Abstract
Immunity in vertebrates is well established to develop with time, but the ontogeny of defence in invertebrates is markedly less studied. Yet, age-specific capacity for defence against pathogens, coupled with age structure in populations, has widespread implications for disease spread. Thus, we sought to determine the susceptibility of hosts of different ages in an experimental invertebrate host–pathogen system. In a series of experiments, we show that the ability of Daphnia magna to resist its natural bacterial pathogen Pasteuria ramosa changes with host age. Clonal differences make it difficult to draw general conclusions, but the majority of observations indicate that resistance increases early in the life of D. magna, consistent with the idea that the defence system develops with time. Immediately following this, at about the time when a daphnid would be most heavily investing in reproduction, resistance tends to decline. Because many ecological factors influence the age structure of Daphnia populations, our results highlight a broad mechanism by which ecological context can affect disease epidemiology. We also show that a previously observed protective effect of restricted maternal food persists throughout the entire juvenile period, and that the protective effect of prior treatment with a small dose of the pathogen (‘priming’) persists for 7 days, observations that reinforce the idea that immunity in D. magna can change over time. Together, our experiments lead us to conclude that invertebrate defence capabilities have an ontogeny that merits consideration with respect to both their immune systems and the epidemic spread of infection.
KEY WORDS: Ontogeny, Immunity, Age, Epidemiology, Daphnia, Invertebrate
INTRODUCTION
It is well established that the immune system of newborn vertebrates is much less capable than that of adults. Specifically, neonates appear to have little ability to synthesise antibodies endogenously (Brambell, 1970; Lawrence et al., 1981; Roitt et al., 1998; Solomon, 1971), and therefore rely upon maternally derived factors both for defence against pathogens and to condition their immune system (Grindstaff et al., 2003). Maternal antibodies persist in offspring to varying degrees (for example, for months in humans and for days in fish), but in most cases it appears that maternal antibodies disappear just as offspring antibody production begins to increase (Grindstaff et al., 2003; Solomon, 1971), and so the phenomenon is widely considered to be a precise adaptation.
Understanding of the development of the vertebrate immune system in early life is centered on the development of the acquired immune system. It is generally accepted that, even as acquired immunity remains undeveloped or under-developed, young individuals will have full use of their innate immune system. With this in mind, it is tempting to assume that invertebrates, which possess only an innate immune system, would not show an immune system or defence ontogeny similar to that of young vertebrates. Yet, in general, the innate immune system of invertebrates is surprisingly capable, and there is even evidence that it develops through time in response to challenges from pathogens (McTaggart et al., 2012), or can be modified via maternal effects (Ben-Ami et al., 2010; Garbutt et al., 2014; Little et al., 2003; Mitchell and Read, 2005; Stjernman and Little, 2011). What has been little studied, however, is the basic ontogeny of immunity early in the life of invertebrates. Changes in the immune system later in life (and immunosenescence in particular) have received considerable attention (Adamo et al., 2001; Doums et al., 2002; Eleftherianos et al., 2008; Felix et al., 2012; Kurtz, 2002; Laws et al., 2004; Lesser et al., 2006; Piñera et al., 2013; Prasai and Karlsson, 2012; Sorrentino et al., 2002; Whitehorn et al., 2011; Zerofsky et al., 2005), but few studies have included the first days of an invertebrate's life. The main aim of the present study was to shed light on the development of the defence system of an invertebrate, the crustacean Daphnia magna Straus, under tight experimental control. The trait we measured is not an immune response per se, but rather we measured their resistance to a pathogen, the bacterium Pasteuria ramosa. We assumed that pathogen resistance is linked to the immune response.
Additionally, by measuring pathogen resistance, we gain greater opportunity to shed light on the potential consequences of age-specific susceptibility for disease spread in populations. Much work has been carried out on the causes and consequences of variation in age structure (Charlesworth, 1994; De Roos et al., 2003; Sterner, 1998), and the impact of age structure on virulence evolution and epidemic spread (Castillo-Chavez et al., 1989; Duffy et al., 2012). Empirical data on the susceptibility of different age classes could provide insight into how ecological factors that affect age structure could modulate disease resistance and spread.
We tested several independent hypotheses. (1) Most generally, we hypothesised that the immune response will develop with age, leading to the prediction that Daphnia will become more resistant with time, at least in the early stages of life. Later in life, however, Daphnia could conceivably show immunosenescence, leading to the prediction that resistance will then decline. (2) As older individuals will inevitably be larger (Daphnia have indeterminate growth, although growth tapers off after the first week of life), the development of resistance is likely to be linked to body size in the early stages of life. The route of infection in this system is a common one – the pathogen is ingested orally, after which it penetrates the gut or oesophagus of susceptible individuals to begin an infection in the haemolymph (Duneau et al., 2011). Consequently, body size is thought to be closely tied to resistance in this system because larger individuals take up larger quantities of food, and thus pathogen spores. This hypothesis predicts that older individuals, at least in the first week of life when growth is substantial, will be more susceptible to infection. (3) It is plausible, however, that older, larger individuals will have more resources, and thus could launch more robust immune responses, which predicts that older individuals will be less susceptible to infection.
RESULTS
Experimental overview
We performed a series of experiments to evaluate the ontogeny of defence in Daphnia. The first (experiment 1: the development of resistance) exposed Daphnia to the parasite at eight time points spread over 40 days, which represents much of this crustacean's life history, including the entire juvenile period and a substantial period of its adulthood (Ebert, 2005a). Thus, we were able to potentially evaluate both the early-life development of resistance and how levels of resistance might decline with age later in life. Next, we sought to establish whether the pattern of change in resistance with age observed in the first experiment was consistent in different environmental contexts, i.e. how resistance in the early stages of Daphnia life developed under variation in maternal nutrition (experiment 2: maternal effects on the early development of resistance), and with variation in experience of exposure to the pathogen (experiment 3: priming and the early development of resistance). Finally, we repeated experiment 1 with a longer, and we assume more natural, exposure regime (experiment 4: the development of resistance with longer exposure times). Data from these experiments are available in supplementary material Table S1.
Experiment 1: the development of resistance
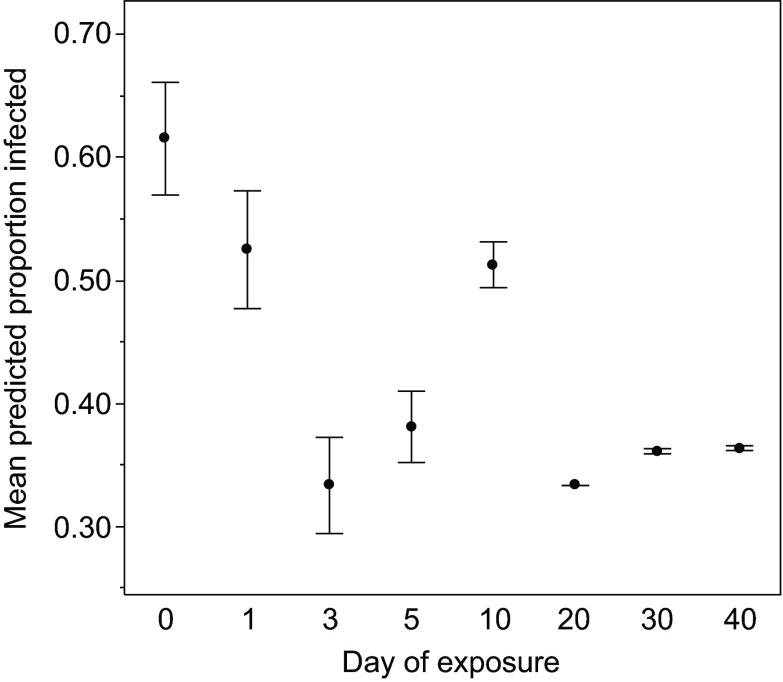
The change with age in the proportion of hosts from two clones (designated FS24 and GG4; see Materials and methods) infected was marginally non-significant (P=0.07; Table 1, Fig. 1); however, the two clones did not respond in the same way, leading to a significant clone × age effect (Table 1; supplementary material Fig. S1). In both genotypes, susceptibility declined early in life (days 0–5; Fig. 1; supplementary material Fig. S1), but after day 5, changes of susceptibility with age differed between the two genotypes: the proportion of clone FS24 hosts infected appears to increase from day 5 to day 40 whilst the proportion of clone GG4 hosts infected appears to fall during the same period (supplementary material Fig. S1).
Table 1.
Statistical details of four experiments testing how the proportion of Daphnia hosts infected is dependent upon the age at exposure of hosts
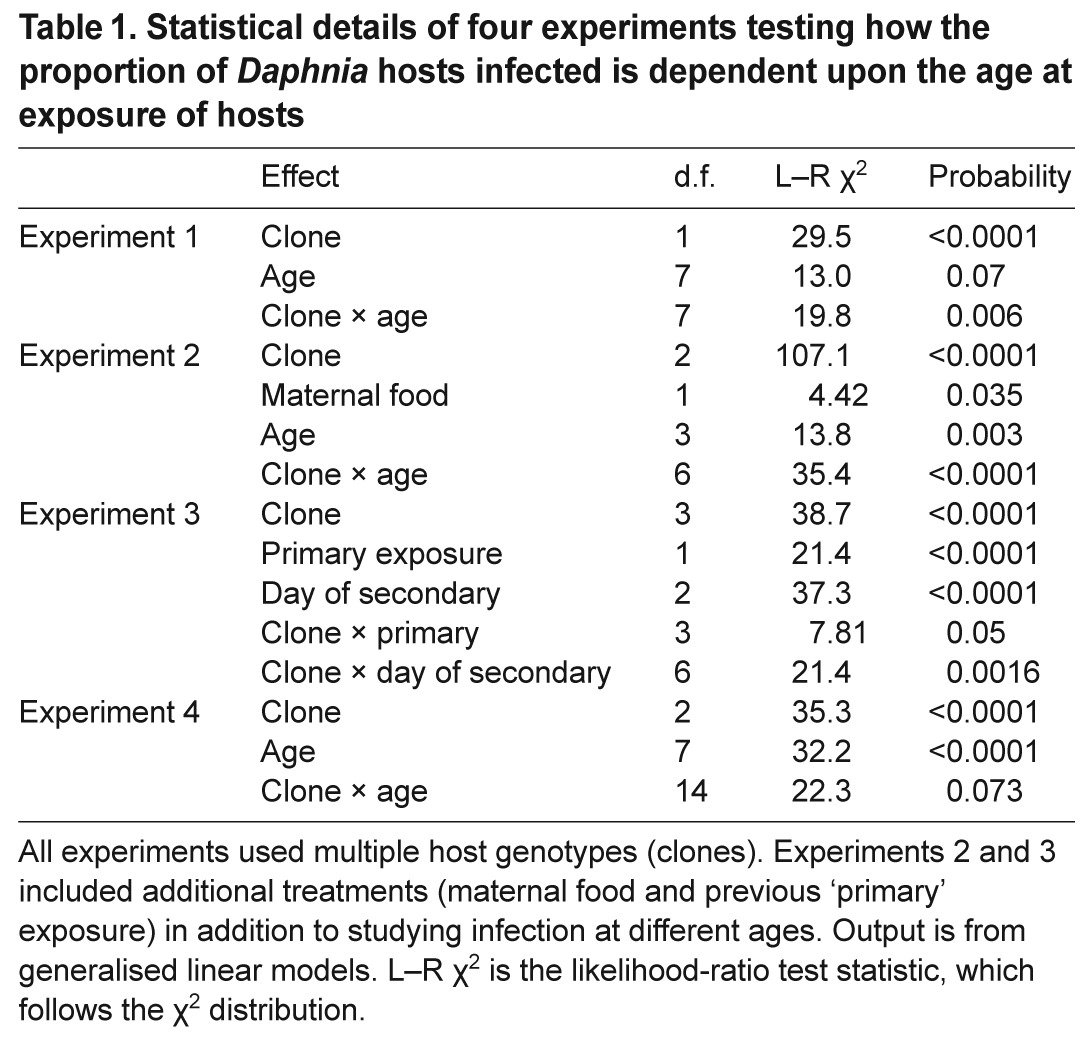
Fig. 1.
The development of resistance in Daphnia magna. The mean predicted proportion of D. magna infected following a 4 h exposure to the pathogen Pasteuria ramosa at one of eight ages (in days). Each data point represents the mean proportion of infected Daphnia across both clones, as predicted by the generalised linear model. Error bars show the variation between clones, and are the standard errors of the predicted values for each clone. Note that 30 and 40 day old Daphnia were exposed on the same day as the 0 and 10 day olds.
Experiment 2: maternal effects on the early development of resistance
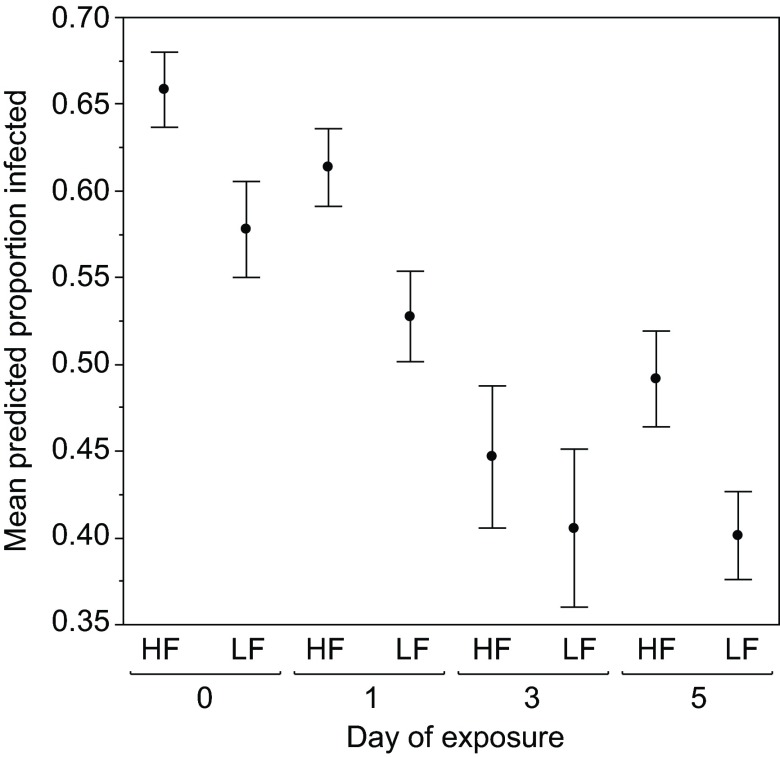
Consistent with experiment 1, susceptibility declined with age early in a daphnid's life, and this pattern was consistent across maternal treatments (i.e. under both high and low maternal food; Table 1, Fig. 2). In this experiment, there was also a clone × age interaction (Table 1; supplementary material Fig. S2). Infection levels declined from day 0 to day 5 in clones FS24 and GG4, while this pattern appears absent from a third clone, KB5A. A previously observed pattern, that low maternal food imparts high parasite resistance (Ben-Ami et al., 2010; Garbutt et al., 2014; Mitchell and Read, 2005; Stjernman and Little, 2011), was also observed here.
Fig. 2.
Maternal effects on the early development of resistance. The mean predicted proportion of D. magna infected following exposure to P. ramosa at one of four ages (in days) early in life. The mothers of these Daphnia were raised under either high food (HF) or low food (LF). Each data point represents the mean proportion of infected Daphnia across three clones, as predicted by the generalised linear model. Error bars show the variation between clones, and are the standard errors of the predicted values for each clone.
Experiment 3: priming and the development of resistance
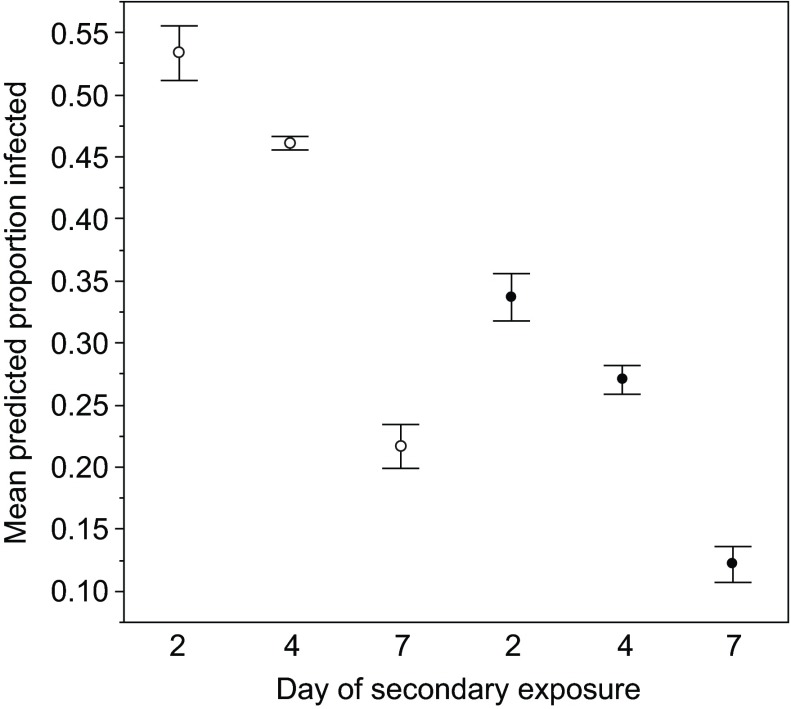
Consistent with the other experiments, susceptibility initially declined, and this pattern was consistent across primary exposure treatments (i.e. with and without previous priming with a low dose of the parasite; Table 1, Fig. 3). In this experiment, there was also a clone × age of secondary exposure interaction (Table 1; supplementary material Fig. S3), probably reflecting the very strong decrease in susceptibility with age in three out of four clones (GG4, Kc200a and Kc49a), but the ‘humped’ relationship in clone FS24. A pattern documented in another study, that previous exposure to the pathogen (i.e. priming) improves pathogen resistance (McTaggart et al., 2012), was also seen here (Table 1, Fig. 3) – individuals in the ‘primed’ treatment were less likely to become infected (comparing control and primed individuals exposed at the same age).
Fig. 3.
Priming and the development of resistance. The mean predicted proportion of D. magna infected after exposure to P. ramosa at three different time points following a primary (and non-infective) exposure to the pathogen (filled circles) or a control exposure (open circles). Each data point represents the mean proportion of infected Daphnia across four clones, as predicted by the generalised linear model. Error bars show the variation between clones, and are the standard errors of the predicted values for each clone.
Experiment 4: the development of resistance with longer exposure times
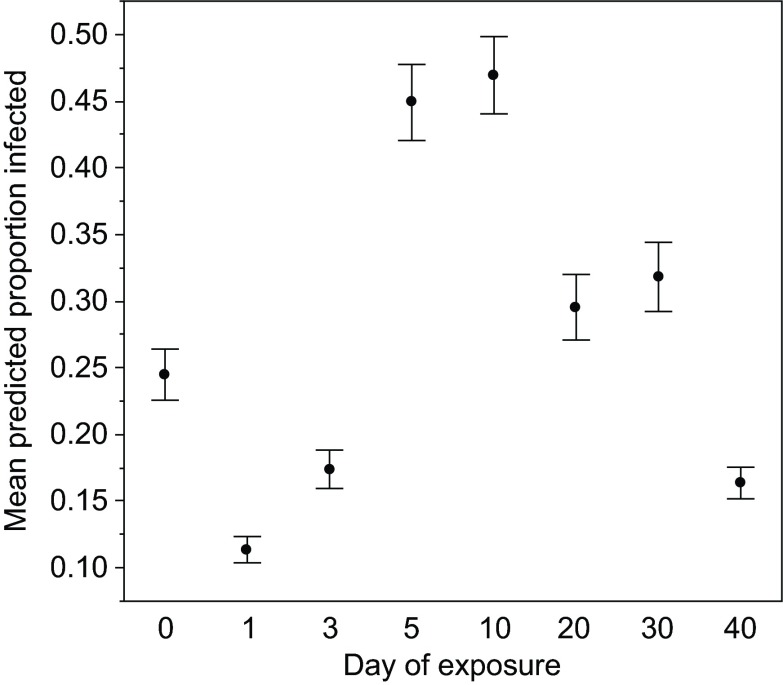
In this experiment, we again found that the proportion of hosts infected significantly changed with age (Table 1, Fig. 4), and also found a near-significant (P=0.073) clone × age effect (Table 1; supplementary material Fig. S4). Susceptibility appears to decline early in life in clones FS24 and Kc49a, but not clone Kc200a. All clones show an increase in susceptibility on day 5, after which susceptibility appears to decline again.
Fig. 4.
The development of resistance with longer exposure times. The proportion of D. magna infected following exposure to the pathogen P. ramosa at one of eight ages (in days). Each exposure period lasted 5 days, and thus the day shown is the first day of a relatively long exposure period.
DISCUSSION
The ability of D. magna to resist P. ramosa can change substantially with host age, as indicated by the significant main effect of age in three of four experiments, and the prevalent clone × age interactions (Table 1). However, the amount of genetic variation we observed makes it difficult to draw general conclusions. For example, our data highlight that there is extensive genetic variation for a late-life decline in defensive ability, which might reflect immunosenescence. Studies on other organisms have supported the theory that immunocompetence generally decreases with advanced age in invertebrates (Adamo et al., 2001; Doums et al., 2002; Kurtz, 2002; Laws et al., 2004; Prasai and Karlsson, 2012; Whitehorn et al., 2011; Zerofsky et al., 2005), but, as with the current results, there also exist examples of genetic variation for immunosenescence or where immunocompetence increases with age (Felix et al., 2012; Khan and Prasad, 2013; Lesser et al., 2006). Assuming that, in the wild, different strategies might be favoured at different times, or in different locations, this genotype × age interaction indicates a mechanism by which genetic variation may be maintained in natural populations. It would be intriguing to gather further data on the different genotypes that follow such strikingly different patterns of age-related resistance. For example, do clones that show patterns of apparent immunosenescence (e.g. clone GG4 in experiment 1; see supplementary material Fig. S1) have exceptionally strong investment in early-life resistance or reproduction (and have thus ‘traded-off’ early life traits with immunocompetence later in life)? Answers to such questions require the extensive collection of further data, but for the moment we emphasise that it is a strength of the current report to have revealed that, although resistance clearly changes with age, gaining a complete characterisation of this will require the study of a great many genotypes.
Despite the strong clone-specific effects, a slight majority of observations consistently indicate that resistance to the pathogen P. ramosa increases in the earliest stages of life (roughly the first week; Figs 1, 2, 3 and 4). This pattern was less clear in our experiment 4 (Fig. 4), but it is important to bear in mind that this experiment used longer exposures (5 days, compared with just hours for the other experiments) and thus, for example, the ‘day 5’ exposures include days 5 to 10, when we might expect resistance to be declining after an initial rise, as it does in experiment 1 (which is the experiment that mirrors experiment 4, aside from exposure time). The observation of increasing resistance early in life is compatible with the idea that the defence system of D. magna develops with time, and does not fit with the idea that larger Daphnia will be more susceptible to an orally acquired pathogen because they feed more. Simultaneous measurement of age, size and feeding rate will be required to fully disentangle the cause(s) of the changes in resistance early in life, but for the moment our observations support the idea that older and therefore larger hosts, perhaps because they have more resources at their disposal and/or have a more developed immune system, have the best defences. The priming effect observed in experiment 3 reinforces that defences can develop in this organism, showing as it does the development of improved immunity following prior exposure to a low dose of the pathogen.
The clear implication of our results is that epidemiological parameters, for instance pathogen prevalence, will be strongly dependent on the age and genetic structure of the population. The age structure of daphnid populations is well studied, with the most prominent influence on age structure apparently being predation by fish, which selectively removes the largest, and presumably oldest, individuals (Galbraith, 1967; Gibson, 1980). High levels of fish predation could result in populations dominated by juvenile Daphnia, which tend to be more susceptible to infection. Fish predation has been shown to limit the size of epidemics of the yeast parasite Metschnikowia bicuspidata (Duffy et al., 2012); it is thus possible that in addition to the selective culling by fish of parasitised Daphnia or reductions in overall host density (Duffy and Hall, 2008; Duffy et al., 2005), fish predation also alters epidemic size through changes in the age structure, and thus resistance competence, of the population. Of course, predators that select the smallest, and presumably youngest, individuals [like the invertebrate Chaoborus (Riessen and Young, 2005)] will have the opposite effect, resulting in populations dominated by older Daphnia. Likewise, other ecological factors that alter the age structure of Daphnia populations are likely to affect disease dynamics; there is some evidence that food availability alters age structure, with declining food increasing the proportion of adults (van Leeuwen et al., 1986). Our observations of changed resistance with age represent an important starting point for further exploration of these factors.
Early declines in susceptibility were generally followed by a rise, and the timing of the rise varied from around 5 days (experiment 4; Fig. 4) to around 10 days (experiment 1; Fig. 1) and, again, between clones, so we generalise with caution. This rise approximately corresponds with the peak of reproductive activity in these organisms. Daphnia typically begin to deposit eggs in the brood chamber (depending on food conditions) around days 5 to 10 of life, and their first clutch is released (again, depending on food conditions) around 3 days later (Ebert, 2005a). Subsequent clutches are released 2–3 days later, and after the first few clutches the inter-clutch interval gradually increases. Thus, the most intense period of reproduction is approximately when susceptibility is highest. Other lines of evidence support a connection (or, perhaps, a trade-off) between reproduction and resistance in this system (Schlotz et al., 2013; Stjernman and Little, 2011), though a study explicitly designed to correlate reproduction and immunity across time and age is still required. Given that the main effect of infection in this system is fecundity reduction, this would essentially be a study of age-specific virulence, and an important contribution to our understanding of the interplay between immunity and reproduction. Again, clones tend to vary in how quickly they reach maturity, and in their inter-clutch interval, and this variation may explain why clones vary in defence ability later in life.
Our results also extend studies of how resistance relates to experience of previous exposure and to maternal nutritional state. In particular, we show that the protective effect evident in the offspring of food-restricted mothers (Ben-Ami et al., 2010; Garbutt et al., 2014; Mitchell and Read, 2005; Stjernman and Little, 2011) persists through much of their juvenile period. That the maternal effect persists until the time that the daphnid nearly reaches reproductive maturity (the time that eggs begin to develop) highlights the potential for the effect to be transmitted one generation further (i.e. to granddaughters). We also found that a protective effect of prior treatment with a small dose of the pathogen (‘priming’) extends for 7 days [previous experiments in this system had only looked for immediate effects and those 2 days following priming exposure (McTaggart et al., 2012)]. This is comparable with the observations of priming after 8 days in beetles (Roth et al., 2009) and 6 days in moths (Tidbury et al., 2011) and shows that priming of the innate system is relatively persistent. This persistent priming also has broad implications for understanding the epidemiological consequences of priming. For example, Tidbury et al. (Tidbury et al., 2012) showed that immune priming may increase the persistence of the pathogen and destabilise host–pathogen dynamics. We suggest that the linked effects of the phenomena reported here – age effects, maternal effects and priming – merit further study as major drivers of disease spread and host–pathogen evolution in this and other systems.
MATERIALS AND METHODS
Organisms
The pathogen P. ramosa is a spore-forming bacterium that greatly reduces reproduction in infected hosts (Ebert et al., 1996). Transmission of P. ramosa is horizontal (transovarial transmission has never been observed), achieved when spores are released from dead hosts and subsequently picked up by Daphnia during filter feeding (Ebert et al., 1996). Thus, to perform experimental infections with this system, infected Daphnia are crushed to form a spore suspension. Spore suspensions were stored frozen at −20°C, and diluted for use in exposures according to determination of density using a counting chamber (Neubauer-improved, 0.1 mm, Marienfield). The host D. magna (Crustacea: Cladocera) is a planktonic crustacean commonly found in small freshwater ponds. Pasteuria ramosa infections in Daphnia are easy to diagnose with the naked eye because red spore growth is visible through the carapace, and because infected Daphnia cease to reproduce (Ebert, 2005b) (supplementary material Fig. S5). In this study, we used D. magna genotypes from two populations: the Kaimes pond near Leitholm (Auld et al., 2012; Auld et al., 2014) in the Scottish Borders, and the Gaarzerfeld population from Germany (Carius et al., 2001). All genotypes were exposed to parasites from their own population that they were susceptible to.
Experiment 1: the development of resistance
This experiment was conducted on two D. magna clones (a clone designated FS24 from the Kaimes population in Scotland, and GG4 from the German population). Initially, 24 replicates of each of host clone were acclimatised for three generations under standardised conditions on a 12 h:12 h light:dark cycle in controlled climate chambers at 20°C. Daphnia were kept in synthetic pond medium (Klüttgen et al., 1994), and were fed on Chlorella spp., a green algae cultured in chemostats with Chu B medium. Food quantity during this period was 1 absorbance unit per jar per day (1 absorbance unit is the optical absorbance of 650 nm light by the Chlorella culture, which represents about 5×106 algal cells). Medium was changed when offspring were observed in the jar or, if none were present, every third day. Acclimating all replicates for three generations is a process designed to ensure that each replicate is independent (see Ebert et al., 1998).
Second-clutch offspring from the third-generation Daphnia were the experimental animals and were exposed to the pathogen at 0, 1, 3, 5, 10, 20, 30 and 40 days old. The experimental set-up was based on a split-clutch design, but with an important extra step to account for time effects. Specifically, each replicate mother contributed one offspring to each of the first six age classes, but mothers destined to contribute offspring to the exposures done on day 30 or 40 were set up prior to the other mothers (though run through the same acclimation process), such that we carried out the day 0 exposures and the day 30 exposures on the same day, while the day 40 exposures were set up on the same day as the day 10 exposures. This allowed us, to some degree, to disentangle day effects from age effects. There were no repeated measures on the same individuals, and the split-brood design meant that individuals that shared a mother were always placed into different treatments (i.e. were infected at different ages). In total, there were 384 Daphnia in this experiment (24 replicates×2 clones×8 exposure times).
Exposures were carried out in 2 ml of medium in the wells of a 24 well plate (Costar Corning, Corning, NY, USA), one Daphnia per well. Each host received 7.5×105 spores for 4 h, after which time they were returned to 60 ml jars and maintained under the same conditions experienced during the acclimation period described above for 25 days following exposure. Daphnia were checked for signs of infection daily and at the end of the 25 day exposure period were scored as either ‘infected’ or ‘uninfected’.
Analysis
We analysed the proportion of hosts infected with a generalised linear model (link=logit, dist=bin) and the explanatory variables ‘age’ (an eight-level factor), ‘clone’ (a two-level factor) and their interaction. This and all models were reduced by removing non-significant interaction terms until only main effects or significant interaction terms remained.
Experiment 2: maternal effects on the early development of resistance
This experiment was conducted on the same two D. magna clones as above, but an additional clone, KB5A, from the Kaimes population, was also studied. Again, 24 replicates of each of host clone were acclimatised for three generations under standardised conditions as above. The essential design feature of this experiment was to raise the mothers (called the F0 generation) of the focal animals (called the F1 generation) under either high or low food conditions and then expose F1 Daphnia of ages 0, 1, 3 and 5 days to the pathogen. Again, each age treatment was composed of different individuals (in a split-clutch design) and therefore there were no repeated measures on the same individuals.
To set up the maternal (F0) generation, we took two third-clutch offspring from each of the 24 acclimatised replicates for each clone and assigned one to a low food treatment (0.3 absorbance units per jar per day) and the other to a high food treatment (1.5 absorbance units per jar per day). Medium was changed when offspring were observed or, alternatively, every third day. At this stage of the experiment, there were 144 Daphnia (24 replicates × two food levels × three clones=144 Daphnia). From the third clutch of these F0 females, we took four (one for each exposure day: 0, 1, 3, 5) offspring from each replicate jar to set up the offspring or F1 generation, which were exposed to the pathogen. Thus, there were 576 F1 Daphnia (144×4 exposure days). For pathogen exposure, F1 offspring were placed individually in 2 ml of medium in the wells of a 24 well plate (Costar Corning) containing 1×105 P. ramosa spores for 4 h. Following exposure, hosts were maintained in 60 ml jars for 30 days to allow infections to be diagnosed. We recorded daily whether a clutch was present and changed medium every other day or when clutches were present. Daphnia were kept at 1.0 absorbance units of food per day for the remainder of the experiment; thus, the variation in food level occurred in the maternal generation and not in the offspring generation.
Analysis
We analysed the proportion of hosts infected with a generalised linear model (link=logit, dist=bin) and the explanatory variables ‘age’ (a four-level factor), ‘clone’ (a three-level factor), ‘maternal food’ (a two-level factor) and their interactions.
Experiment 3: priming and the development of resistance
This experiment used the two clones studied in the first experiment, but added two further clones, designated Kc200a and Kc49a, from the Kaimes population. As above, initially, 24 replicates of each host clone were acclimatised for three generations. Six of the offspring from the third clutch of the third generation were selected at random from each Daphnia on the day of birth and assigned to one of six treatment paths. This experiment studied the development of resistance, but also included two exposures: a primary exposure that occurred on day 5, and a later secondary exposure that occurred 2, 4 or 7 days after the primary exposure (i.e. when the Daphnia were 7, 9 or 12 days old).
For the primary exposure, half of the Daphnia were exposed to a non-infectious dose of 500 spores, with the other half being exposed to a control solution. The spore solution was prepared as above (i.e. from crushed infected Daphnia), while the control solution was crushed uninfected Daphnia. On the day of primary exposure, each Daphnia was placed in an individual 2 ml well, and a non-infectious dose of 500 spores was added to half of the wells. The other half received the same volume of control solution. After 4 h, the Daphnia were returned to their original jars. For the secondary exposures, hosts were exposed to 5×105 spores, which is an infectious dose, 2, 4 or 7 days after the primary exposure. Post-secondary exposure Daphnia were maintained as they were during the acclimation period and observed for infection for 28 days. In this experiment there were 576 Daphnia [24 replicates × 4 clones × 2 primary exposure levels (exposed or control) × 3 secondary exposure times].
Analysis
We analysed the proportion of hosts infected with a generalised linear model (link=logit, dist=bin) and the explanatory variables ‘primary exposure’ (a two-level factor), ‘clone’ (a four-level factor), ‘day of secondary exposure’ (a three-level factor) and their interactions.
Experiment 4: the development of resistance with longer exposure times
This experiment was conducted on three D. magna clones (designated FS24, Kc200a and Kc49a) from the Kaimes population in Scotland. Methods were identical to experiment 1, except that exposures were carried out in 60 ml jars with 5×105 spores and lasted for 5 days. Thus, for example, the day 0 exposure lasted from day 0 to day 4, the day 1 exposure began on day 1 and ended on day 5, etc. Following exposure, hosts were moved to fresh jars and maintained as they were during the acclimation period. All Daphnia were checked daily to determine infection status (infected or not) for 25 days following exposure.
Analysis
We analysed the proportion of hosts infected with a generalised linear model (link=logit, dist=bin) and the explanatory variables ‘age’ (an eight-level factor), ‘clone’ (a three-level factor) and their interaction.
Supplementary Material
ACKNOWLEDGEMENTS
We thank two anonymous referees for their helpful comments on the manuscript.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This work was supported by the Natural Environment Research Council (NE/I026405/1). S.J.M. was supported by a strategic award from the Wellcome Trust to the Centre for Immunity, Infection and Evolution (grant reference no. 095831).
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.111260/-/DC1
References
- Adamo S. A., Jensen M., Younger M. (2001). Changes in lifetime immunocompetence in male and female Gryllus texensis (formerly G. integer): trade-offs between immunity and reproduction. Anim. Behav. 62, 417-425 [Google Scholar]
- Auld S. K. J. R., Graham A. L., Wilson P. J., Little T. J. (2012). Elevated haemocyte number is associated with infection and low fitness potential in wild Daphnia magna. Funct. Ecol. 26, 434-440 [Google Scholar]
- Auld S. K. J. R., Wilson P. J., Little T. J. (2014). Rapid change in parasite infection traits over the course of an epidemic in a wild host–parasite population. Oikos 123, 232-238 [Google Scholar]
- Ben-Ami F., Ebert D., Regoes R. R. (2010). Pathogen dose infectivity curves as a method to analyze the distribution of host susceptibility: a quantitative assessment of maternal effects after food stress and pathogen exposure. Am. Nat. 175, 106-115 [DOI] [PubMed] [Google Scholar]
- Brambell F. W. R. (1970). Transmission of immunity in birds. In The Transmission of Passive Immunity from Mother to Young (ed. Neuberger A., Tatum E. L.), pp. 20-41. New York, NY: Elsevier; [Google Scholar]
- Carius H. J., Little T. J., Ebert D. (2001). Genetic variation in a host-parasite association: potential for coevolution and frequency-dependent selection. Evolution 55, 1136-1145 [DOI] [PubMed] [Google Scholar]
- Castillo-Chavez C., Hethcote H. W., Andreasen V., Levin S. A., Liu W. M. (1989). Epidemiological models with age structure, proportionate mixing, and cross-immunity. J. Math. Biol. 27, 233-258 [DOI] [PubMed] [Google Scholar]
- Charlesworth B. (1994). Evolution in Age-Structured Populations. Cambridge: Cambridge University Press; [Google Scholar]
- De Roos A. M., Persson L., McCauley E. (2003). The influence of size-dependent life-history traits on the structure and dynamics of populations and communities. Ecol. Lett. 6, 473-487 [Google Scholar]
- Doums C., Moret Y., Benelli E., Schmid-Hempel P. (2002). Senescence of immune defence in Bombus workers. Ecol. Entomol. 27, 138-144 [Google Scholar]
- Duffy M. A., Hall S. R. (2008). Selective predation and rapid evolution can jointly dampen effects of virulent parasites on Daphnia populations. Am. Nat. 171, 499-510 [DOI] [PubMed] [Google Scholar]
- Duffy M. A., Hall S. R., Tessier A. L., Huebner M. (2005). Selective predators and their parasitized prey: Are epidemics in zooplankton under top-down control? Limnol. Oceanogr. 50, 412-420 [Google Scholar]
- Duffy M. A., Ochs J. H., Penczykowski R. M., Civitello D. J., Klausmeier C. A., Hall S. R. (2012). Ecological context influences epidemic size and parasite-driven evolution. Science 335, 1636-1638 [DOI] [PubMed] [Google Scholar]
- Duneau D., Luijckx P., Ben-Ami F., Laforsch C., Ebert D. (2011). Resolving the infection process reveals striking differences in the contribution of environment, genetics and phylogeny to host-parasite interactions. BMC Biol. 9, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D. (2005a). Introduction to Daphnia biology. In Ecology, Epidemiology and Evolution of Parasitism in Daphnia. Bethesda, MD: The National Center for Biotechnology Information National Library of Medicine; [Google Scholar]
- Ebert D. (2005b). Some parasites of Daphnia, Chapter 3. In Ecology, Epidemiology, and Evolution of Parasitism in Daphnia [Internet]. Bethesda (MD): National Center for Biotechnology Information (US). Available from: http://www.ncbi.nlm.nih.gov/books/NBK2043/ [Google Scholar]
- Ebert D., Rainey P., Embley T. M., Scholz D., Ebert D., Rainey P., Embley T. M., Scholz D. (1996). Development, life cycle, ultrastructure and phylogenetic position of Pasteuria ramosa Metchnikoff 1888: rediscovery of an obligate endoparasite of Daphnia magna Straus. Philos. Trans. R. Soc. B 351, 1689-1701 [Google Scholar]
- Ebert D., Zschokke-Rohringer C. D., Carius H. J. (1998). Within- and between-population variation for resistance of Daphnia magna to the bacterial endoparasite Pasteuria ramosa. Proc. R. Soc. B 265, 2127-2134 [Google Scholar]
- Eleftherianos I., Baldwin H., ffrench-Constant R. H., Reynolds S. E. (2008). Developmental modulation of immunity: changes within the feeding period of the fifth larval stage in the defence reactions of Manduca sexta to infection by Photorhabdus. J. Insect Physiol. 54, 309-318 [DOI] [PubMed] [Google Scholar]
- Felix T. M., Hughes K. A., Stone E. A., Drnevich J. M., Leips J. (2012). Age-specific variation in immune response in Drosophila melanogaster has a genetic basis. Genetics 191, 989-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith M. G. (1967). Size-selective predation on Daphnia by rainbow trout and yellow perch. Trans. Am. Fish. Soc. 96, 1-10 [Google Scholar]
- Garbutt J. S., Scholefield J. A., Vale P. F., Little T. J. (2014). Elevated maternal temperature enhances offspring disease resistance in Daphnia magna. Funct. Ecol. 28, 424-431 [Google Scholar]
- Gibson R. M. (1980). Optimal prey-size selection by three-spined sticklebacks (Gasterosteus aculeatus): a test of the apparent-size hypothesis. Z. Tierpsychol. 52, 291-307 [Google Scholar]
- Grindstaff J. L., Brodie E. D., III, Ketterson E. D. (2003). Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. B 270, 2309-2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I., Prasad N. G. (2013). The aging of the immune response in Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 68, 129-135. PubMed [DOI] [PubMed] [Google Scholar]
- Klüttgen B., Dülmer U., Engels M., Ratte H. (1994). ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 28, 743-746 [Google Scholar]
- Kurtz J. (2002). Phagocytosis by invertebrate hemocytes: causes of individual variation in Panorpa vulgaris scorpionflies. Microsc. Res. Tech. 57, 456-468 [DOI] [PubMed] [Google Scholar]
- Lawrence E. C., Arnaud-Battandier F., Grayson J., Koski I. R., Dooley N. J., Muchmore A. V., Blaese R. M. (1981). Ontogeny of humoral immune function in normal chickens: a comparison of immunoglobulin-secreting cells in bone marrow, spleen, lungs and intestine. Clin. Exp. Immunol. 43, 450-457 [PMC free article] [PubMed] [Google Scholar]
- Laws T. R., Harding S. V., Smith M. P., Atkins T. P., Titball R. W. (2004). Age influences resistance of Caenorhabditis elegans to killing by pathogenic bacteria. FEMS Microbiol. Lett. 234, 281-287 [DOI] [PubMed] [Google Scholar]
- Lesser K. J., Paiusi I. C., Leips J. (2006). Naturally occurring genetic variation in the age-specific immune response of Drosophila melanogaster. Aging Cell 5, 293-295 [DOI] [PubMed] [Google Scholar]
- Little T. J., O'Connor B., Colegrave N., Watt K., Read A. F. (2003). Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 13, 489-492 [DOI] [PubMed] [Google Scholar]
- McTaggart S. J., Wilson P. J., Little T. J. (2012). Daphnia magna shows reduced infection upon secondary exposure to a pathogen. Biol. Lett. 8, 972-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S. E., Read A. F. (2005). Poor maternal environment enhances offspring disease resistance in an invertebrate. Proc. R. Soc. B 272, 2601-2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñera A. V., Charles H. M., Dinh T. A., Killian K. A. (2013). Maturation of the immune system of the male house cricket, Acheta domesticus. J. Insect Physiol. 59, 752-760 [DOI] [PubMed] [Google Scholar]
- Prasai K., Karlsson B. (2012). Variation in immune defence in relation to age in the green-veined white butterfly (Pieris napi L.). J. Invertebr. Pathol. 111, 252-254 [DOI] [PubMed] [Google Scholar]
- Riessen H. P., Young J. D. (2005). Daphnia defense strategies in fishless lakes and ponds: one size does not fit all. J. Plankton Res. 27, 531-544 [Google Scholar]
- Roitt I., Brostoff J., Male D. K. (1998). Immunology, 5th edn. London: Mosby; [Google Scholar]
- Roth O., Sadd B. M., Schmid-Hempel P., Kurtz J. (2009). Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc. R. Soc. B 276, 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz N., Ebert D., Martin-Creuzburg D. (2013). Dietary supply with polyunsaturated fatty acids and resulting maternal effects influence host–parasite interactions. BMC Ecol. 13, 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J. B. (1971). Maternal antibody II: protective effects and immunological hazards. In Foetal and Neonatal Immunology (ed. Neuberger A., Tatum E. L.), pp. 115-138. London: North Holland Publishing Company; [Google Scholar]
- Sorrentino R. P., Carton Y., Govind S. (2002). Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 243, 65-80 [DOI] [PubMed] [Google Scholar]
- Sterner R. W. (1998). Demography of a natural population of Daphnia retrocurva in a lake with low food quality. J. Plankton Res. 20, 471-489 [Google Scholar]
- Stjernman M., Little T. J. (2011). Genetic variation for maternal effects on parasite susceptibility. J. Evol. Biol. 24, 2357-2363 [DOI] [PubMed] [Google Scholar]
- Tidbury H. J., Pedersen A. B., Boots M. (2011). Within and transgenerational immune priming in an insect to a DNA virus. Proc. Biol. Sci. 278, 871-876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidbury H. J., Best A., Boots M. (2012). The epidemiological consequences of immune priming. Proc. R. Soc. B 279, 4505-4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen C. J., Rijkeboer M., Niebeek G. (1986). Population dynamics of Daphnia magna as modified by chronic bromide stress. Hydrobiologia 133, 277-285 [Google Scholar]
- Whitehorn P. R., Tinsley M. C., Brown M. J. F., Darvill B., Goulson D. (2011). Genetic diversity, parasite prevalence and immunity in wild bumblebees. Proc. R. Soc. B 278, 1195–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerofsky M., Harel E., Silverman N., Tatar M. (2005). Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4, 103-108 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.