It is just over 20 years since the initial cloning of periostin was reported, and already there are over 600 articles ascribing various roles and functions to this matricellular protein. In humans, the periostin gene (POSTN) encodes 835 amino acids, and its full-length isoform is a 90-kDa secreted protein that contains four tandem Fas domains, so called because they are homologous to insect Fasciclin-I. First perceived as a highly conserved osteoblast-restricted adhesion molecule, over the past 8 years, periostin has been recognized as an important regulator of matricellular dynamics relevant to cancer biology, cardiovascular diseases, pulmonary morphogenesis, and dental and periodontal conditions, among others. Recently, the capacity to measure serum periostin levels has triggered noteworthy efforts to establish its value as a biomarker for numerous diseases. Recently, Yamada and collaborators reported a PDL-specific mechanism by which this multifaceted protein orchestrates cytodifferentiation and mineralization in the periodontium (Yamada et al., 2014).
Although various alternatively spliced isoforms of periostin have been described, little is known of their specific functions (Takeshita et al., 1993; Horiuchi et al., 1999; Litvin et al., 2004; Kim et al., 2008; Bai et al., 2010). In general, it has been shown that the larger, full-length 90- and 87-kDa isoforms are normally secreted by cells such as neuro-ectodermal-derived fibroblasts. Tissue-specific isoforms are thought to be modulators of respective tissues. Periostin is one of those peculiar matricellular ECM proteins that is transiently up-regulated during cell fate changes, either physiologic or pathologic. It is speculated that the larger isoforms are associated with the pathological responses, rather than the smaller matrix-associated isoforms, which appear more physiologic and stable. These isoforms can regulate cell function and cell–matrix interactions, as well as act as mechanosensors. The isoform reported by Yamada and collaborators is a novel human PDL-specific variant that can regulate FAK signaling and, potentially, mineralization when over-expressed in vitro. Yamada’s study leads to speculation that this isoform is a downstream responder along the PDL differentiation pathway. Therefore, it should be transient (i.e., switched off after differentiation or as PDL inflammation diminishes), and if it stays expressed, normal tissue function could be compromised.
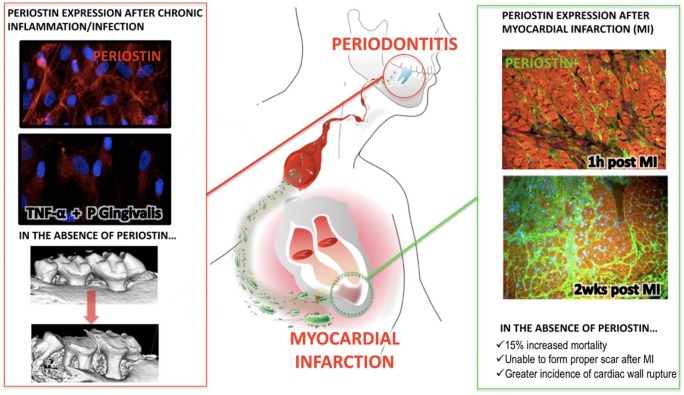
Periostin interacts with several ECM molecules such as type I collagen through its EMI domain, with tenascin-C, and with BMP-1 through its fas I domains to increase the collagen type I fiber diameter, which translates into greater tissue strength. In contrast, deletion of periostin results in a reduction in collagen fiber diameter, leading to a decrease in tissue modulus of elasticity. Periostin high binding affinity to αvβ3 integrin receptors triggers AKT/PKB signaling, increasing fibroblast and epithelial/endothelial migration, and also activates the mTOR signaling pathway, leading to proliferation. Similar to other highly homologous proteins such as βigH3, periostin expression is regulated by TGF-β in response to mechanical stimulation. During craniofacial homeostasis, periostin is essential to maintain the functional and structural integrity of the periodontium to ensure anchorage of the teeth (Rios et al., 2005, 2008; Ma et al., 2011). During normal cardiovascular development, periostin plays an important structural mediator role, whereas in the adult heart, its temporal and spatial distribution supports tissue recovery by modulating repair mechanisms after pathological insults and myocardial infarctions (Dorn, 2007; Kuhn et al., 2007; Shimazaki et al., 2008; Shimazaki and Kudo, 2009; Segers and Lee, 2010). Periostin-deficient animals clearly show both severe deterioration of the tooth-supporting structures and significantly impaired cardiac morphogenesis and healing after induced cardiac ischemia (Fig.). Therefore, periostin plays an important role in both dental and cardiac tissue integrity and function.
Figure.
Model diagram for the effect of periodontal infection and myocardial repair after an AMI. (1) Red outline: Advanced periodontal disease leads to altered local and systemic periostin levels due to chronic microbial and inflammatory challenges. (2) Green outline: Lack of adequate periostin levels compromises the integrity of the periodontal tissues and impedes the necessary myocardial repair and remodeling after MI.
The field of dentistry is recognizing the importance of periostin in dental health, leading to a growing interest in this protein. This has been brought about through the study and characterization of periostin-null mouse models. The rodent phenotype of the continuously growing tooth model clearly illustrates the importance of periostin in tissue stability and response to injury. Chronic exposure to both bacterial by-products and inflammatory cytokines reduces periostin expression by periodontal ligament fibroblasts. As a consequence, defects in the ECM occur and compromise the structural and functional integrity of the tissues. Although no mutation resulting in complete depletion of periostin in patients is known, nor have physiological levels of periostin been reported in patient clinical studies, a normal threshold that modulates the biomechanical competency of the periodontal ECM can be assumed. Additionally, an increase in periostin levels such as after surgical treatment, or by active delivery, can be hypothesized to improve periodontal wound-healing and regeneration due to its properties in stabilizing the ECM and promoting periodontal tissue cell activity. Clearly, studies to test these hypotheses need to be initiated.
In contrast, the scientific evidence regarding the critical modulatory role of periostin for myocardial repair and remodeling is rapidly increasing. Findings from these studies can be used to advance the field of periodontics. It has been shown from these studies that components of the ECM do not passively follow the pathologic alterations of the infarcted heart, but critically modulate inflammatory and reparative pathways by transducing signals that affect cell survival along with phenotype and gene expression at the edges of the infarcted area. Periostin is increased in the infarcted tissue through deposition by circulating fibroblasts and regulates cellular interactions while promoting matrix organization. The proper regulation of these interactions by molecules such as periostin could determine adaptive, reparative, and regenerative responses by modulating function among important growth factors, cytokines, and proteases, thus allowing for proper healing and function.
The study of periostin thus provides a unique opportunity to learn more about these conditions. Novel insights into the role of periodontitis as a potentially modifiable risk determinant of cardiac recovery after acute myocardial infarction might lead to novel therapeutic interventions and treatment. Yamada’s findings could have significant implications in aiding our understanding of periodontal cell-matrix dynamics and homeostasis. This emerging evidence and other lessons learned from periostin help us understand the key, pivotal role of matricellular proteins to control tissue stability under stress. Periostin appears to be an essential and therapeutically promising molecule to provide tissues with the competency to sustain and overcome different challenges over time. Unraveling novel pathways to explore and ultimately develop practical therapeutic protocols will possibly have an impact on the repair and regeneration potential of these physically distant, yet functionally connected, tissues.
Footnotes
This work was supported by National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) grants R56 DE022787 and K23 DE019872.
The authors declare no conflicts of interest with this article.
References
- Bai Y, Nakamura M, Zhou G, Li Y, Liu Z, Ozaki T, et al. (2010). Novel isoforms of periostin expressed in the human thyroid. Jpn Clin Med 1:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW., 2nd (2007). Periostin and myocardial repair, regeneration, and recovery. N Engl J Med 357:1552-1554. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. (1999). Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239-1249. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Isono T, Tambe Y, Chano T, Okabe H, Okada Y, et al. (2008). Role of alternative splicing of periostin in human bladder carcinogenesis. Int J Oncol 32:161-169. [PubMed] [Google Scholar]
- Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. (2007). Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13:962-969. [DOI] [PubMed] [Google Scholar]
- Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, et al. (2004). Expression and function of periostin-isoforms in bone. J Cell Biochem 92:1044-1061. [DOI] [PubMed] [Google Scholar]
- Ma D, Zhang R, Sun Y, Rios HF, Haruyama N, Han X, et al. (2011). A novel role of periostin in postnatal tooth formation and mineralization. J Biol Chem 286:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, et al. (2005). Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25:11131-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios HF, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, et al. (2008). Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol 79:1480-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers VF, Lee RT. (2010). Protein therapeutics for cardiac regeneration after myocardial infarction. J Cardiovasc Transl Res 3:469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki M, Kudo A. (2009). [Periostin, acting in regeneration of periodontal ligament, contributes to cardiac healing and tumor capsule formation]. Fukuoka Igaku Zasshi 100:67-74 [article in Japanese]. [PubMed] [Google Scholar]
- Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, et al. (2008). Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205:295-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S, Kikuno R, Tezuka K, Amann E. (1993). Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294( Pt 1):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Tauchi T, Toshihito A, Maeda K, Kajikawa T, Yanagita M, et al. (2014). Characterization of a novel periodontal ligament-specific periostin isoform. J Dent Res 93:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]