Abstract
Oral mucositis is a significant problem in cancer patients treated with radiation or chemotherapy, often hindering definitive cancer treatment. For patients with oral mucositis, pain is the most distressing symptom, leading to loss of orofacial function and poor quality of life. While oral mucositis has been well-described, its pathophysiology is poorly understood. Oral health professionals treating patients with mucositis have almost no effective therapies to treat or prevent oral mucositis. The purpose of this review is to (1) describe the current preclinical models of oral mucositis and their contribution to the understanding of mucositis pathophysiology, (2) explore preclinical studies on therapies targeting mucositis and discuss the clinical trials that have resulted from these preclinical studies, and (3) describe the proposed pathophysiology of oral mucositis pain and preclinical modeling of oral mucositis pain.
Keywords: mucositis treatment, stomatitis, cancer complications, oral pain, cancer symptoms, mucositis models
Introduction
Mucositis is painful ulceration of the alimentary mucosa. It develops in virtually all head and neck cancer patients receiving radiation or chemotherapy, in patients with hematologic malignancies undergoing stem cell transplant, and in up to 40% of cancer patients receiving chemotherapy (Sonis, 2004). While mucositis could occur throughout the digestive tract, mucositis in the oral cavity is particularly distressing and painful (Sonis, 2013). Severe oral mucositis hinders definitive treatment of the cancer (Scully et al., 2003). Furthermore, oral pain resulting from mucositis drastically reduces oral function and nutritional intake, often requiring percutaneous endoscopic gastrostomy (PEG) tube insertion, systemic analgesics, and a prolonged hospital stay (Sonis, 2004). Although oral mucositis has been well-described, there is no effective therapy for mucositis or its associated pain. Current supportive measures to reduce pain and discomfort for patients with oral mucositis include improved oral hygiene, soft bland diet, bicarbonate/saline rinses, mucosal coating agents, cryotherapy, and systemic analgesics. Aside from these minimally effective supportive measures, oral health professionals treating patients with oral mucositis have almost no effective therapeutic options.
Oral mucositis pathogenesis is multifactorial. The current pathogenesis model includes cytotoxicity from chemotherapeutic drugs, loss of epithelial junctional integrity, inflammation, myelosuppression due to treatment, and changes in the oral flora. A five-phase chronological process has been proposed to explain mucositis, involving (1) cell death, (2) the generation of reactive oxygen species, (3) the activation of biological pathways, (4) ulceration, and (5) mucosalization (Sonis, 2004), and has been reviewed extensively in previous publications. In this review we explore recent advances in mucositis research. We focus on studies using preclinical mucositis models and novel therapeutics that effectively prevent, provide relief against, or reduce the severity of mucositis. Furthermore, since pain is the most distressing symptom in patients, we explore the pathophysiology of oral pain in mucositis and current advances in mucositis pain modeling.
Preclinical Models of Mucositis
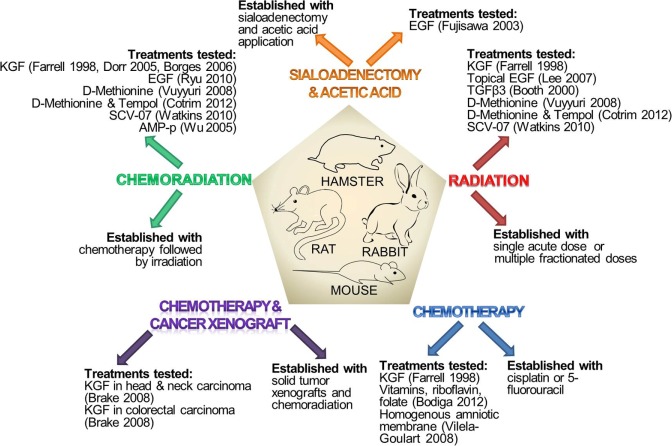
Animals used in preclinical studies of mucositis include rats, mice, hamsters, and rabbits [reviewed previously in Bowen et al. (2011)]. Mucositis is generated by radiation or chemotherapeutic drugs, which replicate cancer treatment in patients (Table 1, Fig. 1). Wolfgang Dorr developed a mouse radiation model (Dorr and Kummermehr, 1990), while Stephen Sonis developed the golden Syrian hamster radiation model (Hwang et al., 2005). Over the years, the radiation model has been refined; currently, radiation challenge could be either a single acute dose or multiple fractionated doses (Watkins et al., 2010). In addition, the radiation model has evolved to include chemotherapy, which replicates chemoradiation treatment in cancer patients (Alvarez et al., 2003). Aside from radiation or chemoradiation mucositis models, chemotherapy-induced mucositis models are frequently used. The first chemotherapy mucositis animal model was published in 1990 and used repeated intraperitoneal injections of 5-fluorouracil (5-FU) in hamsters, (Sonis et al., 1990), followed by mechanical injury to the buccal mucosa to induce ulceration. Currently, common chemotherapeutics used to induce mucositis include cisplatin and 5-FU (Dorr et al., 2005). A less common model involves mucosal injury with acetic acid. Acetic acid is applied after either a sialoadenectomy or intravenous treatment with cisplatin and peplomycin sulfate. Both procedures create a state of impaired healing from the acetic acid injury and, ultimately, ulceration at the injury site (Fujisawa et al., 2003). Most preclinical studies focus on oral mucositis; however, a few do measure intestinal mucositis and loss of structural integrity of intestinal crypts. The focus on oral, rather than intestinal, mucositis could be attributed to the fact that oral mucositis is significantly debilitating and painful in patients. In studies where the investigators are also exploring possible effects of the mucositis treatment on tumor activity, xenografts of squamous cell carcinoma are concurrently established in the animal mucositis models (Brake et al., 2008).
Table 1.
Methods to Establish Animal Models of Mucositis
| Mucositis Model Type | Species Used | Mechanism | Described by |
|---|---|---|---|
| Radiation | Golden Syrian hamster | Buccal cheek pouch radiation in single dose or fractionated doses | Hwang et al., 2005 |
| Mouse | Tongue and snout radiation in single dose or fractionated doses | Dorr et al., 1990 | |
| Chemotherapy | Golden Syrian hamster | Injection of 5-FU followed by buccal mucosa injury | Sonis et al., 1990 |
| Mouse | Injection of 5-FU | Farrell et al., 1998 | |
| Rat | Injection of 5-FU followed by mucosal injury | Vilela-Goulart et al., 2008 | |
| Rabbit | Injection of cisplatin/peplomycin sulfate followed by mucosal injury with acetic acid | Fujisawa et al., 2003 | |
| Chemoradiation | Golden Syrian hamster | Radiation with cisplatin or 5-FU injection | Alvarez et al., 2003 |
| Mouse | Radiation with cisplatin and/or 5-FU injection | Dorr et al., 2005 | |
| Chemoradiation and cancer xenograft | Mouse | Inoculation with cancer followed by chemotherapy | Brake et al., 2008 |
| Sialoadenectomy | Rabbit | Sialoadenectomy followed by mucosal injury with acetic acid | Fujisawa et al., 2003 |
Figure 1.
Methods of establishing preclinical mucositis models and treatments tested in these models. The diagram illustrates the established preclinical mucositis models used to test the efficacy of different mucositis treatments. These preclinical models are created in rat, mouse, hamster, or rabbit species. Methods used to induce mucositis include radiation, chemotherapy, chemoradiation, or sialoadenectomy. To investigate the effects of mucositis treatments on proliferation of solid tumors, researchers also use a solid tumor xenograft animal model, in which mucositis is induced by chemotherapy and/or radiation. EGF, epidermal growth factor; KGF, keratinocyte growth factor; SCV-07, gamma-D-glutamyl-L-tryptophan; TGFβ3, transforming growth factor-β3.
Preclinical Studies on Mucositis Treatments
Growth Factors
Keratinocyte Growth Factor
Since mucositis pathogenesis involves damage to the epithelial and submucosal cells, which results in ulceration and breakdown of mucosal barrier, many preclinical trials focus on the use of growth factors to regenerate the mucosal barrier. Growth factors are thought to regenerate mucosa by stimulating cellular proliferation. Of the growth factors that have been studied, recombinant human keratinocyte growth factor (KGF) is possibly the most widely investigated. KGF is a member of the fibroblast growth factor family. Its receptor, KGFR, is predominantly expressed on epithelial cells. KGF activation of its receptor results in increased proliferation, migration, differentiation, and survival of epithelial cells (Finch and Rubin, 2004). The therapeutic efficacy of KGF has been studied in multiple preclinical mucositis models (Farrell et al., 1998). These models were established with chemotherapy alone, radiotherapy alone, or chemoradiation. In this study, KGF was used as a pretreatment; models receiving KGF had significantly less weight loss and longer survival than in controls. These benefits were attributed to increased mucosal thickness in response to palifermin pretreatment (see Table 2 for a summary of efficacy of treatments discussed in this review). The promising preclinical results were upheld in clinical studies, and in 2005 a truncated form of the recombinant human KGF, named ‘palifermin’, was approved for use to decrease the incidence and duration of severe mucositis in patients with hematologic malignancies receiving intensive chemoradiotherapy for autologous stem cell transplant (Sonis, 2010). Since then, preclinical studies have also been conducted to expand the use of palifermin for mucositis in solid malignancies. In a study by Dorr et al., palifermin was injected into a mouse model before and during chemoradiation. This treatment schedule of palifermin injection resulted in increased dose tolerance of radiation and chemotherapy (Dorr et al., 2005). Since tumor cells of epithelial origin express KGFR, there was concern that palifermin could protect tumors against antitumor drugs. A study by Brake et al. determined whether palifermin decreased antitumor activity of several chemotherapeutics and biologics in mouse xenograft models of head and neck carcinoma and colorectal carcinoma. Results of this study showed that palifermin did not promote proliferation in the carcinoma models and also had no effect on antitumor activity of the drugs (Brake et al., 2008). In a mouse model of chemoradiation-induced mucositis, palifermin injection prior to chemoradiation promoted cell proliferation and increased epithelial thickness of the oral mucosa, salivary glands, and digestive tract (Borges et al., 2006). Despite promising results in preclinical models of solid malignancies, evidence to support the use of palifermin for mucositis prevention in patients with solid malignancies is still incomplete (Sonis, 2010).
Table 2.
Oral Mucositis Treatments and Mechanisms of Action
| Therapy | Method of Application | Species Tested | Reduces Severity | Accelerates Healing |
|---|---|---|---|---|
| Amniotic membrane biological dressing | Oral | Rat | X | |
| Antral mucosal protein peptide (AMP-p) | Subcutaneous | Hamster | X | X |
| D-Methionine | Intravenous or topical | Mouse, rat | X | |
| Epidermal growth factor (EGF) | Subcutaneous or topical | Mouse, rabbit | X | X |
| Gamma-D-glutamyl-L-tryptophan (SCV-07) | Subcutaneous | Hamster | X | X |
| Keratinocyte growth factor (KGF) | Subcutaneous | Mouse | X | X |
| Tempol | Intraperitoneal or topical | Mouse | X | |
| Transforming growth factor-β3 (TGFβ3) | Intraperitoneal | Mouse | X | |
| Vitamins, riboflavin, and folate | Oral | Rat | X | X |
Epidermal Growth Factor
Another growth factor that has been investigated in preclinical models of mucositis is epidermal growth factor (EGF). EGF is produced by platelets, macrophages, and monocytes. It acts on its receptor EGFR on epithelial cells, smooth-muscle cells, and fibroblasts to increase their proliferation and accelerate wound healing. In a mouse model of mucositis that was generated by cisplatin and radiation, systemic administration of EGF reduced the severity of oral mucositis. Histologically EGF-treated mice showed significantly increased epithelial cell layer thickness, increased basal cell number, and expression of Ki-67 (proliferative marker) compared with controls (Ryu et al., 2010). Unlike studies where palifermin was administered prior to chemoradiation, in this study EGF was administered after chemoradiation-induced mucositis. Another study from the same group tested the efficacy of topical rather than systemic EGF administration and showed that topical EGF had similar wound-healing benefits in a radiation mucositis model (Lee et al., 2007). EGF was used in a rabbit model of mucositis that was induced by sialoadenectomy and acetic acid mucosal injury. In this study, the authors injected EGF systemically after mucositis lesions developed. They showed that systemic EGF accelerated mucosal healing. However, topical EGF application did not significantly accelerate wound healing (Fujisawa et al., 2003). A clinical trial evaluated a recombinant human EGF oral spray in 58 patients with oral mucositis induced by intensive chemotherapy followed by stem cell transplant. Patients with mucositis of grade 3 or higher (according to the World Health Organization scale) who were treated with the EGF oral spray had significantly shorter duration of mucositis, reduced limitations in swallowing and drinking, shorter duration of hospital stays, total parenteral nutrition, and opioid usage (Kim et al., 2013).
Transforming Growth Factor-β3
Transforming growth factor-β3 (TGF-β3) has been found to reduce radiation-induced intestinal cell damage in a mouse model (Booth et al., 2000). In a phase I clinical trial on 11 breast cancer patients, a TGF-β3 mouthwash was administered at the start of chemotherapy and showed good tolerability (Wymenga et al., 1999). A larger clinical trial using the TGF-β3 mouthwash in patients with solid malignancies undergoing high-dose chemotherapy was started. However, the trial was stopped when results of preclinical studies were published suggesting suboptimal formulation and dosing. Interim analysis of the halted clinical trial showed that the TGF-β3 mouthwash did not decrease the incidence or duration of mucositis (Foncuberta et al., 2001). The negative results from this clinical trial highlight the importance of well-designed preclinical trials to establish appropriate dosage and frequency of the intervention to treat oral mucositis.
Amino Acids and Peptides
D-Methionine and Tempol
D-Methionine is a dextro-isomer of the common amino acid L-Methionine. In preclinical models, it has been shown to protect normal tissues, but not tumor tissue, from chemotherapy- and radiation-induced cell death. D-Methionine treatment did not alter tumor control following cisplatin and radiation. Pharmacokinetics showed that liquid suspension of D-Methionine in rats had 68% bioavailability relative to IV administration (Vuyyuri et al., 2008). In a mouse model of radiation-induced mucositis, D-Methionine showed a dose-dependent increase in protection from mucositis. Furthermore, D-Methionine did not compromise chemoradiation-mediated tumor control (Vuyyuri et al., 2008). A phase I clinical trial was conducted to assess the pharmacokinetics of MRX-1024, the orally bioavailable formulation of D-Methionine, in healthy individuals and head and neck cancer patients undergoing radiation therapy and/or chemotherapy. D-Methionine treatment was associated with a modest increase in emesis, with one incidence of grade 3 emesis (Hamstra et al., 2010).
D-Methionine has also been used in combination with Tempol in a preclinical model of radiation with and without cisplatin-induced mucositis. A D-Methionine gavage was given with either an intraperitoneal injection or a topical application of Tempol prior to chemoradiation. While either drug alone provided partial protection from ulcerative mucositis, the combination of the two drugs provided complete protection (Cotrim et al., 2012).
AMP-p
A 21-amino-acid peptide derived from antrum mucosal protein-18 (AMP-p) has been used in a hamster model of oral mucositis that was established with radiation and cisplatin. Daily subcutaneous injections of Amp-p reduced the incidence and duration of mucositis. This effect was attributed to the peptide’s ability to limit tight junction loss and cellular apoptosis in the presence of tumor necrosis factor-alpha (a secreted mediator in the oral mucositis microenvironment that induces cell death) (Wu et al., 2012).
Gamma-D-Glutamyl-L-Tryptophan (SCV-07)
SCV-07 is a synthetic water-soluble peptide that acts as an immune modulator by stimulating T-helper 1 (Th1) cells and inhibiting T-helper 2 (Th2) cells. The homeostatic disruption of Th1:Th2 cells is hypothesized to contribute to mucositis pathogenesis, and SCV-07 functions to restore the imbalance. While SCV-07 was clinically developed to treat infectious diseases, it was applied to mucositis treatment in a preclinical model in golden Syrian hamsters. SCV-07 was found to reduce the severity and duration of oral mucositis resulting from acute or fractionated radiation, and the duration of mucositis resulting from chemoradiation (Watkins et al., 2010). Subsequently, a phase IIa dose-ranging study was conducted on 59 head and neck cancer patients receiving chemoradiation. In this trial, only some patients treated with SCV-07 had a delay in the onset of mucositis. This non-uniform response led the investigators to perform a gene expression array and identify 107 genes to differentiate between the responders and non-responders (Alterovitz et al., 2011). An additional phase II trial was initiated in 2010, but was halted after a Data and Safety Monitoring Board review in 2012 demonstrated no drug effect.
Vitamin Supplements and Antioxidants
The pathogenesis of mucositis involves the release of reactive oxygen species, which activate multiple biological pathways and result in cell death; as such, the efficacy of antioxidants in reducing mucositis has been explored. A multiple vitamin mixture in combination with riboflavin and folate has been tested for their ability to reduce the oxidative burden in a rat model of chemotherapy-induced mucositis. ‘Oxidative burden’ was quantified as the formation of thiobarbituric-acid-reactive substances and protein carbonyls. The vitamin and antioxidant mixture was able to improve the functional and structural integrity of intestinal crypts and reduced apoptotic indices (Bodiga et al., 2012).
Amniotic Membrane
Aside from treatment with peptides or growth factors that could accelerate epithelial healing, some studies have taken a completely different approach by protecting the mucositis lesion with a physical barrier. Vilela-Goulart et al. used a rat model of 5-FU-induced mucositis to test the efficacy of a homogenous amniotic membrane (HAM) as a biological dressing for oral mucositis lesions (Vilela-Goulart et al., 2008). The amniotic membrane in this study was taken from rats, had a thin epithelial layer, thick basement membrane layer, and an avascular stroma of collagen. Studies prior to this preclinical trial showed that amniotic membranes have antibacterial properties and low immunogenicity, promote re-epithelialization, and decrease inflammation. In addition, they also produce growth factors such as KGF, EGF, and TGFβ (Alviano et al., 2007). Rats treated with HAM showed a histologically lower quantity of inflammatory cells.
Pathophysiology and Preclinical Modeling of Oral Mucositis Pain
Oral Mucositis Pain
While oral mucositis pain causes dysfunction and significant distress in patients, preclinical and clinical studies evaluating the efficacy of novel treatments for mucositis rarely measure the ability of the treatment to reduce pain. Weight loss is often measured and used as a proxy for oral function. A trial on doxepin quantified systemic analgesic use between trial visits as an index of pain. The study participants were also asked to rate their pain on a visual analog scale at study visits (Epstein et al., 2007). A few clinical trials measured oral function. One trial that used benzadymine chloride for mucositis management measured pain on swallowing; patients in the treatment group had decreased pain on swallowing compared with those in the placebo group. Another trial using tetrachlorodecaoxide measured oral intake in 62 patients (Malik et al., 1997). The treatment group reported improved oral intake and a shorter duration of oral pain (3.1 days in the treatment group vs. 3.6 days in the placebo group). The lack of attention to pain in clinical trials carries over to clinical practice, where oral mucositis pain is often under-recognized and inadequately managed (Epstein et al., 2012).
Lack of progress in the treatment of oral mucositis pain could be attributed to a poor understanding of the pathogenesis of mucositis pain. Just as there are few clinical studies measuring pain and loss of function, there are even fewer preclinical studies to elucidate the molecular pathways of mucositis pain. Our understanding of oral mucositis pain is derived from animal models of other oral pain conditions, such as oral cancer pain or temporomandibular joint pain. Here we review receptors and pathways with known roles in these oral pain conditions. While their role in oral mucositis pain is not yet established, future preclinical mucositis studies could focus on these specific receptors and pathways, since they are likely to result in novel mucositis pain treatments.
Nociceptive Component of Mucositis Pain
Oral mucositis pain includes both nociceptive and neuropathic etiologies. In all oral pain conditions, nociceptive stimuli are conveyed through the trigeminal system and terminate in the subnucleus caudalis. Nociceptive afferents include A-delta fibers, which convey sharp pain, and C fibers, which convey burning pain. C fibers are further divided into peptidergic and non-peptidergic populations. Nociceptive pain in the oral cavity is modulated by several different receptors, including the transient receptor potential (TRP) family of sensory ion-channel proteins, the endothelin-1 (ET-1) receptors, the tumor necrosis factor alpha (TNFα) receptor, and receptors for nerve growth factor (NGF) (Fig. 2).
Figure 2.
Mediators, receptors, and nociceptor fiber types within the trigeminal system that likely contribute to oral mucositis pain. TRP receptors associated with mechanical hyperalgesia include TRPV1, TRPA1, TRPV4, and TRPM8. Epithelial cells within the oral mucositis microenvironment mediate inflammation by secreting IL-1β, IL-2, IL-6, TNFα, and NGF. TNFα activates TNFR2, producing a nociceptive response. NGF binds to either the low-affinity p75 receptor or high-affinity TrkA receptor on peptidergic neurons. Peptidergic C fibers and A-delta fibers secrete substance P (SP) and calcitonin gene-related peptide (CGRP) in the periphery, and SP, CGRP, and glutamate in the nucleus caudalis, to mediate neuronal sensitization and nociception. ET-1 and cyclooxgenase-2 (COX-2) production are induced by the transcription factor NF-κB. Secretion of ET-1 is hypothesized based on the up-regulation of NF-κB in the oral mucositis microenvironment. The COX-2 enzyme produces prostaglandins (PG). TNFR2, TRPV4, and prostaglandin receptors (PR) have not been localized to specific fiber types, and are shown here on multiple fiber types.
The transient receptor potential (TRP) family of sensory ion-channel proteins is expressed on trigeminal ganglion neurons that convey thermal and mechanical nociception in the orofacial region (Alessandri-Haber et al., 2009). Mechanical nociception results in oral dysfunction in patients with oral mucositis. Within the TRP family, receptors conveying mechanical nociception include TRPV1 (Nilius et al., 2007), TRPA1, TRPM8 (Colburn et al., 2007; Basbaum et al., 2009), and TRPV4 (Alessandri-Haber et al., 2009). TRP receptor sensitization by peripheral signaling molecules (Nilius et al., 2007; Basbaum et al., 2009) further contributes to mechanical pain. TRPV4 activation also results in the release of endothelin-1 (Moore et al., 2013), a vasoactive and nociceptive peptide.
Endothelin-1 (ET-1) mediates nociception in all trigeminal branches (Chichorro et al., 2010). The two ET-1 receptor subtypes, ETAR and ETBR, are expressed in human trigeminal ganglia (Uddman et al., 2006). These receptor subtypes are also expressed in rat trigeminal ganglia (Yamamoto et al., 2013). Therefore, preclinical models exploring the role of ET-1 in orofacial pain have involved rats. ET-1 injection into the upper lip or temporomandibular joint of the rat results in nociception (Chichorro et al., 2010). In addition, both ETAR and ETBR receptors in rat trigeminal ganglia mediate orofacial thermal hyperalgesia (Chichorro et al., 2009). ET-1 is elevated in the saliva of oral cancer patients with pain (Pickering et al., 2007), and blockade of the endothelin receptors alleviates oral cancer pain in a preclinical model (Quang and Schmidt, 2010a,b). The possible link between ET-1 and oral mucositis is the transcription factor NF-κB. The current working hypothesis for oral mucositis involves an initial stage of endothelial cell damage by reactive oxygen species (ROS). ROS species activate NF-κB, stimulating pathways that result in mucositis (Bierhaus et al., 1997). ROS species are thought to produce pain by potentiating inflammation and increasing the production of proalgesic agents (Basbaum et al., 2009). ET-1 is also activated by NF-κB during oxidative stress. These collective results suggest that ET-1 contributes to oral mucositis pathogenesis and pain.
In addition to ET-1, other factors induced by NF-κB could contribute to oral mucositis pathogenesis and pain. TNFα and cyclooxygenase-2 (COX-2) are two examples. TNFα induces cancer pain by binding to the TNFR2 receptor on nociceptors. In oral mucositis patients, TNFα mRNA in buccal samples correlates with oral mucositis pain (Fall-Dickson et al., 2007). TNFα blockade reduces oral mucositis in head and neck cancer patients undergoing chemoradiation (Watanabe et al., 2010). COX-2 expression is also up-regulated in oral mucositis. COX-2 is responsible for converting arachidonic to prostaglandin H2. However, a controlled clinical trial showed no effect of a prostaglandin antagonist oral rinse on chemotherapy-induced oral mucositis pain (Lalla et al., 2011).
Aside from the factors discussed thus far, a separate class of factors termed ‘neurotrophic factors’ is secreted in different oral pain states to mediate nociception. Neurotrophic factors are secreted by neurons, inflammatory cells, or cancer cells (Ye et al., 2011). They mediate pain by binding to either the low-affinity p75 receptor or high-affinity “Trk” family of receptors, which are confined to peptidergic C fibers. NGF is possibly the most well-studied neurotrophic factor. NGF is produced at high levels by oral cancer cells and mediates oral cancer pain. NGF blockade with anti-NGF produced significant mechanical pain relief, return of oral function, and reduction in weight loss in a mouse model of oral cancer (Ye et al., 2011).
Neuropathic Component of Mucositis Pain
The second component of mucositis pain is neuropathic pain. Neuropathic pain is caused by neuronal sensitization by chemotherapeutic agents and inflammatory mediators. Examples of inflammatory mediators include glutamate and neuropeptides (e.g., substance P, calcitonin gene-related peptide, and NGF), which are secreted by trigeminal A-delta and C neuronal fibers in the nucleus caudalis and periphery. Peripheral release from peptidergic C fibers produces the neurogenic inflammation component of the complex pain response seen with oral mucositis. Glutamate release strongly contributes to inflammation (Haas et al., 2010). Other pro-inflammatory cytokines (e.g., TNFα, IL-2, IL-6, IL-1beta) at the site of mucosal injury result in neuronal sensitization. Given these findings, the effects of anti-inflammatory cytokines on reducing mucositis pathogenesis and pain have been explored. Although they have shown promise in multiple preclinical models, these results have not been supported by clinical studies (Fall-Dickson et al., 2007).
Assays for Quantifying Oral Mucositis Pain
The key to understanding mechanisms of mucositis pain is in objective preclinical nocifensive assays. Orofacial nocifensive assays quantify either reflexive nocifensive responses or operant responses. Reflexive nocifensive responses include withdrawal reflexes or innate, brainstem-derived nocifensive behaviors, such as licking, guarding, grooming, vocalization, or face-wash strokes (Mogil, 2009). Operant assays quantify voluntary behavior in response to a stimulus, allowing for the assessment of central processing of pain. The dolognawmeter is an operant assay that quantifies the amount of time required for a mouse to gnaw through a series of dowels. The gnaw time has been validated as a behavioral index of orofacial dysfunction in mouse models of temporomandibular disorder, masticatory myositis, and oral cancer (Dolan et al., 2010). Another operant assay is the Orofacial Pain Assessment Device (OPAD), in which an animal is trained to place its face against a thermal or mechanical stimulus to gain access to positive reinforcement. Numbers and durations of contacts and reward intake are quantified as indices of thermal or mechanical hypersensitivity (Nolan et al., 2011). Other operant assays include quantification of meal duration or bite force, both of which are influenced by the animal’s appetite (Ro, 2005; Kramer et al., 2010). Both reflexive nocifensive responses and operant assays are commonly used to quantify orofacial pain, and could be used to quantify nociceptive or neuropathic components of oral mucositis pain.
The Future of Mucositis Pain Management
Currently, opioids are the most effective treatment for mucositis pain. However, patients rapidly develop tolerance to opioids or have variable analgesic responses. Variable responses have been attributed to genetic variability (e.g., single-nucleotide polymorphisms, which result in alternative splicing) of the mu-opioid receptor (MOR) gene (OPRM1). To improve the analgesic effect, investigators are developing new drug compounds that specifically target certain mu-opioid receptor isoforms. The rs1799971 (A118G) single-nucleotide polymorphism (SNP) is the most well-investigated SNP; this SNP is associated with altered opioid response in different regions of the brain (Oertel et al., 2009). Another SNP, the rs563649 SNP, was recently discovered in a comparative genomic analysis study. This SNP is located within a structurally conserved internal ribosome entry site (IRES) in the 5′UTR of a novel exon-13-containing MOR-1K isoform. Presence of the SNP affects both mRNA levels and translation efficiency and results in increased levels of MOR-1K, a six-transmembrane (6TM) isoform of MOR. Activation of MOR-1K produces neuronal excitation, rather than inhibition, as seen with the canonical MOR-1 (Gris et al., 2010). This SNP is observed in 6% of individuals and is the strongest independent contributor to quantified pain-sensitivity responses in those individuals (Shabalina et al., 2009). A group led by Pasternak synthesized analgesics to target six-transmembrane isoforms of MOR such as MOR-1K. In their preclinical trials, these new compounds were more potent than morphine and lacked the side effects of respiratory depression and physical dependence seen with traditional opioids (Majumdar et al., 2011).
Fink and colleagues took a different approach to develop novel analgesics. In a series of preclinical and clinical trials, they used modified herpes simplex viruses (HSV) to deliver genes for endogenous opioids (i.e., pro-enkephalin gene, which produces met-enkephalin and leu-enkephalin) to treat chronic pain (Wolfe et al., 2009). The results from preclinical trials showed that delivery of the endogenous opioid genes produced an analgesic effect in facial pain and cancer pain models. Similarities between the etiology of cancer pain and mucositis pain suggest that this treatment could be effective against mucositis pain.
Oral mucositis and mucositis pain are complex problems. Accurate preclinical modeling of mucositis is key to a deeper understanding of mucositis pathogenesis. Current preclinical models have inherent limitations. For example, some models require mechanical injury to induce ulceration; in patients, the mucosa does not need to be superficially injured for mucositis to develop. Dose and scheduling of chemotherapy or radiation are also not necessarily translatable between animals and humans. Despite the limitations of animal models, preclinical studies on mucositis treatment are far ahead of preclinical studies of oral mucositis pain. Although pain is the most distressing symptom for patients, few studies on mucositis directly address pain. Future preclinical studies should quantify pain using existing orofacial nocifensive assays. The candidate pain mediators proposed in this review should be addressed.
Footnotes
This study is supported by National Institutes of Health (NIH) grant R01 DE19796 and by an Oral and Maxillofacial Surgery Foundation Research Support Grant.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alessandri-Haber N, Dina OA, Chen X, Levine JD. (2009). TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci 29:6217-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterovitz G, Tuthill C, Rios I, Modelska K, Sonis S. (2011). Personalized medicine for mucositis: Bayesian networks identify unique gene clusters which predict the response to gamma-D-glutamyl-L-tryptophan (SCV-07) for the attenuation of chemoradiation-induced oral mucositis. Oral Oncol 47:951-955. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Fey EG, Valax P, Yim Z, Peterson JD, Mesri M, et al. (2003). Preclinical characterization of CG53135 (FGF-20) in radiation and concomitant chemotherapy/radiation-induced oral mucositis. Clin Cancer Res 9:3454-3461. [PubMed] [Google Scholar]
- Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, et al. (2007). Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. (2009). Cellular and molecular mechanisms of pain. Cell 139:267-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T, et al. (1997). Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes 46:1481-1490. [DOI] [PubMed] [Google Scholar]
- Bodiga VL, Bodiga S, Surampudi S, Boindala S, Putcha U, Nagalla B, et al. (2012). Effect of vitamin supplementation on cisplatin-induced intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition 28:572-580. [DOI] [PubMed] [Google Scholar]
- Booth D, Haley JD, Bruskin AM, Potten CS. (2000). Transforming growth factor-B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling. Int J Cancer 86:53-59. [DOI] [PubMed] [Google Scholar]
- Borges L, Rex KL, Chen JN, Wei P, Kaufman S, Scully S, et al. (2006). A protective role for keratinocyte growth factor in a murine model of chemotherapy and radiotherapy-induced mucositis. Int J Radiat Oncol Biol Phys 66:254-262. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Gibson RJ, Keefe DM. (2011). Animal models of mucositis: implications for therapy. J Support Oncol 9:161-168. [DOI] [PubMed] [Google Scholar]
- Brake R, Starnes C, Lu J, Chen D, Yang S, Radinsky R, et al. (2008). Effects of palifermin on antitumor activity of chemotherapeutic and biological agents in human head and neck and colorectal carcinoma xenograft models. Mol Cancer Res 6:1337-1346. [DOI] [PubMed] [Google Scholar]
- Chichorro JG, Zampronio AR, Cabrini DA, Franco CR, Rae GA. (2009). Mechanisms operated by endothelin ETA and ETB receptors in the trigeminal ganglion contribute to orofacial thermal hyperalgesia induced by infraorbital nerve constriction in rats. Neuropeptides 43:133-142. [DOI] [PubMed] [Google Scholar]
- Chichorro JG, Fiuza CR, Bressan E, Claudino RF, Leite DF, Rae GA. (2010). Endothelins as pronociceptive mediators of the rat trigeminal system: role of ETA and ETB receptors. Brain Res 1345:73-83. [DOI] [PubMed] [Google Scholar]
- Colburn RW1, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, et al. (2007). Attenuated cold sensitivity in TRPM8 null mice. Neuron 54:379-386. [DOI] [PubMed] [Google Scholar]
- Cotrim AP, Yoshikawa M, Sunshine AN, Zheng C, Sowers AL, Thetford AD, et al. (2012). Pharmacological protection from radiation +/− cisplatin-induced oral mucositis. Int J Radiat Oncol Biol Phys 83:1284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan JC, Lam DK, Achdjian SH, Schmidt BL. (2010). The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J Neurosci Methods 187:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr W, Kummermehr J. (1990). Accelerated repopulation of mouse tongue epithelium during fractionated irradiations or following single doses. Radiother Oncol 17:249-259. [DOI] [PubMed] [Google Scholar]
- Dorr W, Bassler S, Reichel S, Spekl K. (2005). Reduction of radiochemotherapy-induced early oral mucositis by recombinant human keratinocyte growth factor (palifermin): experimental studies in mice. Int J Radiat Oncol Biol Phys 62:881-887. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Epstein JD, Epstein MS, Oien H, Truelove EL. (2007). Management of pain in cancer patients with oral mucositis: follow-up of multiple doses of doxepin oral rinse. J Pain Symptom Manage 33:111-114. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, Kolnick L, et al. (2012). Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 62:400-422. [DOI] [PubMed] [Google Scholar]
- Fall-Dickson JM, Ramsay ES, Castro K, Woltz P, Sportes C. (2007). Oral mucositis-related oropharyngeal pain and correlative tumor necrosis factor-alpha expression in adult oncology patients undergoing hematopoietic stem cell transplantation. Clin Therapeutics 29 (Suppl):2547-2561. [DOI] [PubMed] [Google Scholar]
- Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL, et al. (1998). Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res 58:933-939. [PubMed] [Google Scholar]
- Finch PW, Rubin JS. (2004). Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res 91:69-136. [DOI] [PubMed] [Google Scholar]
- Foncuberta MC, Cagnoni PJ, Brandts CH, Mandanas R, Fields K, Derigs HG, et al. (2001). Topical transforming growth factor-beta3 in the prevention or alleviation of chemotherapy-induced oral mucositis in patients with lymphomas or solid tumors. J Immunother 24:384-388. [DOI] [PubMed] [Google Scholar]
- Fujisawa K, Miyamoto Y, Nagayama M. (2003). Basic fibroblast growth factor and epidermal growth factor reverse impaired ulcer healing of the rabbit oral mucosa. J Oral Pathol Med 32:358-366. [DOI] [PubMed] [Google Scholar]
- Gris P, Gauthier J, Cheng P, Gibson DG, Gris D, Laur O, et al. (2010). A novel alternatively spliced isoform of the mu-opioid receptor: functional antagonism. Mol Pain 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HS, Linecker A, Pfragner R, Sadjak A. (2010). Peripheral glutamate signaling in head and neck areas. Head Neck 32:1554-1572. [DOI] [PubMed] [Google Scholar]
- Hamstra DA, Eisbruch A, Naidu MU, Ramana GV, Sunkara P, Campbell KC, et al. (2010). Pharmacokinetic analysis and phase 1 study of MRX-1024 in patients treated with radiation therapy with or without cisplatinum for head and neck cancer. Clin Cancer Res 16:2666-2676. [DOI] [PubMed] [Google Scholar]
- Hwang D, Popat R, Bragdon C, O’Donnell KE, Sonis ST. (2005). Effects of ceramide inhibition on experimental radiation-induced oral mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:321-329. [DOI] [PubMed] [Google Scholar]
- Kim KI, Kim JW, Lee HJ, Kim BS, Bang SM, Kim I, et al. (2013). Recombinant human epidermal growth factor on oral mucositis induced by intensive chemotherapy with stem cell transplantation. Am J Hematol 88:107-112. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Kerins CA, Schneiderman E, Bellinger LL. (2010). Measuring persistent temporomandibular joint nociception in rats and two mice strains. Physiol Behav 99:669-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla RV, Gordon GB, Schubert M, Silverman S, Jr, Hutten M, Sonis ST, et al. (2011). A randomized, double-blind, placebo-controlled trial of misoprostol for oral mucositis secondary to high-dose chemotherapy. Support Care Cancer 20:1797-1804. [DOI] [PubMed] [Google Scholar]
- Lee SW, Jung KI, Kim YW, Jung HD, Kim HS, Hong JP. (2007). Effect of epidermal growth factor against radiotherapy-induced oral mucositis in rats. Int J Radiat Oncol Biol Phys 67:1172-1178. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, et al. (2011). Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA 108:19778-19783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik IA, Moid I, Haq S, Sabih M. (1997). A double-blind, placebo-controlled, randomized trial to evaluate the role of tetrachlorodecaoxide in the management of chemotherapy-induced oral mucositis. J Pain Symptom Manage 14:82-87. [DOI] [PubMed] [Google Scholar]
- Mogil JS. (2009). Animal models of pain: progress and challenges. Nat Rev Neurosci 10:283-294. [DOI] [PubMed] [Google Scholar]
- Moore C, Cevikbas F, Pasolli HA, Chen Y, Kong W, Kempkes C, et al. (2013). UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci USA 110:E3225-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. (2007). Transient receptor potential cation channels in disease. Physiol Rev 87:165-217. [DOI] [PubMed] [Google Scholar]
- Nolan TA, Hester J, Bokrand-Donatelli Y, Caudle RM, Neubert JK. (2011). Adaptation of a novel operant orofacial testing system to characterize both mechanical and thermal pain. Behav Brain Res 217:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel BG, Kettner M, Scholich K, Renne C, Roskam B, Geisslinger G, et al. (2009). A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J Biol Chem 284:6530-6535. [DOI] [PubMed] [Google Scholar]
- Pickering V, Jordan RC, Schmidt BL. (2007). Elevated salivary endothelin levels in oral cancer patients—a pilot study. Oral Oncol 43:37-41. [DOI] [PubMed] [Google Scholar]
- Quang PN, Schmidt BL. (2010a). Endothelin-A receptor antagonism attenuates carcinoma-induced pain through opioids in mice. J Pain 11:663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quang PN, Schmidt BL. (2010b). Peripheral endothelin B receptor agonist-induced antinociception involves endogenous opioids in mice. Pain 149:254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro JY. (2005). Bite force measurement in awake rats: a behavioral model for persistent orofacial muscle pain and hyperalgesia. J Orofac Pain 19:159-167. [PubMed] [Google Scholar]
- Ryu SH, Kang KM, Moon SY, Chai GY, Hong JP, Cho KO, et al. (2010). Therapeutic effects of recombinant human epidermal growth factor (rhEGF) in a murine model of concurrent chemo- and radiotherapy-induced oral mucositis. J Radiation Res 51:595-601. [DOI] [PubMed] [Google Scholar]
- Scully C, Epstein J, Sonis S. (2003). Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: Part 1, pathogenesis and prophylaxis of mucositis. Head Neck 25:1057-1070. [DOI] [PubMed] [Google Scholar]
- Shabalina SA, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, et al. (2009). Expansion of the human mu-opioid receptor gene architecture: novel functional variants. Hum Mol Genet 18:1037-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonis ST. (2004). Oral mucositis in cancer therapy. J Support Oncol 2(6 Suppl 3):3S-8S. [PubMed] [Google Scholar]
- Sonis ST. (2010). Efficacy of palifermin (keratinocyte growth factor-1) in the amelioration of oral mucositis. Core Evid 4:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonis ST. (2013). Oral mucositis in head and neck cancer. Am Soc Clin Oncol Educ Book 2013:236-240. [DOI] [PubMed] [Google Scholar]
- Sonis ST, Tracey C, Shklar G, Jenson J, Florine D. (1990). An animal model for mucositis induced by cancer chemotherapy. Oral Surg Oral Med Oral Pathol 69:437-443. [DOI] [PubMed] [Google Scholar]
- Uddman R, Tajti J, Cardell LO, Sundler F, Uddman E, Edvinsson L. (2006). Endothelin ETA and ETB receptor expression in the human trigeminal ganglion. Neuro Endocrinol Lett 27:345-349. [PubMed] [Google Scholar]
- Vilela-Goulart M, Teixeira RT, Rangel DC, Niccoli-Filho W, Gomes MF. (2008). Homogenous amniotic membrane as a biological dressing for oral mucositis in rats: histomorphometric analysis. Arch Oral Biol 53:1163-1171. [DOI] [PubMed] [Google Scholar]
- Vuyyuri SB, Hamstra DA, Khanna D, Hamilton CA, Markwart SM, Campbell KC, et al. (2008). Evaluation of D-methionine as a novel oral radiation protector for prevention of mucositis. Clin Cancer Res 14:2161-2170. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Ishihara M, Matsuura K, Mizuta K, Itoh Y. (2010). Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer 127:1984-1990. [DOI] [PubMed] [Google Scholar]
- Watkins B, Pouliot K, Fey E, Tuthill C, Sonis S. (2010). Attenuation of radiation- and chemoradiation-induced mucositis using gamma-D-glutamyl-L-tryptophan (SCV-07). Oral Dis 16:655-660. [DOI] [PubMed] [Google Scholar]
- Wolfe D, Mata M, Fink DJ. (2009). A human trial of HSV-mediated gene transfer for the treatment of chronic pain. Gene Ther 16:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Chen P, Sonis ST, Lingen MW, Berger A, Toback FG. (2012). A novel peptide to treat oral mucositis blocks endothelial and epithelial cell apoptosis. Int J Radiat Oncol Biol Phys 83:e409-e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymenga AN, van der Graaf WT, Hofstra LS, Spijkervet FK, Timens W, Timmer-Bosscha H, et al. (1999). Phase I study of transforming growth factor-beta3 mouthwashes for prevention of chemotherapy-induced mucositis. Clin Cancer Res 5:1363-1368. [PubMed] [Google Scholar]
- Yamamoto T, Ono K, Hitomi S, Harano N, Sago T, Yoshida M, et al. (2013). Endothelin receptor-mediated responses in trigeminal ganglion neurons. J Dent Res 92:335-339. [DOI] [PubMed] [Google Scholar]
- Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, et al. (2011). Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther 10:1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]