Abstract
The epibranchial organ (EO) is an enigmatic tubular organ found in the pharyngeal cavity of many filter-feeding fishes. We investigated whether it might function as a taste organ that mediates aggregation and ingestion of planktonic food within the buccal cavity. The EO and associated structures of bighead and silver carps, two successful and invasive planktivorous fishes, were examined using histological and electrophysiological techniques. Both species possess finely structured gill rakers that extend directly via a series of protrusions into each of the four blind canals which are organized as the muscular EO, suggesting that the gill rakers and EO probably function in an integrated manner. Both the interior and exterior surfaces of the EOs of both species are covered with high densities of taste buds and solitary chemosensory cells (SCCs) as well as mucous cells. Conversely, taste buds are scarce in both the buccal cavities and external portions of the head and mouth of both species. Electrophysiological recordings from a caudal branch of the vagus nerve (cranial nerve X) found to innervate the EO showed it to be sensitive to chemicals found in a planktonic diet. l-Amino acids accounted for some, but not all of the neural activity. We conclude that taste buds and SCCs located on the EO and gill rakers probably serve to chemically detect food particles, which the EO then aggregates by mucus secretion before eventually expelling them onto the floor of the pharynx for ingestion. This specialized, pharyngeal chemosensory structure may explain the feeding success of these, and perhaps other planktivorous, filter-feeding fishes.
KEY WORDS: Epibranchial organ, Taste system, Taste bud, Solitary chemosensory cell
INTRODUCTION
Many species of filter-feeding teleostean fishes have extremely well-developed and seemingly specialized tubular structures at the posterior portion of their pharynges, which are often thought to function as accessory feeding structures that aid in accumulating tiny food particles for consumption (Nelson, 1967; Bauchot et al., 1993). These bilaterally paired structures, commonly called epibranchial organs (EOs), assume at least seven different morphologies in at least half a dozen unrelated families of filter-feeding fishes (Bertmar et al., 1969; Miller, 1969), suggesting their importance to a filter-feeding, planktivorous lifestyle. In most instances, the EO comprises a series of blind sacs or tubes, but sometimes these tubes are either open (continuous) or vestigial (Nelson, 1967). The tubes always align with the gill arches and are supported by modified epibranchial bones (Bertmar et al., 1969). In many cases the EO is exclusively associated with the fourth gill arch (Miller, 1969). However, the precise function(s) of the EO, which is also known as the suprabranchial organ, gill snail, accessory gill organ and accessory branchial organ (Nelson, 1967), remains enigmatic and the subject of a century-long debate.
Anecdotal observations of food boli found in the EO canals of many fishes suggest the EO probably receives food particles that collect on the gill rakers. How and why it might do so is unclear, although the speculation is that it aggregates planktonic food so that it can be swallowed (Bertmar et al., 1969; Wilamovski, 1972). Less clear is whether this putative feeding function might be accompanied by sensory, respiratory and/or digestive functions that are involved with food recognition and/or sorting (Bertmar et al., 1969). How the EO might aggregate food is also unknown, but some speculate that it is related to a possible pumping function and cross-flow filtration based on its well-developed musculature and fine gill rakers (Wilamovski, 1972; Bauchot et al., 1993; Sanderson et al., 2001). However, neither of these possible functions, nor their relationship to each other, have been systematically described. Furthermore, while several investigators have noted structures on the EO that resemble taste buds (D'Aubenton, 1955; Bauchot et al., 1993) and have shown that nerve fibers innervating the EO are connected to the vagal lobe of the brain (Hyrtl, 1854; Kapoor, 1954; Bertmar et al., 1969; Braford, 1986), chemosensory function of the EO has not yet been determined. Sensitivity to food chemicals could give this organ the ability to discern desirable food particles amongst the plethora of debris commonly encountered by filter feeders, and then aggregate these food particles for consumption. The physiological function of the EO has yet to be determined in any fish.
Two congeneric species of carp from Asia, the silver carp (Hypophthalmichthys molitrix Valenciennes 1884) and the bighead carp (H. nobilis Richardson 1845), grow large (tens of kilograms) quickly, are well-known for their ability to efficiently feed on tiny plankton, and possess highly developed EOs (Boulenger, 1901; Fang, 1928, Wilamovski, 1972). Members of the genus Hypophthalmichthys are also known as ‘bigheaded carp’ which, together with other carp species from Asia are commonly called ‘Asian carp’. The bigheaded carps (i.e. both the silver and bighead carp) are excellent models to examine EO function. The bigheaded carps are also of great economic importance as they are one of the most highly cultured and consumed fishes in the world (Michielsens et al., 2002). In North America, however, these species escaped from captivity and have become highly invasive in the Mississippi River where they can comprise up to 75% of the fish biomass. Tens of millions of dollars are spent annually trying to control these invasive carp species (Kolar et al., 2005). The silver carp feeds on mixtures of detritus, bacteria, phytoplankton and zooplankton down to a size of about 10 μm; they grow to about 20 kg, and when startled, jump to heights of about 3 m, often injuring boaters (Kolar et al., 2005). The bighead carp feeds largely on phytoplankton, zooplankton and detritus down to a size of about 50 μm; they may reach 80 kg, and do not jump (Kolar et al., 2005). Both species possess extremely fine gill rakers with inter-gill raker distances of only 35 μm for silver carp and 50 μm for bighead carp (Fang, 1928). Silver carp gill rakers also have a sponge-like filtering apparatus with small pores about 100 μm in diameter (Boulenger, 1901; Fang, 1928). The ability of both these species to capture tiny phytoplankton and zooplankton efficiently is well known, leading them to being used to clean up eutrophic bodies of water (Smith, 1985; Spataru and Gophen, 1985; Smith, 1989; Kolar et al., 2005) and giving them some of the highest growth rates noted for any fish (Abdelghany and Ahmad, 2002). It is generally assumed, but unproven, that their ability to feed on tiny particles is attributable to their EO which also produces copious amounts of mucus and has often been found to contain boli of food mixed with mucus (Wilamovski, 1972; Spataru and Gophen, 1985; Kolar et al., 2005). The bigheaded carps feed using buccal pumping (active ingestion of water though their gills by opercular flaring) (Dong and Li, 1994), but how that might be connected to food identification, filtration and EO function is unknown. However, as in other species with EOs, it has been suggested that the EO of bigheaded carp aggregates food from (or off) the gill rakers (Wilamovski, 1972; Bauchot et al., 1993; Kolar et al., 2005), but how this might be accomplished is also unknown. No study has yet examined the EOs or buccal-pharyngeal cavities of the bigheaded carps for either taste buds or solitary chemosensory cells (SCC; another type of chemosensory cell common to fish whose function is very poorly understood) (Whitear and Kotrschal, 1988; Whitear, 1992; Hansen, 2005), or attempted to test whether this structure is chemosensitive using electrophysiological recording.
The present study was designed to understand the organization and function of the EO in the bigheaded carps. We specifically tested the hypothesis that their EO functions as a taste organ that is chemosensitive to planktonic food. First, we examined the gross anatomy of the EO and gill structures of both species to assess overall function and the relationship between the two. Second, we used scanning electron microscopy and immunohistochemistry to determine whether the EO has taste buds and/or SCCs, and if so, where they might be and what their structure is. Third, we conducted electrophysiological experiments to test directly chemosensory function of the EO to planktonic food. To our knowledge, this is the first study to demonstrate chemosensory function in an EO.
RESULTS
Gross anatomy of the EO shows that it is intimately associated with specialized gill rakers
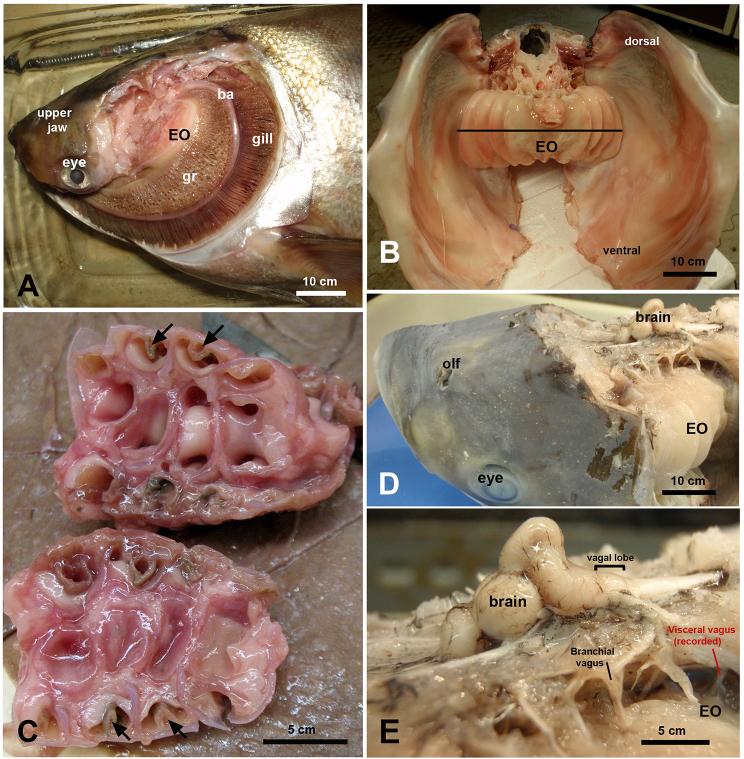
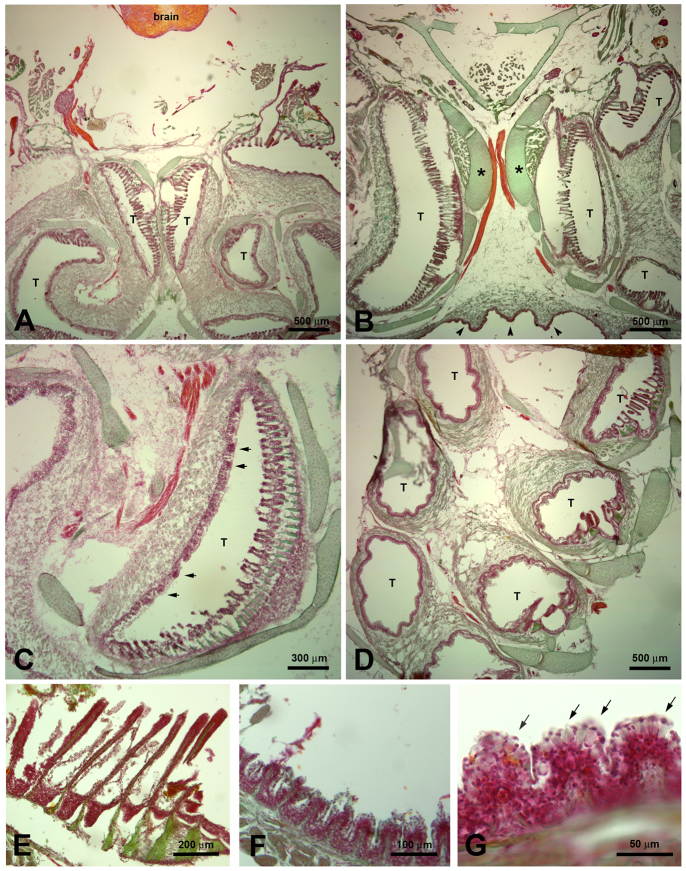
How might the EO function in conjunction with the gill rakers to capture food? To answer this question, juvenile and large adult silver and bighead carps were carefully dissected and the size and structure of their EOs and how they aligned with the gill rakers carefully examined along with their innervation. The EOs of both species are large, paired structures located at the caudal portion of their pharyngeal cavities. In each case, their gill rakers were found to fit closely along the outside of their EOs and were continuous with the interior coils of each of their paired EOs (Fig. 1A). In each species, each half of the organ had four external ridges (Fig. 1B,C), with each ridge corresponding to one of four coiled tubes within each organ (Fig. 1C). All four coils in both species were supported by robust musculature and ended blindly (dye squirted into them did not leave). After being dissected, we noted that the four coil lateral units quickly sprang back into shape after being compressed due to supporting cartilaginous structures. In many cases, we found the tubes contained boli of mucus and food. Upon dissecting the opercula and the tissue overlying the brain (Fig. 1D,E), we found that a caudal branch of the vagus nerve (cranial nerve X), located just caudal of several other branches which ran into each of the gill rakers, innervated the EO (Fig. 1D,E). Close inspection of this structure suggested that this nerve branch arborized and terminated within this organ. Neither the vagal lobe nor the facial lobes were found to be highly developed. The opening to the alimentary canal was V-shaped and small (only a few tens of micrometers in diameter; not shown).
Fig. 1.
Gross morphology of the epibranchial organ and brain in H. molitrix and H. nobilis. (A) The epibranchial organ (EO) of H. molitrix is located behind the eye adjacent to the upper jaw and surrounded by the branchial arches (ba). The gill rakers (gr) are closely associated with the EO. (B) Upper portion of the body of H. molitrix dissected to show paired EOs with four ridges on each side of the paired organs. The line depicts the cutting plane for panel C. (C) Horizontal section through one half of the EO of H. molitrix showing the four coiling tubes within the organ. These tubes end blindly. The arrows point out little flaps (see also Fig. 4C and Fig. 5F) that line parts of the tubes. (D) Overview of a head of H. nobilis. Tissue was dissected to show the brain and the EO. Note how small the brain is compared with the head; olf: inlet and outlet of the olfactory organ. (E) Fibers of the vagal nerve radiating into the EO of H. nobilis. The branchial arches were dissected to have a better view of the EO. In the electrophysiological preparation these nerve branches were accessed by removing the operculum and branchial arches on one side of the anesthetized subject.
Scanning electron microscopy demonstrates a high level of fine-scale specialization for food acquisition
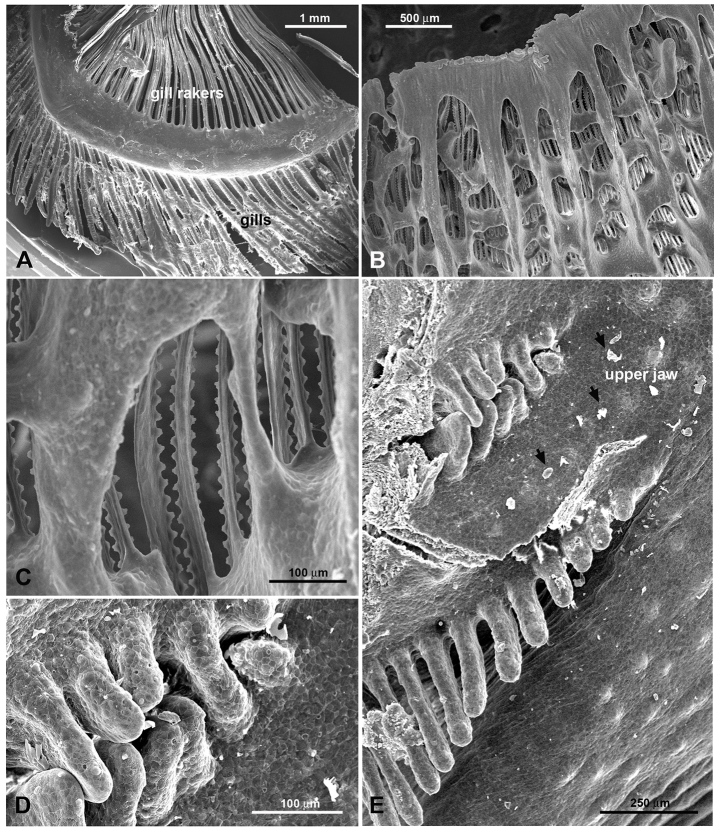
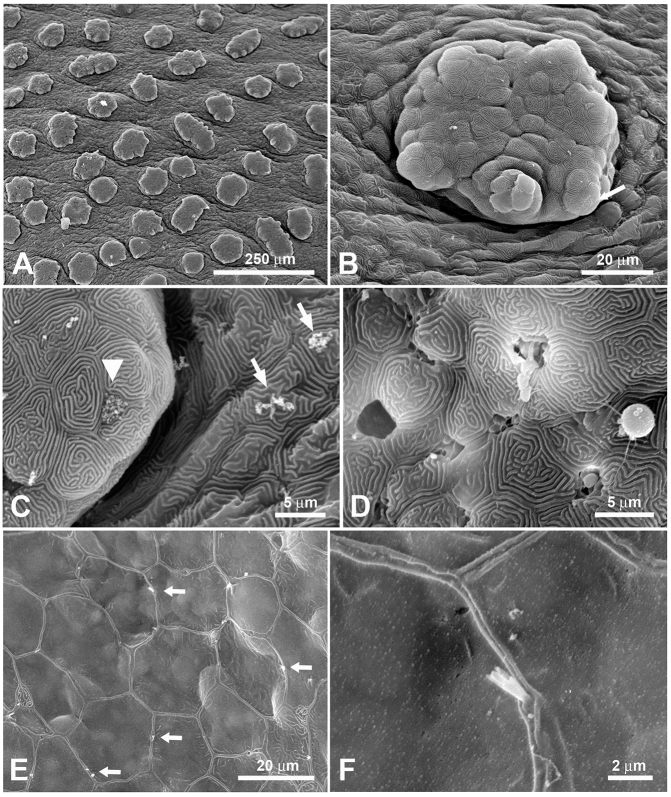
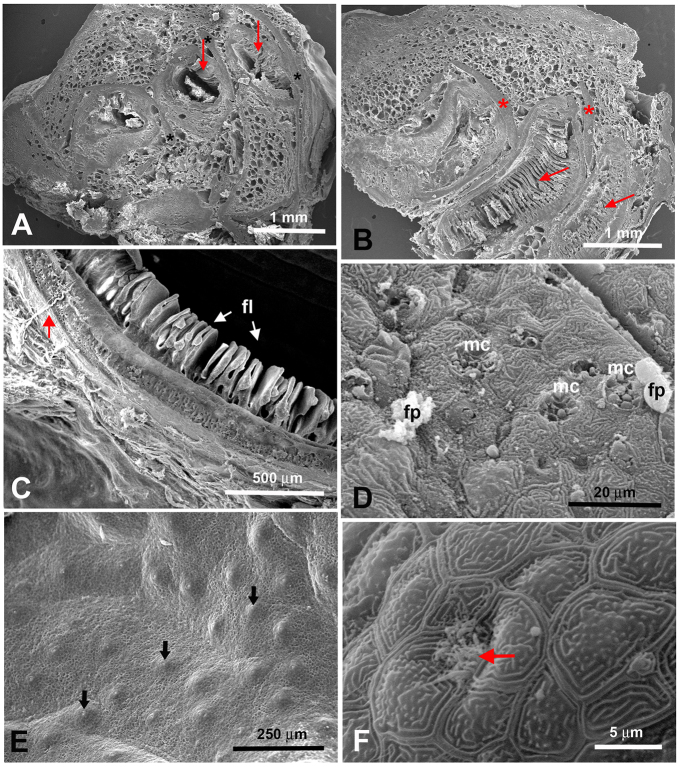
How might food particles be captured by the gill rakers, discerned, and then transported to the EO? We addressed this question by examining the gill rakers and EO of both species using scanning electron microscopy (SEM). We were interested in possible differences between species because of their different food habits. SEM showed the gill rakers of the bighead carp to be unattached (Fig. 2A), while those of the silver carp were fused together into a sieve-like structure that appeared capable of capturing very small particles (Fig. 2B). The dorsal ends of each of the gill rakers of both species were continuous with its corresponding EO coil, and modified gill rakers extended into the internal coils, demonstrating an intimate relationship between the two. The entrances to these coils were about 1 mm in width in juveniles [total length (TL) 13–15 cm] and up to 1 cm in large adults (TL>50 cm) and were guarded by small finger-like protuberances (Fig. 2D,E). The general gross morphology of the EO did not appear to be overtly influenced by fish size or species. The external surface of the EO of each species was lined with cells that showed a ‘fingerprint’ pattern typical of fish epithelia (Fig. 3). The EO of both species was covered with small protrusions (Fig. 3A), each of which had smaller protrusions (Fig. 3B) with many small structures that appeared to be taste buds inserted into them (Fig. 3B,C). Mucus cells were extremely abundant over the outside of each EO (Fig. 3D) along with many SCCs (Fig. 3E,F). The apical endings of SCCs protruded between the epithelial cells and varied in morphology. Each SCC had either one stout villus or two or more smaller villi (Fig. 3E,F). These smaller villi sometimes extended from a common base (Fig. 3F). The insides of the blind coiling tubes of both species were also lined with epithelial cells that showed a ‘fingerprint’ pattern (Fig. 4F). In sections through the tubes (Fig. 4A), the modified gill rakers were visible in some areas (Fig. 4B), and some smaller flap-like structures were present (Fig. 4A,C). The inside of the tubes also contained numerous mucus cells (Fig. 4D), and sometimes tiny food particles. In the EO epithelium opposite to the modified gill rakers, small taste bud-like structures were often evident on small hillocks (Fig. 4E,F). Inspection of the outer lips of both species as well as their buccal cavities revealed only a few possible taste buds and no SCCs. No taste buds were found on the gill arches and rakers, although a few SCCs were observed on the latter.
Fig. 2.
Scanning electron microscope images of gills and gill rakers in H. nobilis and H. molitrix. (A) Branchial arch with gills and gill rakers in H. nobilis. The gill rakers are free. (B) Gill rakers in H. molitrix. The gill rakers are widely fused and build a sieve-like structure. (C) Higher magnification of fused gill rakers in H. molitrix. (D,E) Entrance to the EO tubes at the posterior end of the upper jaw of H. nobilis. Small food particles (arrows) litter the epithelium.
Fig. 3.
Scanning electron microscope images of the outside of the epibranchial organ in H. molitrix. (A) The outside epithelium of the epibranchial organ is densely covered with small protrusions. (B) Each protrusion itself has further protrusions. Taste buds are inserted in these protrusions (arrow). (C) Higher magnification of one of these small protrusions showing the taste pore of a taste bud (arrowhead). The surface of the epithelium is littered with small food particles (arrows). (D) Abundant mucus cells are present throughout the epithelium. This image shows the various stages of mucus cells: discharged (left), discharging (middle) and discharged droplets of mucus (right). Almost all epithelial cells of the epibranchial organ show the ‘finger-print’ pattern typical for fishes. (E) Numerous solitary chemosensory cells (arrows) are scattered between the epithelial cells. (F) Higher magnification of a solitary chemosensory cell. The apex of these cells varies between oligovillous as seen here and monovillous.
Fig. 4.
Scanning electron microscope images of the inside of the epibranchial organ in H. nobilis and H. molitrix. (A) Horizontal section through the epibranchial organ of H. nobilis. The tubes contain food particles. The arrows depict small flaps that line some parts of the tubes. (B) Cross-section through the epibranchial organ of H. nobilis. The tubes are supported by cartilaginous structures (*). Modified gill rakers that continue into the tubes are visible (arrows). Note how much longer they are than the small flaps shown in panel A. (C) Higher magnification of the small flaps (fl) of H. molitrix. Note the nerve fiber (arrow) travelling towards the flaps. (D) Abundant mucus cells (mc) discharging small round mucus droplets are also present within the tubes of H. nobilis. Food particles (fp), some of them covered in mucus, are seen everywhere. (E) Parts of the tubes in H. molitrix are equipped with small hillocks. Each hillock contains a taste bud (arrows). (F) Higher magnification of the taste pore of a taste bud in H. molitrix (arrow).
Light microscopy confirms the presence of many taste buds and SCCs in, and on, the EO
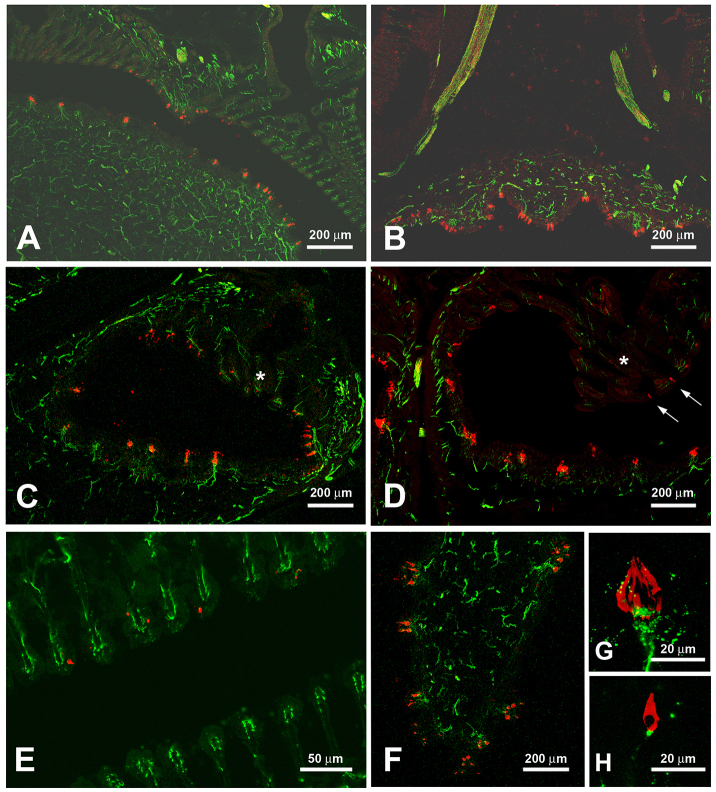
Were the specialized cell types we noted previously using SEM taste buds and SCCs? To answer this question, we employed immunohistochemistry and molecular markers. Cryosections of cross-sections of H. molitrix and H. nobilis EOs showed taste buds and/or SCCs visible as small end organs or single cells both in the epithelium covering the EO (Fig. 5B) as well as within the epithelium lining the tubes (Fig. 5C). Some areas of the coiling tubes also had small flap-like protrusions (Fig. 5F) along with abundant mucus cells (Fig. 5G). Arborized branches of the vagus nerve pathway followed the coils of the tube which was supported by a system of muscle and cartilage (Fig. 5A–D). Immunohistochemical studies using calretinin and acetylated tubulin confirmed the presence of both taste buds and SCCs both inside and outside the EO (Fig. 6A–D). Although both were labeled in red, SCCs were clearly distinguished from taste buds as single cells. The SCCs were mostly on, or in the vicinity of the modified gill rakers (Fig. 6C–E). Taste buds were about 20–25 μm in height and 16–18 μm in width with 4 μm pores (Fig. 6F,G). Taste cells had a round cell body and a long, slender apical portion reaching into the taste pore. The taste buds (Fig. 6G) as well as SCC (Fig. 6H) were contacted by nerve fibers.
Fig. 5.
Histological staining with nuclear red/light green/orange of 14 μm cryosections of the EO. Cartilage and bone were stained green, the brain, nerves and nerve fiber bundles were stained orange, and epithelia including taste buds were stained purple. These stainings were done for a general overview on sections adjacent to the sections used for immunohistochemistry. Images A, B and C show H. molitrix; images D, E, F and G show H. nobilis. A, B and C depict cross-sections through the epibranchial organ and its tubes (T). Image B shows the supporting cartilaginous structures (*) and the ridges at the outside of the epibranchial organ (arrowheads). (D) Horizontal section: the tubes are lined with epithelium that contains taste buds (arrows in C). In some areas modified gill rakers face the areas with taste buds. (E) Higher magnification of the modified gill rakers. (F) Some areas of the tubes contain small flaps as shown in Fig. 4C. (G) Higher magnification of the small flaps, which are lined with abundant mucus cells (arrows).
Fig. 6.
Histology of the epibranchial organ. Sections adjacent to those of Fig. 5 were treated with antibodies against calretinin (red), a marker for taste buds and solitary chemosensory cells, and acetylated tubulin (green), a marker for nerve fibers. Images A, B, D and E show H. molitrix, images C, F, G and H show H. nobilis. (A) As seen in Fig. 5, parts of the tubes are lined with epithelium containing taste buds whereas taste buds in other areas are scarce. (B) The epithelium outside the EO has ridges that contain taste buds (cf. Fig. 5B). (C) Taste buds innervated by small tubulin-positive nerve fibers lie opposite modified gill rakers (*), which usually do not have taste buds. (D) The modified gill rakers (*) contain few solitary chemosensory cells (arrow). (E) Higher magnification of the modified gill rakers. The red dots depict a few calretinin-positive solitary chemosensory cells. (F) The ridges on the outside of the EO have small protrusions that contain several taste buds. (G) Higher magnification of a taste bud (red) contacted by small nerve fibers (green). (H) Higher magnification of a solitary chemosensory cell also contacted by small nerve fibers.
Gustatory electrophysiology shows the EO to be chemosensitive
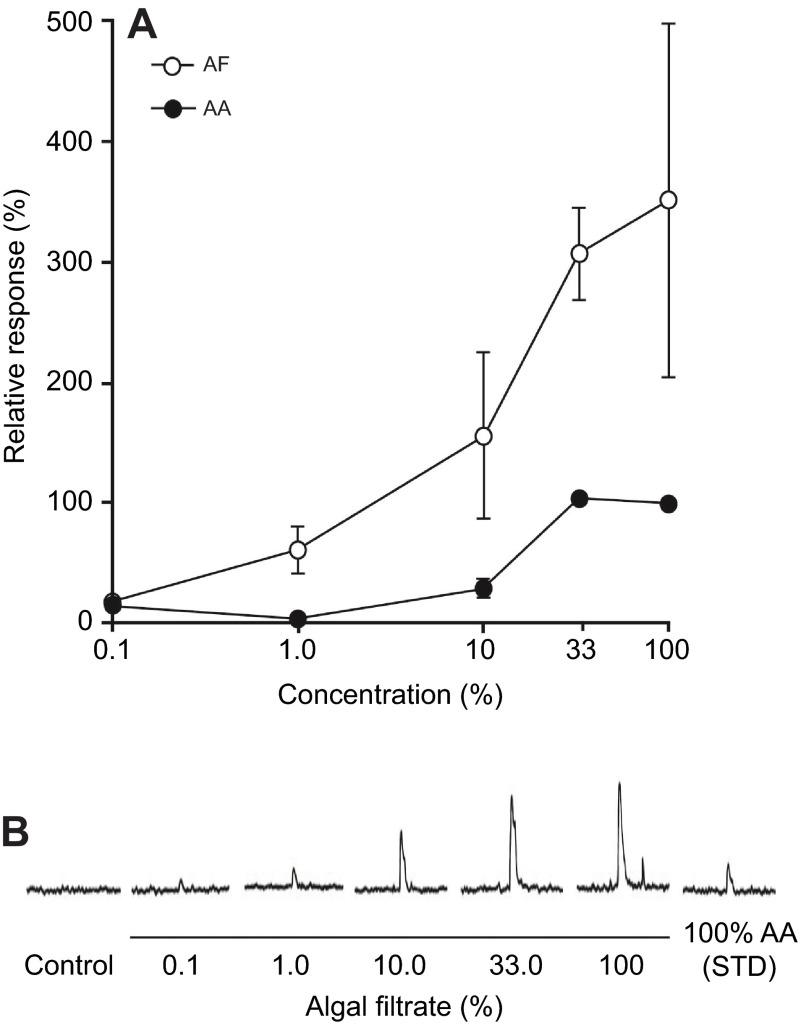
To determine whether the taste buds and SCCs found on, and in, the EO were functional, we conducted extracellular electrophysiological recordings from a caudal branch of the vagus nerve (cranial nerve X) in bigheaded carp while exposing their EO to food chemicals. Recordings were obtained from the caudal branch of the vagus nerve running to the EO of 15 animals, of which three produced multi-hour records that included various chemical and pressure stimuli as well as controls that could be analyzed. Visual and auditory display of the neural activity indicated that our recordings were from a large population of nerve fibers (there appeared to be dozens or perhaps hundreds of action potentials) that innervated the EO. While preparations from this nerve branch were insensitive to tactile stimulation, they did respond to a filtrate of the algae fed to the fish (AF), as well as the l-amino acids [commonly viewed as the primary feeding stimuli in fish (Sorensen and Caprio, 1998)] which we found in that diet (AA) (Fig. 7A). [HPLC analysis of AF (see Materials and methods) had found it to be composed of (mmol l−1): 0.62 l-glutamic acid, 0.62 l-glutamine, 0.01 l-aspartic acid, 0.12 l-asparagine, 0.16 l-serine, 0.10 l-histidine, 0.39 l-glycine, 0.12 l-threonine, 1.01 l-alanine, 0.49 l-arginine, 0.17 l-tyrosine, 0.19 l-valine, 0.09 l-methionine, 0.19 l-phenylalanine, 0.16 l-isoleucine, 0.30 l-leucine, 0.26 l-lysine and 0.14 l-proline]. We chose to use the concentration of the amino acid mixture (AA) found in the AF as our standard (STD) while well water alone (WW) served as a control (i.e. 100%AA=STD). Initial recordings often showed the EO to contract strongly in response to chemical test stimuli, expelling water and mucus in a forceful manner out of the internal coils. Consequently, subsequent work (i.e. all that described herein) used a muscle relaxant. Integrated electrophysiological responses to AF were about three times greater than those elicited by the AA it contained (i.e. STD; Fig. 7B). Integrated nerve responses to a 5 s pulse of chemical stimulus typically peaked within 2 s of contacting the sensory field and returned to baseline after the stimulus was washed out (i.e. within 15 s for the STD). Neural responses recovered fully within the 2 min inter-stimulus interval. The lowest electrophysiological detectable concentration of AF was 0.1% of full strength AF, and the dose–response relationship showed a sigmoidal relationship, saturating at about 50% full strength AF. A 10% dilution of AF elicited a response about 1.5 times that of the STD (Fig. 7A). Integrated phasic nerve responses elicited by the AA mixture were similar in form and duration to those elicited by the AF, but the magnitude of the integrated phasic nerve response to the AA mixture was only about a third of the magnitude elicited by a matching concentration of AF (Fig. 7B). The electrophysiological detection threshold of the AA mixture was about 1% of the STD. Responses to AAs saturated at about a third of the concentration of the STD.
Fig. 7.
Electrophysiological responses of a branch of the vagus nerve which innervates the epibranchial organ of bighead carp to chemical feeding stimuli. (A) Integrated gustatory electrophysiological responses from a branch of the vagus nerve in bighead carp to increasing concentrations of the filtrate of the algal food (AF) and the l-amino acids this food contains (AA). Mean responses (± s.e.m.) are expressed relative to those elicited by the AA mixture (STD). Data represent three preparations. (B) Representative integrated traces from one of the carp whose data are represented in panel A. Responses are shown to 0.1, 1.0, 10.0, 33.3 and 100% AF (Control: well water control; STD: AA mixture at full strength). The responses elicited to 100% AA and AF differed (P<0.10, paired t-test; N=3).
DISCUSSION
This study expanded our understanding of the anatomy and function of the EO in two species of planktivorous fishes of special importance, H. molitrix and H. nobilis, by demonstrating that the EO is an important chemosensory organ in these species. We suggest that it uses this sense to accumulate tiny food particles. In addition, this study provides the first detailed description of the morphology of the gill rakers and EO in these important species and shows that their extremely fine gill rakers and EO canals are closely associated and capable of functioning as an integrated unit. While differences in gill raker morphology and spacing were noted between species, their EOs seem much the same (if not identical). We also present histological evidence suggesting that the EO functions as a sophisticated pharyngeal chemosensory organ with both taste buds and SCCs. Our electrophysiological recordings, the first reported from an EO, demonstrate that the EO detects food-related chemicals including l-amino acids and likely other yet unknown chemicals. Together, these data suggest that the EO via pump filtering identifies and packages planktonic food that bigheaded carp have become specialized to consume. This ability might explain the extreme efficiency with which these carp species feed and thus their invasiveness in eutrophic waters.
The primary finding of this study is that the EO of bigheaded carps functions as a chemoreceptive organ. It has large numbers of taste buds and SCCs and is innervated by vagal nerve fibers that respond to relevant chemical stimuli. Specifically, we found large numbers of both taste buds and SCCs both within and outside the EO in both species. The taste buds stained with calretinin, typical of taste buds described in other fish (Reutter et al., 1974). In contrast, we found few taste buds and no SCCs either on the lips or within the buccal cavity of either species, consistent with our inability to record neural activity to chemical stimuli applied to these areas. Together, these data strongly suggest that the EO is the primary taste organ in these species. Although rather small, taste buds of the bigheaded carps resembled those of other fish species (Reutter et al., 1974). For comparison, taste buds in catfish of sizes comparable to the size of our specimens are about 50–80 μm high (Kirino et al., 2013) and in other fish species reach heights even up to 80 μm and widths 40–60 μm (Hansen and Reutter, 2004). The taste buds in the bigheaded carps were only 22–25 μm high and 10–18 μm wide. That these taste buds responded to AAs is typical of those on other organs in other fishes (Sorensen and Caprio, 1998) (see below). Our experiments also described the presence of SCCs on the EO as well as on the internal flaps within the EO and the gill rakers. SCCs have been observed on gill rakers in other teleosts (Hansen, 2005) as well as other organs of other vertebrates (Finger et al., 2003), but their function is not well understood. SCC morphology varies with respect to the apical endings of the cells and may vary even in the same fish species (Kotrschal et al., 1997), as seen here in the bigheaded carps. Only in the sea robin, Prinotus carolinus (Silver and Finger, 1984), is there direct evidence for the types of chemical stimuli detected by SCCs in fish, and l-amino acids have been implicated. It is possible, but unknown, whether our electrophysiological recordings included responses from SCCs.
In addition to presenting clear histological results that the EO serves as a specialized pharyngeal taste organ, we present electrophysiological evidence that it is responsive to the chemical stimuli found in their planktonic foods. It is notable that the detection threshold of the EO was about 1% that of stock concentration because this concentration would be relevant within the buccal cavity, which lacks other chemosensory structures: the EO appears to be the primary taste organ in these species. The mixture of l-amino acids found in their algal food was only partly responsible for the responsiveness, strongly suggesting that additional unidentified stimuli exist. This is notable because it is commonly thought that l-amino acids are the primary feeding cues in fishes, although most work has focused on carnivores (Sorensen and Caprio, 1998); likely some not yet tested chemostimulatory metabolites are present in their specialized planktonic diet which includes cyanobacteria. Although future studies should examine the physiological function of the EO in greater detail, we believe our work establishes the EO as a new type of internal, pharyngeal taste organ.
Our study extends our understanding of the gross morphology of the EO in bigheaded carps while elaborating on its remarkable anatomical specialization. In particular, we confirmed Boulenger's (Boulenger, 1901) century-old observation that the EO in bigheaded carp contains four blind tubes and demonstrate that the gill rakers of these species directly continue into these tubes through a series of specialized protrusions, probably allowing it to serve as an integrated feeding system. These protrusions, which have not been noted before, may keep larger undesirable particles from entering the EO. As long suspected, but not previously demonstrated, our histological results show that the EO contains large numbers of mucus cells and food boli inside the EO, suggesting that it does indeed aggregate food particles (Wilamovski, 1972). No other specialized secretory cell types were found in the EO, adding no support to a previous conjecture that the organ may also have a digestive function (Bertmar et al., 1969). While confirming an earlier report that the EO is muscular (Wilamovski, 1972; Bauchot et al., 1993), we found new evidence that the EO is reinforced with cartilage, which probably facilitates its ability to forcefully intake and expel water and food particles. Additionally, we illustrated gill raker morphology in both species in a detail not previously shown (Boulenger, 1901; Fang, 1928). Their fine structure is consistent with the likelihood that the gill rakers function with the EO to direct food for aggregation at the entrance of the alimentary canal via cross-flow filtration (Sandersen et al., 2001).
Finally, our study adds new insight into the function of the EO. We show that the EO contains numerous mucus and chemosensory cells, its canals are continuous with the gill rakers, and it contracts strongly when exposed to chemical stimuli. These findings directly support conjecture by Wilamovski (Wilamovski, 1972) that the EO in the bigheaded carps aggregate food from the gill rakers by secreting mucous and pumping and expelling it as boli, to the floor of the pharynx near the tiny alimentary canal for consumption (see Fig. 8 for schematic detail). From this study it now appears that the EO detects the presence of accumulating, desirable food particles in its canals using food chemicals detected by its taste buds and SCCs. Given the huge mass of fine particles that frequently exist in eutrophic (and dimly lit) waters, many of which would not be expected to be edible, but which would tend to accumulate in the gill rakers, the presence of chemosensory cells to discern food consumption would be highly adaptive. The fact that EO detects compounds other than AAs is intriguing given that many phytoplankton species (i.e. cyanobacteria) contain toxins (Beveridge et al., 1993; Leflaive and Ten-Hage, 2007), which might also be detected as part of a possible role of the EO in food selection. Whether mucus production in the EO might be directly stimulated by appropriate food chemicals is unknown. The precise connection between the presence of food particles and their chemicals, and EO pumping will be critical to unravel. Analogies may exist between the EO and the palatal organ, a specialized internal food recognition and sorting system in Eurasian carps including the goldfish, Carassius auratus (Finger, 2008), and common carp (Sibbing, 1982). It is interesting that taste buds and SCCs both occur on the EO, but any functional consequences of this association are unknown at present.
Fig. 8.
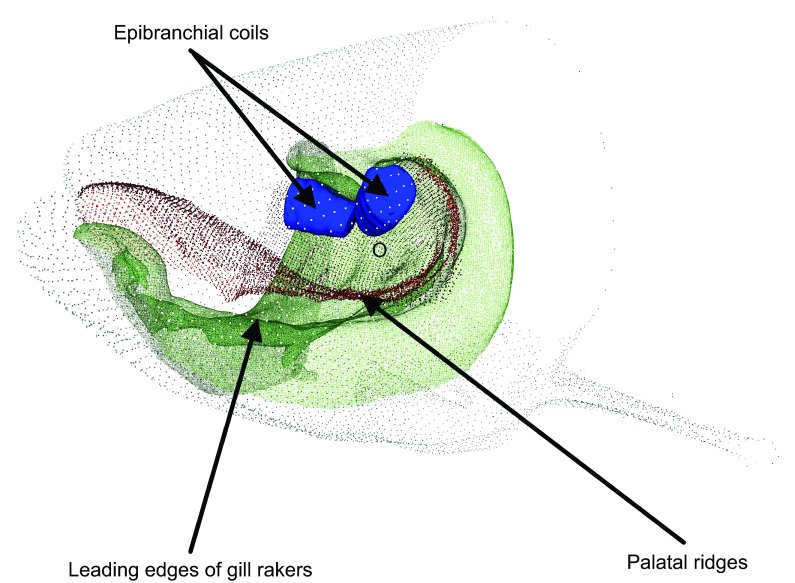
Oblique view of the epibranchial organ, which shows its relationship to other morphological features in the mouth of a bighead carp. A three-dimensional point cloud model of the head of an adult bighead carp shows internal EO coil structures in blue, the palatal ridges making up the dorsal surface of its buccal-pharyngeal cavity in red, and the opposing gill rakers making up the ventral surface of its buccal-pharyngeal cavity in green. Note the intimate relationship between the EO and gill rakers. The small opening to the alimentary canal is also noted (o). Well-developed pharyngeal teeth and opposing keratinous pad reside in fascia caudal to ‘o’. The branch of the vagus nerve we recorded from innervates the EO, a region of high sensory cell abundance. The morphology shown here is very similar in the silver carp except for differences in gill raker support structure and inter-gill raker distance as described by Fang (Fang, 1928) and this study.
In conclusion, this study demonstrates new aspects of the function of the EO in fishes, and in bigheaded carps in particular. Our results show that the EO is chemosensitive, and suggest that it plays a role in ingestion and food selection. It is possible that the chemosensitivity of the EO function might be exploited using flavored nanoparticles that are now being considered as means to selectively deliver toxins to these species for control (Hinterthuer, 2012). Further studies will need to determine the full range of chemical classes detected by the EO and its precise role in food ingestion in these species and other species that possess this intriguing organ. How this system might work together with the sense of smell (which is seemingly well developed) to locate, select and ingest novel planktonic food will also be interesting to determine.
MATERIALS AND METHODS
Animals
Juvenile bighead and silver carps were obtained from an experimental research facility [US Geological Survey (USGS), Columbia, MO, USA] where they were raised in ponds and then shipped by air courier to the University of Minnesota. Fish ranged in size from 5 cm (total length, TL) to 114 cm (TL). In Minnesota, carps were maintained in flowing well-water (20°C) and fed a planktonic diet (see below) until needed. In addition, several large adult carp (TL>50 cm) were obtained from commercial fisheries in the Illinois River for studies of their gross morphology. All experimental procedures followed the National Institutes of Health (NIH) Guidelines for the Care and Use of Animals and were in compliance with the Guidelines of the University of Minnesota Animal Care and Use Committee (IACUC). All necessary federal and state permits for shipping and holding prohibited species were also obtained.
Gross anatomy
Juvenile (8–15 cm TL) carp from an experimental research facility (USGS, Columbia, MO, USA) and large adult carps (TL>50 cm) obtained from commercial fisheries (N=7) were carefully dissected. We examined the size and structure of the EOs of silver and bighead carps while paying special attention to how the gill rakers are aligned with the canals in the EO and possible species differences. We also examined the innervation of the EO by branches of the vagus nerve (cranial nerve X). Gross anatomy of the brain was also examined. Structures were photographed with a digital camera (Canon Powershot A630, Olympus Stylus Tough 810). Lastly, a 3-dimensional point cloud model of an adult bighead carp's head (80 cm TL) was constructed using a Carmine 1.08 structured light sensor (PrimeSense; Tel Aviv, Israel), Skanect scanning software (skanect.occipital.com; version 1.6), and MeshLab open source mesh processing software (meshlab.sourceforge.net; version 1.3.3) to elucidate the relationship of EO with other structures.
Scanning electron microscopy
We examined the finer structures of the EO and gill rakers of each species to discern its possible function and to evaluate whether chemosensory cells might be present. Because the gross anatomy of the EO did not appear to vary with fish size or maturity, we used juvenile fishes (8–15 cm TL, N=8), which were easier to handle in the microscope. We searched for taste buds and SCCs in both species for their presence on gill rakers, opening to the EO, the mouth, lips and oral cavity. Lastly, both the exterior surfaces of several representative EOs as well as the interior surfaces of several EOs were carefully examined for taste buds, SCCs and secretory (mucus) cells. Specimens of both species were anesthetized and then fixed by immersion either in 5% glutaraldehyde in 0.05 mol l−1 phosphate buffer (pH 7.2) or in 2.5% glutaraldehyde + 1.0% paraformaldehyde in 0.05 mol l−1 phosphate buffer (pH 7.2) with post-fixation of 12 h in 1% osmium tetroxide. After rinsing in phosphate buffer, specimens were cut into smaller pieces and then dehydrated in a graded series of ethanol in a dehydrating microsystem (Leica, Buffalo Grove, IL, USA), critical-point-dried with CO2 in an Autosamdri-814 (Tousimis, Rockville, MD, USA), and coated with gold-palladium in a Fullam sputter coater (now Ted Pella, Inc., Redding, CA, USA). Samples of these specimens were then examined with a Hitachi S3500N scanning electron microscope (Hitachi, Schaumburg, IL, USA).
Light microscopy
The histology of putative chemosensory structures was examined to confirm the presence of taste buds or SCCs. We used both conventional histological staining and immunohistochemistry to focus on the EO, because chemosensory structures had not been noted elsewhere with SEM. Juvenile specimens (5–8 cm TL, N=4) of both species were fixed overnight in 10% formalin, cryoprotected in 20% sucrose and embedded in Tissue-Tek, cryosectioned (12–14 μm) and stained with Kernechtrot-Lichtgrün-Orange (KLO) (nuclear red-light green-orange). For staining, the slides were washed in distilled water (dH2O) for 2 min, immersed in a solution of nuclear red in 5% aluminium sulphate for 15 min, washed in dH2O for 5 s, and immersed in a mixture of light green and orange G in phosphotungstic acid for 2 min. After the staining process, slides were dehydrated in ethanol (96% for 10 s, 96% for 20 s, 2×100% for 5 min each), and xylene (twice for 10 min each). Slides were coverslipped with Permount mounting medium (Fisher Scientific, Pittsburgh, PA, USA) and examined under a light microscope (Olympus, Center Valley, CA, USA). KLO stains nuclei red, collagenous connective tissue and basal lamina green, erythrocytes yellow to orange, neuron somata red (due to nuclear staining), and neuropil slightly greenish-gray (Romeis, 1989).
For immunohistochemistry, cryosections adjacent to the ones used for KLO staining were processed with antisera against calretinin, a marker for taste buds and SCCs, and acetylated tubulin, a marker for nerve fibers. Standard immunohistochemical procedures were used. Briefly, cryosections were washed in 0.1 mol l−1 phosphate-buffered saline (PBS), blocked in blocking solution containing 1% BSA, 3–5% normal serum, and 0.3% Triton X-100 in PBS for 2 h, and then incubated in the primary antisera for 3 days (rabbit calretinin, dilution 1:2000, Swant catalog no. 7699/4, lot 18299, and mouse acetylated tubulin, dilution 1:5000, Sigma catalog no. T7451, lot 118K4821). After three washes (20 min each), the sections were incubated in the secondary antibodies (donkey anti-mouse Alexa 488, catalog no. A21202, lot 11/3537, donkey anti-rabbit Alexa 568 catalog no. A10042, lot 1235798, both 1:400; Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. After incubation, sections were washed three times for 20 min and coverslipped with Fluormount-G (Fisher Biotech, Birmingham, AL, USA). Control slides were treated either without the primary antibody or with normal rabbit serum replacing the primary antiserum. Control sections showed no labelling. Sections were viewed under a fluorescence microscope or a confocal laser microscope (Olympus, Center Valley, PA, USA). All anatomical figures were created in Adobe Photoshop, version CS2 (Adobe Systems Inc., San Jose, CA, USA).
Electrophysiology
Extracellular electrophysiological recordings were obtained from a visceral branch of the vagus nerve (X) that innervated the caudal portion of the EO (Fig. 1G). We tested juvenile bighead carp (TL 18–26 cm, N=15 of which three produced useful data), because this species was more plentiful in our laboratory. Briefly, an individual juvenile bighead carp was anesthetized in an aerated anesthetic bath (0.01% MS-222; Syndel, CO, USA), wrapped in moist tissue paper, and moved onto a groove in a beeswax block where it was braced in position with metal dissecting pins. The gills were continuously perfused with 0.01% MS-222 dissolved in aerated well water at 21°C. Their exposed operculum was removed and arterial severances clamped off with a microhemostat after dorso-caudal regions of the first to fourth branchial arches were removed, leaving a length of the vagus nerve exposed. A caudal branch of the vagus nerve that innervated the EO was selected, and a 1 cm length isolated from its surrounding fascia was transected at its central end just peripheral to the ganglion. The cut nerve branch was bathed in Cortland freshwater teleost Ringer's solution and inserted into a glass capillary (0.2–0.35 mm inside tip diameter, which had been fire polished and bent to a ~45 deg angle) connected to a suction electrode (no. 573000, A-M Systems, Sarasota, FL, USA). The electrode was fitted with a syringe (Gilmont, A-M Systems, no. 728000) which held Ringer solution and facilitated the precise regulation of suction pressure (which was released after a seal was formed). The subject fish was grounded via a stainless-steel catheter located in the dorsal musculature, which was also used to deliver an initial dose of 0.1 mg (0.1 mg ml−1) of the neuromuscular blocking agent Flaxedil (gallamine triethiodide; Sigma, St Louis, MO, USA). Additional doses were administered (up to 0.5 mg) as necessary to suppress epibranchial muscle contractions. A stainless-steel electrode served as the reference electrode and was placed in exposed connective fascia ~4 mm from the recording electrode. The resulting signal was processed by a Humbug (Quest Scientific) noise cancellation device, amplified via a high gain AC amplifier (Grass P511; Warwick, RI, USA) with high and low pass filters set at 30 and 3000 Hz, respectively. The neural signal was monitored on an oscilloscope and audio monitor, integrated (0.5 s) and plotted on chart paper where the magnitude of the phasic response was measured. The EO was exposed to test chemicals via a constant flow (15 ml min−1) of well water which bathed the caudal surface of the EO and then ran into the organ through a custom-built pipette delivery system into which the chemical stimuli were added for 5 s using a pneumatic switching device that minimized temperature and pressure fluctuations (Irvine and Sorensen, 1993). We tested whether the EO was sensitive to chemicals found in their algal food, including amino acids (Sorensen and Caprio, 1998). This algal food was developed by Robin Calfee (USGS, Columbia, MO, USA) and consisted of: 19.76 g l−1 dried spirulina algae (www.bulkfoods.com), 11.4 g l−1 dried chlorella algae (www.bulkfoods.com), 0.7 g l−1 Oncor FW trout pellet crumble (www.skretting.us), 1.1 g l−1 tropical flake food (www.aquaticeco.com), 1.64 g l−1 Otohime C1marine larval food (www.reed-mariculture.com), 0.7 g l−1 nannochloropsis 3600 condensed micro-algal culture, 0.7 g l−1 shellfish 1800 condensed micro-algal culture (www.reed-mariculture.com), 0.6 g l−1 Cyclopeeze freeze-dried decapod crustaceans (www.argent-labs.com), and 0.6 g l−1 soluble vitamin mixture (www.aquaticeco.com). Algal filtrate (AF) was prepared from the algal food formula by centrifugation and vacuum filtered to a 6 μm size threshold. AF and well water (WW, control) were analysed using an Agilent 1200 series HPLC–DAD and Agilent 1100 series fluorescence detector (Agilent Technologies, Santa Clara, CA, USA), to determine the absolute concentration of l-amino acids (Buha et al., 2011). Stock stimuli were prepared 24 h prior to use and were stored refrigerated in 250 ml Pyrex bottles (Schott Duran). The pH of the algal filtrate (100%) was between 7.69 and 7.89, while the pH of the well water was between 8.07 and 8.14. Each trial commenced by establishing a stable baseline of integrated nervous activity after which a solution of AF was tested several times to establish the responsiveness of the preparation. Well water was also tested to confirm a lack of sensitivity to pressure and carriers. If only tactile responses were noted, then the position of stimulus addition device was adjusted. Data were obtained only from those preparations in which the integrated baseline nerve activity was stable, and repeated responses to AF were within 20% of each other while eliciting no responses to blank water control. Experiments began by testing the mixture of l-amino acids (AA) found in their food which also served as our standard (STD). Responses to AA were tested at the standard concentration and at four dilutions: 0.1, 1.0, 10 and 33%. AF was also tested at matching concentrations. All stimuli were tested at least twice with 2 min inter-stimulus intervals. The mean response magnitude for each stimulus per preparation was calculated relative to the mean STD response magnitude. Means and standard errors were calculated from these standardized data and the data were plotted.
ACKNOWLEDGEMENTS
We thank Dr Ed Little and Ms Robin Calfee for their advice, enthusiastic support, and for providing the fish. We also acknowledge University Imaging Centers at the University of Minnesota, Twin Cities, for help with sample preparation and analyses for scanning electron microscopy. We also thank Dr Thomas Finger, University of Colorado Anschutz Medical Campus (AMC), Aurora, for helpful comments on our manuscript.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
Funding was provided by the United States Geological Survey (P.W.S.), the Environment and Natural Resources Trust Fund (P.W.S.), and the National Institutes of Health (P30 DC 04657 to Dr Diego Restrepo and Dr Thomas E. Finger, University of Colorado AMC]. Deposited in PMC for release after 12 months.
References
- Abdelghany A. E., Ahmad M. H. (2002). Effects of feeding rates on growth and production of Nile tilapia, common carp and silver carp polycultured in fertilized ponds. Aquac. Res. 33, 415-423 [Google Scholar]
- Bauchot R., Ridet J. M., Diagne M. (1993). The epibranchial organ, its innervation and its probable functioning in Heterotis niloticus (Pisces, Teleostei, Osteoglossidae). Environ. Biol. Fishes 37, 307-315 [Google Scholar]
- Bertmar G., Kapoor B. G., Miller R. V. (1969). Epibranchial organs in lower teleostean fishes – an example of structural adaptation. Int. Rev.Gen. Exp. Zool. 4, 1-48 [Google Scholar]
- Beveridge M. C. M., Baird D. J., Rahmatullah S. M., Lawton L. A., Beattie K. A., Codd G. A. (1993). Grazing rates on toxic and non-toxic strains of cyanobacteria by Hypophthalmichthys molitrix and Oreochromis niloticus. J. Fish Biol. 43, 901-907 [Google Scholar]
- Boulenger G. A. (1901). On the presence of a superbranchial organ in the cyprinoid fish Hypophthalmichthys. Ann. Mag. Nat. Hist. 8, 186-188 [Google Scholar]
- Braford M. R., Jr (1986). De gustibus non est disputandem: a spiral center for taste in the brain of the teleost fish, Heterotis niloticus. Science 232, 489-491 [DOI] [PubMed] [Google Scholar]
- Buha S. M., Panchal A., Panchal H., Chambhare R., Kumar S., Jain M., Patel P. R. (2011). HPLC-FLD for the simultaneous determination of primary and secondary amino acids from complex biological sample by pre-column derivatization. J. Chromatogr. Sci. 49, 118-123 [DOI] [PubMed] [Google Scholar]
- D'Aubenton E. (1955). Sur le role de l'organe suprabranchial d'Heterotis niloticus Ehrenberg (Téléostéen). C.R. Acad. Sci. Paris 241, 113-114 [Google Scholar]
- Dong S., Li D. (1994). Comparative studies on the feeding selectivity of silver carp Hypophthalmichthys molitrix and bighead carp Aristichthys nobilis. J. Fish Biol. 44, 621-626 [Google Scholar]
- Fang P. W. (1928). Notes on the gill rakers and their related structures of Hypophthalmichthys nobilis and H. molitrix. Cont. Biol. Lab.Sci. Soc. China 4, 1-30 [Google Scholar]
- Finger T. E. (2008). Sorting food from stones: the vagal taste system in goldfish, Carassius auratus. J. Comp. Physiol. A 194, 135-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger T. E., Böttger B., Hansen A., Anderson K. T., Alimohammadi H., Silver W. L. (2003). Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc. Natl. Acad. Sci. USA 100, 8981-8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A. (2005). The system of solitary chemosensory cells. In Fish Chemosenses (ed. Kapoor B. G., Reutter K.), pp. 165-176. New Delhi: Oxford and IBH Publishing Co. [Google Scholar]
- Hansen A., Reutter K. (2004). Chemosensory systems in fish: structural, functional and ecological aspects. In The Senses of Fish: Adaptations for the Reception of Natural Stimuli (ed. von der Emde G., Mogdans J., Kapoor B. G.), pp. 55-89. New Delhi: Narosa Publishing House Pvt. Ltd. [Google Scholar]
- Hinterthuer A. (2012). The explosive spread of Asian carp. Bioscience 62, 220-224 [Google Scholar]
- Hyrtl J. (1854). Beitrag zur anatomie von Heterotis ehrenbergii C.V. Denkschriften der Kaiserlichen Akademie der Wissenschaften 8, 73-88 [Google Scholar]
- Irvine I. A. S., Sorensen P. W. (1993). Acute olfactory sensitivity of wild common carp, Cyprinus carpio, to goldfish hormonal sex pheromones is influenced by gonadal maturity. Can. J. Zool. 71, 2199-2210 [Google Scholar]
- Kapoor B. G. (1954). The pharyngeal organ and its associated structures in the Milk-fish, Chanos chanos (Forskal). J. Zool. Soc. India 6, 51-58 [Google Scholar]
- Kirino M., Parnes J., Hansen A., Kiyohara S., Finger T. E. (2013). Evolutionary origins of taste buds: phylogenetic analysis of purinergic neurotransmission in epithelial chemosensors. Open Biol. 3, 130015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar C. S., Chapman D. C., Courtenay W. R., Jr, Housel C. M., Williams J. D., Jennings D. P. (2005). Asian Carps of the Genus Hypophthalmichthys: A Biological Synopsis and Environmental Risk Assessment. Washington, DC: US Fish and Wildlife Service; [Google Scholar]
- Kotrschal K., Krautgartner W.-D., Hansen A. (1997). Ontogeny of the solitary chemosensory cells in the zebrafish, Danio rerio. Chem. Senses 22, 111-118 [DOI] [PubMed] [Google Scholar]
- Leflaive J., Ten-Hage L. (2007). Algal and cyanobacterial secondary metabolites in freshwaters: a comparison of allelopathic compounds and toxins. Freshw. Biol. 52, 199-214 [Google Scholar]
- Michielsens C. G. J., Lorenzen K., Phillips M. J., Gauthier R. (2002). Asian carp farming system: towards a typology and increased resource use efficiency. Aquacult. Res. 33, 403-413 [Google Scholar]
- Miller R. V. (1969). Constancy of epibranchial organs and fourth epibranchial bones within species groups of clupeid fishes. Copeia 1969, 308-312 [Google Scholar]
- Nelson G. J. (1967). Epibranchial organs in lower teleostean fishes. J. Zool. 153, 71-89 [Google Scholar]
- Reutter K., Breipohl W., Bijvank G. J. (1974). Taste bud types in fishes. II. Scanning electron microscopical investigations on Xiphophorus helleri heckel (Poeciliidae, Cyprinodontiformes, Teleostei). Cell Tissue Res. 153, 151-165 [DOI] [PubMed] [Google Scholar]
- Romeis B. (1989). Mikroskopische Technik. München; Wien; Baltimore, MD: Urban und Schwarzenberg; [Google Scholar]
- Sanderson S. L., Cheer A. Y., Goodrich J. S., Graziano J. D., Callan W. T. (2001). Crossflow filtration in suspension-feeding fishes. Nature 412, 439-441 [DOI] [PubMed] [Google Scholar]
- Sibbing F. A. (1982). Pharyngeal mastication and food transport in the carp (Cyprinus carpio L.): a cineradiographic and electromyographic study. J. Morphol. 172, 223-258 [DOI] [PubMed] [Google Scholar]
- Silver W. L., Finger T. E. (1984). Electrophysiological examination of a non-olfactory, non-gustatory chemosense in the searobin, Prionotus carolinus. J. Comp. Physiol. A 154, 167-174 [Google Scholar]
- Smith D. W. (1985). Biological control of excessive phytoplankton growth and the enhancement of aquacultural production. Can. J. Fish. Aquat. Sci. 42, 1940-1945 [Google Scholar]
- Smith D. W. (1989). The feeding selectivity of silver carp, Hypophthalmichthys molitrix Val. J. Fish Biol. 34, 819-828 [Google Scholar]
- Sorensen P. W., Caprio J. (1998). Chemoreception in fish. Chapter 15 in The Physiology of Fishes, 2nd edn (ed. Evans R. E.), pp. 375-406. Boca Raton, FL: CRC Press; [Google Scholar]
- Spataru P., Gophen M. (1985). Feeding behaviour of silver carp Hypophthalmichthys molitrix Val. and its impact on the food web in Lake Kinneret, Israel. Hydrobiologia 120, 53-61 [Google Scholar]
- Whitear M. (1992). Solitary chemosensory cells. In Fish Chemoreception (ed. Hara T. J.), pp. 103-125. London; New York, NY: Chapman & Hall; [Google Scholar]
- Whitear M., Kotrschal K. (1988). The chemosensory anterior dorsal fin in rocklings (Gaidropsarus and Ciliata, Teleostei, Gadidae): activity, fine structure and innervation. J. Zool. (Lond.) 216, 339-366 [DOI] [PubMed] [Google Scholar]
- Wilamovski A. (1972). Structure of the gill apparatus and suprabranchial organ of Hypophthalmichthys molitrix Val. (silver carp). Bamidgeh 24, 87-98 [Google Scholar]