, Vera-Caraballo V, Ruth LE, Eissenstat DM. (2008) Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). Am J Bot 95: 1506–1514 [DOI] [PubMed] [Google Scholar]
- Veen BW. (1981) Relation between root respiration and root activity. Plant Soil 63: 73-76 [Google Scholar]
- Wang ZQ, Guo DL, Wang XR, Gu JC, Mei L. (2006) Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 288: 155–171 [Google Scholar]
- Wells CE, Eissenstat DM. (2003) Beyond the roots of young seedlings: the influence of age and order on fine root physiology. J Plant Growth Regul 21: 324–334 [Google Scholar]
- Winzeler H, Hunt LA, Mahon JD. (1976) Ontogenetic changes in respiration and photosynthesis in a uniculm barley. Crop Sci 16: 786–790 [Google Scholar]
- Zogg GP, Zak DR, Burton AJ, Pregitzer KS. (1996) Fine root respiration in northern hardwood forests in relation to temperature and nitrogen availability. Tree Physiol 16: 719–725 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.