Abstract
Background
Both little and excessive physical activity (PA) may relate to chronic musculoskeletal pain. The primary objective of this study was to characterize the relationship of PA levels with chronic low back pain (CLBP) and chronic knee pain (CKP).
Methods
We evaluated 4559 adults aged 40–79 years in a community-based cross-sectional survey conducted in 2009 in Shimane, Japan. We used self-administered questionnaires to assess sociodemographics and health status: PA was assessed by the International Physical Activity Questionnaire, and CLBP and CKP were assessed by a modified version of the Knee Pain Screening Tool. We examined relationships of PA with prevalence of CLBP and CKP using Poisson regression, controlling for potential confounders.
Results
CLBP and CKP were both prevalent (14.1% and 10.7%, respectively) and associated with history of injury, medication use, and consultation with physicians. PA was not significantly related to CLBP or CKP (P > 0.05) before or after adjustment for potential confounders. For example, compared with adults reporting moderate PA (8.25–23.0 MET-hours/week), prevalence ratios for CKP adjusted for sex, age, education years, self-rated health, depressive symptom, smoking, chronic disease history, and body-mass index were 1.12 (95% confidential interval [CI] 0.84–1.50) among those with the lowest PA and 1.26 (95% CI 0.93–1.70) among those with the highest PA (P quadratic = 0.08). The prevalence ratios were further attenuated toward the null after additional adjustment for history of injury, medication use, and consultation (P quadratic = 0.17).
Conclusions
This cross-sectional study showed that there were no significant linear or quadratic relationships of self-reported PA with CLBP and CKP. Future longitudinal study with objective measurements is needed.
Key words: exercise, musculoskeletal pain, arthritis, epidemiology, public health
Abstract
【背景】
身体活動の不足あるいは過剰な実施は慢性的な運動器の痛みと関連している可能性がある。本研究の目的は、身体活動量と慢性腰痛および慢性膝痛の関係を明らかにすることである。
【方法】
地域在住の40から79歳住民を対象として、2009年に島根県で横断的調査を実施した。本研究では、その回答者4559人のデータについて解析を行った。自記式質問紙によって、社会人口統計学的変数および健康に関する項目を調査した。身体活動量は国際身体活動質問票(International Physical Activity Questionnaire)によって、慢性腰痛および膝痛は 改訂版Knee Pain Screening Toolを用いて評価した。身体活動量と慢性腰痛・膝痛有病率の関係は他の要因を調整したポアソン回帰分析によって検討した。
【結果】
慢性腰痛と慢性膝痛の有病率はそれぞれ14.1%と10.7%であり、過去の受傷、医薬品の使用、医療機関の受診と有意に関連していた。共変量で調整する前・後ともに、身体活動量と慢性腰痛・膝痛との間に有意な関連は見られなかった(P>0.05)。性、年齢、教育年数、健康度自己評価、抑うつ症状、喫煙、慢性疾患既往歴、体格指数(Body-Mass Index)で調整した慢性膝痛の有病率比は、中程度の身体活動量(8.25-23.0 MET-時/週)の群と比較した場合、身体活動量が最も低い群で1.12(95%信頼区間、0.84-1.50)であり、 最も高い群で1.26 (0.93-1.70)であった(2次トレンドP値=0.08)。これらの有病率比は、過去の受傷、医薬品の使用および医療機関の受診を調整変数に加えると、さらに1へと近づき縮小した(2次トレンドP値=0.17)。
【結論】
本研究では、自己申告による身体活動量と慢性腰痛・膝痛の間に、1次(直線的)および2次(曲線的)いずれの形でも有意な関係が見られなかった。客観的な評価指標を用いた縦断的研究による今後の検証が望まれる。
INTRODUCTION
Musculoskeletal disorders are a major burden on individuals, health systems, and society, contributing meaningfully to indirect costs1 and disability worldwide.2 Further, chronic musculoskeletal pain (CMP), a major symptom of musculoskeletal disorders,3–6 worsens quality of life and physical functioning later in life.7,8 In the United States, 28.8% of men and 26.6% of women reported feeling some pain.9 The lifetime risk of low back pain in Japan is estimated to be 83%.10 However, despite its importance to public health, evidence linking lifestyle to CMP remains to be established.
Physical activity (PA), including exercise therapy, is recommended as a non-pharmacological intervention for CMP.11,12 Pharmacological treatments, including nonsteroidal anti-inflammatory drugs, are also commonly prescribed. Considering the expense of prescriptions and side effects of such treatments,13 increasing PA should receive greater priority both as a therapeutic agent and as preventative action against CMP. However, the relationship between PA levels and CMP has not been established yet.
Recently, both too little PA and too much PA were found to be hazardous to spinal health,14,15 indicating a U-shaped relationship between PA and chronic low back pain (CLBP). However, few studies have examined the dose-response relationship between PA and CMP.15–18
When examining the relationship between PA and CMP, weight status and musculoskeletal injury need to be accounted for, since adiposity is an established risk factor for knee osteoarthritis and CMP.19–22 Among overweight individuals, excessive PA may cause high physical load on the knee joint, leading to chronic knee pain (CKP).23 This mechanism suggests that excess PA may cause CMP, especially among overweight adults. Injury is also an established risk factor for CMP.23,24 Excess PA increases the probability of experiencing injury,25,26 and musculoskeletal injury may reduce PA levels,27,28 potentially leading to weight gain.29 For these reasons, it is important to consider both weight status and injury history when investigating the association of PA with CMP. To our knowledge, a history of injury has been accounted for only in studies examining the risk of knee osteoarthritis,23,30,31 while studies of the relationship between PA and CMP in the general population typically have not taken injury history into account. In addition, adults who have history of injury are likely to take medications and consult physicians, and these pain management factors may also affect pain itself as well as daily habits (such as PA). Thus, consideration of these factors is also important.
To fill the gap in knowledge on the potential role of PA in the development of CMP, we examined cross-sectional associations of PA with CLBP and CKP among adults in a community-based survey in Japan, taking into account potential confounding by body weight, history of joint injuries, and pain management factors. We also examined post hoc how these factors could influence associations between common demographic variables with CLBP and CKP.
METHODS
Data collection
We cross-sectionally evaluated observations from an ongoing community-based intervention study for community-level improvement in levels of PA.32 In October 2009, invitation letters, consent forms, and questionnaires were mailed to 6000 residents randomly selected from the city registry in Unnan City (population 43 520, area 553.4 km2), a rural mountainous region in Shimane, Japan. Men and women aged 40 to 79 years were invited to participate; excluded were those in assisted living facilities, those who required long-term care, and those who could not complete the questionnaires by themselves. We took a pragmatic approach to increase our survey response rate, including the use of personalised and relatively short questionnaires33 and sending postcard reminders to non-responders.
A total of 4559 adults (76.0%) responded to the initial survey of the trial and were considered eligible for the present study. Written informed consent was obtained from each participant. This study was approved by the research ethics committee of the Physical Education and Medicine Research Center UNNAN (H21-10-13-1).
Measures
Sex and age were derived from the city registry, and other sociodemographic variables were obtained from self-administered questionnaires. We inquired about weight and height (used for calculating body mass index [BMI] in kg/m2), years of education, self-rated health (very good, good, poor, or very poor),34 depressive symptom (yes or no),35 smoking (never, past, or current), and chronic disease history (hypertension, hyperlipidaemia, diabetes, hyperuricaemia, stroke, heart disease, kidney and urologic diseases, liver disease, gastrointestinal disease, endocrine disease, or cancer). These covariates were selected because they previously have been reported to be associated with PA, musculoskeletal morbidity, or both.23,36
Musculoskeletal pain
CLBP and CKP were assessed using a questionnaire (available as web-only supplemental material eQuestionnaire 1) that has questions similar to those in the Knee Pain Screening Tool (KNEST), except for questions about use of health services (which were not examined in this study).37,38 The KNEST was previously developed to screen and identify individuals who have knee pain in a general population. CLBP and CKP were defined as current pain (ie, episodes of pain at the time of the questionnaire) that had lasted longer than 3 months in the past year.39 We assessed the test-retest reliability of CLBP and CKP in study subjects by mailing the questionnaire twice to 500 randomly-selected adults aged 40–84 years, separated by an interval of 10 days. These were individuals living in Unnan who were not invited to participate in the main trial/survey. Evaluating the 206 respondents (response rate 41.2%; mean and standard deviation [SD] of age 63.4 and 11.9 years; 51.4% women) to both questionnaires, we observed moderate reliability (Cohen’s kappa 0.49 for CLBP and 0.72 for CKP).
We also obtained information on a visual analogue scale (VAS) for intensity of pain. We defined “severe chronic pain” as chronic pain with a VAS pain score ≥75 on a 100-point scale.40 However, the prevalence of severe chronic pain was low (low back: n = 96, 2.4%; knee: n = 83, 2.0%) in this general population. Thus, we were unable to analyze this outcome in detail in the current study. We also asked about a history of low back injury and knee injury, medication use, and consultation with physicians for low back or knee pain. These factors were included in analyses as dichotomous variables (yes or no for each item).
Physical activity
We used the Japanese short version of the International Physical Activity Questionnaire (IPAQ),41 for which external reliability and validity have been reported elsewhere.42,43 The IPAQ asks separate questions about time spent on walking, moderate physical activity (MPA), and vigorous physical activity (VPA) in a typical week.
We estimated total weekly PA by multiplying the reported duration (hours) per week of walking, MPA, and VPA by their Metabolic Equivalent of Tasks (METs; walking = 3.3 METs; MPA = 4.0 METs; and VPA = 8.0 METs) to obtain estimated energy expenditure in MET-hours per week.41 Using these values, total moderate-to-vigorous physical activity (MVPA) was defined as 7 days × (3.3 METs × walking hours/day + 4.0 METs × MPA hours/day + 8.0 METs × VPA hours/day). The internal reliability over 10 days of the IPAQ was tested within our study, and found to be acceptable (Spearman correlation r = 0.64 among adults aged 40–84 years in the forementioned reliability study). In a validation study conducted among a sample of 95 subjects (40 men and 55 women) aged 62 to 85 (mean [SD], 74.9 [4.5]) years living in Unnan, we compared energy expenditure derived from the IPAQ with that objectively measured by a uniaxial accelerometer (Lifecorder, Suzuken Co., Ltd., Nagoya, Japan44,45). The validity (r = 0.33) was comparable to that observed in other studies.42,43
Statistical analyses
We compared the prevalence of CLBP and CKP in adults with different PA levels, estimating prevalence ratios (PR) by multivariable-adjusted Poisson regression.46 Poisson regression was used because the prevalence of CLBP and CKP was relatively high (>10% each). We examined CLBP and CKP separately as well as simultaneously using generalized estimating equations because these outcomes were correlated (kappa = 0.20).47
We evaluated total MVPA levels both continuously and categorically. To define categories, we chose an MVPA cutpoint of 8.25, corresponding to the WHO recommendation of 2.5 hours/week of MVPA (brisk walking in this case).48 For those with ≥8.25 MET-hours/week, we used tertiles within this sufficiently active group to determine further cutpoints (23.1, 75.4). Thus, the participants were divided into five categories: 0, 0.01–8.24, 8.25–23.00, 23.01–75.39, and ≥75.40 MET-hours/week. The adjusted PR and 95% confidence intervals (CIs) were then estimated using the middle category (8.25–23.0 MET-hours/week) as the reference category to assess potential non-linear relationships between MVPA and CMP.
When we evaluated MVPA as a continuous variable, we truncated the variable at the 95th percentile value (180 MET-hours/week) and log-transformed the variable to minimize effects of outliers and right-skewed distribution; analyses without truncation and log-transformation produced similar results, although whether the homoscedasticity assumption was met was uncertain (data not shown). In the regression analyses, we separately tested linear and quadratic relationships between MVPA and CMP.
We adjusted for the following potential confounders: sex, age, years of education, self-rated health, depressive symptoms, smoking habit, and chronic disease history (Model 1). In a separate model, we further adjusted for BMI (Model 2), past history of joint injuries (Model 3), and medication use and consultation with physicians (Model 4). Prevalence ratios by each covariate were additionally evaluated. We also assessed whether excess PA was associated with CKP, especially among adults with greater weight, by testing for an interaction between MVPA and BMI for CKP prevalence, and by examining joint categories of BMI (<20, 20–24.9, and ≥25 kg/m2) and MVPA (<8.25, 8.25–39.59, and ≥39.6 MET-hours/week). For these analyses, we used the median value of MVPA in adults with sufficient PA (39.5 MET-hours/week) for the cutpoint. We further assessed interactions between MVPA and history of injuries (low back or knee) for the combined outcome of either CLBP or CKP. While a prior review recommended exclusion of adults previously experiencing joint injuries in such analyses,23 our sample size would have been substantially reduced by excluding adults with a history of injury (33% of total). In a sensitivity analyses, we examined only adults without such a history and findings were little changed. Thus, in the present analyses, we included them, treating history of injury as a potential confounder and an effect-modifier.
We examined the associations of the different PA intensities with CLBP and CKP. In these analyses, VPA, MPA, and walking (in minutes per week) were entered into the same model simultaneously. Categorical and continuous analyses were performed separately for each PA intensity.
Missing information was imputed to minimize bias due to missing information and repeated four times, under the assumption that values were missing at random.49,50 Each imputation was based on regression models including variables used in the main regression analyses. The five imputed datasets were analysed independently and combined for inference, accounting for variability of imputation.49,50 We also repeated our analyses evaluating adults with complete information only, including 3329 participants. Analyses (two-sided α < 0.05) were carried out using SAS version 9.3 (Cary, NC, USA).
RESULTS
Of the 4559 participants, 46.3% were men, and participants had a mean (SD) age of 60.9 (10.6) years (Table 1). The median (interquartile range) level of MVPA was 10.6 (0–46.2) MET-hours/week. A total of 55% engaged in the recommended level of MVPA (≥8.25 MET-hours/week), whereas 25.6% did not engage in any MVPA. Adults with greater MVPA were more likely to be men, older, smokers, less educated, less depressed, and more likely to have prevalent chronic diseases and history of low back or knee injury (all P < 0.05); however, MVPA was not associated with BMI (P = 0.7) (data not shown).
Table 1. Characteristics of adults in a community-based survey in Shimane, Japan, 2009 (n = 4559).
| Total | Participants who had CLBP |
Participants who had CKP |
|
| Number of participants | 4559 | 605 | 471 |
| Physical activitya | |||
| MVPA, MET-hours/week | 10.6 (0–46.2) | 11.6 (0–49.5) | 11.6 (0–56.3) |
| Vigorous physical activity, min/week | 0 (0–0) | 0 (0–0) | 0 (0–10) |
| Moderate physical activity, min/week | 0 (0–40) | 0 (0–40) | 0 (0–60) |
| Walking, min/week | 120 (0–420) | 120 (0–420) | 123 (0–510) |
| Men, % | 46.3 | 49.9 | 39.5 |
| Age, years | 60.9 (10.6) | 62.8 (10.6) | 65.9 (10.0) |
| 40s, % | 17.6 | 13.2 | 7.0 |
| 50s, % | 26.8 | 24.3 | 20.4 |
| 60s, % | 29.8 | 29.6 | 28.5 |
| 70s, % | 25.8 | 32.9 | 44.2 |
| Self-rated health | |||
| Excellent or good, % | 81.8 | 61.6 | 68.9 |
| Education status, years | 11.4 (2.4) | 11.2 (2.4) | 10.8 (2.3) |
| Chronic disease history, %b | 62.0 | 68.4 | 64.8 |
| Depressive symptom, % | 47.6 | 52.4 | 72.8 |
| Smoking | |||
| Past smoker, % | 8.8 | 11.4 | 9.2 |
| Current smoker, % | 16.9 | 18.9 | 9.6 |
| Body mass index, kg/m2 | 22.5 (3.1) | 22.7 (3.2) | 23.6 (3.1) |
| Past low back injury, % | 23.2 | 45.1 | 29.1 |
| Past knee injury, % | 16.0 | 24.1 | 42.5 |
| Medication use for low back pain, % | 18.5 | 50.2 | 35.5 |
| Medication use for knee pain, % | 11.8 | 20.6 | 51.0 |
| Consultation with physicians for low back pain, % | 16.3 | 43.7 | 26.9 |
| Consultation with physicians for knee pain, % | 11.6 | 17.7 | 53.0 |
CLBP, chronic low back pain; CKP, chronic knee pain; MET, metabolic equivalent; MVPA, moderate-to-vigorous physical activity.
Means (standard deviations) for continuous variables and proportions for categorical variables are presented unless stated otherwise.
aMedian (interquartile range).
bReporting history of any of the following diseases: hypertension, hyperlipidemia, diabetes, hyperuricemia, cerebrovascular disease, heart disease, kidney and urologic diseases, liver disease, gastrointestinal disease, endocrine disease, cancer.
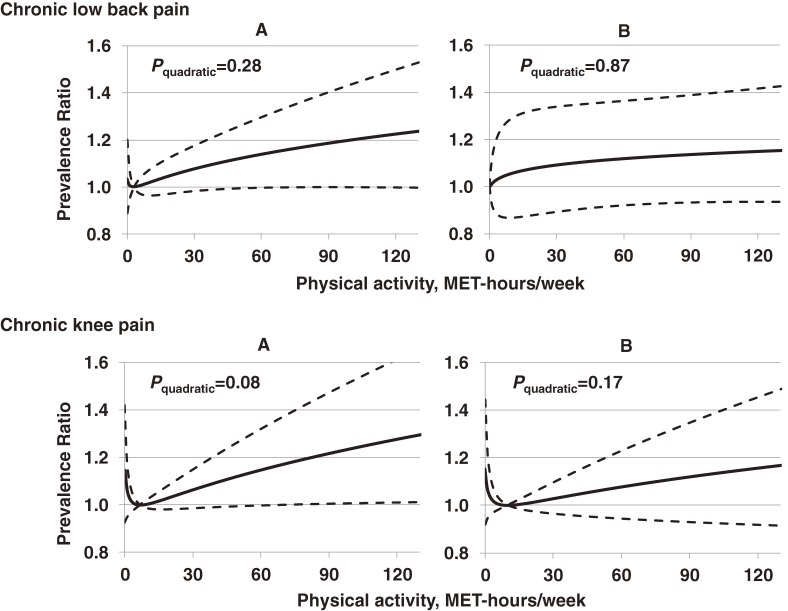
CLBP was present in 14.1% of adults (n = 605), CKP was present in 10.7%, and both pain conditions were present in 3.7%. Fair or poor self-rated health, history of injury, medication use, and consultation with physicians were significantly associated with CLBP (Table 2). The relationship between MVPA and CLBP was not significant (P > 0.10 for both linear and quadratic associations). Although CKP was more prevalent in adults with the lowest (0 MET-hours/week) and the highest (≥75.4 MET-hours/week) PA (10.8% and 12.2%, respectively) than in those with average PA (9.7% in those with 8.25–23.0 MET-hours/week), PRs adjusted for potential confounders including BMI (Model 2) were not significantly different from 1.00 (lowest MVPA: PR 1.12, 95% CI 0.84–1.50; highest PA: PR 1.26, 95% CI 0.93–1.70) (Table 3). The non-significant quadratic association between PA and CKP (P = 0.08) in Model 2, further attenuated (to P = 0.17) in Model 4 after additional adjustment for history of injury and pain management (ie, medication use and consultation) (Figure 1). The pattern of results were similar to the above results with CLBP and CKP evaluated separately when we evaluated CLBP and CKP together as a combined outcome (P quadratic trend > 0.3; data not shown).
Table 2. Cross-sectional associations of energy expended on moderate to vigorous physical activity with chronic low back pain among Japanese adults (n = 4559).
| Adults with CLBP, % |
PR (95% CI)a | ||||
| Model 1a | Model 2b | Model 3c | Model 4d | ||
| PA levels, MET-hours/week | |||||
| 0 | 14.9 | 0.94 (0.72–1.23) | 0.93 (0.71–1.22) | 0.95 (0.73–1.24) | 0.93 (0.72–1.21) |
| 0.1–8.24 | 12.8 | 0.86 (0.66–1.13) | 0.86 (0.65–1.13) | 0.89 (0.68–1.18) | 0.86 (0.66–1.13) |
| 8.25–23.0 | 15.0 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 23.1–75.3 | 13.7 | 0.94 (0.68–1.31) | 0.94 (0.67–1.30) | 0.95 (0.69–1.32) | 0.98 (0.72–1.33) |
| ≥75.4 | 16.1 | 1.09 (0.84–1.41) | 1.10 (0.851.42) | 1.04 (0.80–1.35) | 1.02 (0.79–1.32) |
| P for linearity | 0.14 | 0.13 | 0.30 | 0.20 | |
| P for quadratic | 0.29 | 0.28 | 0.53 | 0.87 | |
| Sex, female | 13.2 | 0.99 (0.82–1.19) | 1.01 (0.84–1.21) | 1.07 (0.89–1.28) | 0.93 (0.78–1.12) |
| Age | |||||
| 50s | 12.5 | 1.16 (0.88–1.52) | 1.16 (0.88–1.52) | 1.18 (0.90–1.55) | 1.13 (0.86–1.49) |
| 60s | 14.0 | 1.33 (1.00–1.78) | 1.34 (1.01–1.79) | 1.39 (1.05–1.86) | 1.19 (0.89–1.60) |
| 70s | 19.0 | 1.62 (1.19–2.20) | 1.64 (1.21–2.24) | 1.63 (1.20–2.23) | 1.26 (0.92–1.74) |
| Self-rated health, fair or poor | 30.3 | 2.59 (2.18–3.08) | 2.59 (2.18–3.08) | 2.36 (1.98–2.81) | 1.75 (1.46–2.09) |
| Education years, per year | —e | 0.98 (0.94–1.03) | 0.99 (0.95–1.03) | 0.99 (0.95–1.03) | 1.01 (0.96–1.05) |
| Chronic disease history | 15.6 | 1.03 (0.86–1.22) | 1.01 (0.85–1.21) | 1.01 (0.85–1.21) | 1.03 (0.86–1.23) |
| Depressive symptom | 15.2 | 1.06 (0.90–1.26) | 1.07 (0.90–1.27) | 1.01 (0.86–1.20) | 1.03 (0.86–1.23) |
| Smoking | |||||
| Past smoker | 18.2 | 1.29 (0.98–1.70) | 1.30 (0.99–1.71) | 1.23 (0.93–1.62) | 1.17 (0.88–1.54) |
| Current smoker | 15.9 | 1.23 (0.98–1.55) | 1.25 (0.99–1.57) | 1.21 (0.96–1.52) | 1.14 (0.91–1.44) |
| BMI, per 5 kg/m2 | —e | —f | 1.09 (0.97–1.23) | 1.07 (0.95–1.22) | 1.03 (0.91–1.17) |
| History of low back injury | 27.6 | — | — | 2.38 (2.03–2.79) | 1.60 (1.35–1.90) |
| Medication use for LBP | 40.9 | — | — | — | 2.66 (2.17–3.27) |
| Consultation for LBP | 39.8 | — | — | — | 1.88 (1.54–2.29) |
BMI, body mass index; CI, confidence interval; CLBP, chronic low back pain; LBP, low back pain; MET, metabolic equivalent; PA, physical activity; PR, prevalence ratio.
aModel 1 adjusted for sex, age, education years, self-rated health, chronic disease history, depressive symptom, and smoking. Reference categories were male, 40s of age, excellent or good self-rated health, no chronic disease history, no depressive symptom, and never smoker. Linear and quadratic relationships were tested separately.
bModel 2 adjusted for variables in the Model 1 and BMI.
cModel 3 adjusted for variables in the Model 2 and history of joint injuries (two indicator variables for injury of the knee and of the low back; yes, no for each).
dModel 4 adjusted for variables in the Model 3 and pain management (medication use and consultation with physicians).
ePrevalence is not shown for continuous variable.
fNot included in the models.
Table 3. Cross-sectional associations of energy expended on moderate to vigorous physical activity with chronic knee pain among Japanese adults (n = 4559).
| Adults with CKP, % |
PR (95% CI)a | ||||
| Model 1a | Model 2b | Model 3c | Model 4d | ||
| PA levels, MET-hours/week | |||||
| 0 | 10.8 | 1.15 (0.86–1.54) | 1.12 (0.84–1.50) | 1.14 (0.85–1.53) | 1.14 (0.85–1.53) |
| 0.1–8.24 | 9.9 | 1.02 (0.74–1.41) | 0.99 (0.72–1.37) | 0.98 (0.70–1.39) | 0.98 (0.71–1.34) |
| 8.25–23.0 | 9.7 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 23.1–75.3 | 10.3 | 1.09 (0.78–1.50) | 1.06 (0.77–1.47) | 1.03 (0.73–1.43) | 0.97 (0.70–1.34) |
| ≥75.4 | 12.2 | 1.26 (0.93–1.71) | 1.26 (0.93–1.70) | 1.19 (0.88–1.60) | 1.15 (0.85–1.56) |
| P for linearity | 0.53 | 0.43 | 0.79 | 1.00 | |
| P for quadratic | 0.07 | 0.08 | 0.09 | 0.17 | |
| Sex, female | 12.1 | 1.22 (0.98–1.52) | 1.31 (1.05–1.64) | 1.25 (1.00–1.56) | 0.98 (0.79–1.22) |
| Age, years | |||||
| 50s | 8.0 | 1.85 (1.24–2.76) | 1.88 (1.26–2.80) | 1.84 (1.23–2.74) | 1.51 (1.01–2.26) |
| 60s | 10.3 | 2.23 (1.49–3.32) | 2.30 (1.54–3.44) | 2.24 (1.50–3.34) | 1.75 (1.16–2.62) |
| 70s | 19.1 | 3.77 (2.51–5.68) | 4.14 (2.75–6.22) | 3.56 (2.37–5.37) | 2.06 (1.36–3.13) |
| Self-rated health, fair or poor | 18.7 | 1.67 (1.36–2.06) | 1.65 (1.34–2.03) | 1.51 (1.22–1.86) | 1.21 (0.98–1.49) |
| Education years | —e | 0.96 (0.92–1.01) | 0.97 (0.93–1.01) | 0.97 (0.93–1.01) | 1.01 (0.96–1.05) |
| Chronic disease history | 12.7 | 1.18 (0.96–1.47) | 1.07 (0.86–1.33) | 1.06 (0.86–1.32) | 0.98 (0.79–1.22) |
| Depressive symptom | 11.2 | 1.19 (0.98–1.44) | 1.24 (1.02–1.51) | 1.20 (0.99–1.46) | 1.17 (0.97–1.41) |
| Smoking | |||||
| Past smoker | 11.1 | 1.11 (0.77–1.60) | 1.15 (0.80–1.66) | 1.17 (0.82–1.69) | 1.17 (0.82–1.67) |
| Current smoker | 6.0 | 0.73 (0.52–1.02) | 0.78 (0.55–1.09) | 0.80 (0.57–1.12) | 0.87 (0.62–1.23) |
| BMI per 5 kg/m2 | —e | —f | 1.68 (1.47–1.91) | 1.57 (1.37–1.80) | 1.28 (1.11–1.48) |
| History of knee injury | 29.0 | — | — | 3.23 (2.65–3.94) | 1.67 (1.35–2.07) |
| Medication use for KP | 49.4 | — | — | — | 2.99 (2.29–3.89) |
| Consultation for KP | 51.5 | — | — | — | 3.11 (2.44–3.96) |
BMI, body mass index; CI, confidence interval; CKP, chronic knee pain; KP, knee pain; MET, metabolic equivalent; PA, physical activity; PR, prevalence ratio.
aModel 1 adjusted for sex, age, education years, self-rated health, chronic disease history, depressive symptom, and smoking. Reference categories were male, 40s of age, excellent or good self-rated health, no chronic disease history, no depressive symptom, and never smoker. Linear and quadratic relationships were tested separately.
bModel 2 adjusted for variables in the Model 1 and BMI.
cModel 3 adjusted for variables in the Model 2 and history of joint injuries (two indicator variables for injury of the knee and of the low back; yes, no for each).
dModel 4 adjusted for variables in the Model 3 and pain management (medication use and consultation with physicians).
ePrevalence is not shown for continuous variable.
fNot included in the models.
Figure 1. Associations between moderate-to-vigorous physical activity and the prevalence of chronic low back pain and chronic knee pain among Japanese adults (n = 4559). Solid lines represent prevalence ratios (PRs), and dashed lines indicate 95% confidence intervals estimated by Poisson regression, estimated by a quadratic function of physical activity levels (metabolic equivalent of task [MET]-hours/week). Panels on the left (A) display PR adjusted for sex, age, education years, self-rated health, depressive symptoms, smoking habit, chronic disease history, and body mass index; while on the right (B), PRs are further adjusted for history of joint injuries, medication use, and consultation with physicians for pain management. The reference value for each was fixed to the values giving the lowest prevalence of each outcome. P for each quadratic function is displayed.
Associations of age and history of injury with CLBP and CKP were found, but these associations attenuated when adjusted for medical treatment and consultation. A significant positive association of BMI with CKP, but not CLBP, persisted; per 5 kg/m2, PRs were 1.03 (95% CI 0.91–1.17) for CLBP and 1.28 (95% CI 1.11–1.48) for CKP, based on Model 4 (Tables 2 and 3). History of injury was also associated with each CMP outcome: PR 1.60 (95% CI 1.35–1.90) for CLBP and PR 1.67 (95% CI 1.35–2.07) for CKP (Tables 2 and 3).
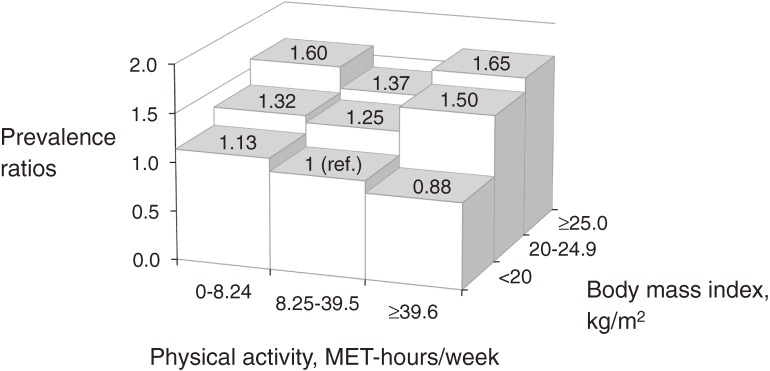
The interaction between BMI and MVPA levels for CKP was not significant (P > 0.9 for linear and quadratic trends). When BMI and total MVPA levels were examined jointly, a non-significant U-shaped relationship between MVPA and CKP was observed in the high-BMI category (Model 4, Figure 2). The interaction between MVPA and joint injuries was also not significant (P = 0.88).
Figure 2. Associations of moderate-to-vigorous physical activity (metabolic equivalent of task [MET]-hours/week) and weight status with chronic knee pain among Japanese adults (n = 4559). Prevalence ratios were estimated with adjustment for sex, age, education years, self-rated health, depressive symptoms, smoking habit, chronic disease history, past joint injuries, medication use, and consultation with physicians for pain management. After adjustment, no significant prevalence ratios were observed (all P > 0.05). Interactions between body-mass index and physical activity levels in models, considering linear as well as non-linear associations, were also not significant (all P > 0.1).
When we evaluated PA of different intensities, VPA, MPA, and walking were neither linearly nor non-linearly significantly associated with CLBP and CKP evaluated separately (all P > 0.05; data not shown) or with CLBP and CKP evaluated simultaneously as a combined outcome (Table 4).
Table 4. Cross-sectional associations between physical activity of different intensity and either chronic low back pain or chronic knee pain among Japanese adults (n = 4559).
| Physical activity type | n | PR (95% CI)a | |||
| Model 1b | Model 2c | Model 3d | Model 4e | ||
| Vigorous PA, min/week | |||||
| 0 | 3200 | 1.13 (0.80–1.59) | 1.15 (0.81–1.63) | 1.15 (0.83–1.59) | 1.15 (0.91–1.45) |
| >0–40.6 | 453 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 40.9–180 | 458 | 1.27 (0.84–1.90) | 1.26 (0.83–1.90) | 1.18 (0.80–1.75) | 1.19 (0.91–1.57) |
| >180 | 448 | 1.05 (0.67–1.66) | 1.06 (0.67–1.67) | 0.98 (0.64–1.49) | 0.93 (0.67–1.29) |
| Plinearityf | 0.94 | 0.89 | 0.43 | 0.21 | |
| Pnon-linearityf | 0.93 | 0.83 | 0.97 | 0.45 | |
| Moderate PA, min/week | |||||
| 0 | 2990 | 1.05 (0.80–1.38) | 1.02 (0.78–1.33) | 1.04 (0.82–1.32) | 1.16 (0.88–1.53) |
| >0–58.8 | 504 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 60.0–240 | 558 | 1.20 (0.87–1.65) | 1.20 (0.88–1.63) | 1.16 (0.87–1.55) | 1.28 (0.96–1.69) |
| >240 | 507 | 1.13 (0.82–1.54) | 1.14 (0.84–1.55) | 1.13 (0.85–1.51) | 1.29 (0.91–1.82) |
| Plinearityf | 0.18 | 0.07 | 0.17 | 0.22 | |
| Pnon-linearityf | 0.56 | 0.61 | 0.52 | 0.28 | |
| Walking, min/week | |||||
| 0 | 1271 | 1.10 (0.92–1.32) | 1.11 (0.93–1.34) | 1.09 (0.90–1.31) | 1.08 (0.91–1.29) |
| >0–119 | 1055 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 120–404 | 1053 | 1.03 (0.85–1.25) | 1.04 (0.86–1.26) | 1.04 (0.86–1.26) | 1.06 (0.88–1.26) |
| >404 | 1180 | 1.11 (0.91–1.36) | 1.12 (0.91–1.38) | 1.11 (0.90–1.36) | 1.13 (0.93–1.36) |
| Plinearityf | 0.99 | 0.98 | 0.84 | 0.57 | |
| Pnon-linearityf | 0.08 | 0.06 | 0.13 | 0.18 | |
CI, confidence interval; PA, physical activity; PR, prevalence ratio.
aPrevalence ratios (PR) and 95% confidence intervals were estimated by Poisson regression. We examined chronic low back pain (CLBP), chronic knee pain (CKP), or both as the outcome of interest simultaneously by generalized estimating equation that accounted for the correlations between CLBP and CKP (kappa = 0.20). The models also included all PA measures simultaneously. Correlations among these PA categories were moderate (Spearman r = 0.48 between vigorous and moderate PA; 0.31 between vigorous PA and walking; 0.28 between moderate PA and walking). For each type of physical activity, we categorized adults into four groups by treating adults with 0 min/week as a single category and by splitting the others into tertiles.
bModel 1 adjusted for sex, age, education years, self-rated health, chronic disease history, depressive symptom, and smoking.
cModel 2 adjusted for variables in the Model 1 and body mass index.
dModel 3 adjusted for variables in the Model 2 and history of joint injuries (two indicator variables for injury of the knee and of the low back; yes or no for each).
eModel 4 adjusted for variables in the Model 3 and pain management (medication use and consultation with physicians).
fLog-linear and quadratic relationships were tested separately, using log-transformed variables.
All of these results from multiple imputed analyses were similar to those from complete-case analyses, with the exception of the complete-case analyses having less precision and wider confidence intervals; the variability of 5-time imputation was <10% of total variance (data not shown), while the variability due to multiple imputation was incorporated into estimations of precision and significant testing in all presented analyses.
DISCUSSION
This study examined the associations of PA with CLBP and CKP among middle-aged and older Japanese. We found that there were no significant cross-sectional relationships of PA with CLBP and CKP. While neither a U-shaped association nor interactions by body mass and prior injury were statistically significant, our analysis indicate the importance of accounting for body mass, history of injury, medication use, and consultation with physicians in research on PA and CMP.
Few previous studies have examined a potential non-linear relationship between PA and CMP, especially for CKP. Some studies suggested U-shaped relationships between PA and CLBP.15,17,18 An occupational cohort study showed that the lowest and highest tertiles of minutes of MVPA yielded statistically significantly higher risks of low back pain than the middle tertile.18 However, our cross-sectional investigation did not detect any significant linear or quadratic associations of PA and CLBP or CKP.
Both positive and negative effects of excess PA on knee joint are conceivable. A systematic review concluded that there was strong evidence for an inverse relationship between PA and cartilage defects of the knee joint.51 However, the authors also concluded that there was a positive relationship between tibiofemoral osteophytes and PA. The results of previous studies on PA and joint health have been inconsistent, and many of the prior studies did not assess non-linear relationships or were too underpowered to do so.51 Therefore, future longitudinal investigations examining a potential non-linear relationship between PA and CMP are of value.
Our results also showed the importance of taking into account BMI, past injuries, and factors related to pain management, which were all significantly associated with CMP. Higher BMI level in this study was significantly associated with higher prevalence of CKP but not CLBP, in line with the postulation that a greater body mass causes physical burden on the knee joint.23 Our failure to show an interaction of PA and BMI on CKP may reflect the limited statistical power of the present study and also the limited range of BMI in our population, which predominantly comprised normal-weight adults with BMI < 25 kg/m2 (80%). Only a few prior studies took a history of injury into account.18,23 One third of the adults in our study reported a history of injury, and we observed a significant positive association of history of injury with CMP; it is possible that prior excess PA could have caused joint injury, which led to CMP. On the other hand, PA is recommended as a non-pharmacological intervention for CMP.11,12 Thus, adults who had history of injury, and possibly CMP, might engage in more PA for treatment and rehabilitation.
Our results showed that there were strong associations of CMP with medication use and consultation with physicians and that adjusting for these factors attenuated the quadratic association between PA and CKP. As seeking medications and undergoing outpatient treatment is directly associated with not only pain but also PA, these results are plausible. Our findings thus emphasize that future research on the relationship between PA and CMP should consider effects of BMI, injury, and pain management factors.
Globally, disability due to musculoskeletal disorders is estimated to have increased by 45% from 1990 to 2010, related to the aging of the population.2 It remains unknown what the most effective and affordable strategies are to reduce the global burden of musculoskeletal disorders.52 Although we detected little indication of benefits of PA for CMP, potential beneficial effects of PA on CMP still deserve discussion. Possible pathways linking greater PA to a reduced risk of CMP include but are not limited to reduction of mechanical stress through improving muscle strength, range of movement, and joint structure; improvement of blood flow to painful regions; relief of psychological stress, such as distraction and depression7,53–55; and elevation of tolerance to pain associated with increased serum concentrations of endocannabinoids that reduce pain sensation.56 Our community-based research in Japan, which has one of the most aged societies in the world, provides important insights into the studies on PA and musculoskeletal health.
Our study has several limitations. In our cross-sectional study, reverse causation and recall bias might have occurred. Individuals with CMP may reduce levels of recreational PA and PA intensities, leading to null findings for MVPA and CMP. Limitations are likely to be present in our assessment of injury, because this was ascertained retrospectively. We also had a limited sample size to tease out independent relations among PA levels, CMP, and potential confounders. Future research should adopt a longitudinal design, assessing PA prior to the development of injuries or pains. Considering potential biases due to self-reported PA, objective measures of PA, as well as anthropometrics, injuries, and pain, should be incorporated in future research.
In conclusion, this cross-sectional study showed that there were no significant linear or quadratic relationships of PA with CLBP and CKP. Our findings indicate the importance of evaluating PA, CMP, body mass, injuries, and pain management factors simultaneously.
ONLINE ONLY MATERIALS
ACKNOWLEDGMENTS
We appreciate the cooperation of the participants and staff members of this study. In this research work we used the supercomputer of ACCMS, Kyoto University. This study was supported by a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan (H20-Junkankitou-Ippan-001) and Meiji Yasuda Life Foundation of Health and Welfare (2010–2011). MK received a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science. FI received funding from the Medical Research Council Epidemiology Unit, UK, MC_UU_12015/1 and MC_UU_12015/5.
Conflicts of interest: None declared.
REFERENCES
- 1.World Health Organization Scientific Group. The burden of musculoskeletal conditions at the start of the new millennium. Geneva: World Health Organization; 2003 [cited 2014 Jan 13]. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_919.pdf [PubMed]
- 2.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suka M, Yoshida K. Musculoskeletal pain in Japan: prevalence and interference with daily activities. Mod Rheumatol. 2005;15:41–7. 10.3109/s10165-004-0362-x [DOI] [PubMed] [Google Scholar]
- 4.Suka M, Yoshida K. Burden of musculoskeletal pain in Japan. Mod Rheumatol. 2005;15:48–51. 10.3109/s10165-004-0363-9 [DOI] [PubMed] [Google Scholar]
- 5.Tokuda Y, Ohde S, Takahashi O, Shakudo M, Yanai H, Shimbo T, et al. Musculoskeletal pain in Japan: prospective health diary study. Rheumatol Int. 2007;28:7–14. 10.1007/s00296-007-0368-8 [DOI] [PubMed] [Google Scholar]
- 6.Harkness EF, Macfarlane GJ, Silman AJ, McBeth J. Is musculoskeletal pain more common now than 40 years ago?: Two population-based cross-sectional studies. Rheumatology (Oxford). 2005;44:890–5. 10.1093/rheumatology/keh599 [DOI] [PubMed] [Google Scholar]
- 7.Brooks P. Issues with chronic musculoskeletal pain. Rheumatology (Oxford). 2005;44:831–3. 10.1093/rheumatology/keh648 [DOI] [PubMed] [Google Scholar]
- 8.Leveille SG, Jones RN, Kiely DK, Hausdorff JM, Shmerling RH, Guralnik JM, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302:2214–21. 10.1001/jama.2009.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krueger AB, Stone AA. Assessment of pain: a community-based diary survey in the USA. Lancet. 2008;371:1519–25. 10.1016/S0140-6736(08)60656-X [DOI] [PubMed] [Google Scholar]
- 10.Fujii T, Matsudaira K. Prevalence of low back pain and factors associated with chronic disabling back pain in Japan. Eur Spine J. 2013;22:432–8. 10.1007/s00586-012-2439-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776–85. 10.7326/0003-4819-142-9-200505030-00014 [DOI] [PubMed] [Google Scholar]
- 12.American Geriatrics Society Panel on Exercise and Osteoarthritis . Exercise prescription for older adults with osteoarthritis pain: consensus practice recommendations. A supplement to the AGS Clinical Practice Guidelines on the management of chronic pain in older adults. J Am Geriatr Soc. 2001;49:808–23. 10.1046/j.1532-5415.2001.00496.x [DOI] [PubMed] [Google Scholar]
- 13.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–99. 10.1056/NEJM199906173402407 [DOI] [PubMed] [Google Scholar]
- 14.Campello M, Nordin M, Weiser S. Physical exercise and low back pain. Scand J Med Sci Sports. 1996;6:63–72. 10.1111/j.1600-0838.1996.tb00073.x [DOI] [PubMed] [Google Scholar]
- 15.Heneweer H, Vanhees L, Picavet HS. Physical activity and low back pain: a U-shaped relation? Pain. 2009;143:21–5. 10.1016/j.pain.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 16.Vuori IM. Dose-response of physical activity and low back pain, osteoarthritis, and osteoporosis. Med Sci Sports Exerc. 2001;33(6 Suppl):S551–86. 10.1097/00005768-200106001-00026 [DOI] [PubMed] [Google Scholar]
- 17.Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152:2241–7. 10.1016/j.pain.2011.04.029 [DOI] [PubMed] [Google Scholar]
- 18.Thiese MS, Hegmann KT, Garg A, Porucznik C, Behrens T. The predictive relationship of physical activity on the incidence of low back pain in an occupational cohort. J Occup Environ Med. 2011;53:364–71. 10.1097/JOM.0b013e31820d1633 [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane GJ, de Silva V, Jones GT. The relationship between body mass index across the life course and knee pain in adulthood: results from the 1958 birth cohort study. Rheumatology (Oxford). 2011;50:2251–6. 10.1093/rheumatology/ker276 [DOI] [PubMed] [Google Scholar]
- 20.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. [DOI] [PubMed] [Google Scholar]
- 21.Tsuritani I, Honda R, Noborisaka Y, Ishida M, Ishizaki M, Yamada Y. Impact of obesity on musculoskeletal pain and difficulty of daily movements in Japanese middle-aged women. Maturitas. 2002;42:23–30. 10.1016/S0378-5122(02)00025-7 [DOI] [PubMed] [Google Scholar]
- 22.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. 10.7326/0003-4819-109-1-18 [DOI] [PubMed] [Google Scholar]
- 23.Urquhart DM, Soufan C, Teichtahl AJ, Wluka AE, Hanna F, Cicuttini FM. Factors that may mediate the relationship between physical activity and the risk for developing knee osteoarthritis. Arthritis Res Ther. 2008;10:203. 10.1186/ar2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ; Leap Study Group . Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–9. 10.1016/j.pain.2006.04.020 [DOI] [PubMed] [Google Scholar]
- 25.Hootman JM, Macera CA, Ainsworth BE, Martin M, Addy CL, Blair SN. Association among physical activity level, cardiorespiratory fitness, and risk of musculoskeletal injury. Am J Epidemiol. 2001;154:251–8. 10.1093/aje/154.3.251 [DOI] [PubMed] [Google Scholar]
- 26.Sampere M, Gimeno D, Serra C, Plana M, Martínez JM, Delclos GL, et al. Effect of working conditions on non-work-related sickness absence. Occup Med (Lond). 2012;62:60–3. 10.1093/occmed/kqr141 [DOI] [PubMed] [Google Scholar]
- 27.Resnick B, Galik E, Boltz M, Hawkes W, Shardell M, Orwig D, et al. Physical activity in the post-hip-fracture period. J Aging Phys Act. 2011;19:373–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceroni D, Martin X, Lamah L, Delhumeau C, Farpour-Lambert N, De Coulon G, et al. Recovery of physical activity levels in adolescents after lower limb fractures: a longitudinal, accelerometry-based activity monitor study. BMC Musculoskelet Disord. 2012;13:131. 10.1186/1471-2474-13-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crane DA, Little JW, Burns SP. Weight gain following spinal cord injury: a pilot study. J Spinal Cord Med. 2011;34:227–32. 10.1179/2045772311Y.0000000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers LQ, Macera CA, Hootman JM, Ainsworth BE, Blair SN. The association between joint stress from physical activity and self-reported osteoarthritis: an analysis of the Cooper Clinic data. Osteoarthritis Cartilage. 2002;10:617–22. 10.1053/joca.2002.0802 [DOI] [PubMed] [Google Scholar]
- 31.Spector TD, Harris PA, Hart DJ, Cicuttini FM, Nandra D, Etherington J, et al. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996;39:988–95. 10.1002/art.1780390616 [DOI] [PubMed] [Google Scholar]
- 32.Kamada M, Kitayuguchi J, Inoue S, Ishikawa Y, Nishiuchi H, Okada S, et al. A community-wide campaign to promote physical activity in middle-aged and elderly people: a cluster randomized controlled trial. Int J Behav Nutr Phys Act. 2013;10:44. 10.1186/1479-5868-10-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008. doi:10.1002/14651858.MR000008.pub4. 10.1002/14651858.MR000008.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichida Y, Kondo K, Hirai H, Hanibuchi T, Yoshikawa G, Murata C. Social capital, income inequality and self-rated health in Chita peninsula, Japan: a multilevel analysis of older people in 25 communities. Soc Sci Med. 2009;69:489–99. 10.1016/j.socscimed.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 35.Hamano T, Yamasaki M, Fujisawa Y, Ito K, Nabika T, Shiwaku K. Social capital and psychological distress of elderly in Japanese rural communities. Stress Health. 2011;27:163–9. 10.1002/smi.1324 [DOI] [PubMed] [Google Scholar]
- 36.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. 10.1097/00005768-200212000-00020 [DOI] [PubMed] [Google Scholar]
- 37.Jinks C, Lewis M, Ong BN, Croft P. A brief screening tool for knee pain in primary care. 1. Validity and reliability. Rheumatology (Oxford). 2001;40:528–36. 10.1093/rheumatology/40.5.528 [DOI] [PubMed] [Google Scholar]
- 38.Jinks C, Jordan K, Ong BN, Croft P. A brief screening tool for knee pain in primary care (KNEST). 2. Results from a survey in the general population aged 50 and over. Rheumatology (Oxford). 2004;43:55–61. 10.1093/rheumatology/keg438 [DOI] [PubMed] [Google Scholar]
- 39.Wijnhoven HA, de Vet HC, Picavet HS. Explaining sex differences in chronic musculoskeletal pain in a general population. Pain. 2006;124:158–66. 10.1016/j.pain.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 40.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–14. 10.1016/S1526-5900(03)00716-8 [DOI] [PubMed] [Google Scholar]
- 41.International Physical Activity Questionnaire (IPAQ) [homepage on the Internet] [cited 2013 Dec 28]. Available from: http://www.ipaq.ki.se/ipaq.htm
- 42.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 43.Murase N, Katsumura T, Ueda C, Inoue S, Shimomitsu T. Reliability and validity study of the Japanese version of the International Physical Activity Questionnaire (IPAQ). J Health Welfare Stat. 2002;49:1–9(in Japanese) [Google Scholar]
- 44.Kumahara H, Schutz Y, Ayabe M, Yoshioka M, Yoshitake Y, Shindo M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–43. 10.1079/BJN20031033 [DOI] [PubMed] [Google Scholar]
- 45.Rafamantanantsoa HH, Ebine N, Yoshioka M, Higuchi H, Yoshitake Y, Tanaka H, et al. Validation of three alternative methods to measure total energy expenditure against the doubly labeled water method for older Japanese men. J Nutr Sci Vitaminol (Tokyo). 2002;48:517–23. 10.3177/jnsv.48.517 [DOI] [PubMed] [Google Scholar]
- 46.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 47.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–75. 10.1093/aje/kwf215 [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization (WHO). Global recommendations on physical activity for health. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 49.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163–94. 10.7326/0003-4819-147-8-200710160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 50.Barnard J, Meng XL. Applications of multiple imputation in medical studies: from AIDS to NHANES. Stat Methods Med Res. 1999;8:17–36. 10.1191/096228099666230705 [DOI] [PubMed] [Google Scholar]
- 51.Urquhart DM, Tobing JF, Hanna FS, Berry P, Wluka AE, Ding C, et al. What is the effect of physical activity on the knee joint? A systematic review. Med Sci Sports Exerc. 2011;43:432–42. 10.1249/MSS.0b013e3181ef5bf8 [DOI] [PubMed] [Google Scholar]
- 52.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 53.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46:397–411. 10.1016/j.ypmed.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 54.van Dijk GM, Dekker J, Veenhof C, van den Ende CH; Carpa Study Group . Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheum. 2006;55:779–85. 10.1002/art.22244 [DOI] [PubMed] [Google Scholar]
- 55.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine. 2002;27:E109–20. 10.1097/00007632-200203010-00017 [DOI] [PubMed] [Google Scholar]
- 56.Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38:536–41. 10.1136/bjsm.2004.011718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.