Abstract
INTRODUCTION
Atrial fibrillation (AF) recurrence after ablation is associated with left atrial (LA) fibrosis on late gadolinium enhanced (LGE) magnetic resonance imaging (MRI). We sought to determine pre-ablation, clinical characteristics that associate with the extent of LA fibrosis in patients undergoing catheter ablation for AF.
METHODS AND RESULTS
Consecutive patients presenting for catheter ablation of AF were enrolled and underwent LGE-MRI prior to initial AF ablation. The extent of fibrosis as a percentage of total LA myocardium was calculated in all patients prior to ablation. The cohort was divided into quartiles based on the percentage of fibrosis. Of 60 patients enrolled in the cohort, 13 had <5% fibrosis (Group 1), 15 had 5–7% fibrosis (Group 2), 17 had 8–13% fibrosis (Group 3), and 15 had 14–36% fibrosis (Group 4). The extent of LA fibrosis was positively associated with time in continuous AF, and the presence of persistent or longstanding persistent AF. However, no statistically significant difference was observed in the presence of comorbid conditions, age, BMI, LA volume, or family history of AF among the four groups. After adjusting for diabetes and hypertension in a multivariable linear regression model, paroxysmal AF remained independently and negatively associated with the extent of fibrosis (−4.0 ± 1.8, P = 0.034).
CONCLUSION
The extent of LA fibrosis in patients undergoing AF ablation is associated with AF type and time in continuous AF. Our results suggest that the presence and duration of AF are primary determinants of increased atrial LGE.
Keywords: MRI, atrial fibrillation, fibrosis
Introduction
Catheter ablation of atrial fibrillation (AF) is an important treatment modality for patients with symptomatic, drug- refractory AF. Ablation success appears to be associated with multiple variables such as AF type, duration of continuous AF, left atrial (LA) dilation, and presence or absence of comorbid conditions.1 Recent advances in magnetic resonance imaging (MRI) with late gadolinium enhancement (LGE) have enabled the non-invasive quantification of LA fibrosis.2–5 The extent of left pre-existing LA fibrosis assessed by LGE-MRI appears to be a strong predictor of ablation failure.5–9 In a landmark publication in 1995, Wijffels and colleagues demonstrated that continuous AF results in atrial remodeling.10 The concept that “AF begets AF” is now widely accepted.6,11–15 Recent evidence from Kottkamp suggests that atrial fibrosis may also result from a process independent of AF persistence, termed “fibrotic atrial cardiomyopathy (FACM).”16–18 According to Kottkamp, the structural atrial disease associated with FACM may act as the substrate for atrial tachy- and brady-arrhythmias.16–18 In this study, we sought to (a) describe the extent of atrial fibrosis in a series of patients undergoing a first AF ablation procedure, and (b) define the association of the extent of atrial fibrosis with clinical predictors including atrial volume, and the type and duration of AF.
Methods
Patient population
The Johns Hopkins Institutional Review Board approved the protocol, and all participants provided written informed consent. The patient population included 60 consecutive patients referred for an initial AF ablation, who underwent pre-procedural LGE-MRI. Paroxysmal AF was defined as recurrent (≥2 episodes) AF that terminated spontaneously within 7 days.1 Persistent AF was defined as AF sustained beyond 7 days or lasting less than 7 days, but necessitating pharmacologic and electrical cardioversion.1 Longstanding persistent AF was defined as continuous AF of greater than 1 year in duration as defined by the 2012 Heart Rhythm Society Consensus Document.1
Magnetic resonance imaging
All subjects underwent pre-ablation MRI using a 1.5 Tesla magnetic resonance scanner (Avanto, Siemens, Erlangen, Germany) with a phased array cardiac coil. Contrast enhanced three-dimensional fast low angle shot angiography images were used to define LA and pulmonary vein (PV) anatomy. LGE-MRI scans were acquired approximately 18 minutes following 0.2 mmol/kg gadolinium injection (gadopentetate dimeglumine; Bayer Healthcare Pharmaceuticals, Montville, NJ, USA). We utilized a three-dimensional inversion recovery prepared fast spoiled gradient recalled sequence that is respiratory triggered and navigated, ECG gated, and fat suppressed (repetition time of 2.5–5.5 ms, echo time of 1.52 ms, flip angle at 10 degrees, in-plane resolution of 1.3 × 1.3, slice thickness of 2.0 mm, and inversion time (TI) 240–290 ms). A parallel imaging technique, generalized auto-calibrating partially parallel acquisition (GRAPPA, reduction factor 2), was used. Of total 60 patients, seven (11.7%) were in AF during MRI acquisition. The extent of fibrosis was not significantly different between those imaged during sinus rhythm versus AF (10.5 ± 7.6 versus 5.6 ± 3.9%, P = 0.100).
LA fibrosis measurement and quantification
The LGE-MRI three-dimensional images were processed using multiplanar reconstruction (MPR) to obtain 3.5 mm thick axial image planes. The LA myocardium was defined by placing epi- and endocardial contours using QMass MR software (Version 7.2, Leiden University Medical Center, Leiden, The Netherlands). The image intensity ratio (IIR), a previously described LGE-MRI technique that normalizes myocardial image intensities by the mean blood pool intensity was used to identify fibrosis.2,19 The methodology for calculation of the IIR and its association with regional voltage by electroanatomic mapping were previously validated.2 The mean IIR of the entire LA and extent of fibrosis (as a percentage of the LA myocardium) were measured for all patients. The cohort was divided into quartiles corresponding to four stages of LA fibrosis as follows: Group 1 or minimal fibrosis (less than 5% fibrosis), Group 2 or mild fibrosis (5–7% fibrosis), Group 3 or moderate fibrosis (8–13% fibrosis), and Group 4 or extensive fibrosis (14–36% fibrosis).
Statistical analysis
Continuous variables were summarized as mean ± standard deviation and categorical variables as counts and percentages. The univariable association of fibrosis extent quartiles with continuous variables was assessed using analysis of variance (ANOVA). The chi-square test was utilized to examine the univariable association of fibrosis extent quartiles with other categorical variables. The univariable association of fibrosis extent as a continuous variable with other continuous and categorical variables was assessed using the Spearman Correlation test and Student’s t-test, or ANOVA, where appropriate. The independent association of clinical variables with fibrosis extent was assessed utilizing multivariable linear regression. Statistical tests were performed using STATA Statistical Software (Version 12, StataCorp, LP, College Station, TX, USA). A P-value of <0.05 was considered statistically significant.
Results
Clinical characteristics
The clinical characteristics of the 60 patients included in the study are summarized in Table 1. Among the 60 patients, 46 (76.7%) were male and the mean age was 59.2 ± 8.9 years. AF was paroxysmal in 35 (58.3%) patients, persistent in 22 (36.7%) patients, and longstanding persistent in three (5%) patients. The mean LA volume was 152.2 ± 47.3 ml and the mean LA volume index was 73.6 ± 22.8 ml/m2. Five (8.3%) patients had congestive heart failure, six (10%) had diabetes mellitus, five (8.3%) had a history of transient ischemic attack (TIA) or stroke, seven (11.7%) had vascular disease, 28 (46.7%) had hypertension, and 17 (28.3%) had obstructive sleep apnea. The median CHADS2 score was 1, with a range of 0–5. Additionally, the median time of continuous AF prior to AF ablation was 0.76 (0.01–6.02) months.
Table 1.
Clinical characteristics based on extent of fibrosis.
| TOTAL (n = 60) |
GROUP 1 (n = 13) |
GROUP 2 (n = 15) |
GROUP 3 (n = 17) |
GROUP 4 (n = 15) |
P-VALUE | |
|---|---|---|---|---|---|---|
| Extent of fibrosis (%) | 9.9 ± 7.4 | 2.3 ± 1.3 | 5.8 ± 0.7 | 10.2 ± 1.8 | 20.3 ± 6.2 | |
| LA volume index (mL/m2) | 73.6 ± 22.8 | 76.1 ± 25.7 | 71.6 ± 19.0 | 73.9 ± 24.8 | 73.0 ± 23.5 | 0.965 |
| Age (years) | 59.2 ± 8.9 | 60.5 ± 9.9 | 58.7 ± 8.1 | 58.6 ± 7.9 | 59.2 ± 10.5 | 0.941 |
| Male gender | 46 (76.7) | 9 (69.2) | 11 (73.3) | 14 (82.3) | 12 (80.0) | 0.816 |
| Body mass index (kg/m2) | 29.3 ± 6.1 | 29.0 ± 6.3 | 27.5 ± 4.3 | 30.2 ± 6.9 | 30.1 ± 6.6 | 0.600 |
| Congestive heart failure | 5 (8.3%) | 0 (0%) | 2 (13.3%) | 2 (11.8%) | 1 (6.7%) | 0.745 |
| Diabetes mellitus | 6 (10%) | 0 (0%) | 2 (13.3%) | 1 (5.9%) | 3 (20%) | 0.379 |
| Vascular disease | 7 (11.7%) | 1 (7.7%) | 1 (7.1%) | 3 (17.7%) | 2 (13.3%) | 0.862 |
| Hypertension | 28 (46.7%) | 6 (46.2%) | 5 (33.3%) | 7 (41.2%) | 10 (66.7%) | 0.328 |
| Sleep apnea | 17 (28.3%) | 5 (38.5%) | 5 (33.3%) | 4 (23.5%) | 3 (20%) | 0.701 |
| Time in continuous AF (months) | 1.3 ± 1.4 | 0.95 ± 1.2 | 1.0 ± 1.5 | 1.7 ± 1.6 | 1.5 ± 1.1 | 0.402 |
| Paroxysmal AF | 35 (58.3%) | 9 (69.2%) | 11 (73.3%) | 9 (52.9%) | 6 (40%) | 0.232 |
| CHADS2 score | ||||||
| 0 | 28 (46.7%) | 6 (46.15%) | 9 (60%) | 9 (52.9%) | 4 (26.7%) | 0.213 |
| 1 | 21 (35%) | 4 (30.8%) | 2 (13.3%) | 7 (41.2%) | 8 (53.3%) | |
| 2 | 5 (8.3%) | 1 (7.7%) | 2 (13.3%) | 0 (0%) | 2 (13.3%) | |
| 3 | 4 (6.7%) | 2 (15.4%) | 0 (0%) | 1 (5.9%) | 1 (6.67%) | |
| 4 | 1 (1.7%) | 0 (0%) | 1 (6.7%) | 0 (0%) | 0 (0%) | |
| 5 | 1 (1.7%) | 0 (0%) | 1 (6.7%) | 0 (0%) | 0 (0%) | |
Note: Continuous variables are summarized as mean ± standard deviation and categorical variables as number (percentage).
Extent and predictors of fibrosis
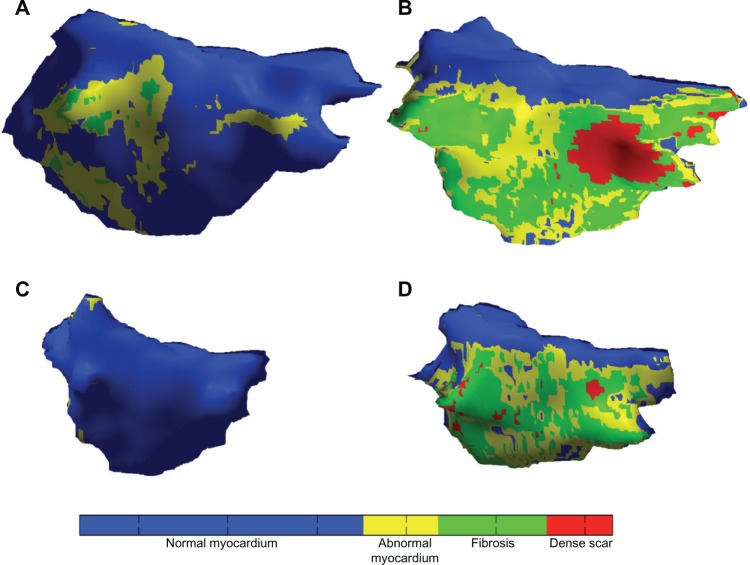
Figure 1 illustrates detailed color fibrosis maps from four patients with variable scar extent and atrial sizes. Among the 60 patients in our study, the mean percentage of fibrosis was 9.9 ± 7.4%. As shown in Table 1, 13 (21.7%) patients were staged in Group 1 with mean fibrosis 2.3 ± 1.3%, 15 (25%) patients were staged in Group 2 with mean fibrosis 5.8 ± 0.7%, 17 (28.3%) patients were staged in Group 3 with mean fibrosis 10.2 ± 1.8%, and 15 (25%) patients were staged in Group 4 with mean fibrosis 20.3 ± 6.2%. The detailed clinical characteristics and results of LGE-MRI for each of the patients enrolled in our study are shown in Table S1 in supplementary material. The patients are organized based on the percentage of fibrosis.
Figure 1.
Fibrosis maps for four patients with variable fibrosis extent and LA size.
Notes: Fibrosis patterns in each of four patients are shown after application of IIR thresholds for LGE-MRI. The colors correspond to IIR thresholds of >0.97, >1.20, and >1.61, corresponding to voltage <0.5, <0.35, and <0.1 mV, respectively.2 Regions with blue color indicate normal myocardium, whereas yellow and green represent a gradation of unhealthy myocardium with increasing fibrosis and red represents dense fibrosis. Patients in the first row (A and B) have dilated atria, whereas those in the bottom row (C and D) have normal atria. Patients in the first column (A and C) have minimal fibrosis depicting the lack of association between atrial volume and fibrosis extent.
To be consistent with prior literature,3 the association of clinical predictors with the extent of fibrosis was first explored using fibrosis extent as a categorical variable (groups 1–4). We then explored the associations with fibrosis extent as a continuous variable. Fibrotic extent as a categorical variable was unassociated with LA volume, age, body mass index, presence of congestive heart failure, presence of diabetes mellitus, presence of vascular disease, CHADS2 score, presence of hypertension, presence of obstructive sleep apnea, time in continuous AF, or AF type (Table 1).
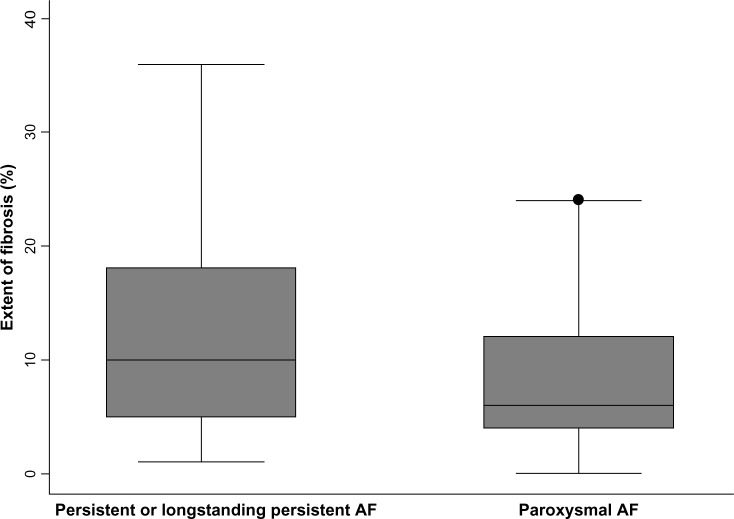
When using fibrosis extent as a continuous variable, no associations were found with LA volume index (P = 0.766), age (P = 0.984), gender (P = 0.219), body mass index (P = 0.360), congestive heart failure (P = 0.820), vascular disease (P = 0.826), sleep apnea (P = 0.269), or CHADS2 score (P = 0.625). However, there was a trend toward greater fibrosis in patients with diabetes mellitus (15.0 ± 4.7 versus 9.4 ± 0.9%, P = 0.078), and hypertension (11.4 ± 1.5 versus 8.7 ± 1.2%, P = 0.167). Additionally, fibrosis extent as a continuous variable had statistically significant univariable associations with time in continuous AF (Spearman’s rho 0.336, P = 0.009, Figure 2), and AF type (12.4 ± 1.8% scar in persistent and longstanding persistent AF versus 8.2 ± 0.9% in paroxysmal AF, P = 0.028, Figure 3). After adjusting for diabetes and hypertension in a multivariable linear regression model, paroxysmal AF remained independently and negatively associated with the extent of fibrosis (−4.0 ± 1.8, P = 0.034). Time in continuous AF was not included as another variable in the multivariable model because it was collinear with AF type.
Figure 2.
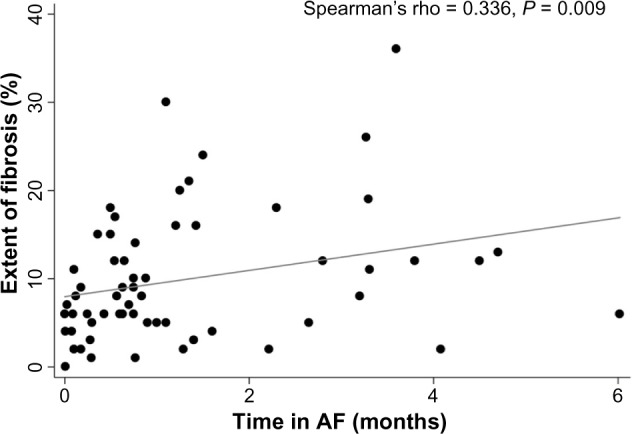
Association between the extent of fibrosis and time in continuous AF.
Notes: The scatter diagram and line of best fit display the association between the extent of LA fibrosis and time in continuous AF.
Figure 3.
Distribution of fibrotic extent among patients with paroxysmal versus persistent AF.
Notes: The figure illustrates the extent of fibrosis in patients with persistent and longstanding persistent versus paroxysmal AF. The box plots display the median and the 25th–75th percentile range (center black line and solid gray box), the lower and upper adjacent values (outer black whiskers), and outlier data points (black dot).
Discussion
The purpose of this study was to describe the extent and clinical predictors of atrial fibrosis in a series of patients undergoing a first AF ablation procedure. We noted univariable associations between the LA fibrosis extent and AF type, and between LA fibrosis extent and the duration of continuous AF. AF type remained associated with the extent of atrial fibrosis in a multivariable model that adjusted for the presence of hypertension and diabetes.
Prior studies of predictors of LA fibrosis
The results of our study confirm and extend the findings of prior investigations of the predictors of LA fibrosis. We found that the extent of LA fibrosis was associated with AF type but independent of patient age and the presence of concomitant comorbidities. Boldt and colleagues examined the association between extracellular matrix proteins and AF in 118 patients with lone AF, mitral valve disease and AF, mitral valve disease and sinus rhythm, or sinus rhythm without mitral valve disease.20 Using quantitative western blotting techniques and immunohistochemical methods, Boldt and colleagues observed greater fibrosis in patients with, when compared to those without AF; however, there was no difference in extracellular matrix expression between patients with paroxysmal and persistent AF.20 Subsequently, Platonov and colleagues reported an association between fibrosis, AF, and age through review of medical records and autopsies from 30 patients without AF, with paroxysmal AF, or with persistent AF.21 No association was observed between age and extent of fibrosis; however, patients with a history of AF had a threefold greater extent of fibrosis compared to patients without prior AF.21 A three-group comparison revealed that fibrosis extent was significantly greater in patients with longstanding persistent AF compared to patients with paroxysmal AF in the superior pulmonary veins, but not in the inferior pulmonary veins or in the posterior left atrium.21 Later, in a study of 24 patients with mitral valve disease, Qian and colleagues reported greater fibrosis in patients with AF compared to those in sinus rhythm.22
Using a noninvasive LGE-MRI method, Oakes and colleagues quantified LA fibrosis in 81 patients with paroxysmal (n = 41) or persistent (n = 40) AF prior to ablation.5 Of the 43 patients with mild enhancement, 28 (65%) patients had paroxysmal AF and 15 (25%) had persistent AF. However, of the 30 patients with moderate fibrotic enhancement, 13 (43%) patients had paroxysmal AF and 17 (57%) had persistent AF, suggestive of a trend toward greater fibrosis in those with persistent AF (P = 0.06).5 Mahnkopf and colleagues later examined the extent of LA fibrosis detected by LGE-MRI in 333 patients undergoing AF ablation, of which 40 patients had lone AF.9 The distribution of patients staged into the Utah I, Utah II, Utah III, and Utah IV fibrosis groups was comparable in patients with lone and non-lone AF, with Utah III or Utah IV fibrosis observed in 25% of lone AF patients and 30.4% of non-lone AF patients.9 Similarly, Akoum and colleagues observed no association between Utah stage of LA fibrosis and age, hypertension, coronary artery disease, congestive heart failure, diabetes, or left ventricular ejection fraction.6
The results of our study add to this growing body of literature in a number of ways. First, we analyzed fibrosis extent using two methods: (a) by categorization by quartiles (similar to the Utah scoring system) and (b) as a continuous variable. Due to preservation of study power using a continuous variable in its native form, associations of AF type and time in continuous AF were observed with fibrosis extent as a continuous variable. However, when the degree of fibrosis was categorized as quartiles, the association between AF type and fibrosis group number was not significant. Patients with paroxysmal and non-paroxysmal AF were observed in each of the fibrosis quartiles. This underscores the importance of study power in determining the association of clinical predictors with fibrosis extent. Categorization of fibrosis extent by quartiles, although convenient from a clinical standpoint, may lead to inadvertent reduction of study power and biased results. Second, we employed the IIR methodology for analysis of fibrosis. This method utilizes normalization of image intensity, which lowers the confounding effect of patient level variables such as contrast dose, bolus timing, patient renal function, and hematocrit.2 By reducing the inter-patient variability in image intensity measurements not attributable to tissue characteristics, the IIR improves the comparison of fibrosis extent across patients.
The association between LA volume and the extent of LA fibrosis
The results of our study provide additional data for the existing discussion of the association between LA volume and the extent of LA fibrosis. Our study revealed no significant association between LA size and the extent of fibrosis. This somewhat counterintuitive finding is consistent with the results of some, but not all prior investigations.5,23 In an early study using LGE-MRI to quantify LA volume and fibrosis, Oakes and colleagues reported that the pre-procedural LA volume was associated with the extent of LA fibrosis in 81 patients presenting for AF ablation.5 However, in a more recent study, Akoum et al found no significant difference in LA volume and extent of fibrosis in 178 patients undergoing LGE-MRI and TEE prior to ablation or cardioversion for AF.23 Our results support the latter study and suggest that the extent of LA fibrosis is independent of LA volume.
Limitations
Our study had several limitations. First, the study had a relatively small sample size. This may have limited study power and the ability to identify other factors that are associated with the extent of LA fibrosis. Additionally, all patients in the study were referred for catheter ablation of AF. Therefore, the study sample is biased toward a healthier subset of all patients with AF. This important bias may limit our ability to adequately examine the associations between scar fibrosis and patient co-morbidities. Also, atrial wall thickness is near the limit of image resolution in some patients. Blood pool or epicardial fat volume averaging may in some cases confound the measurement of fibrosis extent by LGE-MRI. Our cohort participants had a larger mean LA volume index than some prior studies. This may reflect the relatively high percentage of patients with persistent or longstanding persistent AF in our cohort. Finally, time in AF was calculated by summating length of AF episodes from loop recorders, electrocardiogram (EKG)s, patient reports, and clinical notes. Since AF was documented in some patients prior to the use of a loop recorder, the duration of AF may be underestimated in these patients.
Conclusion
The results of this study provide insight into the clinical predictors of an important patient characteristic, LA fibrosis. The extent of LA fibrosis appears to be independent of age, body mass index, congestive heart failure, vascular disease, CHADS2 score, and obstructive sleep apnea. However, the extent of LA fibrosis is positively associated with time in continuous AF, and AF persistence. Additionally, the extent of atrial fibrosis is independent of LA volume. Our results suggest that the presence and duration of AF itself may be a primary determinant of increased atrial LGE in patients with AF.
Supplementary Data
Supplementary Table 1. Individual patient characteristics.
Footnotes
Author Contributions
Conceived and designed the experiments: JD, HC, SN. Analyzed the data: JD, IMK, SN. Wrote the first draft of the manuscript: JD. Contributed to the writing of the manuscript: JD, HC, SN. Agree with manuscript results and conclusions: JD, IMK, FP, DS, JEM, RDB, HA, JR, SLZ, VZ, HC, SN. Jointly developed the structure and arguments for the paper: JD, IMK, FP, DS, JEM, RDB, HA, JR, SLZ, VZ, HC, SN. Made critical revisions and approved final version: JD, IMK, FP, DS, JEM, RDB, HA, JR, SLZ, VZ, HC, SN. All authors reviewed and approved of the final manuscript.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ACADEMIC EDITOR: Thomas E Vanhecke, Editor in Chief
FUNDING: The study was funded by a Biosense-Webster grant and NIH grants K23HL089333 and R01HL116280 to Dr. Nazarian, the Roz and Marvin H. Weiner and Family Foundation, the Dr. Francis P. Chiaramonte Foundation, and The Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund.
COMPETING INTERESTS: Dr. Nazarian is a scientific advisor and principal investigator for research funding to Johns Hopkins University from Biosense-Webster Inc. Other authors disclose no competing interests.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants. Provenance: the authors were invited to submit this paper.
REFERENCES
- 1.Calkins H, Kuck KH, Cappato R, et al. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation Developed in partnership with the european heart rhythm association (ehra), a registered branch of the european society of cardiology (esc) and the european cardiac arrhythmia society (ecas); and in collaboration with the american college of cardiology (acc), american heart association (aha), the asia pacific heart rhythm society (aphrs), and the society of thoracic surgeons (sts). Endorsed by the governing bodies of the american college of cardiology foundation, the american heart association, the european cardiac arrhythmia society, the european heart rhythm association, the society of thoracic surgeons, the asia pacific heart rhythm society, and the heart rhythm society. Heart Rhythm. 2012;9(632–96):e621. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Khurram IM, Beinart R, Zipunnikov V, et al. Magnetic resonance image intensity ratio, a normalized measure to enable inter-patient comparability of left atrial fibrosis. Heart Rhythm. 2014;11(1):85–92. doi: 10.1016/j.hrthm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spragg DD, Khurram I, Zimmerman SL, et al. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm. 2012;9:2003–9. doi: 10.1016/j.hrthm.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Beinart R, Khurram IM, Liu S, et al. Cardiac magnetic resonance t1 mapping of left atrial myocardium. Heart Rhythm. 2013;10:1325–31. doi: 10.1016/j.hrthm.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akoum N, Daccarett M, McGann C, et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a de-mri guided approach. J Cardiovasc Electrophysiol. 2011;22:16–22. doi: 10.1111/j.1540-8167.2010.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teh AW, Kistler PM, Lee G, et al. Long-term effects of catheter ablation for lone atrial fibrillation: progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm. 2012;9:473–80. doi: 10.1016/j.hrthm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 8.McGann C, Kholmovski E, Blauer J, et al. Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J Am Coll Cardiol. 2011;58:177–85. doi: 10.1016/j.jacc.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahnkopf C, Badger TJ, Burgon NS, et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced mri: implications for disease progression and response to catheter ablation. Heart Rhythm. 2010;7:1475–81. doi: 10.1016/j.hrthm.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 11.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–63. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 12.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–31. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 13.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–46. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 14.Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Schotten U, Duytschaever M, Ausma J, Eijsbouts S, Neuberger HR, Allessie M. Electrical and contractile remodeling during the first days of atrial fibrillation go hand in hand. Circulation. 2003;107:1433–9. doi: 10.1161/01.cir.0000055314.10801.4f. [DOI] [PubMed] [Google Scholar]
- 16.Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, av node disease, and thromboembolic complications. J Cardiovasc Electrophysiol. 2012;23:797–9. doi: 10.1111/j.1540-8167.2012.02341.x. [DOI] [PubMed] [Google Scholar]
- 17.Kottkamp H. Atrial fibrillation substrate: the “unknown species”—from lone atrial fibrillation to fibrotic atrial cardiomyopathy. Heart Rhythm. 2012;9:481–2. doi: 10.1016/j.hrthm.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013;34:2731–8. doi: 10.1093/eurheartj/eht194. [DOI] [PubMed] [Google Scholar]
- 19.Khurram IM, Beinart R, Zipunnikov V, et al. Validation of image intensity ratio, a novel magnetic resonance-based metric to facilitate standardized inter-patient comparison of left atrial fibrosis. Heart Rhythm. 2013;10:S124–S5. [Google Scholar]
- 20.Boldt A, Wetzel U, Lauschke J, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–5. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225–32. doi: 10.1016/j.jacc.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 22.Qian Y, Meng J, Tang H, et al. Different structural remodelling in atrial fibrillation with different types of mitral valvular diseases. Europace. 2010;12:371–7. doi: 10.1093/europace/eup438. [DOI] [PubMed] [Google Scholar]
- 23.Akoum N, Fernandez G, Wilson B, McGann C, Kholmovski E, Marrouche N. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patient with atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:1104–9. doi: 10.1111/jce.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Individual patient characteristics.