Abstract
Background
Minimal Change Disease (MCD) is associated with CD80 expression in podocytes and elevated urinary CD80 excretion during active disease. We have evaluated the CTLA-4 and CD80 urinary excretion during different stages of the nephrotic syndrome in patients with MCD to test the hypothesis that the persistent increased urinary CD80 excretion in MCD in relapse is due to an ineffectual CTLA-4 response of the host to curtail the CD80 activation.
Methods
32 children with biopsy-proven MCD were studied during relapse and/or remission. 11 healthy subjects served as controls.
Results
Urinary CD80 excretion was significantly increased in MCD patients in relapse when compared to MCD patients in remission (p<0.001) and controls (p<0.001). Although urinary CTLA-4 excretion was higher in MCD patients in relapse than in MCD patients in remission (p=0.01) and controls (p=0.03), no significant correlation was observed between urinary CD80 and urinary CTLA-4 in MCD patients at the time of relapse (p=0.06). At the time of remission, CD80 has decreased significantly in all patients but CTLA-4 either decreased or remained unchanged in all but five patients and no correlation was observed between urinary CD80 and CTLA-4 (p=0.7).
Conclusions
Urinary CTLA-4 levels do not correlate with urinary CD80 excretion, suggesting the possibility that the CTLA4 response may be suboptimal in this disease during relapse.
Keywords: CD80, CTLA-4, Minimal Change Disease, podocyte
Introduction
Minimal Change Disease (MCD) is the most common nephrotic syndrome in childhood (1). MCD is usually a relapsing disease triggered by an upper respiratory infection (URI) (2). The relapse pattern is variable with some patients relapsing frequently while others enjoy long periods of remission (3).
MCD is considered a podocytopathy (4). We have reported that podocytes in patients with MCD in relapse express CD80 and increased urinary excretion of this molecule in the absence of an increase in circulating soluble CD80 (sCD80) (5, 6). After remission is induced, CD80 expression in podocytes remits and urinary CD80 excretion normalizes (5, 6).
Furthermore, podocytes may express CTLA-4 (cytotoxic T-lymphocyte antigen 4) under certain circumstances (7). CTLA-4 negatively regulates CD80 (8). Increased CD80 expression can be facilitated by a poor CTLA-4 response (8).
We have postulated that the persistent CD80 podocyte expression in MCD patients in relapse could be due to an inadequate censoring of podocyte CD80 expression due to an impaired podocyte CTLA-4 response (9). Therefore, the purpose of this study was to assess the pattern of CD80 and the CTLA-4 response during different stages of the nephrotic syndrome in MCD patients. We hypothesized that intrarenal CTLA-4 production, as reflected by urinary CTLA-4, is unable to regulate podocyte CD80 expression leading to persistently increased glomerular and urinary CD80.
Patients and methods
Patients
Thirty-two children and adolescents with biopsy-proven MCD, defined according to the International Study for Kidney Diseases in Children (10), were studied (Table 1). Twenty-three patients were studied during relapse and remission. Seven patients were studied only during relapse and two only during remission. The majority of patients with MCD during relapse and during remission were receiving variable amounts of immunosuppressive therapy. Eleven healthy subjects, ages 9 to 17 year-old, served as control (Table 2). All patients and control subjects were followed at the University of Florida. Patients studied represent a different set of patients from those previously reported (6).
Table 1.
Characteristics of children with Minimal Change Disease (MCD)
| Table 1a MCD patients in relapse | ||||||
|---|---|---|---|---|---|---|
| Patient (n=30) |
Age (years) |
Urinary Pr/Cr Ratio* |
Serum Albumin (g/dL)* |
Urinary CD80 (ng/g creat)* |
Urinary CTLA-4 (ng/g creat)* |
Urinary CD80 to CTLA-4 ratio* |
| 1 | 16 | 14.5 | 1.1 | 216 | 113 | 1.9 |
| 2 | 9 | 19.6 | 1.6 | 222 | 88 | 2.5 |
| 3 | 4 | 4 | 3 | 341 | 99 | 3.4 |
| 4 | 11 | 12.6 | 2.7 | 472 | 758 | 0.6 |
| 5 | 3 | 40.7 | 2.4 | 464 | 245 | 1.8 |
| 6 | 4 | >300 | 2.4 | 842 | 0.5 | 1684 |
| 7 | 4 | 5.5 | 3.6 | 214 | 90 | 2.3 |
| 8 | 19 | 1.1 | 3.4 | 2114 | 65 | 32.5 |
| 9 | 12 | 9 | 2.1 | 542 | 734 | 0.7 |
| 10 | 3 | 3.8 | 3.2 | 156 | 60 | 2.6 |
| 11 | 4 | 10 | 1.6 | 303 | 59 | 5.1 |
| 12 | 13 | 1.7 | 2.4 | 89 | 18 | 4.9 |
| 13 | 3 | 10 | NA | 497 | 465 | 1 |
| 14 | 16 | 19 | 2 | 228 | 577 | 0.3 |
| 15 | 3 | 6 | 1.7 | 318 | 134 | 2.3 |
| 16 | 7 | 12 | 1.7 | 333 | 1085 | 0.3 |
| 17 | 7 | 18.3 | 1.6 | 380 | 1634 | 0.2 |
| 18 | 11 | 7.8 | 2.8 | 346 | 184 | 1.8 |
| 19 | 7 | 12.5 | 2.4 | 976 | 294 | 3.3 |
| 20 | 6 | 5.3 | 1.7 | 309 | 0.5 | 618 |
| 21 | 4 | 21.5 | 1.4 | 667 | 427 | 1.5 |
| 22 | 7 | 7.6 | 3.7 | 127 | 12 | 10.5 |
| 23 | 2 | 16 | 2.8 | 1282 | 2461 | 0.5 |
| 24 | 7 | 8.5 | 2.6 | 236 | 0.2 | 1180 |
| 25 | 3 | 5.9 | 2.9 | 315 | 0.5 | 630 |
| 26 | 2 | >300 | NA | 496 | 47 | 10.5 |
| 27 | 6 | 3.8 | 1.4 | 161 | 1458 | 0.1 |
| 28 | 1 | 8.7 | 2.1 | 299 | 212 | 1.4 |
| 29 | 2 | 6 | 2.6 | 551 | 235 | 2.3 |
| 30 | 3 | 10.6 | 1.8 | 430 | 2196 | 0.2 |
| mean±s.d. | 6.6±4.6 | 10.7±7.8 | 1.9±0.6 | 464±398 | 458±652 | 140.2±383.7 |
| Table 1b Minimal Change Disease (MCD) patients in remission | ||||||
|---|---|---|---|---|---|---|
| Patient** (n=25) |
Age (years) |
Urinary Pr/Cr Ratio* |
Serum Albumin (g/dL)* |
Urinary CD80 (ng/g creat)* |
Urinary CTLA-4 (ng/g creat)* |
Urinary CD80 to CTLA-4 ratio* |
| 2 | 9 | 0.19 | 4.8 | 31 | 7 | 4.4 |
| 3 | 4 | 0.1 | 4 | 57 | 19 | 3 |
| 5 | 3 | 0.2 | 4.1 | 21 | 1505 | 0.01 |
| 6 | 4 | 0.16 | 2.2 | 92 | 1 | 92 |
| 7 | 4 | NEGATIVE | NA | 7 | 3 | 2.3 |
| 8 | 19 | NEGATIVE | 4.6 | 27 | 0.5 | 54 |
| 9 | 12 | 1.7 | 3.2 | 1 | 415 | 0.002 |
| 10 | 3 | 0.2 | NA | 72 | 51 | 1.4 |
| 11 | 4 | 0.1 | 3.1 | 0.3 | 0.3 | 1 |
| 12 | 13 | 0.6 | 2.2 | 17 | 2 | 8.5 |
| 14 | 16 | 1 | 3.8 | 24 | 3 | 8 |
| 15 | 3 | 0.1 | NA | 35 | 5 | 7 |
| 16 | 7 | 0.1 | NA | 4 | 315 | 0.01 |
| 17 | 7 | 0.1 | 4.8 | 0.7 | 17 | 0.04 |
| 18 | 11 | 0.1 | 4.2 | 22 | 56 | 0.3 |
| 19 | 7 | 0.2 | 3.8 | 37 | 40 | 0.9 |
| 21 | 4 | 4.8 | 3.5 | 155 | 131 | 1.1 |
| 22 | 7 | NEGATIVE | NA | 2 | 296 | 0.006 |
| 24 | 7 | 1.3 | 3.5 | 26 | 83 | 0.3 |
| 25 | 3 | 0.1 | 3.8 | 15 | 200 | 0.07 |
| 26 | 2 | 2 | 3.4 | 46 | 1 | 46 |
| 27 | 6 | NEGATIVE | NA | 0.5 | 17 | 0.02 |
| 28 | 1 | 0.4 | 3.7 | 37 | 88 | 0.4 |
| 31 | 2 | 0.6 | 2.5 | 0.7 | 12 | 0.05 |
| 32 | NA | 0.08 | 4.9 | 28 | 292 | 0.09 |
| mean±s.d. | 6.5±4.5 | 0.6±1 | 3.6±0.8 | 30±34 | 142±302 | 9.2±21.5 |
Data represents average for each patient.
NA, not available.
Numbers correspond to patients in Table 1a.
Table 2.
Characteristics of control subjects
| Subject (n=11) |
Age (years) |
Urinary protein in dipstick |
Serum Albumin (g/dL) |
Urinary CD80 (ng/g creat) |
Urinary CTLA-4 (ng/g creat) |
|---|---|---|---|---|---|
| 33 | 16 | negative | Not done | 35 | 52 |
| 34 | 9 | negative | Not done | 0.5 | 13 |
| 35 | 16 | negative | Not done | 0.1 | 10 |
| 36 | 17 | negative | Not done | 0.2 | 0.2 |
| 37 | 6 | negative | Not done | 106 | 67 |
| 38 | 14 | negative | Not done | 0.2 | 0.2 |
| 39 | 13 | negative | Not done | 0.1 | 0.1 |
| 40 | 14 | negative | Not done | 0.1 | 0.1 |
| 41 | 13 | negative | Not done | 19 | 0.05 |
| 42 | 16 | negative | Not done | 0.1 | 5 |
| 43 | 15 | negative | Not done | 0.1 | 9 |
| mean ±s.d. | 13.5±3.1 | 14.6±30.8 | 14.2±22 |
This study was performed in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of the University of Florida, and written informed consent was obtained before participation.
Definitions
Steroid frequent relapsing patients were defined as having at least 2 relapses in 6 months and at the time of relapse prednisone therapy had been withdrawn for variable period of times (> 2 weeks) (11). Steroid infrequent relapsing patients were defined as having 1 or 2 relapses per year. Steroid dependency was defined as the presence of two consecutives relapses during steroid therapy, or within fourteen days of ceasing therapy.
Relapse of the nephrotic syndrome was defined as the presence of proteinuria of 3+ using the tetrabromophenol-citrate buffer colorimetric qualitative dipstick test, confirmed by a urine protein/creatinine ratio ≥ 2.0, and a concomitant serum albumin < 2.5 g/dL during the course of the episode. Complete remission was defined as no proteinuria using the colorimetric qualitative test and a urinary protein/creatinine ratio of 0.2 on a random morning urine sample.
Methods
Urinary CD80 was measured using a commercially available ELISA kit (Bender MedSystems, Burlingame, CA, USA). We measured urinary CTLA-4 according to Oaks and Hallet with minor modifications (12). CD80 and CTLA-4 results were adjusted for urinary creatinine excretion. Urinary creatinine and protein were measured using an autoanalyzer.
Statistical analysis
Data graphics and statistical analysis were performed using Prism 5 (GraphPad). Kruskal–Wallis tests and Mann–Whitney tests were applied to evaluate differences between the groups and Spearman correlation coefficient was calculated between urinary CD80 and proteinuria, urinary CTLA-4 and proteinuria and urinary CD80 and urinary CTLA-4 at the time of relapse and remission. P < 0.05 was regarded as statistically significant. Values are presented as means ± s.d. unless otherwise stated.
Results
Urinary CD80 excretion in MCD and control subjects
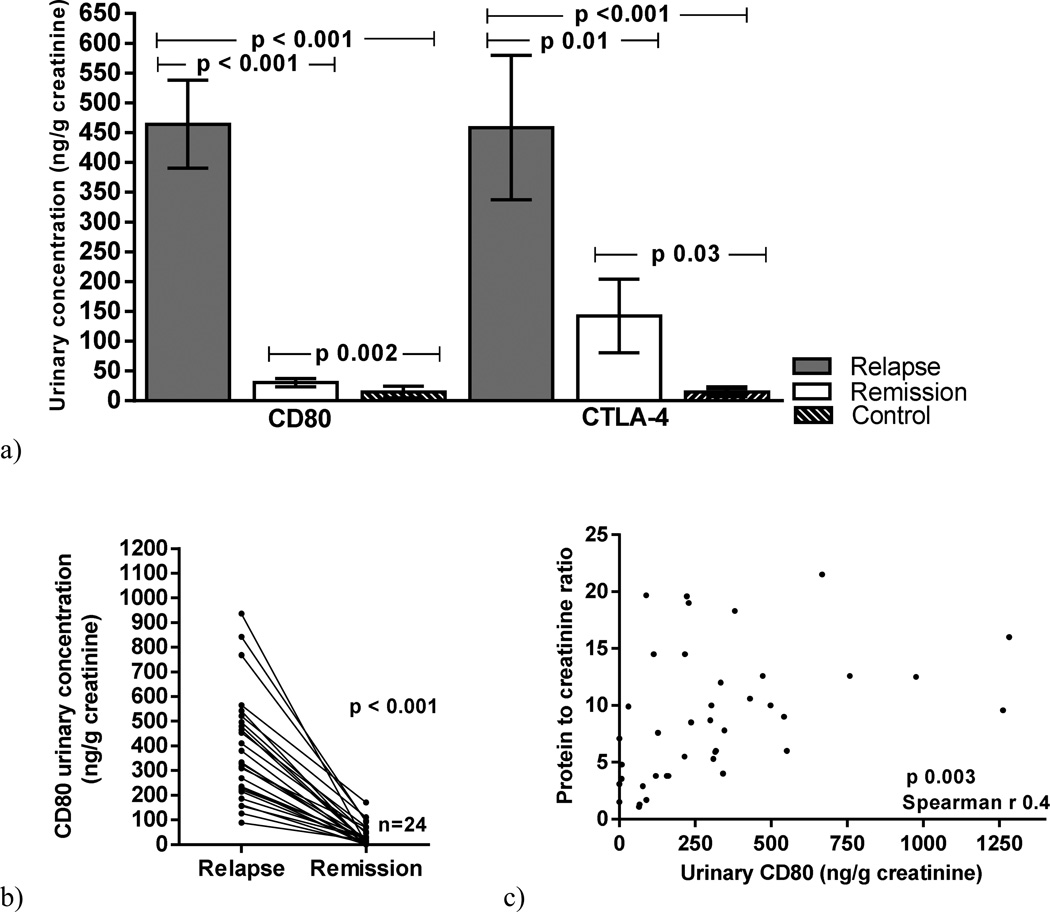
Urinary CD80 excretion was significantly increased in MCD patients in relapse (464±398 ng/g creatinine) when compared to those MCD patients in remission (30±34 ng/g creatinine) (p<0.001) and control subjects (14.6±30.8 ng/g creatinine) (p<0.001) (Figure 1a). Twenty-four patients with MCD were studied at relapse and within 1 month after remission. Urinary CD80 decreased significantly (p<0.001) after remission was induced (Figure 1b). There was a positive correlation between urinary CD80 excretion and proteinuria in MCD patients in relapse (p=0.003) (Figure 1c)
Figure 1.
Urinary CD80 and urinary CTLA-4 concentration in Minimal Change Disease (MCD) patients in relapse, MCD patients in remission and control subjects (a). Serial urinary CD80 in MCD patients at the time of relapse and remission (b). Correlation between urinary CD80 and proteinuria in MCD patients (c).
Urinary CTLA-4 excretion in MCD and control subjects
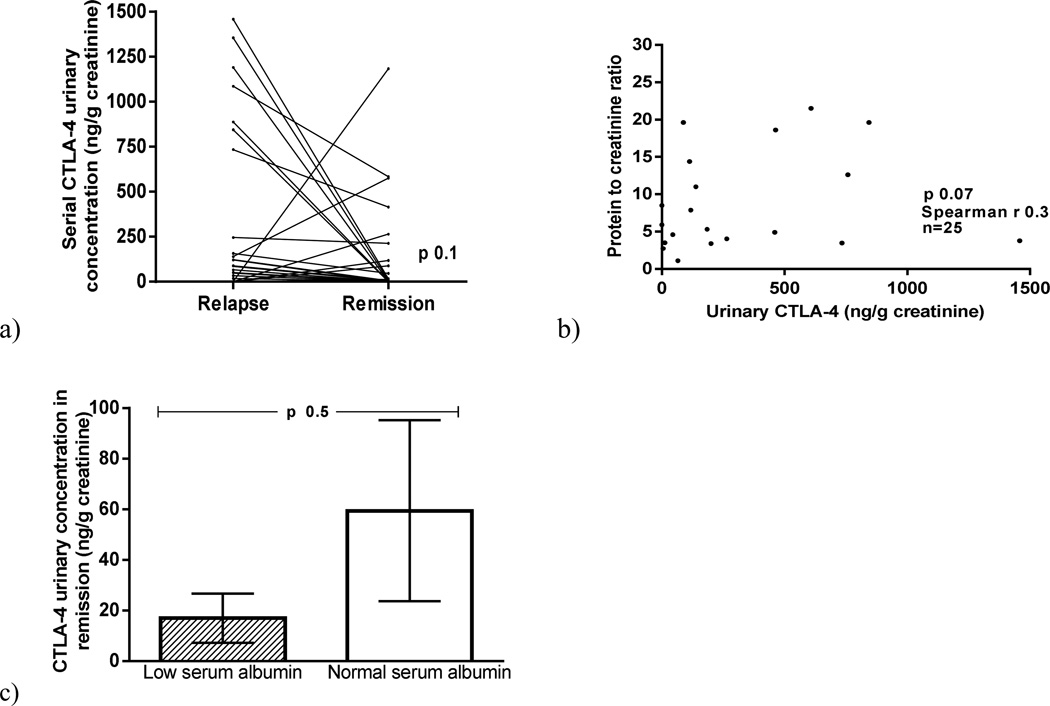
Urinary CTLA-4 excretion was higher in MCD patients in relapse (458±652 ng/g creatinine) than in MCD patients in remission (142±302 ng/g creatinine) (p=0.01) and control subjects (14.2±22 ng/g creatinine) (p=0.03) (Figure 1a). When urinary excretion of CD80 and CTLA-4 were evaluated in the same patients in relapse and in remission, CD80 decreased significantly in all patients at the time of remission but CTLA-4 either decreased or remained unchanged in all but five of the 25 patients (Figure 2a). Five patients, however, showed an increase in urinary CTLA-4 excretion with remission (Figure 2a). There was no significant correlation between urinary CTLA-4 and proteinuria in MCD patients at the time of relapse (p=0.07) (Figure 2b). Urinary CTLA-4 excretion was similar in those patients with urine samples obtained at the time of resolution of the proteinuria but still showing low serum albumin and those who presented with no proteinuria and a normal serum albumin (p=0.6)(Figure 2 c).
Figure 2.
Serial urinary CTLA-4 in Minimal Change Disease (MCD} patients at the time of relapse and remission (a). Correlation between urinary CTLA-4 and proteinuria in MCD patients (b). Urinary CTLA-4 in MCD patients in remission with normal serum albumin and low serum albumin (c).
Urinary CTLA-4 of patients in relapse was analyzed according to time to achieve remission. Although those who responded earlier (< 2 weeks) showed a higher CTLA urinary excretion when compared to those who took > 2 weeks to respond, the difference was not statistically significant between the groups (p=0.9).
We observed no significant differences in the urinary CTLA-4 excretion among patients who were not receiving any treatment and those treated either with steroids and/or calcineurin inhibitors either at the time of relapse or at the time of remission (p=0.4).
The excretion of urinary CTLA-4 in MCD patients in relapse was not significantly different among patients with different patterns of response (infrequent relapse, frequent relapse and steroid-dependent) (p=0.8).
Urinary CD80 and CTLA-4 in MCD
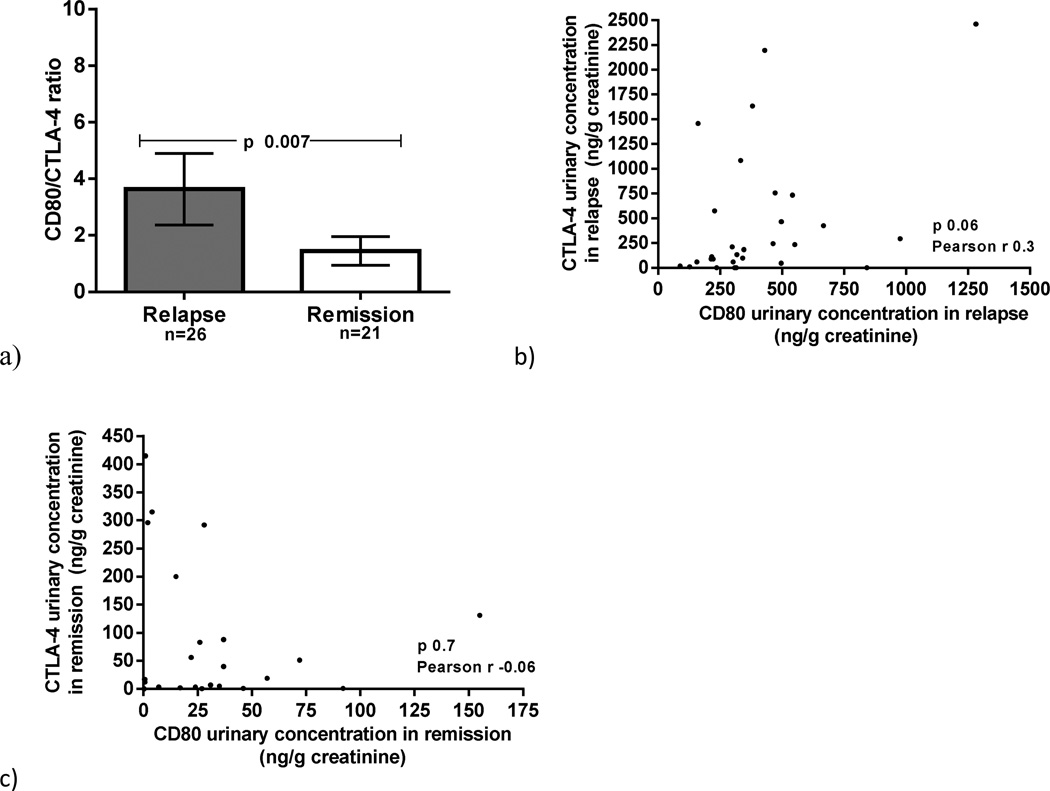
We examined the ratio of urinary CD80 to CTLA-4 in MCD patients in relapse and in remission. We found that the urinary CD80/CTLA-4 ratio was elevated in MCD in relapse when compared to those in remission (p=0.007) (figure 3a). No inverse correlation was observed between urinary CD80 and urinary CTLA-4 in MCD patients at the time of relapse (p=0.06) (figure 3b) nor remission (p=0.7) (figure 3c).
Figure 3.
CD80/CTLA-4 ratio in Minimal Change Disease (MCD) patients in relapse and MCD patients in remission (a). Correlation between urinary CD80 and urinary CTLA-4 in MCD patients in relapse (b) and in remission (c).
Discussion
In this study we evaluated the relationship of urinary CD80 and CTLA-4 excretion in MCD subjects in relapse and remission to test the hypothesis that intrarenal CTLA-4 fails to block podocyte CD80 expression resulting in the persistently elevated CD80 urinary excretion observed in MCD patients in relapse. We have studied the urinary excretion of CD80 and CTLA-4 as their most likely source is the glomerulus.
As in our previous reports (6), and studying a different set of MCD patients, we found a significantly elevated urinary CD80 excretion in MCD patients in relapse compared to those in remission.
CD80, a transmembrane protein, is present in dendritic cells, podocytes, renal tubular cells and B cells (13, 14). The source of the increased urinary CD80 in MCD patients in relapse is the podocyte. This argument is supported by several facts: 1) serum level of sCD80 is similar in patients with MCD during relapse compared to MCD in remission, excluding the idea of over secretion of circulating CD80 by extra renal dendritic cells (6). 2) Immunofluorescence studies in MCD patients in relapse showed that CD80 localized exclusively in the podocyte (5). 3) The molecular weight of the CD80 measured in urine is 53 KDa, consistent with it being the cell-membrane associated CD80, in contrast to soluble CD80 (23 KDa) which represents the extracellular portion of the molecule (5).
CD80 may play a critical role in the development of proteinuria in nephrotic syndrome including MCD (15). The CD80 role is based on the LPS model of nephrotic syndrome in the mice, in which CD80 was de novo expressed on podocytes in association with proteinuria and foot processes effacement. Importantly, no proteinuria was observed when CD80 knock-out mice were administered LPS (15).
CTLA-4, cytotoxic T-lymphocyte antigen 4, is a surface protein expressed by T cells which down-regulates T cell activation after binding to CD80 on the antigen presenting cells (8). The most likely source of urinary CTLA-4 in MCD patients is the podocyte since we previously reported no difference in serum CTLA-4 from patients with MCD in relapse compared to those in remission (6). Therefore, the increased urinary CTLA-4 is not due to filtered circulating CTLA-4. Furthermore, cultured human podocytes exposed to Poly:IC have been shown to express CTLA-4 (7). In addition, there is no evidence of tubular CTLA-4 expression.
We have hypothesized that the persistent CD80 podocyte expression and increased urinary CD80 excretion observed in MCD patients in relapse is due to an impaired podocyte CTLA-4 response which is unable to censor the CD80 expression in the podocyte (9) Since CTLA-4 is the natural inhibitor of CD80 and increased CD80 podocyte expression may induce proteinuria, one might speculate that the urinary levels of CTLA-4 should increase during relapse reflecting the increased intrarenal CTLA-4 production regulating the augmented CD80 podocyte expression.
In this study, 7 out of 30 patients with MCD in relapse were found to have a normal urinary excretion of CTLA-4. Four of these patients were found to have a higher urinary CTLA-4 excretion once remission was achieved. In 23 out of 30 patients with MCD, urinary CTLA-4 excretion was increased during relapse. Urine specimens were available during remission in 17 out of these 23 patients. All these patients but one were found to have lower urinary CTLA-4 excretion after achieving remission compared to relapse. In one patient, the urinary CTLA-4 excretion increased further once remission was achieved.
The excretion of urinary CD80 remained elevated despite increased urinary CTLA-4, as indicated by the CD80/CTLA-4 ratio, finding similarly reported by us in a smaller number of patients (6). In 9 out of 30 patients in relapse the ratio CD80/CTLA-4 was ≤1, but CD80 was rather elevated and proteinuria presented. This suggests that the CTLA-4 response, while present during relapse, was inadequate to down-regulate CD80 podocyte expression. It can be argued that there has been not enough time for the podocyte to increase CTLA-4 expression when the urine samples were collected. However, the CTLA-4 expression was also low during early remission (urine samples taken when proteinuria had resolved but serum albumin was still low) and similarly in late remission (no proteinuria with normalization of serum albumin). Indeed, the inability of urinary CTLA-4 levels to increase higher during relapse could be speculated to explain the low spontaneous remission rate of <10% in MCD patients.
Almost all patients were receiving some type of immunosuppressive therapy at the time of relapse. Two drugs are known to influence CTLA-4 expression. Calcineurin agents are known to decrease CTLA-4 expression in T cells (16). However, the blunted CTLA-4 during relapse could not be attributed to the effect of calcineurin agents because there was no difference in CTLA-4 urinary excretion in patients in relapse when patients on prednisone were compared with those receiving prednisone and tacrolimus. This blunted CTLA-4 expression during remission cannot be due to prednisone effect because steroids have been reported to increase CTLA-4 expression (16).
Many single nucleotide polymorphisms have been identified in the CTLA-4 gene. The +49GG genotype has been associated with decreased expression of CTLA-4 (17).The replacement of threonine by alanine has been thought to induce a conformational change of CTLA-4 protein leading to an insufficient transport of the molecule to the cell surface. Confirming this, a higher ratio of cell surface/total CTLA-4Thr versus CTLA-4 Alan has been observed (17). Of interest in our discussion, the frequency of the +49GG genotype is significantly increased in MCD patients with respect to normal controls (17).
In conclusion, CTLA-4 levels in the urine do not correlate with urinary CD80 excretion, suggesting the possibility that the CTLA4 response to the observed augmented CD80 podocyte expression may be suboptimal in this disease during relapse.
Chimeric CTLA-4 is commercially available. The above findings are the background to justify the administration of the drug to MCD patients in relapse. Well design studies need to be performed before CTLA4-Ig becomes one more drug to treat MCD patients. CTLA4-Ig has been widely used in patients with rheumatoid arthritis with an optimal safety profile (18).
The CTLA-4 administration with resolution of the proteinuria could have two important consequences. It may become therapy available to treat the MCD patient presenting severe steroid dependency or resistance but also will confirm that in MCD patients, the mechanism of the proteinuria is due to an increased and uncontrolled podocyte expression of CD80 triggered by a yet to be discovered circulating factor.
Acknowledgments
Support:
This study was supported by NIH R01DK080764 to E.H.G. and R.J.J.
Footnotes
Financial Disclosures:
Authors declare no conflict of interest.
References
- 1.Feehally J, Floege J, Johnson RJ. Comprehensive clinical nephrology. 3rd edn. Philadephia: Mosby Elsevier; 2007. pp. 209–216. [Google Scholar]
- 2.Alwadhi RK, Mathew JL, Rath B. Clinical profile of children with nephrotic syndrome not on glucorticoid therapy, but presenting with infection. J Paediatr Child Health. 2004;40:28–32. doi: 10.1111/j.1440-1754.2004.00285.x. [DOI] [PubMed] [Google Scholar]
- 3.Ponticelli C, Glassock RJ. Treatment of Primary Glomerulonephritis. 2nd edn. New York: Oxford University Press; 2010. p. 185. [Google Scholar]
- 4.Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007;2:529–542. doi: 10.2215/CJN.04121206. [DOI] [PubMed] [Google Scholar]
- 5.Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, Johnson RJ. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78:296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]
- 6.Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, Johnson RJ. Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol. 2009;20:260–266. doi: 10.1681/ASN.2007080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishimoto T, Shimada M, Gabriela G, Kosugi T, Sato W, Lee PY, Lanaspa MA, Rivard C, Maruyama S, Garin EH, Johnson RJ. Toll-like receptor 3 ligand, polyIC, induces proteinuria and glomerular CD80, and increases urinary CD80 in mice. Nephrol Dial Transplant. 2013;28:1439–1446. doi: 10.1093/ndt/gfs543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 9.Shimada M, Araya C, Rivard C, Ishimoto T, Johnson RJ, Garin EH. Minimal Change Disease: A "Two hit" podocyte immune disorder? Pediatr Nephrol. 2011;26:645–649. doi: 10.1007/s00467-010-1676-x. [DOI] [PubMed] [Google Scholar]
- 10.Pathology of the nephrotic syndrome in children: A report for the International Study of kidney Disease in Children. Lancet. 1970;760:1299–1302. doi: 10.1016/s0140-6736(70)91905-7. [DOI] [PubMed] [Google Scholar]
- 11.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–771. doi: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 12.Oaks MK, Hallet KM. Cutting edge: A soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol. 2000;164:5015–5018. doi: 10.4049/jimmunol.164.10.5015. [DOI] [PubMed] [Google Scholar]
- 13.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 14.Niemann-Masanek U, Mueller A, Yard BA, Waldherr R, van der Woude FJ. B7–1 (CD80) and B7–2 (CD86) expression in human tubular epithelial cells in vivo and in vitro. Nephron. 2002;92:542–556. doi: 10.1159/000064084. [DOI] [PubMed] [Google Scholar]
- 15.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia M, Gasser J, Feige U. Dexamethasone enhances CTLA-4 expression during T cell activation. Cell Mol Life Sci. 1999;55:1649–1656. doi: 10.1007/s000180050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spink C, Stege G, Tenbrock K, Harendza S. The CTLA-4 +49GG genotype is associated with susceptibility for nephrotic kidney diseases. Nephrol Dial Transplant. 2013;28:2800–2805. doi: 10.1093/ndt/gft381. [DOI] [PubMed] [Google Scholar]
- 18.Schiff M, Weinblatt ME, Valente R, van der Heijde D, Citera G, Elegbe A, Maldonado M, Fleischmann R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014;73:86–94. doi: 10.1136/annrheumdis-2013-203843. [DOI] [PMC free article] [PubMed] [Google Scholar]