Abstract
Drinking water is innately rewarding to thirsty animals. In addition, the consumed value can be assigned to behavioral actions and predictive sensory cues by associative learning. Here we show that thirst converts water avoidance into water-seeking in naïve Drosophila. Thirst also permits flies to learn olfactory cues paired with water reward. Water learning requires water taste and <40 water-responsive dopaminergic neurons that innervate a restricted zone of the mushroom body γ lobe. These water learning neurons are different from those that are critical to convey the reinforcing effects of sugar. Naïve water-seeking behavior in thirsty flies does not require water taste but relies on another subset of water-responsive dopaminergic neurons that target the mushroom body β′ lobe. Furthermore, these naïve water-approach neurons are not required for learned water-seeking. Our results therefore demonstrate that naïve and learned water-seeking, and water learning, utilize separable neural circuitry in the brain of thirsty flies.
Introduction
Thirst is a manifestation of an animal’s internal deprivation of water 1. Increasing dehydration promotes whether the animal pursues the goal of finding water and drinking. Serving this need requires foraging behavior that is guided by the collection of sensory cues that are present, and the most meaningful, in the environment. Some of these are innately significant and clear, like water itself, and others are learned as useful signs from knowledge of previous procurement 2-4. Therefore as it forages, an animal needs to integrate the most useful innate and learned cues, with its internal state to direct appropriate motivated or goal-directed behavior. How thirst impacts the nervous system to control water-seeking behavior is largely unknown.
Dopaminergic neurons are generally considered to signal reward value in the mammalian brain 5-7 and recent work in Drosophila has provided in roads to a cellular resolution analysis of reward value coding 8,9. A cluster of approximately 130 dopaminergic neurons that innervate the horizontal lobes of the mushroom body was implicated in conveying positive reinforcement value and to be required for the flies to learn with sugar reward 8,9. Surprisingly, a subset of these rewarding neurons were also described to be required for flies to evaluate the lesser of two voltages during learning 10, suggesting an unforeseen complexity in the function of dopaminergic valuation processes in the fly.
Here we further probed Drosophila reward coding by developing new assays to study water valuation in thirsty flies. We found that valuation of water vapor in naïve flies utilizes a different population of rewarding dopaminergic neurons than those that are required for ingested water to provide reinforcing value during reward learning. Furthermore, the water learning neurons are apparently separate from those required for sugar reward learning. The type of rewarding stimulus therefore seems to functionally subdivide the fly dopaminergic system.
Results
Thirsty flies seek water
Water-sated flies actively avoid water, preferring to enter a dry tube rather than one that is humid 11. Since thirsty flies should seek water rather than avoid it, we first determined a time of deprivation in which water became attractive to naïve flies. Significant approach was evident after 6 hours of water deprivation and 90% of flies entered the water tube following 14 h without drinking (Fig. 1a). These data demonstrate that thirsty flies seek water. Thirst therefore changes the flies’ valuation of water from something they consider to be aversive into something they seek, or want.
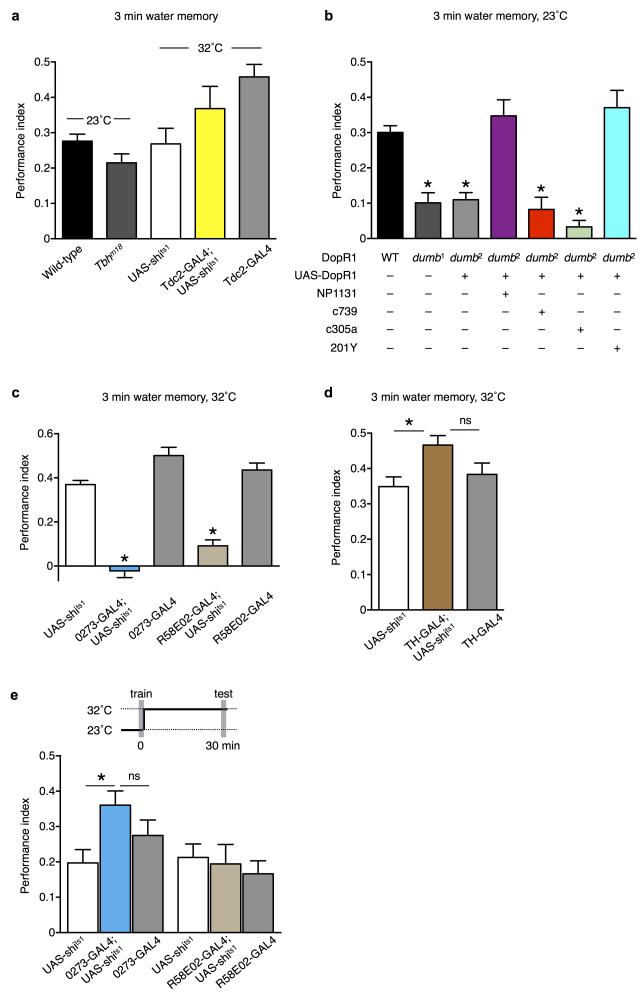
Fig. 1. Thirsty flies approach water and can be trained with water reward.
(a) Increasing time of water deprivation in naïve flies converts avoidance of humidity into attraction (n=4 for each time point). (b) Water memory performance after training at 23°C (magenta line) or 32°C (blue line). Allowing thirsty flies to drink water for 1 h before training significantly diminished water learning at both temperatures (23°C - open brown circle and 32°C - open black circle). N=4 for the 1 h time point of the thirsty-32°C group; others n≥8, P<0.005 (t-test) for the difference between thirsty and sated groups. (c) Flies consume more water in 2 min at 32°C than at 23°C (P<0.0001, n=4; t-test). (d) Water memory is retrieved more robustly in thirsty than hungry flies (*P<0.01, n=8; ns, P=0.74, n=8; ANOVA followed by post hoc Tukey’s HSD test). (e) Sugar memory is retrieved more robustly in hungry than thirsty flies (*P<0.001, n=12; ns, P=0.57, n=12; ANOVA followed by post hoc Tukey’s HSD test). (f) PPK28 is required for water learning (P<0.0001, n=12; t-test). (g) PPK28 is not required for naïve water approach in thirsty flies (P=0.51, n=8; t-test).. (h) Flies exhibit thirst-dependent attraction to water vapor. All data are mean ± standard error of the mean (s.e.m.) and asterisks denote significant difference between marked groups and the relevant controls.
Thirsty flies can learn using water-reward
To determine whether water was rewarding to thirsty flies we conditioned 16 h water-deprived flies by presenting one of two odors with water in a variant of an established olfactory appetitive learning paradigm 12,13. A robust but short-lived olfactory memory was formed when flies were trained at 23°C (Fig. 1b, magenta line). When flies were trained at 32°C, initial memory scores increased and significant performance could be measured at least 24 h later (Fig. 1b, blue line). We reasoned that the increased performance might result from the flies being more motivated to drink at higher temperature. Indeed, thirsty flies given 2 min to drink consumed statistically more blue-dyed water at 32°C than 23°C (Fig. 1c). Allowing flies to drink to satiety for 1 h before training significantly decreased water memory at both temperatures (Fig. 1b, brown and black circles). Therefore thirst and drinking is required for the acquisition of water memory.
We also tested whether thirst is required for behavioral expression of water memory (Fig. 1d and Supplementary Fig. 1a-b). Thirsty flies trained with water displayed significantly greater conditioned odor approach performance than when they were hungry or water sated. (Fig. 1d). A similar specificity of deprivation state dependence was also observed with sugar-reinforced memory. Hungry flies trained with dry sugar, expressed significantly greater memory performance when hungry than when thirsty or food sated (Fig. 1e and Supplementary Fig. 1c-d). Water reinforced appetitive memory performance therefore exhibits a thirst-state dependence that is analogous to that for hunger and carbohydrate memory 13,14. In addition, the two types of appetitive memory are independently controlled by the appropriate deprivation states of thirst or hunger.
PPK28 is required for water learning but not naïve water-seeking
We next investigated the neural circuitry of water-directed behaviours. Flies can taste water via the osmosensitive ion channel Pickpocket 28 (PPK28) that is expressed in gustatory neurons on the proboscis 15. Thirsty flies homozygous for ppk28 were defective in water learning (Fig. 1f), despite displaying normal olfactory acuity (Supplementary Fig. 2) and naïve water-seeking behaviour (Fig. 1g). Finding that performance in our water-choice assay did not require water taste lead us to test whether the flies were instead directed by water vapour. We gave water-sated or thirsty flies the choice between entering a tube containing dry air, or one with an inaccessible water source at the end. Whereas sated flies preferred the dry tube, thirsty flies approached the water vapour (Fig. 1h). Water-seeking therefore utilizes water vapour detection. In contrast, our experiments suggest that water learning requires the flies to taste water in order to stimulate drinking.
Octopamine is not required for water learning
Octopamine has long been considered to signal reward in insects 16-19 and recent studies suggest that in Drosophila it exclusively conveys the reinforcing effects of the sweet taste of sugars 9. Given the requirement for water taste neurons in learning, we tested whether octopamine was required for water-reinforced learning. Both Tyramine β-hydroxylase (TbhM18) mutant flies that lack octopamine 20 and flies in which octopaminergic neurons were blocked using Tdc2-GAL4 21 to express UAS-shits1 22, displayed water learning that was indistinguishable from control flies (Fig. 2a). Therefore octopamine is not critical for water reinforcement. These results suggest that the neural pathways used to learn with sugar and water reward are different.
Fig. 2. Water reinforced learning is independent of octopamine and is supported by dopaminergic signaling to mushroom body gamma neurons.
(a) Water learning performance is normal for TbhM18 flies that lack octopamine (P=0.0528, n=24; t-test) and flies in which transmission from Tdc2-GAL4 octopaminergic neurons is blocked by UAS-shits1 (P>0.34, n=8; ANOVA followed by post hoc Tukey’s HSD test). (b) Flies harboring the dumb1 and dumb2 mutations in the DopR1 dopamine receptor show impaired water learning (P<0.0001, n≥12; ANOVA followed by post hoc Tukey’s HSD test). Restoring DopR1 expression to γ mushroom body neurons rescues water-learning of dumb mutant flies to a level that is indistinguishable from wild-type flies (P>0.38, n≥8; ANOVA followed by post hoc Tukey’s HSD test). (c) Output from 0273 and R58E02 expressing PAM dopaminergic neurons is required for water learning (P<0.0001, n≥12; ANOVA followed by post hoc Tukey’s HSD test). (d) Blocking output from TH-GAL4 expressing PPL1 dopaminergic neurons does not impair water learning (*P=0.02, n≥11; ns, P=0.12, n=12; ANOVA followed by post hoc Tukey’s HSD test). (e) Blocking 0273 and R58E02 PAM dopaminergic neurons after training does not disrupt 30 min water memory (P=0.76 for R58E02-GAL4;UAS-shits1, n=8; ns, P=0.31, n=8; *P=0.03, n=8; ANOVA followed by post hoc Tukey’s HSD test).
Water learning utilizes DopR1 signalling in mushroom body γ neurons
Dopamine signalling is essential for reward learning with sugar 8,9,23, and conveys both the octopamine-dependent sweet taste signal and that for nutrient value 9. We therefore addressed the role of dopamine in water learning. Flies carrying the dumb1 or dumb2 mutation in the D1 dopamine DopR1 receptor that are defective in sugar reward learning 23 were also significantly impaired for water learning (Fig. 2b). We also rescued the performance of dumb2 mutant flies by re-establishing expression of DopR1 in mushroom body neurons. Expressing UAS-DopR1 in mostly the γ (with NP1131-GAL4 and 201Y-GAL4), but not the αβ (with c739-GAL4) or α′β′ neurons (with c305a-GAL4), produced a significant restoration of memory performance (Fig. 2b). The dumb mutant flies are not defective in water drinking (Supplementary Fig. 3a). Furthermore, despite the dumb2 flies having an apparent olfactory acuity defect, restoration of olfaction with the c739-GAL4 and c305a-GAL4 drivers, does not correlate with wild-type learning ability (Supplementary Fig. 3b, Fig. 2b). However, since both NP1131-GAL4 and 201Y-GAL4 driven UAS-DopR1 restores olfaction and learning and that the region of clear overlap in expression in these lines is in the γ lobe 24, we conclude that key water reinforcing dopamine signals might be delivered to the γ lobe.
Rewarding dopaminergic neurons reinforce water memory
Previous studies have established that dopaminergic neurons in the PAM (protocerebral anterior medial) cluster that innervate the horizontal lobes of the mushroom body are critical for sugar-reinforced olfactory memory 8,9. We therefore used 0273-GAL4 9 and R58E02-GAL4 8 driven UAS-shits1 to block during training either the entire population of approximately 130, or ~90 dopaminergic neurons in PAM, respectively. In both cases, water memory formation was significantly impaired at 32°C (Fig. 2c) but not at the permissive 23°C (Supplementary Fig. 4a). The olfactory acuity of all strains was not significantly different (Supplementary Fig. 4b). The 0273-GAL4; UAS-shits1 flies drink significantly less water during the two-minute training cycle (Supplementary Fig. 4c). However, the magnitude of the decreased drinking is unlikely to account for the abolishment of memory performance (Fig. 2c) because 0273-GAL4; UAS- shits1 flies still consume a quantity of water that is comparable to that of wild-type flies at 23°C (Fig. 1c) and that is sufficient to form robust 3 min water memory (Fig. 1b). Moreover, the R58E02-GAL4; UAS-shits1 flies drink normally during training (Supplementary Fig. 4c). We also tested the role of dopaminergic neurons that have been implicated in aversive reinforcement 18,25 by blocking them during training using TH-GAL4 driven UAS-shits1. No defect was observed (Fig. 2d). Lastly, flies in which the PAM neurons were blocked for 30 min after training and during memory testing displayed memory performance that was indistinguishable from that of controls (Fig. 2e). The PAM dopaminergic neurons are therefore required during acquisition, but are apparently dispensable for the expression of water memory.
Water-rewarding dopaminergic neurons innervate γ4
To identify the water-reinforcing dopaminergic neurons we visually isolated seven GAL4 lines that express in subsets of the 0273 and R58E02 populations, and assayed the consequence of blocking these neurons with UAS-shits1. In this screen only R48B04; UAS-shits1 flies revealed a significant defect in water learning (Fig. 3a; also see Fig. 3d). Importantly, the water learning defect of R48B04-GAL4; UAS-shits1 was not observed at the permissive 23°C (Supplementary Fig. 5a) and water consumption (Supplementary Fig. 5b) and olfactory acuity (Supplementary Fig. 5c) was not impaired. Finding a role for R48B04 neurons caught our attention because R48B04 expression is driven by a promoter fragment from the oamb octopamine receptor gene 26. A previous study showed that oamb-dependent signalling in 0104-GAL4 neurons was critical for the short-term memory reinforcing effects of the sweet taste of sugars 9. However, octopamine is not required for water learning (Fig. 2a) and 0104-GAL4 blockade did not impair water learning (Fig. 3a) suggesting that water and sugar reinforcing neurons might be separable within the neurons labelled by R48B04-GAL4. R48B04 expresses in around 55 dopaminergic neurons in the PAM cluster that can be labeled by immunostaining for the tyrosine-hydroxylase enzyme (Fig. 3b and Supplementary Fig. 5d). We verified that it was the R48B04 dopaminergic neurons that were critical for water learning by removing them from the R48B04 expression pattern using an overlapping R58E02-GAL80 transgene 8 (Fig. 3b-c). This manipulation restored wild-type learning performance (Fig. 3d), suggesting that the R48B04 dopaminergic neurons convey the reinforcing effects of water.
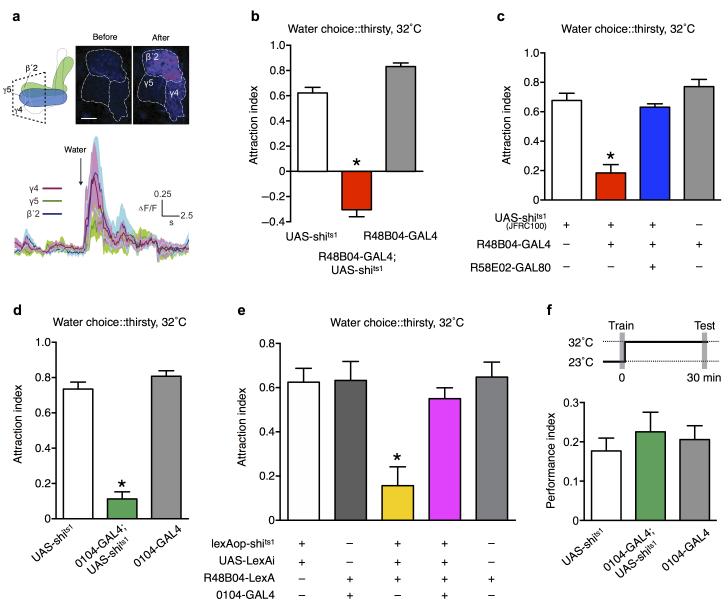
Fig. 3. Water learning requires reinforcing dopamine from specific neurons in the PAM cluster.
(a) Screening GAL4 lines that label subsets of PAM dopaminergic neurons revealed significantly impaired water learning performance in R48B04; UAS-shits1 flies (P<0.0001, n≥8; ANOVA followed by post hoc Tukey’s HSD test). (b) Expression pattern of R48B04-GAL4 driven GFP in the adult fly brain. The open yellow box marks the position of dopaminergic neurons in the PAM cluster. Scale bar 40 μm. (c) R48B04-GAL4 driven GFP expression in the PAM cluster is removed by R58E02-GAL80. Scale bar 40 μm. (d) The water learning defect in R48B04; UAS-shits1 flies (P<0.0001, n=8; ANOVA followed by post hoc Tukey’s HSD test) is rescued by removing expression in dopaminergic neurons with R58E02-GAL80 (P>0.4, n=8; ANOVA followed by post hoc Tukey’s HSD test). (e, f) R48B04 dopaminergic neurons innervate the γ4, γ5 and β′2 zones of the horizontal mushroom body lobes. (g, h) 0104 dopaminergic neurons also innervate the γ4, γ5 and β′2 zones. (i, j) Intersectional genetics removing 0104 neuron expression from R48B04 leaves dopaminergic neurons that innervate γ4 and γ5 and deletes expression in β′2 (dotted lines). The genotype is R48B04-LexA::P65/lexAop-rCD2::GFP; 0104-GAL4/UAS-LexARNAi. Scale bar 40 μm. (k) Blocking the PAM-γ4/5 neurons significantly impairs water learning (P<0.0001, n=12; ANOVA). (l, m) Pairing a 2 min odor presentation with PAM-γ4/5 neuron stimulation forms appetitive memory that is significantly greater than controls in (l) thirsty flies (P=0.01, n=11; ANOVA) but not in (m) water sated flies (P=0.8, n=8; ANOVA). (n) Subtracting R15A04 neuron expression from R48B04 completely removes labeling in neurons innervating γ5 (dotted lines). Scale bar 40 μm. (o) Removing γ5 neurons from R48B04 did not reverse the water learning defect (P<0.0001, n=8; ANOVA).
Dopaminergic neurons in R48B04 innervate the γ4, γ5 and β′2 zones of the horizontal mushroom body lobes 27 (Fig. 3e-f). Although this pattern resembles 0104 innervation (Fig. 3g-h), the discordance of behavioural phenotypes lead us to investigate whether R48B04 and 0104 label distinct neurons that innervate similar areas of the mushroom body lobes. When 0104-GAL4 and R48B04-LexA expression was visualized in the same brain, it was evident that 0104 and R48B04 neurons are largely non-overlapping in γ5, and that R48B04 has more neurons in γ4 than 0104 (Supplementary Fig. 5e). In contrast, 0104 and R48B04 label the same neurons in β′2 (Supplementary Fig. 5f). We therefore generated flies in which 0104 neuron expression was removed from the R48B04 pattern. 0104-GAL4 was used to drive a UAS-LexARNAi transgene in R48B04-LexA flies. Despite the potential caveat of partial interference with RNAi, expressing LexAop-GFP with this combination revealed dopaminergic neurons whose processes are largely confined to the γ4 and γ5 zones of the mushroom body γ lobe (Fig. 3i-j), from here on referred to as PAM-γ neurons. Importantly, blocking only the PAM-γ neurons with UAS-shits1 significantly impaired water learning (Fig. 3k). The defect was not observed at the permissive temperature of 23°C (Supplementary Fig. 5g) and these flies show normal water consumption (Supplementary Fig. 5h) and odor acuity (Supplementary Fig. 5i).
To test whether the PAM-γ4/5 neurons can provide instructive reinforcement, we conditioned flies with odor presentation and remote activation of PAM-γ4/5 neurons, using LexAop-dTrpA19. dTrpA1 encodes a transient receptor potential (TRP) channel that conducts calcium and depolarizes neurons when flies are exposed to temperature >25°C 28. PAM-γ4/5; LexAop-dTrpA1 flies exhibited appetitive memory that was statistically different to all control flies (Fig. 3l). This learning was not observed without the temperature shift during odor presentation (Supplementary Fig. 5j), and the flies displayed no differences in naïve olfactory acuity (Supplementary Fig. 5k). More strikingly, the artificial memory performance was suppressed when the flies were allowed to drink before training, demonstrating that PAM-γ4/5 neuron implanted memory is water-satiable (Fig. 3m). We are currently unable to exclusively manipulate the PAM-γ4 neurons. However, it is notable that blocking R15A04 neurons did not significantly disrupt water learning (Fig. 3a). Combining an R15A04-GAL80 with R48B04-GAL4 revealed that R15A04 expresses in R48B04-labeled dopaminergic neurons that innervate γ5, but not γ4 (Fig. 3n). In addition, removing γ5 expression from R48B04 did not restore wild-type water learning (Fig. 3o). Importantly, the remaining defect in these flies was not observed at the permissive temperature (Supplementary Fig. 5l) and neither water consumption (Supplementary Fig. 5m) nor olfactory acuity (Supplementary Fig. 5n) was different from that of control flies. We therefore conclude that the key water-reinforcement signals come from PAM-γ4 neurons.
Drinking water activates rewarding dopaminergic neurons
We also tested whether drinking evoked a response in dopaminergic neurons in thirsty flies by expressing GCaMP5 29 a genetically encoded indicator of intracellular calcium, with R48B04-GAL4. Drinking water drove a strong increase in GCaMP fluorescence in dopaminergic neuron processes in γ4 and β′2, and to a lesser extent in the γ5 zone of the mushroom body (Fig. 4a). These results support the model that water-reinforcement is conveyed by PAM-γ4 neurons, and they also suggest a possible role for the β′2 and γ5 innervating neurons.
Fig. 4. Naïve thirst-dependent water-seeking requires different dopaminergic neurons than those required for water learning.
(a) Drinking evokes an increase in intracellular Ca2+ in R48B04 dopamine neurons. Time courses of GCaMP5 responses (ΔF/F) to water consumption (solid lines are average traces and shaded areas represent the s.e.m. n=6 flies). Time of water presentation is indicated by the arrow. Panels show pseudo-colored examples of GCaMP5 fluorescence before (left) and after (right) water consumption. Dashed lines outline the distinct anatomical zones of the mushroom body lobes. Scale bar 15 μm. (b) Blocking R48B04 dopaminergic neurons converts naïve water approach in 16h water-deprived flies into significant avoidance (P<0.0001 from other groups and P=0.0009 from zero, n=8; ANOVA and one sample t-test). (c) The naïve water approach defect in thirsty R48B04-GAL4; UAS-shits1(JFRC100) flies (P<0.0001, n≥6; ANOVA followed by post hoc Tukey’s HSD test) is nullified by suppressing expression in dopaminergic neurons using R58E02-GAL80 (P>0.1, n≥6; ANOVA followed by post hoc Tukey’s HSD test). (d) Output from 0104 neurons is required for naïve water approach in thirsty flies (P<0.0001, n≥7; ANOVA). (e) Blocking R48B04 neurons with R48B04-LexA and LexAop-shits1 impairs naïve water approach in thirsty flies (P<0.0005, n=8; ANOVA followed by post hoc Tukey’s HSD test). The impairment was lost by removing expression from 0104 neurons (P>0.7 to the control flies, n=8; P=0.0016 to the non-rescued flies, n=8; ANOVA followed by post hoc Tukey’s HSD test). (f) Blocking 0104 neurons after training does not impair 30 min water memory performance in thirsty flies (P=0.69, n=8; ANOVA).
Naïve water evaluation requires dopaminergic neurons innervating β′2
We reasoned that water-evoked signals in another zone might represent incentive salience that controls naïve water-seeking behaviour. We therefore investigated a role for these dopaminergic neurons in naïve approach to water in thirsty flies. Strikingly, blocking R48B04 neurons converted the behaviour of naïve thirsty flies from water approach into water avoidance (Fig. 4b), like that observed in water sated flies (Fig. 1a). This behavioural reversal was not evident at the permissive temperature (Supplementary Fig. 6a). Furthermore, blocking R48B04 neurons had no effect on water avoidance in sated flies (Supplementary Fig. 6b), suggesting that these flies perceive water normally and that output from R48B04 neurons is only required for water approach in thirsty flies. A weaker but significant water approach defect was also observed when we expressed a different UAS-shits1 transgene (JFRC100 30) with R48B04-GAL4 (Fig. 4c). This defect was not observed at the permissive temperature (Supplementary Fig. 6c) and these flies showed normal water avoidance when they were water sated (Supplementary Fig. 6d). Moreover, using R58E02-GAL808 to suppress expression in the PAM dopaminergic neurons in this combination removed the behavioural defect of blocking R48B04 neurons (Fig. 4c). Unlike with water learning, blocking 0104 neurons also abolished naïve water-seeking behaviour in thirsty flies (Fig. 4d and Supplementary Fig. 6a-b). In addition, using 0104 intersection of R48B04 to suppress expression in β′2 neurons (Fig. 3i-j) restored water-seeking to R48B04; UAS-shits1 flies (Fig. 4e and Supplementary Fig. 6e-f). Taken together our experiments suggest that the β′2 neurons are required for the flies to evaluate water vapour signals in the naïve state, whereas the PAM-γ4 neurons assign water value to odors during learning.
Naïve water evaluation is independent of the DopR1 receptor
Since water learning requires D1 dopamine receptor (Fig. 2b), we also tested its role in naïve water-seeking in thirsty flies (Supplementary Fig. 6g). Surprisingly, the water-seeking behaviour of thirsty dumb1 mutant flies was indistinguishable from that of thirsty wild-type flies. We speculate that dopamine signals from the β′2 neurons to the β′ tip of the mushroom body are interpreted by a different dopamine receptor, or that a co-transmitter of dopamine might regulate naïve water-seeking.
Neurons controlling naïve water-seeking differ from those for learned water-seeking
We also tested whether the β′2 neurons were required for conditioned odor approach by blocking 0104 neurons immediately after training and during testing of water-reinforced memory. No significant defect was observed (Fig. 4f) consistent with the prior results when the entire PAM cluster was blocked with either 0273-GAL4 or R58E02-GAL4 (Fig. 2e). Paradoxically blocking R48B04 neurons after training and during testing of water-reinforced memory significantly enhanced learned odor approach (Supplementary Fig. 7) – a trend that was also apparent when blocking 0273 but not 0104 neurons (Fig. 2e). Since blocking 0104 neurons did not have any effect and the R48B04 enhancement is the opposite of the effect observed with naïve water-seeking, we conclude that the β′2 neurons are not required for water-seeking using learned odor cues. However, the data suggest that other R48B04 expressing neurons may play a role in limiting the efficiency of water-reinforced memory expression.
Discussion
Psychologists have split reward into wanting, learning and liking components, that can be assessed using drinking and feeding behaviors in animal models 31. Wanting denotes an animal’s desire to seek the resource, whereas learning assigns the consumed food or water value to associated sensory stimuli. An animal is considered to like a substance if it is accepted as palatable. Our results clearly demonstrate that separate PAM dopaminergic neurons in the fly are required for naïve water-seeking behavior (wanting) and to learn with water reward. In addition, our data suggest a different neural mechanism controls learned water-seeking. What about liking? Investigators have used acceptance and facial expression such as tongue protrusion as a sign that mammals like a given tastant 31. Flies extend their proboscis to palatable substances and retract it when presented with something bitter 32.
Furthermore, proboscis extension is controlled by the motivational state of the fly 33 . Interestingly, none of our neural manipulations that impaired naïve water-seeking or water learning, disrupted proboscis extension to water in thirsty flies (Supplementary Fig. 8). Hunger responsive dopaminergic neurons in the subesophageal ganglion have been shown to regulate proboscis extension to sugar 33. It therefore seems possible that analogous thirst responsive neurons will control responses to water. Nevertheless, it appears that manifestations of thirst in the fly that resemble wanting, learning and liking are supported by separate neural circuitry, at least some of which involve dopaminergic neurons (Supplementary Fig. 9).
Taken with previous work, our results here demonstrate an elaborate level of neural circuitry onto the mushroom body that allows independent control of naïve and learned appetitive behaviors in the fly 8,9,14,34. Water reinforcement involves different dopaminergic neurons, and independent mechanisms, to those required for sugar learning 8,9. Water-reward can be associated with odors through the PAM-γ4 neurons whereas sugar memory is reinforced by other rewarding dopaminergic neurons in the PAM cluster. Thirst motivates naïve water-seeking through the activity of the PAM-β′2 neurons. Although the behavioural expression of learned approach to water-associated odors is also specifically regulated by thirst, the PAM-β′2 neurons are not essential for learned approach. Hunger releases the mushroom body-MP1 dopaminergic neurons to permit expression of sugar-seeking memory14. It will therefore be interesting to determine whether other dopaminergic neurons provide a similar inhibitory control over the expression of water-seeking memory. Our data indicate that some R48B04 labeled neurons may play a role. Having mechanisms to separately learn food and water information and retrieve these memories appropriately permits efficient foraging behaviour. In addition, segregating the control of naïve water-seeking from water learning and memory expression likely permits the fly to seek water using learned distance cues that may predict the presence of water, in addition to the most reliable signal of vapor from the water source itself.
Online Methods
Fly strains
Drosophila melanogaster strains, were raised on standard cornmeal-agar food at 25°C and 60% humidity under 12/12 hr light/dark cycle. In all the behavioral studies, 5-7 days old flies of both sexes were used and experiments were performed between 9 am and 6 pm. The wild-type strain is Canton-S. The ppk28, TbhM18 and dumb1 mutant strains are described 15,20,23. The UAS-shits1(X;3), UAS-shits1 (JFRC100), Tdc2-GAL4, R58E02-GAL4, R58E02-GAL80, 0273-GAL4 and 0104-GAL4 transgenic strains are described 8,9,21,22,30 . R48B04-GAL4 and R15A04-GAL4 were obtained from the Bloomington stock centre. LexAop-rCD2::GFP, LexAop-shits1 and LexAop-TrpA1 strain are described 9,35,36. The dumb2 rescue flies: UAS-dDA1;dumb2, NP1131;dumb2, c305a;dumb2, 201Y;dumb2 and c739;dumb2 are those used previously24. R48B04-LexA::P65 construct was made by inserting the enhancer fragment of R48B04-GAL4 into the pBP-LexA::P65Uw vector (Addgene plasmid 26231). R15A04-GAL80 construct was made by inserting the enhancer fragment of R15A04-GAL4 into the pBPGAL80Uw-6 vector (Addgene plasmid 26236). The R48B04 and R15A04 enhancer fragments are from the JFRC FlyLight database 26. The R48B04-LexA::P65 and R15A04-GAL80 fly strains were made commercially (BestGene) by site-specific insertion into the attP40 and attP2 landing sites, respectively. The UAS-LexA RNAi was made as described 37, with two miRNA targeting sites: 5′-CGACAGCAGTCCTTTACTATCG-3′ and 5′-CTTAGCACGATTAACTATGATG-3′. UAS-LexA RNAi flies were raised commercially (BestGene) using routine P-element directed transformation.
Water deprivation
Approximately 80 flies per vial were water deprived by housing them for a defined time period with a 2cm × 6cm piece of dry sucrose-coated filter paper at 25°C and 60% humidity. For 6h quick desiccation, flies were kept in vials containing a 2cm × 3cm piece of dry sucrose-coated filter paper above a thick layer of drierite (Sigma-Aldrich). The flies and sugar paper were separated from the drierite by a layer of cotton wool. The vials were kept in a sealed box containing a thin layer of drierite for 6h.
Naïve water choice test
Water sated or deprived flies were given 2 min to choose between a dry filter paper lined tube and one containing a water-soaked filter paper. The water attraction index was calculated as the number of flies in the wet tube minus the number of flies in the dry tube, divided by the total number of flies in each experiment. For water vapor choice test, the filter papers were put in an inaccessible compartment at the end of the tube. The flies can therefore detect the vapor but cannot touch the water.
Proboscis Extension Reflex (PER) Assay
PER was performed as described 38 with a few modifications. 16h Water deprived flies were anesthetized on ice and stuck backside down onto nontoxic adhesive fly paper at 23°C, 60% humidity. Immobilised flies were then transferred to 32°C, 60% humidity and left to recover for 30 min. PER was assayed by presenting each fly with a drop of distilled water to either the foreleg or labellum. Water was presented three times per fly. Data represent the percentage of the total water offerings that elicited PER.
Ingestion Assay
To measure water consumption, flies were placed in a training tube used in the learning assay and allowed to drink for 2 min. Tubes were lined with a filter paper coated with a thin layer of 1% non-nutritious agar containing distilled water and 0.4% FD&C Blue No. 1 food dye (Spectrum Chemical). After 2 min, flies were quickly frozen at −20°C to prevent excretion. Twenty flies were homogenized in 500 μl phosphate-buffered saline (PBS) and centrifuged at 14000 rpm for 3 min to clear debris. The supernatant was then mixed with 100 μl PBS and centrifuged again at 14000 rpm for 3 min. The dye in the supernatant was then quantified by measuring the absorbance at 625 nm using a nanodrop. Sugar consumption was measured similarly by replacing water in 1% agar with 3M sucrose.
Water conditioning
The olfactory water conditioning paradigm was modified from the previously described sugar-reinforced olfactory conditioning paradigm 13. Odors were 3-octanol (7 μl in 8 ml mineral oil) and 4-methylcyclohexanol (14 μl in 8 ml mineral oil). Flies were exposed to one odor for 2 min in a tube lined with dry filter paper, followed by 30 s of fresh air. The flies were then transferred to another tube lined with water-soaked filter paper and exposed to a second odor for 2 min, followed by 30 s of fresh air. To measure learning (3 min memory), the flies were transferred to a choice point and given 2 min to choose between the two odors used in training. To assay longer-term memory, the flies were transferred back into water deprivation vials until the time of memory testing. The Performance Index was calculated as the number of flies running toward the conditioned odor minus the number of flies running toward the unconditioned odor, divided by the total number of flies in each experiment. A single Performance Index value is the average score from two experiments where a different population of the same genotype of flies is trained and tested with each odor paired with reinforcement. To satiate flies with water or food, flies were transferred to vials containing 1% agar or standard molasses-based fly food, respectively. Most experiments were performed at 23°C and 60% humidity, except where noted otherwise. For experiments at 32°C the flies were moved to 32°C 30 min before training and maintained at 32°C throughout the experiment. For experiments using UAS-shits1, the permissive temperature was 23°C and restrictive 32°C. To block neurotransmission for 3 min memory, flies were shifted from 23°C to 32°C for 30 min before training and testing. Alternatively, to block neurotransmission after training flies were moved to 32°C for 30 min prior to, and during testing. Artificial learning pairing odor exposure with lexAop-dTrpA1 mediated neural activation was performed as described 9. The relevant groups of flies were trained and tested in parallel and the order of groupings randomized. Data collection and analyses were not performed blind to the conditions of the experiments. To accommodate likely differences in genetic background between strains from different sources, each experiment included all relevant control groups, where transgenic lines were crossed to wild-type flies. Between group comparisons of independent JFRC Flylight lines 26 was assumed to be the best control for GAL4 lines from that source that exhibited behavioural phenotypes.
Imaging
Brains were dissected in PBS and fixed in PBS with 4% paraformaldehyde at room temperature for 20 min. They were washed three times, 20 min each in PBS containing 0.5% Triton-X100 (PBT), followed by 30 min incubation in PBT containing 5% normal goat serum. Anti-GFP (1:1000; Invitrogen, A11122), anti-TH (1:200; Millipore, AB152) and anti-nc8239(1:50; DSHB) antibodies were added to the solution and brains were incubated overnight at 4°C. Brains were then washed in PBT three times, 20 min each at room temperature, followed by incubation in PBT containing Alexa 488 conjugated goat anti-rabbit (1:100; Invitrogen, A11034) and Cy3 conjugated goat anti-mouse (1:200; Jackson ImmunoResearch, 115-165-003) overnight at 4°C. Brains were then washed in PBT three times, 20 min each at room temperature, before being mounted on slides with Gold anti-Fade mounting solution (Invitrogen). Imaging was performed using a Leica TCS SP5X confocal microscope. The resolution of the image stack was 1024 × 1024 with 1μm step size. Images were processed using Fiji. For each genotype, at least two brains were dissected to confirm that they had the same pattern of expression.
2-Photon in vivo calcium imaging
Up to 7-day-old UAS-GCaMP5;R48B04-GAL4 flies were water-deprived for 6-8 hours, briefly anaesthetized on ice and waxed to a custom built imaging chamber. The head capsule was opened under sugar-free HL3-like saline 40. Two-photon imaging was performed using a multiphoton imaging system (Scientifica), with a 40X, 0.8 NA water-immersion objective, controlled by ScanImage 3.8 41 software. Fluorescence was excited at 910 nm, 80 MHz repetition rate, ~ 70 fs, using a Ti:Sapphire laser (Coherent Chameleon). Images (256 × 256 pixels) were acquired at approximately 6 Hz. Water was delivered to the fly (for <10 s) using an automated feeding device, while the flies’ drinking behavior was observed using a Stingray CCD camera (Allied Vision Technologies). Two-photon images were analysed using Fiji/ImageJ. Regions of interest were manually assigned to the anatomically distinct lobe zones. Intensity tables were exported to Microsoft Excel and the ΔF/F was calculated, with an F calculated using the 20th – 30th frames. Traces were generated in Prism 6 (GraphPad Software).
Statistical analysis
Data distribution was assumed to be normal. Data were analyzed using Prism 6 (GraphPad Software). We used unpaired two-tailed T-test with Welch’s correction (not assuming equal standard deviations) to compare the difference between two groups. One-way ANOVA with Geisser-Greenhouse correction (not assuming equal variability of differences) and Tukey’s multiple comparisons tests were used to compare more than two groups. Definition of statistical significance is set at P<0.05. One sample t-test was used to detect the difference between an actual mean and zero. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in many previous publications8-13.
Supplementary Material
Acknowledgements
We thank C. J. Burke for his extensive failings and teachings in the art of water learning. We also thank Y. Huang and R. Brain for technical support and the Bloomington stock centre, T. Clandinin, D. Gohl, M. Silies, G. Rubin and the Janelia Farm Project, Y. Ben-Shahar, K. Scott, T. Lee and J. Dubnau for fly lines. S. L. was supported by an EMBO Long-Term Fellowship. D.O. was supported by an EMBO Long-Term Fellowship and a Sir Henry Wellcome Postdoctoral Fellowship. V. C. was supported by a Andrew Mason Memorial Scholarship. S.W. is funded by a Wellcome Trust Senior Research Fellowship in the Basic Biomedical Sciences and by funds from the Gatsby Charitable Foundation and Oxford Martin School.
References
- 1.Rolls BJ, Rolls ET. Thirst. CUP; Cambridge: 1982. [Google Scholar]
- 2.Skinner BF. The behavior of organisms: An experimental analysis. 1938 doi: 10.1901/jeab.1988.50-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Changizi MA, McGehee RM, Hall WG. Evidence that appetitive responses for dehydration and food-deprivation are learned. Physiol Behav. 2002;75:295–304. doi: 10.1016/s0031-9384(01)00660-6. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto Y, Mizunami M. Context-dependent olfactory learning in an insect. Learn Mem. 2004;11:288–293. doi: 10.1101/lm.72504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat. Rev. Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 9.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perisse E, et al. Different kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron. 2013;79:945–956. doi: 10.1016/j.neuron.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 12.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc. Natl. Acad. Sci. U S A. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J. Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krashes MJ, et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 17.Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Nature. Learn Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 18.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroll C, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Monastirioti M, Linn CEJ, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole SH, et al. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 22.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 23.Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin H, et al. Gamma neurons mediate dopaminergic input during aversive olfactory memory formation in Drosophila. Curr. Biol. 2012;22:608–614. doi: 10.1016/j.cub.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 28.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akerboom J, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. Proc. Natl. Acad. Sci. U S A. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J. Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- 33.Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azanchi R, Kaun KR, Heberlein U. Competing dopamine neurons drive oviposition choice for ethanol in Drosophila. Proc. Natl. Acad. Sci. U S A. 2013;110:21153–21158. doi: 10.1073/pnas.1320208110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parnas M, Lin AC, Huetteroth W, Miesenbock G. Odor discrimination in Drosophila: from neural population codes to behavior. Neuron. 2013;79:932–944. doi: 10.1016/j.neuron.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat. Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 37.Chen CH, et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- 38.Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr. Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laissue PP, et al. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol. 1999;405:543–552. [PubMed] [Google Scholar]
- 40.Yoshihara M. Simultaneous recording of calcium signals from identified neurons and feeding behavior of Drosophila melanogaster. J Vis Exp. 2012 doi: 10.3791/3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.