Abstract
Cardiovascular disease (CVD) contributes to excess long-term mortality after liver transplantation (LT), however little is known about early post-operative CVD mortality in the current era. In addition, there is no model to predict early post-operative CVD mortality across centers. We analyzed adult recipients of primary LT in the Organ Procurement and Transplantation Network (OPTN) database between February 2002 and December 2012 to assess prevalence and predictors of early (30-day) CVD mortality, defined as death from arrhythmia, heart failure, myocardial infarction, cardiac arrest, thromboembolism, and/or stroke. We performed logistic regression with stepwise selection to develop a predictive model of early CVD mortality. Sex and center volume were forced into the final model, which was validated using bootstrapping techniques. Among 54,697 LT recipients, there were 1576 (2.9%) deaths within 30 days. CVD death was the leading cause of 30-day mortality (42.1%), followed by infection (27.9%) and graft failure (12.2%). In multivariate analysis, 9 (6 recipient, 2 donor, 1 operative) significant covariates were identified: age, pre-operative hospitalization, ICU and ventilator status, calculated MELD score, portal vein thrombosis, national organ sharing, donor BMI and cold ischemia time. The model showed moderate discrimination (c-statistic 0.66, 95% CI: 0.63–0.68). We provide the first multicenter prognostic model for the prediction of early post-LT CVD death, the most common cause of early post-LT mortality in the current transplant era. However, evaluation of additional CVD-related variables not collected by the OPTN are needed in order to improve model accuracy and potential clinical utility.
Keywords: risk assessment, MELD score, outcomes, heart disease, OPTN
INTRODUCTION
Cardiovascular disease (CVD), as defined by the American Heart Association(1) and includes ischemic heart disease, stroke, heart failure and thromboembolism, is a leading cause of long-term complications following liver transplantation (LT)(2). However, it is unknown what role CVD plays in early post-transplant mortality, or whether certain features may predict early CVD mortality. In addition, little is known regarding the range of CVD complications that may occur following LT apart from those related to ischemic heart disease. The specific cardiovascular and hemodynamic responses that occur in end-stage liver disease unrelated to traditional coronary risk factors may contribute to increased CVD complications post-transplant(3, 4). Finally, there are liver donor and surgical characteristics, such as donor age or cold ischemic time, which are known to increase the early mortality of the recipient(5). Given the limited availability of donor organs, recipient selection and appropriate monitoring for and prevention of early CVD mortality is of paramount importance(6). Historically, prevalent CVD has been considered a relative, and under certain circumstances absolute, contraindication to LT with estimated post-transplant mortality rates as high as 50%(7). Thus, this study aims to better define early CVD-related mortality and aid in recipient selection and recipient-donor matching in the most recent era of LT.
Among the instruments for clinical risk stratification are decision tools such as the Revised Cardiac Risk Index (RCRI)(8), which uses six variables (history of ischemic heart disease, heart failure, stroke, insulin-dependent diabetes, chronic kidney disease and high risk surgery) to predict cardiac complications after non-cardiac surgery. In addition, both noninvasive (dobutamine stress echocardiography) and invasive (coronary angiography) cardiac tests may be used preoperatively to assist in risk stratification. However, these risk algorithms and tests have poor discriminative ability to predict early cardiac mortality post-LT(9, 10). Current guidelines for preoperative cardiac evaluations before noncardiac surgery are based on studies of patient cohorts not undergoing liver transplantation, so the optimal preoperative cardiac evaluation for liver transplant patients remains unknown(9, 11, 12). Although many studies have reported on the increased incidence of long-term CVD complications following transplantation, few have focused on predictors of early CVD outcomes(13, 14). In addition, despite the high prevalence of cirrhotic cardiomyopathy, studies on early CVD outcomes after LT have predominantly focused on complications of ischemic heart disease(7, 14). Finally, attempts at describing LT-specific CVD risk predictors have been inconsistent in their findings, are limited by single center data with inherent variability in candidate selection, and often predate recent advances in surgical technique and anesthesia (2, 14–16).
Using a large multi-center national database, we hypothesized that there are unique characteristics in end-stage liver disease, and LT-specific risk factors that are associated with excess early CVD mortality. In the long term, incorporation of these factors into validated risk algorithms may improve patient outcomes and organ utilization.
PATIENTS AND METHODS
Study Population
Adults (age ≥ 18 years) who were listed for LT between February 1, 2002 and December 31, 2012 and who underwent transplantation within the same time period were identified from the Organ Procurement Transplantation Network (OPTN) Standard Transplant Analysis and Research files (created on March 15, 2013, n=56,914). Those listed as status 1 and those who underwent re-transplantation or who received simultaneous heart, lung, intestine or pancreas transplants were excluded (n= 2,217). The Institutional Review Board of Northwestern University approved the study.
Definitions and Outcomes
Recipient cause of death was determined by a physician’s review (L.B.V.) of primary and contributory causes of death (including all free text inputs) listed in the OPTN database. Any potential case with death due to CVD, defined as primary cause of death from arrhythmia, heart failure, myocardial infarction, primary cardiac arrest, thromboembolism, and/or stroke, was then manually reviewed by an independent panel of three physicians (2 cardiologists, 1 surgeon) in order to attempt to adjudicate CVD case mortality. The primary study outcome was early (30-day) CVD mortality. This standardized time period was chosen due to its use as a outcomes-based quality indicator(17). The time period also allows a fair assessment of transplant outcomes across centers and minimizes differences in variations in length of post-transplant stay from affecting the measurement. Since some operative factors, such as electrolyte flux, are not captured within the OPTN dataset and may have a differential effect on cardiac events, analyses were also categorized into perioperative CVD mortality, defined as CVD mortality within the first 24 hours of transplant, and early postoperative CVD mortality, defined as CVD mortality occurring between 1 and 30 days. Secondary outcomes included overall patient and graft survival. Patients were censored at time of death, date of last follow up, time of re-transplantation or at 30 days.
Potential risk factors for CVD-related mortality after LT were examined based on a priori clinical hypotheses. Covariates included known traditional CVD risk factors (e.g. diabetes status) as well as transplant-specific critical illness indicators known to contribute to competing mortality risk. Recipient risk factors evaluated included age at transplant, sex, race/ethnicity (Black, non-Hispanic White, Asian and Hispanic), socioeconomic status, BMI, etiology of liver disease (including diseases known to increase CVD risk, such as nonalcoholic steatohepatitis (NASH) and hepatitis C), history of comorbid CVD conditions (diabetes, angina, cerebrovascular disease, hypertension, pulmonary embolism, peripheral vascular disease, renal failure), laboratory values at time of transplant (creatinine, albumin, sodium, INR, alanine aminotransferase (ALT), bilirubin), hepatocellular carcinoma (HCC), calculated model for end-stage liver disease (MELD) score at the time of transplant, waitlist time, functional capacity prior to transplant, complications of end-stage liver disease (ascites, encephalopathy, portal vein thrombosis (PVT), etc.), hospitalization and ventilator status at transplant. Donor risk factors included age, gender, race, BMI, cause of death, donor type (living, deceased, donation after cardiac death), donor risk index, procurement medications (inotropic support, vasopressin, antihypertensives, steroids, thyroid replacement, desmopressin), history of CVD comorbidity (diabetes, hypertension, renal disease, myocardial infarct), and health behaviors (smoking status, alcohol/cocaine/other drug use). Transplant related variables included transplant center location and volume, region, organ allocation type, cold ischemia time (CIT), steroid induction, and use of a calcineurin inhibitor.
Statistical Methods
Clinical characteristics and causes of death of primary LT recipients from the 2002–2012 OPTN dataset were described using frequency counts and percentages for categorical variables and means ± standard deviations for continuous variables. Logistic regression models were first fitted for each variable separately to determine associations with 30-day CVD mortality following LT as the dependent variable. Odds ratios with 95% confidence intervals, as well as their corresponding p-values, are shown. Twenty-two candidate variables that were significant in univariate analysis were entered into a multivariable logistic regression model. Stepwise regression was performed with entry and exit criteria set to p=0.05. Nine covariates were selected for the final model based on significance and additive contribution to the model. Further covariates did not improve model fit. It was determined a priori that center and recipient sex should be forced into the final model. The performance of the logistic regression model was then internally validated using 1000 bootstrap resamples. Bootstrapping is a nonparametric method for assigning measures of accuracy to sample estimates. Bootstrapping essentially resamples (multiple times) from the study population to approximate how precise statistical estimates (e.g. C statistic, confidence intervals) are related to the true population of interest. Kaplan-Meier analysis with log-rank test assessed time to CVD mortality, and all-cause graft and patient survival. All analysis was performed using SAS 9.3 (SAS institute, Cary, NC).
RESULTS
Patient Characteristics
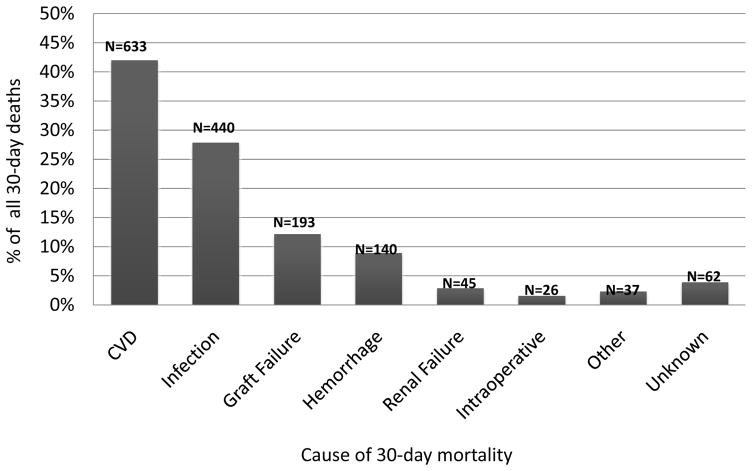
The baseline characteristics of 54,697 orthotopic liver transplant recipients who were included in the final analysis are shown in Table 1. Mean age of the study sample was 54.0 ± 9.5 years, 31% were female, and 73% were non-Hispanic White. All-cause early mortality was 2.9% (n=1576). CVD accounted for 42.1% of all deaths within 30 days, followed by infection (27.9%) and graft failure (12.2%, Figure 1). Mean time to early CVD death was 6.2 ± 8.2 days, with a median of 2.0 days (range 0–30 days). The leading underlying cause of early CVD mortality was cardiac arrest (47.8%), followed by stroke (12.5%), heart failure (12.3%) and pulmonary embolism (9.1%). The prevalence of cardiac arrest as an underlying cause of CVD mortality did not differ significantly between those deaths that occurred perioperatively compared to those that occurred in the early postoperative period (46 vs. 54%, p=0.08). Since a significant proportion of events were coded as cardiac arrest, we also examined secondary causes of death listed in OPTN, which may have significantly contributed to the cardiac arrest (Table 2). There were no significant differences in the secondary causes of death in those recipients with cardiac arrest versus other causes of early CVD mortality in the 25% of recipients who had a secondary cause of death listed.
Table 1.
Clinical characteristics of first orthotopic liver transplant recipients in the Organ Procurement and Transplantation Network Database (OPTN) 2002–2012
| Characteristic | N = 54,697 |
|---|---|
| Age, mean ± SD, years | 54.0 ± 9.5 |
| Sex (women), No (%) | 17,198 (31.4) |
| Race & ethnicity, No (%) | |
| Non-Hispanic White | 39,747 (72.7) |
| Black | 4820 (8.8) |
| Hispanic | 7165 (13.1) |
| Asian | 2435 (4.5) |
| Other | 530 (0.97) |
| Socioeconomic Status, No (%) | |
| Less than high school education | 2405 (4.4) |
| Working for income at time of transplant | 11,463 (21.0) |
| Etiology of Liver Disease, No (%) | |
| Hepatitis C | 15,529 (33.5) |
| Alcohol | 11,220 (24.2) |
| NASH | 3085 (6.7) |
| Other | 16,545 (30.2) |
| Calculated MELD score at transplant, mean ± SD | 19.0 ± 10.4 |
| Waitlist time, mean ± SD, days | 273.6 ± 493.5 |
| Hepatocellular Carcinoma, No (%) | 12,694 (23.2) |
| Simultaneous liver-kidney transplant, No (%) | 3206 (5.9) |
| BMI (kg/m2) at transplant, mean ± SD | 28.2 ± 5.6 |
| Pretransplant Comorbid CVD Conditions, No (%) | |
| Angina | 790 (3.4) |
| Cerebrovascular Disease | 137 (0.6) |
| Diabetes | 13902 (25.4) |
| Hypertension | 4399 (19.6) |
| Functional status at transplant, No (%) | |
| Independent | 24411 (49.7) |
| Partially dependent | 12229 (24.9) |
| Totally dependent | 12528 (25.5) |
| Hospitalization status at transplant, No (%) | |
| Not hospitalized | 41090 (75.2) |
| Hospitalized not in ICU | 9032 (16.5) |
| In ICU | 4489 (8.2) |
| Revised Cardiac Risk Index (RCRI), mean | 1.79 ± 1.21 |
Abbreviations: SD, standard deviation; NASH, nonalcoholic steatohepatitis; MELD, model for end-stage liver disease; BMI, body mass index; ESLD, end-stage liver disease; CVD, cardiovascular disease; RRT, renal replacement therapy; ICU, intensive care unit; TIPS, transjugular intrahepatic portosystemic shunt
Figure 1.
Distribution of cause of death for 1,576 adult first liver transplant recipients who died within 30 days of liver transplantation (OPTN data 2002–2012). CVD=cardiovascular disease
Table 2.
Secondary causes of death in those patients who died of cardiac arrest within 30 days of liver transplantation
| Secondary cause of CVD-related mortality | All early CVD deaths N=633 | All cardiac arrests N=303 | Perioperative cardiac arrest N=140 (46%) | Postoperative cardiac arrest N=163 (54%) |
|---|---|---|---|---|
| Infectious/Sepsis | 46 (7.3) | 20 (6.6) | 3 (2.1) | 17 (10.4) |
| Graft Failure | 30 (4.7) | 16 (5.3) | 4 (2.9) | 12 (7.4) |
| Hemorrhage | 32 (5.1) | 19 (6.3) | 9 (6.4) | 10 (6.1) |
| Operative | 14 (2.2) | 7 (2.3) | 5 (3.6) | 2 (1.2) |
| Renal Failure | 18 (2.8) | 11 (3.6) | 3 (2.1) | 8 (4.9) |
| Unknown/None | 475 (75.0) | 225 (74.3) | 113 (80.7) | 112 (68.7) |
| Other* | 18 (2.8) | 5 (1.7) | 3 (2.1) | 2 (1.2) |
Predictors of Early Cardiovascular Disease Mortality
Univariate predictors of early CVD mortality are shown in Table 3. Recipients who died from CVD within 30-days of LT were slightly older (55.3 ± 9.7 vs. 54.0 ± 9.5 years), with higher calculated MELD scores (22.8 vs. 19.0) and a higher prevalence of medical comorbidity (e.g. renal and pulmonary disease) than those without early CVD mortality (p<0.05 for all). There were no statistically significant differences in recipient race, ethnicity, sex, or pre-transplant cerebrovascular disease between those with either perioperative or early postoperative CVD mortality and those recipients without early CVD mortality. However, older age, presence of NASH (vs. hepatitis C), and pre-transplant recipient diabetes, hypertension, or chronic obstructive pulmonary disease (COPD) were all more prevalent in recipients with perioperative CVD mortality compared to those without early CVD mortality (p<0.05 for all). Donor factors related to early perioperative CVD mortality included higher BMI and greater likelihood of deceased donor donation (versus living donor) and donation after cardiac death (p<0.05 for all). These recipient and donor factors did not appear to affect early postoperative outcomes (Table 3). Operative factors included increased cold ischemia time (CIT) where the odds of overall early CVD mortality was 1.04 (95% CI 1.02–1.06) higher for every 1-hour increase in CIT (p<0.001). We also examined univariate predictors of cardiac arrest compared to other causes of early CVD mortality (Supplemental Table). Older age and a higher prevalence of pre-transplant diabetes and COPD were seen in recipients with cardiac arrest compared to those without early CVD mortality (p<0.05 for all). These factors did not predict non-cardiac arrest early CVD mortality (Supplemental Table). No other significant univariate differences were seen.
Table 3.
Univariate predictors of perioperative (within 24 hours) and early postoperative (1–30 days) mortality following orthotopic liver transplantation
| Characteristic | No CVD Mortality (N = 54,064) | Any early CVD mortality (N=633) | Perioperative CVD mortality (N=235) |
Early postoperative CVD mortality (N=398) |
||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR (95% CI)* | P value | OR (95% CI)* | P value | |||||
|
| ||||||||
| Recipient Age, mean ± SD, years | 54.0 ± 9.5 | 55.3 ± 9.7 | 56.2 ± 9.7 | 1.03 (1.01, 1.04) | <0.001 | 54.7 ± 9.7 | 1.01 (0.99, 1.02) | 0.134 |
|
| ||||||||
| Recipient Sex (women), No (%) | 16977 (31.4) | 221 (34.9) | 87 (37.0) | 1.28 (0.99, 1.68) | 0.065 | 134 (33.7) | 1.11 (0.90, 1.37) | 0.332 |
|
| ||||||||
| Recipient Race & ethnicity, No (%) | ||||||||
|
| ||||||||
| Non-Hispanic White | 39292 (72.7) | 455 (71.9) | 174 (74.0) | Reference | Reference | 281 (70.6) | Reference | Reference |
| Black | 4767 (8.8) | 53 (8.4) | 21 (8.9) | 0.99 (0.63, 1.57) | 0.982 | 32 (8.0) | 0.94 (0.65, 1.36) | 0.735 |
| Hispanic | 7079 (13.1) | 86 (13.6) | 28 (11.9) | 0.89 (0.60, 1.33) | 0.580 | 58 (14.6) | 1.15 (0.86, 1.52) | 0.348 |
| Asian | 2401 (4.4) | 34 (5.4) | 12 (5.1) | 1.13 (0.63, 2.03) | 0.686 | 22 (5.5) | 1.28 (0.83, 1.98) | 0.264 |
| Other | 525 (1.0) | 5 (0.8) | 0 (0.0) | n/a | n/a | 5 (1.3) | 1.33 (0.55, 3.24) | 0.526 |
|
| ||||||||
| Recipient Socioeconomic Status, No (%) | ||||||||
| Less than high school education | 2369 (5.4) | 36 (7.0) | 9 (3.8) | 0.87 (0.45, 1.69) | 0.680 | 27 (6.8) | 1.59 (1.07, 2.35) | 0.021 |
| Working for income | 11391 (26.1) | 72 (13.7) | 28 (14.5) | 0.48 (0.32,0.72) | <0.001 | 44 (13.3) | 0.43 (0.32, 0.60) | <0.001 |
|
| ||||||||
| Etiology of Liver Disease, No (%) | ||||||||
| Hepatitis C | 15375 (33.6) | 154 (27.8) | 47 (23.3) | Reference | Reference | 107 (30.4) | Reference | Reference |
| Alcohol | 11092 (24.2) | 128 (23.1) | 42 (20.8) | 1.24 (0.82, 1.88) | 0.314 | 86 (24.4) | 1.14 (0.84, 1.48) | 0.457 |
| NASH | 3046 (6.7) | 39 (7.0) | 20 (9.9) | 2.15 (1.27, 3.63) | 0.004 | 19 (5.4) | 0.90 (0.55, 1.46) | 0.662 |
| Others | 16312 (35.6) | 233 (42.1) | 93 (46.0) | 1.87 (1.31, 2.65) | <0.001 | 140 (39.8) | 1.23 (0.96, 1.59) | 0.104 |
|
| ||||||||
| Calculated MELD score at transplant, mean | 19.0 ± 10.4 | 22.8 ± 11.5 | 22.3 ± 11.9 | 1.03 (1.02, 1.04) | <0.001 | 23.1 ± 11.2 | 1.04 (1.03, 1.04) | <0.001 |
|
| ||||||||
| Waitlist time, mean, days | 273.4 ± 492.8 | 288.5 ± 548.8 | 342.7 ± 637.8 | 1.00 (1.00, 1.00) | 0.032 | 256.6 ± 486.7 | 1.00 (1.00, 1.00) | 0.498 |
|
| ||||||||
| Organ Allocation Type, No (%) | ||||||||
| Local | 41223 (76.3) | 456 (72.0) | 157 (66.8) | Reference | Reference | 299 (75.1) | Reference | Reference |
| Regional | 10001 (18.5) | 127 (20.1) | 54 (23.0) | 1.42 (1.04, 1.93) | 0.027 | 73 (18.3) | 1.02 (0.78, 1.30) | 0.962 |
| National | 2840 (5.3) | 50 (7.9) | 24 (10.2) | 2.20 (1.44, 3.42) | <0.001 | 26 (6.5) | 1.26 (0.84, 1.89) | 0.256 |
|
| ||||||||
| Hepatocellular Carcinoma, No (%) | 12584 (23.3) | 110 (17.4) | 48 (20.4) | 0.85 (0.62, 1.16) | 0.303 | 62 (15.6) | 0.61 (0.46, 0.80) | <0.001 |
|
| ||||||||
| Simultaneous liver-kidney transplant, No (%) | 3180 (5.9) | 26 (4.1) | 0 (0.0) | n/a | n/a | 26 (6.5) | 1.12 (0.75, 1.67) | 0.583 |
|
| ||||||||
| Recipient BMI (kg/m2) at transplant, mean | 28.2 ± 5.6 | 28.5 ± 5.8 | 28.9 ± 5.8 | 1.02 (0.99, 1.04) | 0.081 | 28.4 ± 5.7 | 1.00 (0.99, 1.02) | 0.665 |
|
| ||||||||
| Laboratory Values at transplant, mean ± SD | ||||||||
| Creatinine (mg/dL) | 1.51 ± 1.33 | 1.87 ± 1.49 | 1.90 ± 1.44 | 1.15 (1.08, 1.23) | <0.001 | 1.85 ±1.51 | 1.14 (1.08, 1.20) | <0.001 |
| Sodium (mEq/L) | 135.9 ± 5.1 | 136.3 ± 5.8 | 136.0 ± 5.8 | 1.00 (0.98, 1.03) | 0.784 | 136.6 ± 5.8 | 1.03 (1.00, 1.05) | 0.022 |
|
| ||||||||
| Recipient Complications of ESLD, No (%) | ||||||||
| Ascites at transplant | 42289 (79.1) | 530 (84.4) | 197 (84.2) | 1.40 (0.99, 2.00) | 0.059 | 333 (84.5) | 1.44 (1.10, 1.89) | 0.009 |
| Spontaneous Bacterial Peritonitis | 1779 (8.0) | 34 (11.6) | 15 (13.8) | 1.84 (1.07, 3.18) | 0.029 | 19 (10.4) | 1.34 (0.83, 2.16) | 0.233 |
| Encephalopathy | 36219 (67.5) | 485 (77.0) | 177 (75.6) | 1.49 (1.11, 2.01) | 0.009 | 308 (77.8) | 1.68 (1.33, 2.14) | <0.001 |
| Portal Vein Thrombosis | 4117 (7.7) | 78 (12.5) | 34 (14.8) | 2.08 (1.44, 2.99) | <0.001 | 44 (11.2) | 1.50 (1.10, 2.06) | 0.011 |
| TIPS | 5576 (10.5) | 87 (14.0) | 36 (15.7) | 1.60 (1.12, 2.28) | 0.010 | 51 (12.9) | 1.27 (0.95, 1.71) | 0.108 |
| Variceal Bleed | 1300 (5.9) | 32 (10.9) | 11 (9.8) | 1.73 (0.93, 3.24) | 0.085 | 21 (11.6) | 2.09 (1.32, 3.30) | 0.002 |
|
| ||||||||
| Recipient Comorbid CVD Conditions, No (%) | ||||||||
| Angina | 768 (3.3) | 22 (7.1) | 10 (8.3) | 2.62 (1.37, 5.03) | 0.004 | 12 (6.3) | 1.95 (1.08, 3.52) | 0.026 |
| Cerebrovascular Disease | 135 (0.6) | 2 (0.7) | 1 (0.9) | 1.47 (0.20, 10.62) | 0.701 | 1 (0.5) | 0.88 (0.12, 6.33) | 0.899 |
| Diabetes | 13727 (25.4) | 175 (27.7) | 75 (31.9) | 1.38 (1.05, 1.81) | 0.022 | 100 (25.1) | 0.99 (0.79, 1.24) | 0.902 |
| Hypertension | 4339 (19.6) | 60 (20.3) | 32 (28.3) | 1.63 (1.08, 2.45) | 0.021 | 28 (15.3) | 0.74 (0.50,1.13) | 0.150 |
|
| ||||||||
| Other Recipient Comorbid Conditions | ||||||||
| Chronic Obstructive Pulmonary Disease | 329 (1.5) | 11 (3.7) | 5 (4.5) | 3.14 (1.27, 7.75) | 0.013 | 6 (3.2) | 2.22 (0.98, 5.04) | 0.057 |
| Respiratory Failure on ventilator | 2152 (4.0) | 90 (14.2) | 24 (10.2) | 2.74 (1.80, 4.20) | <0.001 | 66 (16.6) | 4.80 (3.67, 6.27) | <0.001 |
| Renal failure requiring RRT | 5530 (10.2) | 126 (19.9) | 40 (17.0) | 1.80 (1.28, 2.53) | <0.001 | 86 (21.6) | 2.42 (1.90, 3.08) | <0.001 |
|
| ||||||||
| Functional status at transplant, No (%) | ||||||||
| Independent | 24235 (49.8) | 176 (32.5) | 68 (34.5) | Reference | Reference | 108 (31.4) | Reference | Reference |
| Partially dependent | 12111 (24.9) | 118 (21.8) | 44 (22.3) | 1.30 (0.89, 1.89) | 0.183 | 74 (21.5) | 1.37 (1.02, 1.84) | 0.037 |
| Totally dependent | 12281 (25.3) | 247 (45.7) | 85 (43.2) | 2.47 (1.79, 3.40) | <0.001 | 162 (47.1) | 2.96 (2.32, 3.78) | <0.001 |
|
| ||||||||
| Hospitalization Status at transplant, No (%) | ||||||||
| Not hospitalized | 40726 (75.5) | 364 (57.5) | 140 (59.6) | Reference | Reference | 224 (56.3) | Reference | Reference |
| Hospitalized not in ICU | 8900 (16.5) | 132 (20.9) | 49 (20.8) | 1.60 (1.16, 2.22) | 0.005 | 83 (20.8) | 1.70 (1.32, 2.18) | <0.001 |
| In ICU | 4352 (8.1) | 137 (21.6) | 46 (19.6) | 3.08 (2.20, 4.30) | <0.001 | 91 (22.9) | 3.80 (2.97, 4.86) | <0.001 |
|
| ||||||||
| Revised Cardiac Risk Index (RCRI), mean | 1.79 ± 1.21 | 2.13 ± 1.38 | 2.21 ± 1.45 | 1.25 (1.15, 1.36) | <0.001 | 2.08 ± 1.33 | 1.18 (1.10, 1.26) | <0.001 |
|
| ||||||||
| Donor Factors | ||||||||
| Age, mean, years | 41.4 ± 16.9 | 41.8 ± 15.8 | 43.5 ± 14.7 | 1.01 (1.00, 1.02) | 0.064 | 40.8 ± 16.3 | 0.99 (0.99, 1.00) | 0.479 |
| Sex (female), No (%) | 21988 (40.7) | 258 (40.8) | 99 (42.1) | 1.06 (0.82, 1.38) | 0.650 | 159 (40.0) | 0.97 (0.79, 1.19) | 0.771 |
| Ethnicity, No (%) | ||||||||
| Non-Hispanic White | 37091 (68.6) | 442 (69.8) | 189 (80.4) | Reference | Reference | 253 (63.6) | Reference | Reference |
| Black | 8449 (15.6) | 71 (11.2) | 19 (8.1) | 0.44 (0.28, 0.71) | <0.001 | 52 (13.1) | 0.90 (0.67, 1.22) | 0.501 |
| Hispanic | 6590 (12.2) | 91 (14.4) | 21 (8.9) | 0.63 (0.40, 0.98) | 0.042 | 70 (17.6) | 1.56 (1.19, 2.03) | 0.001 |
| Donor Risk Index, mean | 1.33 ± 0.35 | 1.34 ± 0.34 | 1.38 ± 0.35 | 1.48 (1.00, 2.18) | 0.050 | 1.32 ± 0.33 | 0.87 (0.64, 1.18) | 0.365 |
| Donor BMI (kg/m2), mean | 26.9 ± 6.1 | 27.6 ± 6.3 | 29.1 ± 6.5 | 1.03 (1.01, 1.04) | <0.001 | 26.7 ± 6.0 | 0.99 (0.98, 1.01) | 0.491 |
| Donation after Cardiac Death, No (%) | 5422 (10.0) | 72 (11.4) | 37 (15.7) | 1.68 (1.18, 2.38) | 0.004 | 35 (8.8) | 0.87 (0.61, 1.23) | 0.414 |
| Living Donor, No (%) | 2344 (4.3) | 17 (2.7) | 0 (0.0) | n/a | n/a | 17 (4.3) | 0.99 (0.61, 1.60) | 0.950 |
|
| ||||||||
| Donor Medications within 24 hours of procurement, No (%) | ||||||||
| Inotropic support | 29361 (58.8) | 325 (54.2) | 121 (53.3) | 0.80 (0.62, 1.04) | 0.097 | 204 (54.7) | 0.85 (0.69, 1.04) | 0.113 |
| Antihypertensives | 11024 (21.4) | 135 (21.9) | 48 (20.4) | 0.95 (0.69, 1.30) | 0.730 | 87 (22.8) | 1.09 (0.86, 1.39) | 0.482 |
| Steroids | 38287 (74.9) | 451 (73.9) | 175 (75.1) | 1.01 (0.75, 1.36) | 0.948 | 276 (73.2) | 0.92 (0.73, 1.15) | 0.445 |
| Thyroid replacement | 28369 (55.5) | 306 (50.0) | 122 (52.1) | 0.88 (0.68, 1.13) | 0.309 | 184 (48.7) | 0.76 (0.62, 0.93) | 0.009 |
|
| ||||||||
| Donor Comorbidities | ||||||||
| Diabetes | 5268 (10.2) | 63 (10.3) | 32 (13.6) | 1.38 (0.95, 2.01) | 0.089 | 31 (8.2) | 0.78 (0.54, 1.13) | 0.190 |
| Hypertension | 17295 (33.7) | 222 (36.2) | 97 (41.5) | 1.40 (1.08,1.81) | 0.012 | 125 (33.0) | 0.97 (0.78, 1.20) | 0.781 |
| Myocardial Infarct | 1808 (3.9) | 23 (4.2) | 10 (4.8) | 1.23 (0.65, 2.33) | 0.524 | 13 (3.8) | 0.97 (0.56, 1.69) | 0.916 |
| Renal Disease | 22539 (44.1) | 293 (47.8) | 121 (51.7) | 1.36 (1.05, 1.76) | 0.020 | 172 (45.4) | 1.05 (0.86, 1.29) | 0.615 |
|
| ||||||||
| Donor Health Behaviors | ||||||||
| Smoker | 15823 (30.1) | 195 (31.6) | 75 (32.2) | 1.11 (0.84, 1.46) | 0.479 | 120 (31.3) | 1.06 (0.85, 1.31) | 0.611 |
| Alcohol dependency | 1887 (19.6) | 18 (14.2) | 6 (13.0) | 0.62 (0.26, 1.46) | 0.271 | 12 (14.8) | 0.72 (0.39, 1.32) | 0.287 |
| Cocaine | 6828 (13.4) | 72 (12.0) | 26 (11.3) | 0.82 (0.55, 1.24) | 0.345 | 46 (12.4) | 0.91 (0.67, 1.24) | 0.547 |
|
| ||||||||
| Graft cold ischemia time, mean, min | 7.0 ± 3.6 | 7.6 ± 3.4 | 8.3 ± 3.3 | 1.06 (1.03, 1.09) | <0.001 | 7.3 ± 3.3 | 1.02 (0.99, 1.04) | 0.186 |
Abbreviations: SD, standard deviation; NASH, nonalcoholic steatohepatitis; MELD, model for end-stage liver disease; BMI, body mass index; ESLD, end-stage liver disease; CVD, cardiovascular disease; RRT, renal replacement therapy; ICU, intensive care unit; TIPS, transjugular intrahepatic portosystemic shunt
compared to no CVD mortality
Derivation and validation of a liver transplant-specific risk model to predict early CVD mortality
Since both age and sex are known strong predictors of CVD we initially examined the incidence of early CVD-mortality for both males and females using age-adjusted univariate analysis. There was no significant difference in early CVD mortality by sex (data not shown), thus a combined age- and sex-adjusted model is shown. Nine (6 recipient, 2 donor, 1 operative) significant predictors of early CVD mortality were identified: age, hospitalization status, ICU status, respiratory failure on a ventilator, MELD score, history of PVT, national organ sharing (versus local/regional), donor BMI and CIT. Sex and transplant center volume were forced into the final model (Table 4). The model showed moderate discrimination (c-statistic 0.66, 95% CI: 0.63–0.68, after bootstrapping, Table 4). There was no significant interaction between transplant region and predicted risk in our model (p=0.56) with regard to CVD mortality, and no significant variation in early CVD events across regions (Table 5). In separate sensitivity analyses, we excluded cardiac arrest, thromboembolism, pulmonary hypertension, stroke or heart failure sequentially from the definition of CVD mortality. The covariates selected for inclusion and fit of the models for prediction of CVD mortality were similar to the main analyses presented above (c-statistic=0.66, 95% CI: 0.64–0.67 for cardiac arrest versus c-statistic=0.65, 95% CI: 0.63–0.68 for non-cardiac arrest CVD mortality). There were also no significant differences noted in the frequency of the underlying cause of CVD mortality or in the covariates selected for inclusion into the prediction models when further separated into perioperative and early postoperative deaths (Table 3). However, model fit was slightly better for perioperative deaths (c-statistic 0.69, 95% CI: 0.66–0.70) than for early postoperative deaths (c-statistic 0.63, 95% CI: 0.62–0.66).
Table 4.
Multivariate predictors of 30-day cardiovascular mortality after orthotopic liver transplantation
| Characteristic | Total Population | 30-day cardiovascular mortality | ||
|---|---|---|---|---|
| N=54,697 | Beta Coefficient | Odds Ratio(95% CI) | P-value | |
| Age, year, mean ± SD | 54.0 ± 9.5 | 0.017 | 1.02 (1.10–1.03) | 0.001 |
| Female (forced in), n (%) | 17,198 (31.4) | 0.184 | 1.20 (1.00–1.45) | 0.052 |
| Medical condition prior to transplant, n (%) | ||||
| Not hospitalized | 41,090 (75.2) | Reference | ||
| Hospitalized not in ICU | 9032 (16.5) | 0.208 | 1.23 (0.95–1.60) | 0.121 |
| In ICU | 4489 (8.2) | 0.623 | 1.86 (1.34–2.60) | <0.001 |
| Respiratory failure, n (%) | 2242 (4.1) | 0.719 | 2.05 (1.46–2.89) | <0.001 |
| MELD score (calculated), mean ± SD | 19.0 ± 10.4 | 0.018 | 1.02 (1.01–1.03) | <0.001 |
| National Organ Allocation (versus Local/Regional), n (%) | 2890 (5.3) | 0.419 | 1.52 (1.08–2.14) | 0.016 |
| Portal vein thrombosis, n (%) | 4195 (7.8) | 0.397 | 1.49 (1.14–1.95) | 0.004 |
| Donor BMI, mean ± SD | 28.2 ± 5.6 | 0.013 | 1.01 (1.00–1.02) | 0.024 |
| Cold ischemia time, hours, mean ± SD | 0.12 ± 0.6 | 0.032 | 1.03 (1.01–1.05) | 0.001 |
| Center volume (forced in) | ||||
| Tertile 1 | n/a | Reference | ||
| Tertile 2 | n/a | 0.176 | 0.84 (0.55–1.27) | 0.404 |
| Tertile 3 | n/a | 0.390 | 0.68 (0.46–1.01) | 0.055 |
Abbreviations: SD, standard deviation, CI, confidence interval; ICU, intensive care unit; BMI, body mass index
Table 5.
Comparison of prevalence of causes of early cardiovascular disease mortality across transplant regions
| Region | Median MELD score | Composite CVD mortality | Cardiac Arrest | Stroke | Thromboembolism | Myocardial Infarction | Heart Failure | Arrhythmia | Other* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17.3 | 1.34% | 0.40% | 0.15% | 0.25% | 0.25% | 0.25% | 0.05% | 0.00% |
| 2 | 17.9 | 1.07% | 0.52% | 0.11% | 0.11% | 0.09% | 0.12% | 0.09% | 0.03% |
| 3 | 18.0 | 0.88% | 0.37% | 0.11% | 0.08% | 0.08% | 0.09% | 0.08% | 0.07% |
| 4 | 18.2 | 0.99% | 0.39% | 0.20% | 0.14% | 0.10% | 0.02% | 0.04% | 0.10% |
| 5 | 19.9 | 1.39% | 0.77% | 0.21% | 0.12% | 0.05% | 0.21% | 0.03% | 0.00% |
| 6 | 16.2 | 1.10% | 0.49% | 0.24% | 0.12% | 0.00% | 0.24% | 0.00% | 0.00% |
| 7 | 19.1 | 1.25% | 0.45% | 0.21% | 0.16% | 0.18% | 0.12% | 0.08% | 0.06% |
| 8 | 18.8 | 1.10% | 0.47% | 0.14% | 0.08% | 0.25% | 0.17% | 0.00% | 0.00% |
| 9 | 17.1 | 1.31% | 0.88% | 0.15% | 0.00% | 0.05% | 0.20% | 0.03% | 0.00% |
| 10 | 16.1 | 1.24% | 0.61% | 0.08% | 0.16% | 0.16% | 0.08% | 0.10% | 0.04% |
| 11 | 17.6 | 1.26% | 0.68% | 0.06% | 0.09% | 0.13% | 0.23% | 0.06% | 0.02% |
Other = ruptured aneurysm, pulmonary hypertension, hypertensive crisis
Abbreviations: MELD, model for end-stage liver disease; CVD, cardiovascular disease
DISCUSSION
This study provides the first liver transplant-specific prognostic model for the prediction of early postoperative CVD mortality, with moderate model accuracy. We identified 9 (6 recipient, 2 donor, and 1 operative) significant predictors of early (30-day) postoperative CVD mortality independent of transplant center. We also observed that in the current era of liver transplantation CVD is now the leading cause of early mortality accounting for over 40% of early deaths, most of which are related to non-coronary CVD events. This highlights the fact that while we are likely appropriately excluding those at high risk of coronary complications prior to transplant, there remains a large proportion of critically ill patients with limited cardiovascular reserve who enter liver transplantation with resultant poor early outcomes.
Early cardiovascular mortality in the current era of transplantation
A preoperative CVD evaluation is undertaken in all potential LT candidates prior to transplant listing, mainly to screen for significant obstructive coronary artery disease, severe heart failure and/or severe pulmonary hypertension which are considered absolute contraindications to liver transplantation(9). Despite exclusion of these high-risk patients from transplantation, we observed a substantial early post-OLT CVD mortality rate of 1.2%. For comparison, early CVD mortality after other types of intraabdominal surgery ranges from 0.2% (laparoscopic cholecystectomy)(18) to 0.3% (Whipple)(19), and is estimated to be as high as 1.7% after coronary artery bypass grafting following acute MI(20). Small single-center studies in the initial era of LT estimated early post-LT CVD mortality anywhere from 0% to 2.7%(21–24). To our knowledge we are the first to provide a multicenter estimate that may provide more accurate and precise data on the true incidence of CVD-related death in the current era of transplantation.
As a result of the increasing average age at which patients are now being transplanted, prevalent CVD comorbidity at the time of transplantation is expected to rise(25), with adverse effects on post-transplant outcomes(26, 27) Age remained a significant predictor in our multivariate model again highlighting concerns about increased cardiac risk associated with the aging transplant recipient population.
Historically, early mortality after LT has been reported to be primarily due to infection or allograft failure and continues to remain a prevalent cause of early death globally(28, 29). However, we observed that in the present transplant era in the United States, CVD has surpassed infection and graft failure as the leading cause of death following LT. We hypothesize that this is a reflection of both improved anti-infective regimens and surgical techniques over time, and also the increasing critical illness burden, with higher median MELD scores among recipients and the high proportion of ventilator-dependent patients at the time of transplantation(30). Such patients likely have a high prevalence of subclinical cardiac disease, and a blunted cardiovascular response to the hemodynamic stress of liver transplantation. As an example, using our study population, if a LT recipient had pretransplant respiratory failure (e.g. on a ventilator) the early CVD death rate was 4% compared with 1% in those without respiratory failure. Thus, our results imply that we may need to reevaluate transplantation practices in high MELD, poor functional status, and intensive care unit-bound potential organ recipients in order to maximize the utilization and longevity of scarce donor organs.
Performance of the current risk model in the context of existing risk scores
Commonly used risk indices can be divided into liver-specific (Childs-Turcott-Pugh (CTP)(31) and MELD(32)), general (Simplified Acute Physiology Score (SAPS) II(33) and Acute Physiology and Chronic Health Evaluation (APACHE)(34)) or organ failure (Sequential Organ Failure Assessment (SOFA)(35)) scores, none of which was specifically developed to assess CVD mortality. In addition, the c-statistics range from 0.5–0.6 for pre-transplant calculation of each of the aforementioned indices to predict early post-LT outcomes, and therefore they have overall poor discriminative ability as useful tools in the pre-transplant setting (36–38). The current risk model performs somewhat more robustly (c-statistic=0.66) in a liver transplant population. However, our model is still not optimal for discrimination, and thus is more appropriate for use as a base model for the development of additional CVD-specific risk models with more refined variables than are available within the OPTN database. We note that in our model, several of the variables are related to either donor or operative factors and therefore would not be available in the pre-transplant setting to use as post-transplant predictors. However, knowledge that these factors—donor BMI, national organ sharing, CIT—collectively affect early CVD mortality may serve as a basis for future modeling aimed at maximizing donor-recipient matching in order to impact patient-centered outcomes.
Although we examined strong cardiac risk factors (e.g., diabetes mellitus, hypertension) for inclusion in the model, none of these were significantly associated with 30-day CVD mortality. Our findings are consistent with prior single-center studies that have also failed to demonstrate that traditional clinical CVD risk factors are associated with early mortality among liver transplant recipients (13, 14). However, we did find several unique predictors of CVD-mortality including history of portal vein thrombosis (PVT), higher donor BMI and higher CIT, all of which have been associated with lower overall survival post LT, though not specifically with CVD mortality (39–41). It is plausible that prior PVT may suggest an underlying hypercoagulable state or endothelial dysfunction, which have been associated with acute coronary syndrome(42). Although hemorrhage has traditionally been regarded as the most significant hemostatic complication of liver disease, there is increasing recognition that hypercoagulability is a prominent aspect of cirrhosis(43, 44). Thus, a pathophysiologic mechanism apart from traditional plaque rupture may be an underlying cause of acute coronary syndrome in a liver transplant population, possibly via de novo thrombotic occlusion(45).
Limitations
Our study has several limitations. First, the current analysis may be limited due to the lack of precise measurement of pre-operative CVD risk variables on individual recipients, such as preoperative cardiovascular testing, laboratory values, medication use, and family history of CVD, not currently available within the OPTN database. Second, transplant centers are not provided with defined criteria for recording a CVD death or comorbid cardiac condition and the OPTN database may be skewed due to reporting bias. A predominant proportion of reported CVD-related death within OPTN was coded as “cardiac arrest.” We acknowledge that there are multiple potential underlying mechanisms of cardiac arrest. However, even when cardiac arrest was removed from the definition of CVD mortality, we observed similar results in the prediction of early CVD mortality with similar model accuracy. These limitations may have led to misclassification of cause of death, which, if non-systematic, could lead to poorer model discrimination than would be found in other settings.
Identifying LT-specific CVD risk factors has important policy implications for both Medicare reimbursement and for organ allocation(46). The SRTR risk prediction models are used by the Centers for Medicare & Medicaid Service (CMS) to certify transplant centers for reimbursement. Transplant centers are required to achieve or exceed 1-year expected graft and patient survival as determined by these risk-adjusted models (47). Therefore, centers may be motivated to restrict access to higher risk patients, who might still benefit from transplantation. Despite the limitations of the OPTN database, the current study has rigorously evaluated the available national data. We provide a novel risk model to serve as a comparator for future more in-depth studies focused on determining which, if in fact any, additional CVD variables should be collected by OPTN and therefore included in the risk-adjusted Scientific Registry of Transplant Recipients (SRTR) performance measures.
Conclusions
CVD mortality is the leading cause of early post-operative deaths in the current era of liver transplantation, reflecting an aging and increasingly sicker transplant population. Future studies using large, multicenter databases are needed to validate and expand on our proposed base model in order to improve patient outcomes and maximize the benefit of scarce organ donors.
Supplementary Material
Acknowledgments
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
GRANTS AND FINANCIAL SUPPORT:
Dr. VanWagner is supported by the National Institutes of Health (1 F32 HL116151-01) and the American Liver Foundation (New York, NY).
Abbreviations (alphabetical)
- ALT
Alanine Aminotransferase
- AST
Aspartate Aminotransferase
- APACHE
Acute Physiology and Chronic Health Evaluation
- AUROC
Area under the receiver operating curve
- BMI
Body mass index
- CVD
Cardiovascular disease
- CIT
Cold ischemic time
- COPD
Chronic Obstructive Pulmonary Disease
- CTP
Childs-Turcott-Pugh
- DDLT
Deceased donor liver transplant
- HCV
Hepatitis C virus
- ICU
Intensive care unit
- LDLT
Living donor liver transplant
- LT
Liver transplantation
- MELD
Model for end-stage liver disease
- NASH
Nonalcoholic steatohepatitis
- OPTN
Organ Procurement and Transplant Network
- PVT
Portal Vein Thrombosis
- SAPS
Simplified Acute Physiology Score
- SOFA
Sequential Organ Failure Assessment
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston SD, Morris JK, Cramb R, Gunson BK, Neuberger J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73(6):901–6. doi: 10.1097/00007890-200203270-00012. [DOI] [PubMed] [Google Scholar]
- 3.Moller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87(1):9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers RP, Lee SS. Cirrhotic cardiomyopathy and liver transplantation. Liver Transpl. 2000;(4 Suppl 1):S44–52. doi: 10.1002/lt.500060510. [DOI] [PubMed] [Google Scholar]
- 5.Gaynor JJ, Moon JI, Kato T, Nishida S, Selvaggi G, Levi DM, et al. A cause-specific hazard rate analysis of prognostic factors among 877 adults who received primary orthotopic liver transplantation. Transplantation. 2007;84(2):155–65. doi: 10.1097/01.tp.0000269090.90068.0f. [DOI] [PubMed] [Google Scholar]
- 6.Lester SJ, Hurst RT. Liver transplantation: do you have the heart for it? Liver Transpl. 2006;12(4):520–2. doi: 10.1002/lt.20577. [DOI] [PubMed] [Google Scholar]
- 7.Plotkin JS, Scott VL, Pinna A, Dobsch BP, De Wolf AM, Kang Y. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg. 1996;2(6):426–30. doi: 10.1002/lt.500020604. [DOI] [PubMed] [Google Scholar]
- 8.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 9.Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, et al. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58(3):223–31. doi: 10.1016/j.jacc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Gupta PK, Gupta H, Sundaram A, Kaushik M, Fang X, Miller WJ, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381–7. doi: 10.1161/CIRCULATIONAHA.110.015701. [DOI] [PubMed] [Google Scholar]
- 11.Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, Kasiske BL, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60(5):434–80. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Palli G, Cardenas A. Pre operative cardio pulmonary assessment of the liver transplant candidate. Ann Hepatol. 2011;10(4):421–33. [PubMed] [Google Scholar]
- 13.Yong CM, Sharma M, Ochoa V, Abnousi F, Roberts J, Bass NM, et al. Multivessel coronary artery disease predicts mortality, length of stay, and pressor requirements after liver transplantation. Liver Transpl. 2010;16(11):1242–8. doi: 10.1002/lt.22152. [DOI] [PubMed] [Google Scholar]
- 14.Safadi A, Homsi M, Maskoun W, Lane KA, Singh I, Sawada SG, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009;120(13):1189–94. doi: 10.1161/CIRCULATIONAHA.108.847178. [DOI] [PubMed] [Google Scholar]
- 15.Fouad TR, Abdel-Razek WM, Burak KW, Bain VG, Lee SS. Prediction of cardiac complications after liver transplantation. Transplantation. 2009;87(5):763–70. doi: 10.1097/TP.0b013e318198d734. [DOI] [PubMed] [Google Scholar]
- 16.Coss E, Watt KD, Pedersen R, Dierkhising R, Heimbach JK, Charlton MR. Predictors of cardiovascular events after liver transplantation: a role for pretransplant serum troponin levels. Liver Transpl. 2011;17(1):23–31. doi: 10.1002/lt.22140. [DOI] [PubMed] [Google Scholar]
- 17.Krumholz HM, Normand SL. Public reporting of 30-day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118(13):1394–7. doi: 10.1161/CIRCULATIONAHA.108.804880. [DOI] [PubMed] [Google Scholar]
- 18.Rao A, Polanco A, Qiu S, Kim J, Chin EH, Divino CM, et al. Safety of Outpatient Laparoscopic Cholecystectomy in the Elderly: Analysis of 15,248 Patients Using the NSQIP Database. J Am Coll Surg. 2013;217(6):1038–43. doi: 10.1016/j.jamcollsurg.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226(3):248–57. doi: 10.1097/00000658-199709000-00004. discussion 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu YL, van der Horst IC, Douglas YL, Svilaas T, Mariani MA, Zijlstra F. Role of coronary artery bypass grafting during the acute and subacute phase of ST-elevation myocardial infarction. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2010;18(7–8):348–54. doi: 10.1007/BF03091790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donovan CL, Marcovitz PA, Punch JD, Bach DS, Brown KA, Lucey MR, et al. Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation. 1996;61(8):1180–8. doi: 10.1097/00007890-199604270-00011. [DOI] [PubMed] [Google Scholar]
- 22.Plotkin JS, Benitez RM, Kuo PC, Njoku MJ, Ridge LA, Lim JW, et al. Dobutamine stress echocardiography for preoperative cardiac risk stratification in patients undergoing orthotopic liver transplantation. Liver Transpl Surg. 1998;4(4):253–7. doi: 10.1002/lt.500040415. [DOI] [PubMed] [Google Scholar]
- 23.Williams K, Lewis JF, Davis G, Geiser EA. Dobutamine stress echocardiography in patients undergoing liver transplantation evaluation. Transplantation. 2000;69(11):2354–6. doi: 10.1097/00007890-200006150-00023. [DOI] [PubMed] [Google Scholar]
- 24.Dec GW, Kondo N, Farrell ML, Dienstag J, Cosimi AB, Semigran MJ. Cardiovascular complications following liver transplantation. Clin Transplant. 1995;9(6):463–71. [PubMed] [Google Scholar]
- 25.Annual Report of the US Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR): Transplant Data 1998–2011. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. [Google Scholar]
- 26.Audet M, Piardi T, Panaro F, Cag M, Ghislotti E, Habibeh H, et al. Liver transplantation in recipients over 65 yr old: a single center experience. Clin Transplant. 2010;24(1):84–90. doi: 10.1111/j.1399-0012.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 27.Zetterman RK, Belle SH, Hoofnagle JH, Lawlor S, Wei Y, Everhart J, et al. Age and liver transplantation: a report of the Liver Transplantation Database. Transplantation. 1998;66(4):500–6. doi: 10.1097/00007890-199808270-00015. [DOI] [PubMed] [Google Scholar]
- 28.Jung B, Cisse M, Chanques G, Arsac E, Bismuth M, Panaro F, et al. Causes of early mortality after liver transplantation: a twenty-years single centre experience. Ann Fr Anesth Reanim. 2011;30(12):899–904. doi: 10.1016/j.annfar.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Azevedo LD, Stucchi RS, Ataide EC, Boin IF. Assessment of causes of early death after twenty years of liver transplantation. Transplant Proc. 2013;45(3):1116–8. doi: 10.1016/j.transproceed.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Wedd JP, Harper AM, Biggins SW. MELD score, allocation, and distribution in the United States. Clinical Liver Disease. 2013;2(4):148–51. doi: 10.1002/cld.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 32.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 33.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 34.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 35.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 36.Wong CS, Lee WC, Jenq CC, Tian YC, Chang MY, Lin CY, et al. Scoring short-term mortality after liver transplantation. Liver Transpl. 2010;16(2):138–46. doi: 10.1002/lt.21969. [DOI] [PubMed] [Google Scholar]
- 37.Reichert B, Becker T, Weismuller TJ, Kleine M, Zachau L, Johanning K, et al. Value of the preoperative SOFT-score, P-SOFT-score, SALT-score and labMELD-score for the prediction of short-term patient and graft survival of high-risk liver transplant recipients with a pre-transplant labMELD-score >/=30. Ann Transplant. 2012;17(2):11–7. doi: 10.12659/aot.883218. [DOI] [PubMed] [Google Scholar]
- 38.Raszeja-Wyszomirska J, Wasilewicz MP, Wunsch E, Szymanik B, Jarosz K, Wojcicki M, et al. Assessment of a modified Child-Pugh-Turcotte score to predict early mortality after liver transplantation. Transplant Proc. 2009;41(8):3114–6. doi: 10.1016/j.transproceed.2009.07.098. [DOI] [PubMed] [Google Scholar]
- 39.Fouzas I, Paul A, Becker C, Vernadakis S, Treckmann JW, Mathe Z, et al. Orthotopic liver transplantation in patients with portal vein thrombosis in the absence of hepatocellular carcinoma. Transplant Proc. 2012;44(9):2734–6. doi: 10.1016/j.transproceed.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Lendoire J, Raffin G, Cejas N, Duek F, Barros Schelotto P, Trigo P, et al. Liver transplantation in adult patients with portal vein thrombosis: risk factors, management and outcome. HPB (Oxford) 2007;9(5):352–6. doi: 10.1080/13651820701599033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20(4 Pt 1):829–38. doi: 10.1002/hep.1840200410. [DOI] [PubMed] [Google Scholar]
- 42.Pineda J, Marin F, Marco P, Roldan V, Valencia J, Ruiz-Nodar JM, et al. Premature coronary artery disease in young (age <45) subjects: interactions of lipid profile, thrombophilic and haemostatic markers. Int J Cardiol. 2009;136(2):222–5. doi: 10.1016/j.ijcard.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–56. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 44.Tripodi A, Primignani M, Chantarangkul V, Dell’Era A, Clerici M, de Franchis R, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137(6):2105–11. doi: 10.1053/j.gastro.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 45.Davidson L, Wilcox J, Kim D, Benton S, Fredi J, Vaughan D. Clinical Features of Precocious Acute Coronary Syndrome. The American Journal of Medicine. 2013 doi: 10.1016/j.amjmed.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 46.VanWagner LB, Skaro AI. Program-specific reports: implications and impact on program behavior. Curr Opin Organ Transplant. 2013;18(2):210–5. doi: 10.1097/MOT.0b013e32835f07f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abecassis MM, Burke R, Cosimi AB, Matas AJ, Merion RM, Millman D, et al. Transplant center regulations--a mixed blessing? An ASTS Council viewpoint. Am J Transplant. 2008;8(12):2496–502. doi: 10.1111/j.1600-6143.2008.02434.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.