This work elucidates the biosynthetic pathway of toxic steroidal glycoalkaloids (SGAs) in potato, revealing that sterol side chain reductase 2 (SSR2) functions as a key enzyme in the biosynthesis of cholesterol and related SGAs. Silencing or disrupting SSR2 yielded potatoes with significantly reduced cholesterol and SGA levels but normal plant growth, making SSR2 an excellent target for breeding.
Abstract
Potatoes (Solanum tuberosum) contain α-solanine and α-chaconine, two well-known toxic steroidal glycoalkaloids (SGAs). Sprouts and green tubers accumulate especially high levels of SGAs. Although SGAs were proposed to be biosynthesized from cholesterol, the biosynthetic pathway for plant cholesterol is poorly understood. Here, we identify sterol side chain reductase 2 (SSR2) from potato as a key enzyme in the biosynthesis of cholesterol and related SGAs. Using in vitro enzyme activity assays, we determined that potato SSR2 (St SSR2) reduces desmosterol and cycloartenol to cholesterol and cycloartanol, respectively. These reduction steps are branch points in the biosynthetic pathways between C-24 alkylsterols and cholesterol in potato. Similar enzymatic results were also obtained from tomato SSR2. St SSR2-silenced potatoes or St SSR2-disrupted potato generated by targeted genome editing had significantly lower levels of cholesterol and SGAs without affecting plant growth. Our results suggest that St SSR2 is a promising target gene for breeding potatoes with low SGA levels.
INTRODUCTION
Steroidal glycoalkaloids (SGAs) are famous as toxic compounds in solanaceous plants that include the world’s major food crops such as potato (Solanum tuberosum) and tomato (Solanum lycopersicum) (Friedman, 2002, 2006). α-Chaconine and α-solanine account for most of the SGAs in cultivated potato and are especially abundant in sprouts, the green peel of tubers, and other aerial parts. To avoid postharvest increases in SGAs, harvested potatoes must be stored in the dark at low temperature (Kozukue and Mizuno, 1990). In potato breeding, introducing wild potato species as a genetic resource could increase the SGA level (Jacobsen and Rousselle, 1993). Thus, reducing the SGA level is an important target for potato breeding.
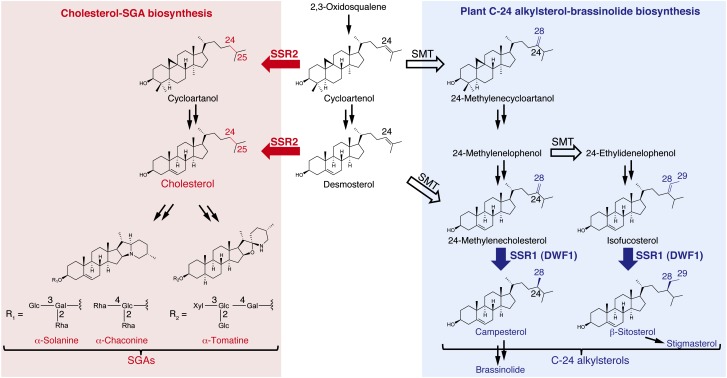
Feeding studies with isotope-labeled substrates suggested that SGAs are biosynthesized via cholesterol as a common precursor (Figure 1) (Tschesche and Hulpke, 1966, 1967; Heftmann et al., 1967). Cholesterol is converted to SGAs by a series of modifications that are predicted to involve oxidation, transamination and glycosylation (Ohyama et al., 2013). The recent releases of the potato (Xu et al., 2011) and tomato (Tomato Genome Consortium, 2012) genome sequences have accelerated the identification of genes encoding these enzymes in the SGA biosynthetic pathway (Moehs et al., 1997; McCue et al., 2005, 2006, 2007; Itkin et al., 2011, 2013). Modifying the SGA biosynthetic pathway by targeting potato genes encoding the oxidases or glycosyltransferases results in decreased levels of SGAs; however, the decrease in SGAs is associated with an increase in levels of intermediates between cholesterol and SGAs or unexpected derivatives of the intermediates (McCue et al., 2005, 2006, 2007; Itkin et al., 2011, 2013). This unintended metabolic change may pose a risk to potato consumers. As a more ideal way of engineering potatoes, reducing the supply of cholesterol, a common precursor for SGA biosynthesis, should result in lower levels of SGAs without a corresponding increase in levels of undesired intermediates between cholesterol and SGAs or their derivatives. However, the biosynthetic mechanism for cholesterol in plants has not been fully elucidated.
Figure 1.
Biosynthetic Pathways of Steroidal Glycoalkaloids and Plant C-24 Alkylsterols.
Plant C-24 alkylsterols and cholesterol, the common intermediates in steroidal glycoalkaloid biosynthesis, are biosynthesized from cycloartenol. Red arrows indicate reduction steps mediated by SSR2. Blue arrows indicate reduction steps mediated by SSR1. Open black arrows indicate transmethylation steps mediated by SMTs.
Generally, plants produce C-24 alkylsterols including campesterol, a precursor of the phytohormone brassinolide (Figure 1). The primary structural difference between the C-24 alkylsterols and cholesterol is the presence of an alkyl group at the C-24 position of the side chain (Benveniste, 1986). The C-24 alkylsterols have a methyl or an ethyl group at C-24 resulting from the addition of one or two methyl groups to Δ24(25)-sterols, such as cycloartenol and desmosterol catalyzed by sterol methyltransferases (SMTs) (Husselstein et al.,1996; Shi et al., 1996; Bouvier-Navé et al., 1997; Grebenok et al., 1997). Diminuto/Dwarf1 (DWF1) (Klahre et al., 1998; Choe et al., 1999) of Arabidopsis thaliana has been proposed to reduce alkylidene groups generated by SMT reactions to alkyl groups (Figure 1). The dwf1 mutant has a dwarf phenotype due to the lack of brassinosteroids (Kauschmann, et al., 1996). In the cholesterol biosynthetic pathway of animals including humans, the C-24 position of Δ24(25)-sterols, such as desmosterol, is reduced by 24-dehydrocholesterol reductase (DHCR24) (Waterham et al., 2001), a homolog of DWF1. Although cholesterol is generally present only in trace amounts in plants, solanaceous plants accumulate considerable amounts of SGAs derived from cholesterol. To meet this high demand for cholesterol, these plants may have a cholesterol biosynthetic mechanism distinct from that for ordinary plant sterol biosynthesis. In this study, a gene encoding sterol side chain reductase 2 (SSR2) committed to cholesterol biosynthesis in plants was identified and used to engineer potatoes with lower levels of SGAs.
RESULTS
Potato and Tomato Have Two Types of Genes Encoding DHCR24 Homologs
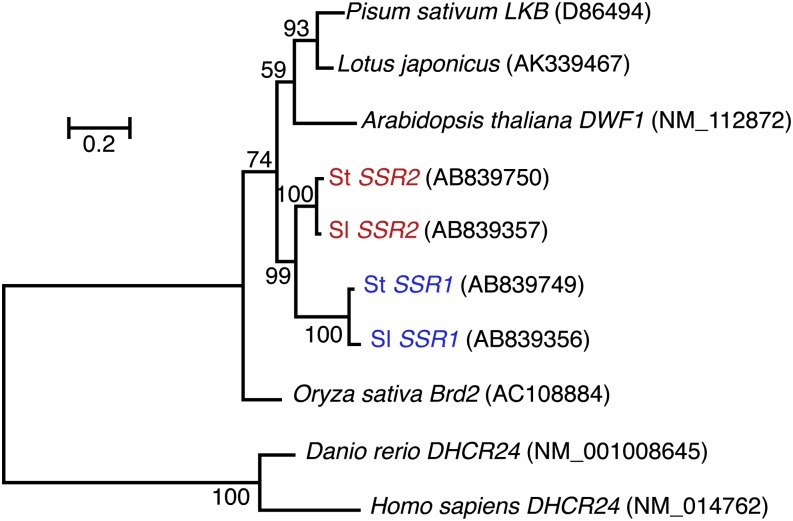
Using the human DHCR24 amino acid sequence as a query, BLAST (W. Gish, http://blast.wustl.edu and http://www.advbiocomp.com/blast/obsolete; Altschul et al., 1997) analysis against potato unigene databases from the Dana-Farber Cancer Institute Potato Gene Index (Quackenbush et al., 2001), and the Sol Genomics Network (Bombarely et al., 2011) allowed us to select two types of cDNA sequences designated as St SSR1 and St SSR2. In contrast, DWF1 is a single copy gene in Arabidopsis. We also found two full-length cDNA sequences (Sl SSR1 and Sl SSR2) similar to DHCR24 from a tomato unigene database from the Sol Genomics Network. The nucleotide sequence identities between St SSR1 and St SSR2 and between Sl SSR1 and Sl SSR2 are 81 and 79%, respectively. Phylogenetic analysis of the coding sequences of DHCR24 homologous genes from plants (Figure 2; Supplemental Data Set 1) indicates that SSR1 and SSR2 are paralogs in the Solanum genus. Although genes associated with SGA biosynthesis in potato and tomato were reported to be clustered on chromosomes 7 and 12 (Itkin et al., 2013), SSR genes in potato and tomato are positioned on chromosome 2 and are more than 10 Mbp away from each other (St SSR1, PGSC0003DMG400011801, and St SSR2, PGSC0003DMG400021142, in Xu et al., 2011; Sl SSR1, Solyc02g030170.2, and Sl SSR2, Solyc02g069490.2, in Tomato Genome Consortium, 2012). All four deduced proteins were predicted to have a flavin adenine dinucleotide binding domain and a transmembrane region by the PROSITE (Sigrist et al., 2002) and SOSUI (Hirokawa et al., 1998) programs, respectively (Supplemental Data Set 2).
Figure 2.
Phylogenetic Relationships among DHCR24 Homologous Genes from Plants.
DHCR24s of Homo sapiens and Danio rerio were used as outgroups. Numbers beside the nodes indicate bootstrap values (in percentages of 1000 replicates). The scale bar indicates the nucleotide substitution ratio. GenBank/EMBL/DDBJ accession numbers for the nucleotide sequences of the genes are given in parentheses.
In Vivo Enzymatic Activity Assays of SSRs
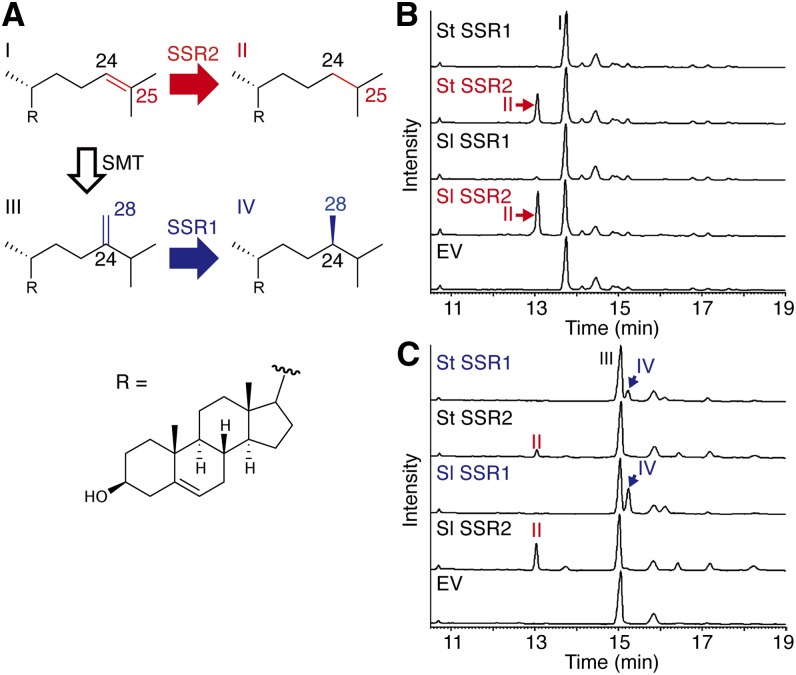
We presumed that one SSR would catalyze the reduction of Δ24(25)-sterol involved in cholesterol biosynthesis and the other SSR would mediate reduction of the Δ24(28)-double bond of C-24 alkylsterol biosynthetic intermediates. To investigate the reduction activities of the four putative SSRs from potato and tomato, the genes were expressed in yeast strains T31 and T21 that have modified ergosterol biosynthesis (Figure 3A; Supplemental Figure 1). The T31 strain accumulates a Δ24(25)-sterol, desmosterol, and the T21 strain accumulates a precursor of campesterol, 24-methylenecholesterol. Ethyl acetate extracts of the yeast cells were analyzed by gas chromatography-mass spectrometry (GC-MS) and compared with standard samples (Supplemental Figure 2). The T31 cells expressing St SSR2 and Sl SSR2 accumulated remarkable amounts of cholesterol compared with cells expressing St SSR1 and Sl SSR1 (Figure 3B). When T21 served as the host strain, the cells expressing St SSR1 and Sl SSR1 accumulated marked amounts of campesterol compared with the cells expressing St SSR2 and Sl SSR2. The T21 cells expressing St SSR2 and Sl SSR2 also accumulated cholesterol (Figure 3C). In the T21 cells, 24-methylenecholesterol is thought to be biosynthesized via Δ24(25)-sterols. The accumulation of cholesterol in the T21 cells expressing St SSR2 and Sl SSR2 may be attributed to the reduction of the Δ24(25)-sterols before C-24 methylation of the Δ24(25)-sterols catalyzed by ERG6 (yeast Δ24-sterol methyltransferase). These results indicate that SSR2 and SSR1 have different substrate specificities. St SSR2 and Sl SSR2 encode the sterol Δ24(25) reductases. In contrast, St SSR1 and Sl SSR1 encode the sterol Δ24(28) reductases. Thus, St SSR1 and Sl SSR1 are orthologous genes of DWF1 in potato and tomato, respectively.
Figure 3.
In Vivo Production of Cholesterol or Campesterol in Yeast Cells Transformed with SSRs.
(A) Reduction of sterol side chains by SSRs from potato and tomato to produce cholesterol (II) or campesterol (IV) in yeasts producing desmosterol (I) or 24-methylencholesterol (III), respectively. Red arrows indicate reduction steps mediated by SSR2. Blue arrows indicate reduction steps mediated by SSR1. The open black arrow indicates a Δ24-methylation step mediated by SMT.
(B) and (C) Analysis of the extracts of SSR-expressing yeast cells by GC-MS (total ion chromatograms). SSRs from potato and tomato were functionally expressed in desmosterol-producing yeast (B) and 24-methylenecholesterol-producing yeast (C). EV, empty vector control. Peak labels refer to (A).
In Vitro Enzymatic Activity Assays of SSRs
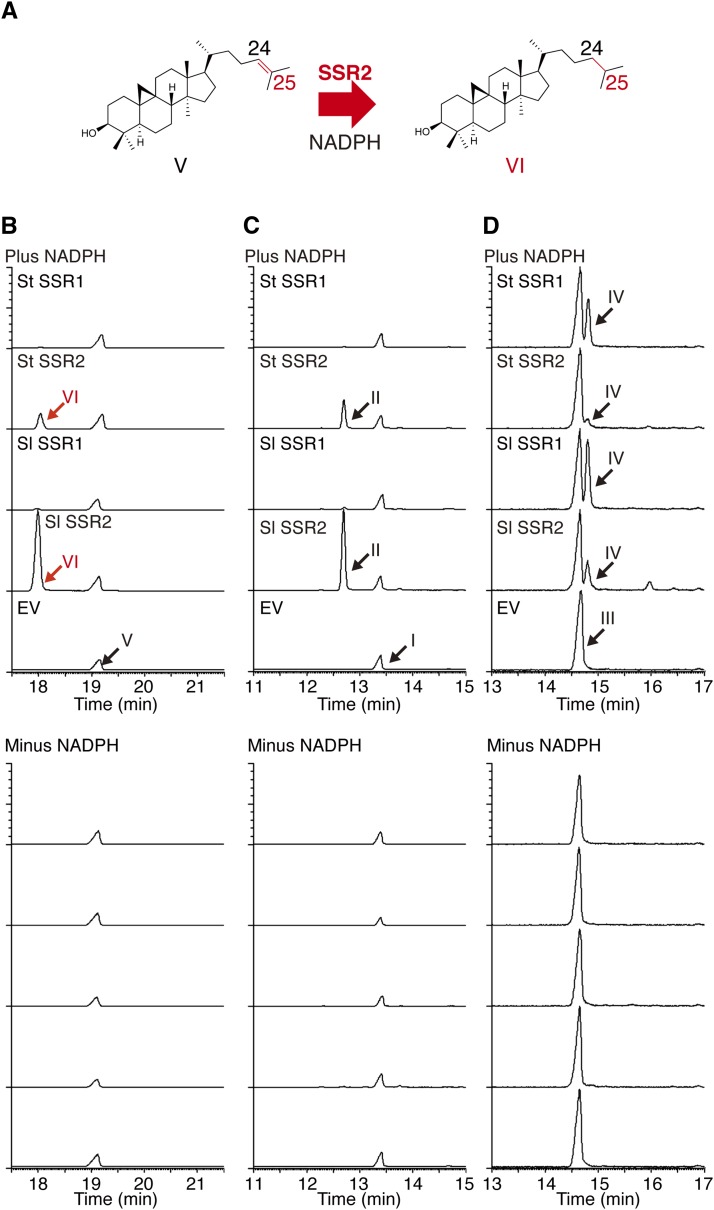
The in vivo cholesterol production by the T31 and T21 yeasts expressing SSR2 suggests that St SSR2 may be able to reduce a variety of the Δ24(25)-sterols, including lanosterol, the first Δ24(25)-sterol in yeast, and desmosterol (Supplemental Figure 1). In plants, cycloartenol is the first Δ24(25)-sterol. If cycloartenol is reduced to cycloartanol by SSR2, the biosynthetic pathway would be efficiently redirected toward cholesterol (Figure 1) (Diener et al., 2000; Schaeffer et al., 2000; Sitbon and Jonsson 2001; Holmberg et al., 2002; Arnqvist et al., 2003). We conducted in vitro assays to examine whether SSR2 has cycloartenol reductase activity and to compare the substrate specificities of SSR1 and SSR2 (Figure 4). St SSR1, St SSR2, Sl SSR1, and Sl SSR2 were heterologously expressed in an erg4 [deficient in sterol Δ24(28) reductase gene] yeast strain to avoid contamination of the endogenous sterol Δ24(28) reductase activity into the in vitro SSR enzymatic assays. Homogenates of the erg4 yeast cells expressing St SSR1, St SSR2, Sl SSR1, or Sl SSR2 were incubated with cycloartenol, desmosterol, or 24-methylenecholesterol, and the n-hexane extracts of the incubated samples were analyzed by GC-MS. St SSR2 and Sl SSR2 efficiently converted cycloartenol to the Δ24(25) reduction product, cycloartanol, and the reaction was NADPH dependent (Figure 4B). St SSR2 and Sl SSR2 also converted desmosterol to cholesterol in an NADPH-dependent manner (Figure 4C), a result consistent with the in vivo assay that indicated the production of cholesterol in yeast cells expressing SSR2 (Figure 3B). On the other hand, St SSR1 and Sl SSR1 efficiently reduced 24-methylenecholesterol to campesterol. The weak activity was also shown by St SSR2 and Sl SSR2 (Figure 4D). St SSR1 and Sl SSR1 also have very weak reductase activity toward cycloartenol and desmosterol (Supplemental Figure 3). These results indicate again that SSR1 and SSR2 have different substrate specificities. In addition, the Δ24(25) reduction activity against cycloartenol of SSR2 suggests that SSR2 effectively directs the sterol biosynthetic flow toward the cholesterol-SGA pathway in potato and tomato.
Figure 4.
In Vitro Enzymatic Assays of SSRs.
(A) SSR2 activity for the Δ24(25) reduction of cycloartenol (V) to cycloartanol (VI).
(B) to (D) In vitro assays using homogenates prepared from erg4 yeast cells expressing tomato or potato SSRs with cycloartenol (B), desmosterol (C), and 24-methylenecholesterol (D) as the substrates. The assays were performed in the absence (minus NADPH) or presence of NADPH (plus NADPH). The reaction products were analyzed by GC-MS. Traces are extracted ion chromatograms at m/z 395, 368 and 382, respectively. EV, empty vector control. Peak labels refer to Figures 3A and 4A.
Analyses of SSR2-Silenced Potato Plants
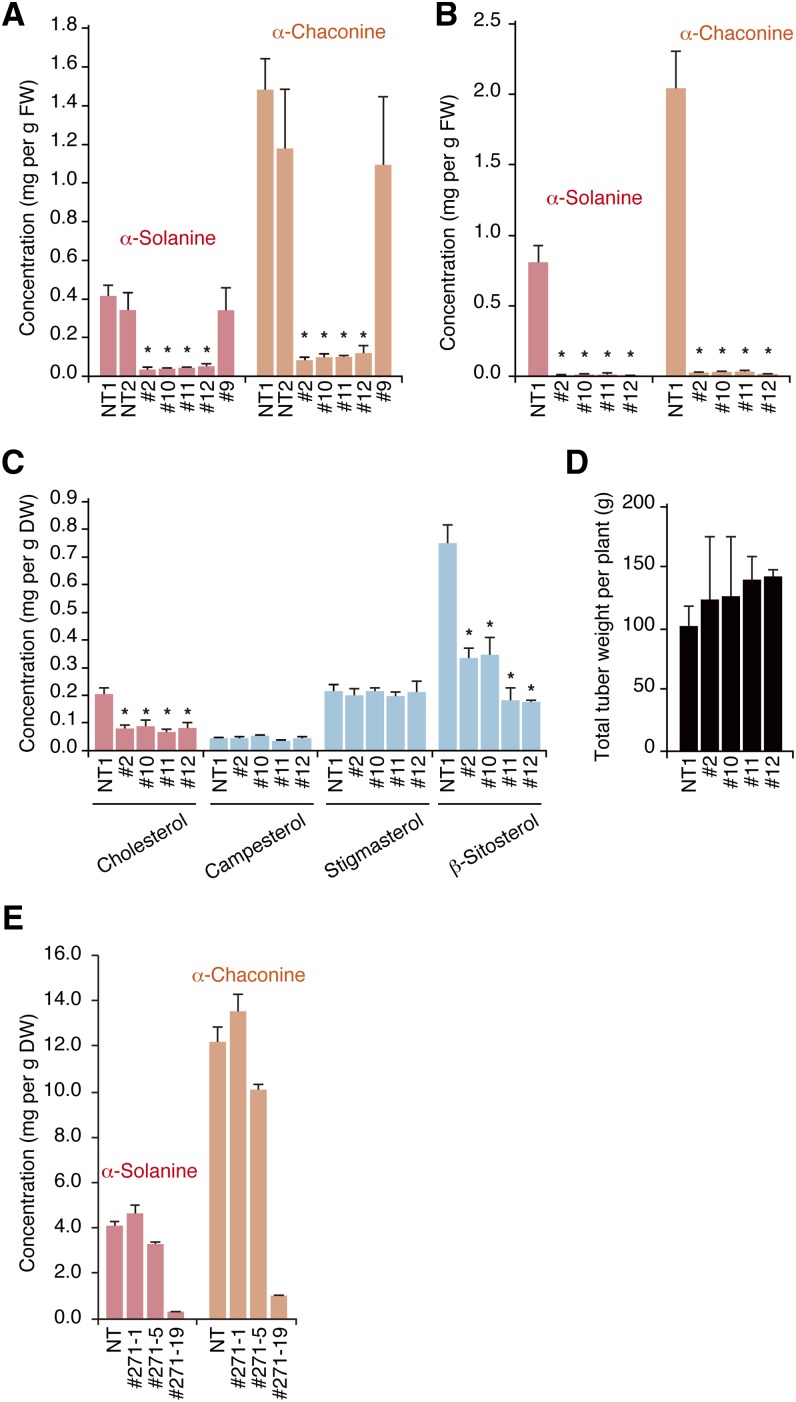
To verify the contribution of St SSR2 in the biosyntheses of cholesterol and SGAs in potato plants, we constructed St SSR2-silenced potato plants by transformation with an RNA interference (RNAi) vector (Supplemental Figure 4A). The RNAi vector was constructed from a 269-bp fragment of St SSR2 cDNA that did not match over 21 consecutive nucleotides with St SSR1. Four independent St SSR2-RNAi potato plant lines (#2, #10, #11, and #12 in Figures 5A to 5D) were identified that had remarkably reduced St SSR2 transcript levels (Supplemental Figure 4B). In these selected RNAi lines, the levels of predominant SGAs (α-solanine and α-chaconine) in the stems of in vitro-grown plants (Figure 5A) and tuber peels (Figure 5B) were significantly lower by 10% or more compared with the nontransformant. The cholesterol content was also significantly lower in the St SSR2-RNAi lines (Figure 5C). The levels of campesterol and stigmasterol were unchanged despite the decrease in β-sitosterol content (Figure 5C). The dwf1 mutant is well known for a dwarfed phenotype caused by a deficiency in brassinosteroids (Kauschmann, et al., 1996); however, the four St SSR2-RNAi lines showed normal growth and harvest yield (Figure 5D; Supplemental Figure 4C). We also analyzed levels of the major biologically active brassinosteroids, brassinolide and castasterone (Kim et al., 2005; Nomura et al., 2005), in two St SSR2-RNAi lines and the nontransformant. The levels of castasterone, which were under one thousandth of the cholesterol level in the nontransformant, were similar in the two transformants, and brassinolide levels were too low to quantify (Supplemental Table 1). These results indicate that St SSR2 is devoted to the production of cholesterol and SGAs in potato plants. The data also suggest that St SSR1 is responsible for C-24 alkylsterols and brassinolide biosyntheses. Levels of C-24 alkylsterol intermediates, such as 14α-methylcholesta-8,24-dien-3β-ol, 4α,14α-dimethylcholesta-8,24-dien-3β-ol, and isofucosterol, in the St SSR2-silenced lines were higher relative to those of the non-transformant (Supplemental Figures 4D and 4E). Similar results were also obtained from Sl SSR2-silenced tomatoes (Supplemental Figure 5).
Figure 5.
St SSR2-Silenced and -Disrupted Potato Plants.
(A) The predominant SGA (α-solanine and α-chaconine) levels in the stems of in vitro-grown nontransformed (NT) and St SSR2-silenced (#2, #10, #11, #12, and #9) plants (mean and sd, n = 3). Asterisks indicate values significantly different from the nontransformant (Dunnett's test, P < 0.05). FW, fresh weight.
(B) The predominant SGA levels in the peels of harvested tubers from nontransformed and St SSR2-silenced (#2, #10, #11, and #12) plants (mean and sd, n = 3). Asterisks indicate values significantly different from the nontransformant (Dunnett’s test, P < 0.05). FW, fresh weight.
(C) Cholesterol and C-24 alkylsterol content in the stems of in vitro grown nontransformed and St SSR2-silenced (#2, #10, #11, and #12) plants (mean and sd, n = 3). Asterisks indicate values significantly different from the nontransformant (Dunnett’s test, P < 0.05). DW, dry weight.
(D) Potato tuber yield of nontransformed and St SSR2-silenced (#2, #10, #11, and #12) plants (mean and sd, n = 3).
(E) The predominant SGA levels in the leaves of nontransformed and St SSR2-TALEN transformed (#271-1, #271-5, and #271-19) plants (mean and sd, n = 3). The predominant SGA levels of the nontransformant, #271-1, and #271-5 were significantly different from those of #271-19 (Dunnett’s test, P < 0.05).
Generation of an St SSR2-Disrupted Tetraploid Potato by Targeted Genome Editing
We also constructed an St SSR2-disrupted potato plant by transformation with a vector bearing a pair of transcription activator-like effector nuclease (TALEN) (Mahfouz et al., 2011; Cermak et al., 2011) constructs targeting St SSR2 (Supplemental Figures 6A and 6B and Supplemental Tables 2 to 4), controlled by an estradiol-inducible promoter (Zuo et al., 2000). Transformants were treated with estradiol to induce TALEN expression. PCR and sequence analyses showed that TALEN could generate St SSR2 alleles with deletions or insertions of varied sizes at all four homologous chromosomes in the tetraploid genome of potato with no influence on St SSR1 loci (Supplemental Figures 6C to 6E). The levels of predominant SGAs in the leaves of the St SSR2-disrupted plant (#271-19 in Figure 5E) were reduced to ∼10% of those of the nontransformant (NT in Figure 5E) or transformants with some intact alleles of St SSR2 (#271-1 and #271-5 in Figure 5E). The cholesterol level was also lower in the St SSR2-disrupted potato (#271-19 in Supplemental Figures 7A and 7B). Levels of some C-24 alkylsterol intermediates and C-24 alkylsterols such as 14α-methylcholesta-8,24-dien-3β-ol, 4α,14α-dimethylcholesta-8,24-dien-3β-ol, campesterol, and β-sitosterol in the St SSR2-disrupted line (#271-19 in Supplemental Figures 7A and 7B) were higher relative to those in the nontransformant (NT in Supplemental Figures 7A and 7B).
DISCUSSION
The data presented here show that St SSR2 is a key enzyme in the biosynthesis of toxic SGAs derived from cholesterol in potato. The methyl addition at C-24 of cycloartenol is the initial step in C-24 alkylsterol biosynthesis in plants. The presence of the alkyl group at C-24 that originates from this reaction is the structural characteristic differentiating C-24 alkylsterol from cholesterol. Therefore, cholesterol levels in plants are hypothesized to be modulated by SMT; this hypothesis is supported by the lower cholesterol level in transgenic plants that overexpress SMT and the higher cholesterol level in SMT knockout plants (Diener et al., 2000; Schaeffer et al., 2000; Sitbon and Jonsson 2001; Holmberg et al., 2002; Arnqvist et al., 2003). However, the considerable amounts of cholesterol and its derivatives, SGAs, in solanaceous plants have suggested that these plants have a mechanism to produce cholesterol that is distinct from other plants. The in vitro and in vivo analyses of both potato and tomato enzymes showed that solanaceous plants have two types of DWF1 homologs, SSR1 and SSR2, with different substrate preferences. SSR1 has Δ24(28) reduction activity required for C-24 alkylsterol biosynthesis. On the other hand, SSR2 has Δ24(25) reduction activity, which is essential for biosynthesis of cholesterol, an SGA precursor, as well as weak Δ24(28) reduction activity. These observations suggest that solanaceous plants have obtained an additional DWF1 homolog and developed it into SSR2 to meet the high demand for cholesterol.
The much reduced levels of cholesterol and SGAs in two distinct loss-of-function potato plants, i.e., silencing by RNAi and disruption by TALEN, strongly support the identification of SSR2 as a key enzyme in their biosyntheses. The increases of some C-24 alkylsterols and their intermediates in the St SSR2-silenced and St SSR2-disrupted potatoes were probably due to switching of the biosynthetic flow from the cholesterol-SGA pathway to the C-24 alkylsterol pathway caused by lowered or abolished SSR2 activity. The different effects of St SSR2 silencing and St SSR2 disruption were observed in the levels of campesterol, β-sitosterol, and isofucosterol produced. These inconsistencies can be attributed to an off-target effect of St SSR2-RNAi on St SSR1 transcription in the silenced lines (Supplemental Figure 4B). The TALEN-disrupted line #271-19 is considered to have only null or strong alleles of the St SSR2 locus because there was no or little intact genomic DNA for this locus (Supplemental Figure 6C). The residual SGA accumulated by this line (Figure 5E) might result from the weak Δ24(25) reduction activity of St SSR1 toward cycloartenol or desmosterol (Supplemental Figure 3), rather than leaky activity of St SSR2. Although roles for cholesterol and SGAs in plant growth and development have not been fully elucidated, the normal growth and development of St SSR2-RNAi and St SSR2-TALEN potatoes also suggests that cholesterol and SGAs may not be essential for the normal growth of potato (at least) under unstressed growth conditions.
In addition to SGAs in solanaceous plants, spirostan and/or furostan saponins such as dioscin in yam (Dioscorea spp; order Liliales) and trigoneosides in fenugreek (Trigonella spp; order Fabales) could also be biosynthesized via cholesterol as an intermediate (Bennett and Heftmann, 1965; Hardman and Fazli, 1972). Whether these plants have any genes homologous to DHCR24 is unknown. The paralogous relationship between SSR1 and SSR2 in the Solanum genus suggests that cholesterol biosynthetic mechanisms in plants were developed by convergent evolution in each order, family, or genus. That is, the cholesterol biosynthetic pathway in other plants may differ from that in Solanum.
In this study, we identified SSR2 as an enzyme that is responsible for the initial reaction from C-24 alkylsterol biosynthesis to cholesterol biosynthesis on the pathway to the production of toxic SGAs in solanaceous plants. In addition, the lower level of SGAs in the St SSR2-disrupted potato by targeted genome editing with TALEN is evidence that the St SSR2 gene identified in this study is an excellent target for breeding potatoes with decreased SGA levels. Breeding an St SSR2-knockout potato may also be possible by targeting induced local lesions in genomes (TILLING) (Muth et al., 2008); however, such methods that involve conventional mutagenesis would not rapidly generate new cultivars of important polyploid food crops, such as tetraploid potato. Our results from the St SSR2-TALEN transformants demonstrated that targeted genome editing with TALEN is able to generate potatoes with no remaining intact allele (Supplemental Figures 8A to 8C). An St SSR2-knockout potato without the transgene will be obtained by self-crossing the transformant (Supplemental Figure 8D). In the engineered potatoes, the absence of any increase in levels of intermediates between cholesterol and SGAs or their unexpected derivatives is expected because of the reduced supply of cholesterol as a common precursor of SGAs. In our analyses, there was no marked increase in the levels of intermediates (Supplemental Figure 7A). Further study of the SSR2-knockout potato will increase our understanding of the roles of cholesterol and SGAs in potato. Furthermore, as one of the possible gain-of-function applications of SSR2, overexpression of SSR2 in plants producing cholesterol-derived saponins could increase the production of such saponins, i.e., dioscin, as a raw material for steroidal drugs.
METHODS
Chemicals
Authentic samples of desmosterol, α-solanine, α-chaconine, and α-tomatine were purchased from Sigma-Aldrich. Desmosterol was also purchased from Avanti Polar Lipids. Cholesterol, campesterol, stigmasterol, and β-sitosterol were purchased from Tama Biochemical Co. Cycloartenol and cycloartanol were prepared by saponification of cycloartenyl ferurate (Wako Pure Chemical Industries) and the catalytic reduction of cycloartenol using H2 and Pd/C, respectively. Isofucosterol, 24-methylenecholesterol, and 24-methylenecycloartanol were prepared by previously described methods (Fagerlund and Idler, 1960; Lee et al., 1992; Fujimoto et al., 1997).
Cloning of SSR cDNAs
The tomato cDNA template was prepared from mRNA isolated from a flowering-stage leaf of Solanum lycopersicum cv Micro-Tom using the SV Total RNA Isolation System (Promega) and SuperScript First-Strand Synthesis System for RT-PCR (Life Technologies). The potato cDNA template was prepared from mRNA isolated from sprouts of Solanum tuberosum cv Sassy using an RNeasy Plant Mini Kit (Qiagen). KOD Plus DNA polymerase (Toyobo), PrimeSTAR Max DNA Polymerase (Takara Bio), and PrimeSTAR HS DNA Polymerase (Takara Bio) were used for PCR. The full open reading frames of St SSR1, St SSR2, Sl SSR1, and Sl SSR2 were PCR amplified with the primer sets 5′-CACCATGACAGATGTTCAGGCTCC-3′/5′-TCAATCTTCAGGCTCATCAACT-3′, 5′-CACCATGTCGGATGCTAAGGCCC-3′/5′-TCAATTCGCAGGTTCATCAG-3′, 5′-CTTGTGGTACCATGACAGATGTTCAGGCTCCCCCCCCTCG-3′/5′-CCATGAGACTCGAGTCAATCTTCAGGCTCATCAACTTCTG-3′, and 5′-AAGTTGGTACCATGTCGGATGCTAAGGCCCCCGTGGC-3′/5′-ATTCTTCTAGATCAATTTGCTTCAGGAGTCTCTTGTTCAG-3′, respectively. The PCR products of St SSR1 and St SSR2 were cloned into the Gateway entry vector pENTR/D-TOPO (Life Technologies) and transferred to the yeast expression vector pYES-DEST52 (Life Technologies) with a Gateway LR clonase reaction (Life Technologies). The PCR product of Sl SSR1 was cloned into KpnI and XhoI sites of the yeast expression vector pYES2 (Life Technologies). The PCR product of Sl SSR2 was cloned into KpnI and XbaI sites of pYES2.
Construction of a Desmosterol-Producing Yeast T21 and a 24-Methylenecholesterol-Producing Yeast T31
The yeast constitutive expression vector pTochigi 101 was constructed from pYO323 (Ohya et al., 1991) by inserting the Saccharomyces cerevisiae GPD promoter followed by a linker with NotI and AscI recognition sites and the PGK1 terminator between the ApaI and SacI sites of the vector (Yoshida et al., 2008). St DWF5 cDNA containing a full open reading frame was PCR amplified with the primer set 5′-CACCAATGGCGGAGTCTCAGTTG-3′/5′-CTAATAAATTCCTGGTATGACCC-3′ and designed based on SGN-U269317 from a unigene database in the Sol Genomics Network (Bombarely et al., 2011). The PCR product was cloned into the pENTR/D-TOPO vector (Life Technologies) and subcloned between the NotI and AscI sites of pTochigi101.
S. cerevisiae strain BY4742 erg4 erg5 was constructed from BY4742 erg4 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 erg4::KanMX4; Open Biosystems) (Kelly et al., 2001) by replacing the ERG5 ORF with a bler gene (Gueldener et al., 2002). The modified cassette was PCR amplified with primer sets 5′-TGTATTTGTTCCGCAATTTCC-3′/5′-CATTTTGTTAAAAGGTATTTATTGTCTATT-3′, 5′-ACATTTTGCTATTCCAAGACAATAAATACCTTTTAACAAAATGCAGCTGAAGCTTCGTACGC-3′/5′-TAAATATGATTTATTGTCTGGACAAAGTTCTGTTTTTCCCCATTAGCATAGGCCACTAGTGGATCTG-3′, and 5′-TAATGGGGAAAAACAGAACTTTG-3′/5′-TTGAACATAACGTCTTCATCTCC-3′.
Strains T21 and T31 were constructed by transforming BY4742 erg4 erg5 and BY4742 erg6 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 erg6::KanMX4; Open Biosystems) (Kelly et al., 2001) with pTochigi101 containing the St DWF5 cDNA.
Yeast in Vivo Assays
Yeast transformation was performed using a Frozen-EZ Yeast Transformation II Kit (Zymo Research) or a Gene Pulser Xcell electroporation system (Bio-Rad) according to the manufacturers’ protocols. Yeast strains T21 and T31 described above were transformed with St SSR1, St SSR2, Sl SSR1, and Sl SSR2 cDNAs cloned into pYES-DEST52 or pYES2. T21 and T31 transformed with intact pYES2 were used as empty vector controls. The transformants were cultured in yeast Synthetic Minimum Medium containing 2% glucose supplemented with -His/-Ura DO supplement (Takara Bio) for 1 d at 30°C. The cells were transferred to new medium of the same composition except for containing 2% (w/v) galactose instead of glucose to induce the recombinant SSRs and cultured for two more days. The harvested cells were lyophilized and extracted with ethyl acetate, and the extracts were analyzed by GC-MS after trimethylsilylation.
In Vitro Assay
S. cerevisiae BY4742 erg4 (Open Biosystems) (Kelly et al., 2001) was transformed with St SSR1, St SSR2, Sl SSR1, and Sl SSR2 cDNAs cloned into pYES-DEST52 or pYES2. BY4742 erg4 transformed with intact pYES2 was used as an empty vector control. The transformants were cultured in yeast Synthetic Minimal Medium containing 2% glucose supplemented with -Ura DO supplement (Takara Bio) for 1 d at 30°C. The cells were transferred to new medium of the same composition except for containing 2% galactose instead of glucose to induce the recombinant SSRs and cultured for one more day. The in vitro enzyme activity assays using yeast homogenates were performed as previously reported (Waterham et al., 2001; Ciufo et al., 2011). Briefly, the harvested cells were suspended in 20 mM Tris-HCl, pH 7.5, containing 50 mM NaCl and disrupted with a Beadbeater (BioSpec Products) containing 0.5-mm glass beads. After removing the glass beads and cell debris by centrifugation at 3000g for 5 min, protein concentrations in the homogenates were measured with Quick Start Bradford Protein Assay Kit 2 (Bio-Rad). The assay mixture contained 100 mM Tris-HCl, pH 7.23, 1 mM DTT, 20 µM flavin adenine dinucleotide, 2 mM NADPH, 0.5 mg mL−1 BSA, 0.56 mg mL−1 homogenate protein, and 168 µM sterol substrate. The substrate was added from a 420 µM stock solution in 100 mM Tris-HCl, pH 7.23, containing 1.25% (w/v) methyl-β-cyclodextrin. After incubation for 24 h at 30°C, samples were saponified with KOH and ethanol for 1 h at 80°C and extracted with n-hexane. The extracts were washed with water and analyzed by GC-MS after trimethylsilylation.
St SSR2-Silenced Potatoes
A 269-bp fragment of St SSR2 cDNA that did not match over 21 consecutive nucleotides with St SSR1 was PCR amplified using the primer set 5′-GAGCTCTAGACCCTAGGAGGAAGATCCAG-3′/5′-GGATCCATATGCGTTTCTCATTCCAACAACA-3′. An RNAi binary vector pKT251 targeting the St SSR2 gene was constructed from the binary vector pKT11 (Umemoto et al., 2001) by locating two St SSR2 fragments in opposite orientations interposing the third intron of the Arabidopsis thaliana At4g14210 gene under the control of the cauliflower mosaic virus 35S (CaMV35S) promoter in the T-DNA region (Supplemental Figure 4A). The RNAi binary vector pKT251 was transformed into Agrobacterium tumefaciens GV3110. Potatoes (S. tuberosum cv Sassy) were transformed using Agrobacterium GV3110 cells with pKT251 as previously reported (Monnma, 1990). In vitro-grown plants were cultured at 20°C under a 16-h-light/8-h-dark cycle. Thirty-nine transformants were individually selected by genomic PCR of the shoots with the primer set 5′-TAAAGCACGAGGAAGCGGT-3′/5′-GCACAACAGACAATCGGCT-3′ targeting the kanamycin resistance gene on the T-DNA region integrated into the potato genome. RT-PCR analyses of St SSR1 and St SSR2 were performed using the primer sets 5′-TTAGGTTTTTCTTTGGATGGGC-3′/5′-TCACCCTGCCTCTTGTGCAG-3′ and 5′-CTCTGCTCAAAGCCACACAA-3′/5′-TCAATTCGCAGGTTCATCAG-3′ and total RNA prepared from stems of five independent lines of in vitro-cultured plants, #2, #10, #11, #12 and #9, and nontransformants. As a control, we used the potato elongation factor 1α gene (EF1α) and the primer pair 5′-ATTGGAAACGGATATGCTCCA-3′/5′-TCCTTACCTGAACGCCTGTCA-3′ (Nicot et al., 2005). For the quantitative comparison of the transcripts, the cycles of PCR reactions were optimized to make sure of both clear visualization of the amplified products on an agarose gel and amplification in the exponential phase. The optimal cycles were 20 and 25 for St SSR1, 25 for St SSR2, and 20 for EF1α (Supplemental Figure 4B).
Sl SSR2-Silenced Tomatoes
A fragment of Sl SSR2 cDNA that did not match over consecutive 21 nucleotides with Sl SSR1 was PCR amplified using the primer set 5′-GAGCTCTAGATGTCGGATGCTAAGGCC-3′/5′-GGATCCATATGCATTCCTCTGGCCAAG-3′. An RNAi binary vector pKT262 targeting Sl SSR2 was constructed with the Sl SSR2 fragment in a way similar to the construction of pKT251. Tomatoes (S. lycopersicum cv Micro-Tom) were transformed using Agrobacterium GV3110 cells with pKT262 as previously reported (Sun et al., 2006). Integration of the T-DNA region into the tomato genomic DNA was investigated by genomic PCR of the tomato leaves targeting the kanamycin resistance gene on the T-DNA region integrated into the tomato genome.
St SSR2-Disrupted Potato
The St SSR2-disrupted potato was generated by targeted genome editing with TALEN (Cermak et al., 2011; Mahfouz et al., 2011). We constructed a new backbone vector, pGW-TAL-NC (Zeo), as follows. The region containing a TALEN scaffold was PCR amplified from pcDNA-TAL-NC (Sakuma et al., 2013) using the primer set 5′-CACCACTAGTAAAAATGGCTTCCTCCCCTCCAAAGAAAA-3′/5′-TTAAAAGTTTATCTCACCGTTATTA-3′ and cloned into the pENTR/D-TOPO vector (Life Technologies). The fragment was transferred to the pDEST17 vector (Life Technologies) with a Gateway LR reaction and subsequently cloned into pDONR/Zeo (Life Technologies) with a Gateway BP reaction to create pGW-TAL-NC (Zeo).
Three TALEN target sequences (SSR2_A, SSR2_B, and SSR2_C) were designed using the TAL effector-Nucleotide Targeter 2.0 website (Doyle et al., 2012; https://tale-nt.cac.cornell.edu) (Supplemental Table 2). TAL effector repeats were constructed by the six-module Golden Gate assembly method (Sakuma et al., 2013). For the single-strand annealing (SSA) assay, left and right regions of each target sequence were incorporated into pcDNA-TAL-NC to create pcDNA-SSA-SSR2_A_L, pcDNA-SSA-SSR2_A_R, pcDNA-SSA-SSR2_B_L, pcDNA-SSA-SSR2_B_R, pcDNA-SSA-SSR2_C_L, and pcDNA-SSA-SSR2_C_R. Among the three TALEN pairs whose activity was evaluated by SSA assays as described below (Supplemental Table 3), the most active TALEN pair, TAL-SSR2_C, was selected for potato transformation. TALEN assembly of SSR2_C was incorporated between the Esp3I sites of pGW-TAL-NC (Zeo) to obtain pGW-TAL-NC-SSR2_C_L and pGW-TAL-NC-SSR2_C_R.
To construct pKT271, an estradiol-inducible TALEN vector, we referred to pER8 (Zuo et al., 2000) and used a custom gene synthesis service to obtain two DNA fragments, N1-2 (AsiSI site-G10-90 promoter-XVE-KpnI site) and N3 (KpnI site-pea rbcS E9 terminator-SmaI site-LexA operator –46 to +12 of the CaMV35S promoter-NotI and AscI sites-Arabidopsis Heat Shock Protein [At5g59720] terminator-PmeI and EcoRI sites). N1-2 digested with AsiSI/KpnI and N3 digested with KpnI/EcoRI were cloned into HindII/EcoRI-digested pKT11 (Umemoto et al., 2001) using an AsiSI-HindIII synthetic linker to produce pESTRA. N3 was digested with KpnI/EcoRI and cloned into KpnI/EcoRI-digested pBluescript SK vector without the PstI-SacI region to produce pN3. The TALEN left region was excised from pGW-TAL-NC-SSR2_C_L as a NotI/AscI fragment and cloned into NotI/AscI-digested pESTRA to obtain pESTRA#23. The TALEN right region was excised from pGW-TAL-NC-SSR2_C_R as a NotI/AscI fragment and cloned into NotI/AscI-digested pN3 to obtain pN3#24. The fragment containing the LexA operator, −46 to +12 of CaMV35S promoter-TALEN right region, was excised from pN3#24 as a SmaI/EcoRI fragment and cloned into PmeI/EcoRI-digested (SmaI and PmeI cleavages produce blunt ends) pESTRA#23 to construct the TALEN vector pKT271 (Supplemental Figure 6A).
The TALEN vector pKT271 was transformed into Agrobacterium GV3110. Potatoes (S. tuberosum cv Sassy) were transformed using Agrobacterium GV3110 cells with pKT271 as previously reported (Monnma, 1990). Twenty-nine transformants were individually selected by genomic PCR of the shoots with the primer set 5′-TAAAGCACGAGGAAGCGGT-3′/5′-GCACAACAGACAATCGGCT-3′ targeting the kanamycin resistance gene on the T-DNA region integrated into the potato genome. The selected transformants were cultured in plant boxes (Murashige and Skoog medium [Murashige and Skoog, 1962] containing 3% sucrose, 100 mg L−1 carbenicillin, and 0.8% agar) at 20°C under a 16-h-light/8-h-dark cycle.
To induce TALEN expression, 6- to 8-cm-tall subcultured shoots grown in plant boxes were dipped in a solution containing 10 μM 17β-estradiol and 0.02% Silwet L-77 and then transferred to soil. They were grown at 23°C under a 16-h-light/8-h-dark cycle.
Two weeks after the transgenic potatoes were transferred to soil, genomic DNA was extracted from TALEN-induced transgenic potato plants to detect somatic mutants in the target site. Regions surrounding the St SSR2 target sites were amplified by PCR using the primer set 5′-TGTTCTCTGACACTGTTGTAGCACT-3′/5′-TCGAAGCATACATACCGGTCATCAT-3′ and PrimeSTAR Max DNA polymerase (Takara Bio). Amplified products were separated on 5% polyacrylamide gels (Ota et al., 2013). Two samples (lines #271-5 and #271-19) had extra bands probably due to the formation of heteroduplexes within the target sites (Supplemental Figure 6C). The PCR products were cloned into the pCR4-Blunt-TOPO (Life Technologies) vector, and 8 and 15 clones were randomly selected for sequencing from lines #271-5 and #271-19, respectively. The presence of targeted deletions or insertions was confirmed in two of eight sequenced clones from line #271-5 and in all 15 sequenced clones from line #271-19 (Supplemental Figures 6D and 6E). The corresponding region of the St SSR1 gene was also PCR amplified from line #271-19 (with the primer set 5′-TGTTCTCAGACACTGTTGTGTCATA-3′/5′-TTGAAGCATATCTACCAGTCATGCA-3′), as the most homologous potential off-target site (Supplemental Figure 6B); however, no mutations were detected in any of the eight sequenced clones.
The levels of sterols and SGAs in TALEN-induced transgenic potato lines were analyzed 6 to 7 weeks after the transgenic potatoes were transferred to soil as described below.
Construction of SSA Reporter Plasmids and the SSA Assay Using HEK293T Cells
Construction of SSA reporter plasmids and the SSA assay were performed as previously described (Sakuma et al., 2013). SSA reporter plasmids for SSR2_A, SSR2_B, and SSR2_C TALENs and pGL4-SSA-SSR2_A, pGL4-SSA-SSR2_B, and pGL4-SSA-SSR2_C were constructed as follows: sense and antisense oligonucleotides (Supplemental Table 4) were annealed and inserted between the BsaI sites of the pGL4-SSA vector. The SSA reporter plasmid for the negative control samples, pGL4-SSA-ZFA36, was constructed as previously described (Ochiai et al., 2010). pGL4-SSA-HPRT1_B-15 (Sakuma et al., 2013) was used as the SSA reporter plasmid for the positive control TALEN.
For the TALEN-positive control, TALEN expression vectors, pcDNA-SSA-HPRT1_B_L and pcDNA-SSA-HPRT1_B_R, were constructed as previously described (Sakuma et al., 2013) and cotransfected with reporter plasmids as described herein.
GC-MS Analysis
GC-MS analyses were conducted with the same conditions as described before (Choi et al., 2014). MS spectra of trimethylsilylated authentic standards are shown in Supplemental Figure 2. 14α-Methylcholesta-8,24-dien-3β-ol, 4α,14α-dimethylcholesta-8,24-dien-3β-ol, 24-ethylcholesta-5,23-dien-3β-ol, and 24-ethyldesmosterol are tentatively identified compounds that were deduced by comparison of their mass spectra (Supplemental Figure 9) with published spectra and the relative retention times (Kornfeldt and Croon, 1981; Berman et al., 1986; Akihisa et al., 1988; Narumi et al., 2001; Wretensjö and Karlberg, 2002). The endogenous levels of sterols in potato and tomato plants were calculated by comparing the peak area values of molecular ions (cholesterol, 14α-methylcholesta-8,24-dien-3β-ol, 24-methylenecholesterol, 4α,14α-dimethylcholesta-8,24-dien-3β-ol, campesterol, stigmasterol, and β-sitosterol), [M–90]+ formed by elimination of a trimethylsilanol (24-ethylcholesta-5,23-dien-3β-ol, 24-ethyldesmosterol, cycloartenol, and 24-methylenecycloartenol), or [M–98]+ formed by McLafferty rearrangement of a side chain (isofucosterol) for the endogenous sterols with that of the molecular ion or [M–90]+ for the internal standard, respectively.
Liquid Chromatography-Mass Spectrometry Analysis of α-Chaconine and α-Solanine in St SSR2-Silenced Potatoes
Fresh plant materials (100 mg) were homogenized with a mixer mill at 4°C in a 1 mL solution containing 80% (v/v) methanol and 0.1% (v/v) formic acid. For analyses of the levels of α-chaconine and α-solanine in the stems of in vitro-grown St SSR2-silenced plants, 10 μg brassinolide was added as an internal standard. After centrifugation, 25 μL of supernatant was diluted with 475 μL 0.1% (v/v) formic acid solution and filtered with a MultiScreen Solvinert (Millipore). An aliquot (10 μL) was analyzed by liquid chromatography-mass spectrometry (LC-MS) using 10 mM ammonium formate in water (pH 10):acetonitrile (2:3, v/v) as eluent at a flow rate of 0.2 mL min−1 at 40°C. LC-MS was performed with a Shimadzu LCMS-2010EV apparatus operating in ESI mode attached to an XBridge Shield RP18-5 column (150 mm × 2.1 mm i.d.; Waters). Quantifications of α-solanine and α-chaconine were calculated from the ratio of peak area at m/z 868 and 852 from positive ion scans using a calibration curve of authentic samples (with both coefficients of determination: r2 > 0.999), respectively.
LC-MS Analysis of α-Chaconine and α-Solanine in St SSR2-Disrupted Potatoes
Extractions and LC-MS analyses of the plant materials were performed with the same method as previously described (Ohyama et al., 2013). Quantifications of α-solanine and α-chaconine were calculated from the ratio of peak area at m/z 868 and 852, respectively, from positive ion scans using a calibration curve of authentic α-solanine (with a coefficient-of determination: r2 > 0.992).
LC-MS Analysis of α-Tomatine
The sample preparation procedures and LC-MS conditions were identical to those for LC-MS analysis of α-chaconine and α-solanine in St SSR2-disrupted potatoes described above. Quantification of α-tomatine was calculated from the ratio of peak areas at m/z 1034 from positive ion scans using a calibration curve of authentic α-tomatine (with a coefficient of determination: r2 > 0.998).
Quantification of Brassinosteroids
Frozen potato leaves (∼1 g fresh weight) were lyophilized and crushed to a fine powder with 10-mm ceramic beads by vortex and then soaked in 10 volume of extraction solvent (80% acetonitrile, 1% acetic acid) with stable isotope-labeled castasterone and brassinolide. After centrifugation, the supernatant was concentrated and reconstituted with 1% acetic acid. To remove interfering compounds, the extract was passed through an Oasis HLB column (Waters), Oasis MCX column, and Oasis WAX column equilibrated with 1% acetic acid, and brassinosteroids-containing fraction was eluted with 80% acetonitrile and 1% acetic acid, 80% acetonitrile and 1% acetic acid, and 80% acetonitrile, respectively. In each column step, the eluate was evaporated and reconstituted with 50% methanol. The castasterone and brassinolide were measured with UHPLC-Q-Exactive (Thermo Fisher Scientific) with an ODS column (AQUITY UPLC BEH C18, 1.7 μm, 2.1 × 100 mm; Waters). Brassinosteroids were separated at a flow rate of 0.25 mL min−1 with linear gradients of solvent A (0.05% formic acid) and solvent B (0.05% formic acid in acetonitrile) set according to the following profile: 0 min, 90% A + 10% B; 20 min, 5% A + 95% B. Data were processed by Xcalibur software (version 2.2; Thermo Fisher Scientific).
Statistical Analysis
Dunnett’s tests (Dunnett, 1955) were performed using the R Project for Statistical Computing software (R Core Team, 2013) with the multcomp package (Hothorn et al., 2008).
Phylogenetic Analysis
Sequence alignments (Supplemental Data Set 1) were constructed in MEGA5 (Tamura et al., 2011) using ClustalW (Thompson et al., 1994). The maximum likelihood tree was inferred in MEGA5 (Tamura et al., 2011) using Kimura's two-parameter model (Kimura, 1980) with a discrete gamma distribution (five categories) and the partial deletion option applied for gaps/missing-data treatment. Bootstrap values were performed with 1000 replications (Felsenstein, 1985). GenBank/EMBL/DDBJ accession numbers for the nucleotide sequences of the DHCR24 homologs are shown in Figure 2.
Multiple Alignments of Deduced SSR Amino Acid Sequences
Multiple alignments of deduced SSR amino acid sequences were constructed in GENETYX-MAC version 16 (Genetyx) with ClustalW2 (Larkin et al., 2007).
Accession Numbers
The sequences reported in this article have been deposited in the GenBank/EMBL/DDBJ database under the following accession numbers: AB839749 (St SSR1), AB839750 (St SSR2), AB839356 (Sl SSR1), AB839357 (Sl SSR2), and AB839751 (St DWF5). The accession numbers of the pER8 and potato EF1α in the GenBank/EMBL/DDBJ database are AF309825 and AB061263, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Modified Ergosterol Biosynthesis in Yeast Strains T21 and T31.
Supplemental Figure 2. MS Spectra of Authentic Sterol Standards.
Supplemental Figure 3. In Vitro Enzymatic Assays of SSRs.
Supplemental Figure 4. St SSR2-Silenced Potatoes.
Supplemental Figure 5. Sl SSR2-Silenced Tomatoes.
Supplemental Figure 6. TALEN Expression Vector Targeting St SSR2.
Supplemental Figure 7. St SSR2-Disrupted Potato.
Supplemental Figure 8. Flow Diagram of TALEN-Induced SSR2 Knockout in Potato.
Supplemental Figure 9. MS Spectra of Sterols in Potato and Tomato.
Supplemental Table 1. Levels of Brassinosteroids in Leaves from Nontransformed and St SSR2-RNAi Transformed Potatoes.
Supplemental Table 2. TALEN Pairs Designed to Target St SSR2.
Supplemental Table 3. Functional Evaluation of Engineered TALENs Targeting the SSR2 Gene by the SSA Assay.
Supplemental Table 4. Nucleotide Sequences of Oligonucleotides Used for Preparation of SSA Reporter Vectors.
Supplemental Data Set 1. Multiple Alignments Used to Construct the Phylogenetic Tree in Figure 2.
Supplemental Data Set 2. Multiple Alignments of Deduced SSR Amino Acid Sequences from Potato and Tomato.
Supplementary Material
Acknowledgments
We thank Yoshinori Fujimoto (Tokyo Institute of Technology) and Shin-ichi Ayabe (Nihon University) for encouragement, Masaharu Mizutani (Kobe University) for helpful discussion, and Tomomi Sawada (RIKEN), Masako Otsuka, Ryo Negoya, Kaori Kuno, and Chie Yoshida (Kirin Co.) for technical support. Tomato cultivar Micro-Tom (TOMJPF00001) was provided by the University of Tsukuba, Gene Research Center, through the National Bio-Resource Project of the Education, Culture, Sports, Science, and Technology (MEXT), Japan. This study was supported in part by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN), Japan; Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science; and the Scientific Technique Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry, Japan.
AUTHOR CONTRIBUTIONS
S.S., K.O., S.Y., H. Seki, T.S., T.Y., M.K., H. Sakakibara, T.A., T.M., K.S., and N.U. designed experiments. S.S., K.O., S.Y., T.S., Y.T., M.K., and N.U. performed the experiments. S.S., K.O., S.Y., H. Seki, T.S., Y.T., M.K., H. Sakakibara, and N.U. analyzed the data. S.S., K.O., S.Y., H. Seki, T.S., M.K., H. Sakakibara, T.A., T.M., K.S., and N.U. wrote the article. All authors discussed the results and approved the article.
Glossary
- SGA
steroidal glycoalkaloid
- SMT
sterol methyltransferase
- GC-MS
gas chromatography-mass spectrometry
- RNAi
RNA interference
- TALEN
transcription activator-like effector nuclease
- CaMV35S
cauliflower mosaic virus 35S
- LC-MS
liquid chromatography-mass spectrometry
Footnotes
Online version contains Web-only data.
Articles can be viewed online without a subscription.
References
- Akihisa, T., Ahmad, I., Singh, S., Tamura, T., Matsumoto, T. (1988). 14α-Methylzymosterol and other sterols from Wrightia tinctoria seeds. Phytochemistry 27: 3231–3234 [Google Scholar]
- Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W., Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist, L., Dutta, P.C., Jonsson, L., Sitbon, F. (2003). Reduction of cholesterol and glycoalkaloid levels in transgenic potato plants by overexpression of a type 1 sterol methyltransferase cDNA. Plant Physiol. 131: 1792–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, R.D., Heftmann, E. (1965). Biosynthesis of Dioscorea sapogenins from cholesterol. Phytochemistry 4: 577–586 [Google Scholar]
- Benveniste, P. (1986). Sterol biosynthesis. Annu. Rev. Plant Physiol. 37: 275–308 [Google Scholar]
- Berman, J.D., Goad, L.J., Beach, D.H., Holz, G.G., Jr. (1986). Effects of ketoconazole on sterol biosynthesis by Leishmania mexicana mexicana amastigotes in murine macrophage tumor cells. Mol. Biochem. Parasitol. 20: 85–92 [DOI] [PubMed] [Google Scholar]
- Bombarely, A., Menda, N., Tecle, I.Y., Buels, R.M., Strickler, S., Fischer-York, T., Pujar, A., Leto, J., Gosselin, J., Mueller, L.A. (2011). The Sol Genomics Network (solgenomics.net): growing tomatoes using Perl. Nucleic Acids Res. 39: D1149–D1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier-Navé, P., Husselstein, T., Desprez, T., Benveniste, P. (1997). Identification of cDNAs encoding sterol methyl-transferases involved in the second methylation step of plant sterol biosynthesis. Eur. J. Biochem. 246: 518–529 [DOI] [PubMed] [Google Scholar]
- Cermak, T., Doyle, E.L., Christian, M., Wang, L., Zhang, Y., Schmidt, C., Baller, J.A., Somia, N.V., Bogdanove, A.J., Voytas, D.F. (2011). Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe, S., Dilkes, B.P., Gregory, B.D., Ross, A.S., Yuan, H., Noguchi, T., Fujioka, S., Takatsuto, S., Tanaka, A., Yoshida, S., Tax, F.E., Feldmann, K.A. (1999). The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 119: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, H., Ohyama, K., Kim, Y.Y., Jin, J.Y., Lee, S.B., Yamaoka, Y., Muranaka, T., Suh, M.C., Fujioka, S., Lee, Y. (2014). The role of Arabidopsis ABCG9 and ABCG31 ATP binding cassette transporters in pollen fitness and the deposition of steryl glycosides on the pollen coat. Plant Cell 26: 310–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciufo, L.F., Murray, P.A., Thompson, A., Rigden, D.J., Rees, H.H. (2011). Characterisation of a desmosterol reductase involved in phytosterol dealkylation in the silkworm, Bombyx mori. PLoS ONE 6: e21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener, A.C., Li, H., Zhou, W., Whoriskey, W.J., Nes, W.D., Fink, G.R. (2000). Sterol methyltransferase 1 controls the level of cholesterol in plants. Plant Cell 12: 853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, E.L., Booher, N.J., Standage, D.S., Voytas, D.F., Brendel, V.P., VanDyk, J.K., Bogdanove, A.J. (2012). TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 40: W117–W122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett, C.W. (1955). A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 50: 1096–1121 [Google Scholar]
- Fagerlund, U.H.M., Idler, D.R. (1960). Marine sterols. VII. Synthesis of 29-isofucosterol and the attempted synthesis of 17-dehydrocholesterol. J. Fish. Res. Board Can. 17: 597–599 [Google Scholar]
- Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791 [DOI] [PubMed] [Google Scholar]
- Friedman, M. (2002). Tomato glycoalkaloids: role in the plant and in the diet. J. Agric. Food Chem. 50: 5751–5780 [DOI] [PubMed] [Google Scholar]
- Friedman, M. (2006). Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J. Agric. Food Chem. 54: 8655–8681 [DOI] [PubMed] [Google Scholar]
- Fujimoto, Y., Ohyama, K., Sato, N., Yamada, J., Morisaki, M. (1997). 13C assignment of diastereotopic C-26 and -27 methyl groups of 24-methylenecholesterol: Steric course of hydrogen migration from C-24 to C-25 during its biosynthesis in higher plants. Chem. Pharm. Bull. (Tokyo) 45: 224–226 [Google Scholar]
- Grebenok, R.J., Galbraith, D.W., Penna, D.D. (1997). Characterization of Zea mays endosperm C-24 sterol methyltransferase: one of two types of sterol methyltransferase in higher plants. Plant Mol. Biol. 34: 891–896 [DOI] [PubMed] [Google Scholar]
- Gueldener, U., Heinisch, J., Koehler, G.J., Voss, D., Hegemann, J.H. (2002). A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 30: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman, R., Fazli, F.R.Y. (1972). Labelled steroidal sapogenins and hydrocarbons from Trigonella foenumgraecum by acetate, mevalonate and cholesterol feeds to seeds. Planta Med. 21: 188–195 [DOI] [PubMed] [Google Scholar]
- Heftmann, E., Lieber, E.R., Bennett, R.D. (1967). Biosynthesis of tomatidine from cholesterol in Lycopersicon pimpinellifolium. Phytochemistry 6: 225–229 [Google Scholar]
- Hirokawa, T., Boon-Chieng, S., Mitaku, S. (1998). SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14: 378–379 [DOI] [PubMed] [Google Scholar]
- Holmberg, N., Harker, M., Gibbard, C.L., Wallace, A.D., Clayton, J.C., Rawlins, S., Hellyer, A., Safford, R. (2002). Sterol C-24 methyltransferase type 1 controls the flux of carbon into sterol biosynthesis in tobacco seed. Plant Physiol. 130: 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn, T., Bretz, F., Westfall, P. (2008). Simultaneous inference in general parametric models. Biom. J. 50: 346–363 [DOI] [PubMed] [Google Scholar]
- Husselstein, T., Gachotte, D., Desprez, T., Bard, M., Benveniste, P. (1996). Transformation of Saccharomyces cerevisiae with a cDNA encoding a sterol C-methyltransferase from Arabidopsis thaliana results in the synthesis of 24-ethyl sterols. FEBS Lett. 381: 87–92 [DOI] [PubMed] [Google Scholar]
- Itkin, M., et al. (2013). Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 341: 175–179 [DOI] [PubMed] [Google Scholar]
- Itkin, M., et al. (2011). GLYCOALKALOID METABOLISM1 is required for steroidal alkaloid glycosylation and prevention of phytotoxicity in tomato. Plant Cell 23: 4507–4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, E., and Rousselle, P. (1993). Potato. In Traditional Crop Breeding Practices: An Historical Review to Serve as a Baseline for Assessing the Role of Modern Biotechnology (Paris: Organisation for Economic Co-operation and Development), pp. 191–204. [Google Scholar]
- Kauschmann, A., Jessop, A., Koncz, C., Szekeres, M., Willmitzer, L., Altmann, T. (1996). Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 9: 701–713 [Google Scholar]
- Kelly, D.E., Lamb, D.C., Kelly, S.L. (2001). Genome-wide generation of yeast gene deletion strains. Comp. Funct. Genomics 2: 236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T.W., Hwang, J.Y., Kim, Y.S., Joo, S.H., Chang, S.C., Lee, J.S., Takatsuto, S., Kim, S.K. (2005). Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 17: 2397–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16: 111–120 [DOI] [PubMed] [Google Scholar]
- Klahre, U., Noguchi, T., Fujioka, S., Takatsuto, S., Yokota, T., Nomura, T., Yoshida, S., Chua, N.H. (1998). The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell 10: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeldt, A., Croon, L.B. (1981). 4-Demethyl-, 4-monomethyl- and 4,4-dimethylsterols in some vegetable oils. Lipids 16: 306–314 [Google Scholar]
- Kozukue, N., Mizuno, S. (1990). Effects of light exposure and storage temperature on greening and glycoalkaloids content in potato tubers. J. Jpn. Soc. Hortic. Sci. 59: 673–677 [Google Scholar]
- Larkin, M.A., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Lee, S.S., Young, L.H., Wang, K.C. (1992). Separation of 24-methylenecycloartanol from cycloartenol via a chemical method. J. Nat. Prod. 55: 644–648 [Google Scholar]
- Mahfouz, M.M., Li, L., Shamimuzzaman, M., Wibowo, A., Fang, X., Zhu, J.K. (2011). De novo-engineered transcription activator-like effector (TALE) hybrid nuclease with novel DNA binding specificity creates double-strand breaks. Proc. Natl. Acad. Sci. USA 108: 2623–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue, K.F., Allen, P.V., Shepherd, L.V., Blake, A., Maccree, M.M., Rockhold, D.R., Novy, R.G., Stewart, D., Davies, H.V.Belknap, W.R. (2007). Potato glycosterol rhamnosyltransferase, the terminal step in triose side-chain biosynthesis. Phytochemistry 68: 327–334 [DOI] [PubMed] [Google Scholar]
- McCue, K.F., Allen, P.V., Shepherd, L.V., Blake, A., Whitworth, J., Maccree, M.M., Rockhold, D.R., Stewart, D., Davies, H.V., Belknap, W.R. (2006). The primary in vivo steroidal alkaloid glucosyltransferase from potato. Phytochemistry 67: 1590–1597 [DOI] [PubMed] [Google Scholar]
- McCue, K.F., Shepherd, L.V.T., Allen, P.V., Maccree, M.M., Rockhold, D.R., Corsini, D.L., Davies, H.V., Belknap, W.R. (2005). Metabolic compensation of steroidal glycoalkaloid biosynthesis in transgenic potato tubers: using reverse genetics to confirm the in vivo enzyme function of a steroidal alkaloid galactosyltransferase. Plant Sci. 168: 267–273 [Google Scholar]
- Moehs, C.P., Allen, P.V., Friedman, M., Belknap, W.R., (1997). Cloning and expression of solanidine UDP-glucose glucosyltransferase from potato. Plant J. 11: 227–236 [DOI] [PubMed] [Google Scholar]
- Monnma, T. (1990). Recent study for genetic engineering of soybean glycinin gene. Plant Tissue Cult. Lett. 7: 57–63 [Google Scholar]
- Murashige, T., Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497 [Google Scholar]
- Muth, J., Hartje, S., Twyman, R.M., Hofferbert, H.R., Tacke, E., Prüfer, D. (2008). Precision breeding for novel starch variants in potato. Plant Biotechnol. J. 6: 576–584 [DOI] [PubMed] [Google Scholar]
- Narumi, Y., Noguchi, T., Fujioka, S., Takatsuto, S. (2001). Identification of 4,4-dimethyl- and 4-monomethylsterols in seeds of Setaria italic and Echinochloa frumentacea. J. Oleo Sci. 50: 53–56 [Google Scholar]
- Nicot, N., Hausman, J.F., Hoffmann, L., Evers, D. (2005). Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 56: 2907–2914 [DOI] [PubMed] [Google Scholar]
- Nomura, T., Kushiro, T., Yokota, T., Kamiya, Y., Bishop, G.J., Yamaguchi, S. (2005). The last reaction producing brassinolide is catalyzed by cytochrome P-450s, CYP85A3 in tomato and CYP85A2 in Arabidopsis. J. Biol. Chem. 280: 17873–17879 [DOI] [PubMed] [Google Scholar]
- Ochiai, H., Fujita, K., Suzuki, K., Nishikawa, M., Shibata, T., Sakamoto, N., Yamamoto, T. (2010). Targeted mutagenesis in the sea urchin embryo using zinc-finger nucleases. Genes Cells 15: 875–885 [DOI] [PubMed] [Google Scholar]
- Ohya, Y., Goebl, M., Goodman, L.E., Petersen-Bjørn, S., Friesen, J.D., Tamanoi, F., Anraku, Y. (1991). Yeast CAL1 is a structural and functional homologue to the DPR1 (RAM) gene involved in ras processing. J. Biol. Chem. 266: 12356–12360 [PubMed] [Google Scholar]
- Ohyama, K., Okawa, A., Moriuchi, Y., Fujimoto, Y. (2013). Biosynthesis of steroidal alkaloids in Solanaceae plants: involvement of an aldehyde intermediate during C-26 amination. Phytochemistry 89: 26–31 [DOI] [PubMed] [Google Scholar]
- Ota, S., Hisano, Y., Muraki, M., Hoshijima, K., Dahlem, T.J., Grunwald, D.J., Okada, Y., Kawahara, A. (2013). Efficient identification of TALEN-mediated genome modifications using heteroduplex mobility assays. Genes Cells 18: 450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush, J., Cho, J., Lee, D., Liang, F., Holt, I., Karamycheva, S., Parvizi, B., Pertea, G., Sultana, R., White, J. (2001). The TIGR Gene Indices: analysis of gene transcript sequences in highly sampled eukaryotic species. Nucleic Acids Res. 29: 159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. (Vienna, Austria: R Foundation for Statistical Computing). [Google Scholar]
- Sakuma, T., et al. (2013). Efficient TALEN construction and evaluation methods for human cell and animal applications. Genes Cells 18: 315–326 [DOI] [PubMed] [Google Scholar]
- Schaeffer, A., Bouvier-Navé, P., Benveniste, P., Schaller, H. (2000). Plant sterol-C24-methyl transferases: different profiles of tobacco transformed with SMT1 or SMT2. Lipids 35: 263–269 [DOI] [PubMed] [Google Scholar]
- Shi, J., Gonzales, R.A., Bhattacharyya, M.K. (1996). Identification and characterization of an S-adenosyl-L-methionine: delta 24-sterol-C-methyltransferase cDNA from soybean. J. Biol. Chem. 271: 9384–9389 [DOI] [PubMed] [Google Scholar]
- Sigrist, C.J.A., Cerutti, L., Hulo, N., Gattiker, A., Falquet, L., Pagni, M., Bairoch, A., Bucher, P. (2002). PROSITE: a documented database using patterns and profiles as motif descriptors. Brief. Bioinform. 3: 265–274 [DOI] [PubMed] [Google Scholar]
- Sitbon, F., Jonsson, L. (2001). Sterol composition and growth of transgenic tobacco plants expressing type-1 and type-2 sterol methyltransferases. Planta 212: 568–572 [DOI] [PubMed] [Google Scholar]
- Sun, H.J., Uchii, S., Watanabe, S., Ezura, H. (2006). A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 47: 426–431 [DOI] [PubMed] [Google Scholar]
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., Gibson, T.J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium (2012). The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschesche, R., Hulpke, H. (1966). Zur Biosynthese von Steroid-Derivaten im Pflanzenreich. 4. Mitt.: Biogenese von Tomatidin aus Cholesterin. Z. Naturforschg. 21b: 893–894 [Google Scholar]
- Tschesche, R., Hulpke, H. (1967). Zur Biosynthese von Steroid-Derivaten im Pflanzenreich. 8. Mitt.: Biogenese von Solanidin aus Cholesterin. Z. Naturforschg. 22b: 791. [PubMed] [Google Scholar]
- Umemoto, N., Tsukahara, M., Yoshioka, M. (2001). JP Patent Publication (Kokai) 2001–161373 A.
- Waterham, H.R., Koster, J., Romeijn, G.J., Hennekam, R.C., Vreken, P., Andersson, H.C., FitzPatrick, D.R., Kelley, R.I., Wanders, R.J. (2001). Mutations in the 3β-hydroxysterol Δ24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 69: 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretensjö, I., Karlberg, B. (2002). Characterization of sterols in refined borage oil by GC-MS. J. Am. Oil Chem. Soc. 79: 1069–1074 [Google Scholar]
- Xu, X., et al. ; Potato Genome Sequencing Consortium (2011). Genome sequence and analysis of the tuber crop potato. Nature 475: 189–195 [DOI] [PubMed] [Google Scholar]
- Yoshida, S., Imoto, J., Minato, T., Oouchi, R., Sugihara, M., Imai, T., Ishiguro, T., Mizutani, S., Tomita, M., Soga, T., Yoshimoto, H. (2008). Development of bottom-fermenting Saccharomyces strains that produce high SO2 levels, using integrated metabolome and transcriptome analysis. Appl. Environ. Microbiol. 74: 2787–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, J., Niu, Q.W., Chua, N.H. (2000). Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24: 265–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.