Abstract
Objectives
Despite different treatments and course of illness, depressive symptoms appear similar in major depressive disorder (MDD) and bipolar I disorder (BP-I). This similarity of depressive symptoms suggests significant overlap in brain pathways underlying neurovegetative, mood, and cognitive symptoms of depression. These shared brain regions might be expected to exhibit similar activation in individuals with MDD and BP-I during functional magnetic resonance imaging (fMRI).
Methods
fMRI was used to compare regional brain activation in participants with BP-I (n = 25) and MDD (n = 25) during a depressive episode as well as 25 healthy comparison (HC) participants. During the scans, participants performed an attentional task that incorporated emotional pictures.
Results
During the viewing of emotional images, subjects with BP-I showed decreased activation in the middle occipital gyrus, lingual gyrus, and middle temporal gyrus compared to both subjects with MDD and HC participants. During attentional processing, participants with MDD had increased activation in the parahippocampus, parietal lobe, and postcentral gyrus. However, among these regions, only the postcentral gyrus also showed differences between MDD and HC participants.
Conclusions
No differences in cortico-limbic regions were found between participants with BP-I and MDD during depression. Instead, the major differences occurred in primary and secondary visual processing regions with decreased activation in these regions in BP-I compared to major depression. These differences were driven by abnormal decreases in activation seen in the participants with BP-I. Posterior activation changes are a common finding in studies across mood states in participants with BP-I.
Keywords: bipolar disorder, emotion, fMRI, major depressive disorder, neurophysiology
Depression is a dysfunctional affective state characterized by persistent negative mood as well as significant neurovegetative and cognitive symptoms (1). Despite different treatments and courses of illness, depressive symptoms are similar in major depressive (MDD) and bipolar disorder type I (BP-I) and these two disorders cannot be distinguished without knowledge of the patient's prior mood episodes (i.e., a history of mania) (2). These common depressive symptoms in both BP-I and MDD suggest significant overlap in brain pathways underlying the neurovegetative, mood, and cognitive symptoms of both disorders (2). Consequently, it is possible that shared neurofunctional abnormalities may underlie the pathophysiology of depressive symptoms in both disorders, and that similar activation of brain structures would be observed in individuals with MDD or BP-I during functional magnetic resonance imaging (fMRI). Yet these two mood disorders have different prognoses, treatments and courses of illness, and there is evidence that they exhibit different alterations within brain circuits that modulate mood (2-4). Differences in brain activation between individuals with BPI and MDD observed during a depressive episode may therefore represent abnormalities unique to each condition. Increased understanding of these pathways would help to clarify the neurophysiology of depression and to advance understanding of the etiology and treatment of depressive disorders.
This is the first study to use fMRI to evaluate attentional and emotional processing together in depressed participants with MDD or BP-I. While the specific neuropathophysiology of BP-I is unknown, neuroimaging studies suggest impairments in cortico-limbic regions responsible for regulating emotion (2-8). Specifically, altered brain activation has been shown in the amygdala, anterior cingulate gyrus (ACC), ventrolateral prefrontal cortex (VLPFC), insula, and medial prefrontal cortex when compared to healthy comparison participants (HC) (2-8). In MDD, the extant neuroimaging data suggests involvement of a different set of brain regions than in BP-I (9-15). Prior imaging studies of patients with MDD have emphasized the roles of abnormalities within hippocampus and dorsolateral prefrontal cortex (DLPFC) (9-15). Moreover, Ketter et al. (16) suggested that hypofunction in DLPFC may lead to over-activation of hippocampus during depression. In addition, functional neuroimaging studies examining the treatment of depression have also found changes in DLFPC and hippocampus and their interaction (9, 13, 17). Structural imaging studies consistently find a decrease in the volume of the hippocampus, and a recent meta-analysis by Videbech and Ravnkilde (10) confirmed these results, but also found no changes in hippocampus in BP-I. In contrast to finding increased striatal volumes in patients with BP-I, several studies found decreased volumes of striatum in subjects with MDD (18-22). Pillay et al. (23) reported volume reductions in striatum in unipolar patients that correlated with illness severity (Hamilton Depression Inventory score).
With these considerations in mind, the current study was designed to examine differences in brain activation during depression between individuals with MDD and BP-I during a dual emotional and cognitive task (24). The task was designed to discriminate between ventral emotional and dorsal cognitive brain networks and requires effective regulation of emotion to complete successfully. When HC subjects performed this task in an MRI scanner, Yamasaki et al. (24) found that dorsal prefrontal regions were activated in response to the attentional component of the task while ventral prefrontal regions were activated in response to the emotional component of the task, consistent with models proposed by Mayberg and colleagues (9, 13-15). Given previous findings in MDD and BP-I, we predicted that the BP-I group would show altered activation in the ACC, VLPFC, insula, and medial prefrontal cortex compared to the MDD group. In contrast, we predicted the MDD group would show altered activation in the hippocampus and DLPFC.
Patients and methods
Participants
Participants with BP-I (n = 25) and MDD (n = 25) were identified and recruited during a depressive episode. Participants were recruited by word of mouth and advertising. Demographically matched HC participants (n = 25) were recruited from the same community as the participants with MDD and BP-I and had no history of Axis I psychiatric disorders in themselves or first-degree relatives. There were no differences in age or sex among the three groups (Table 1). All participants provided written informed consent after study procedures were fully explained and the study was approved by the Institutional Review Board of the University of Cincinnati (Cincinnati, OH, USA).
Table 1.
Demographics, symptom ratings, and behavioral performance data for all groups
| Group | BP-I | MDD | HC | p-value | ||
|---|---|---|---|---|---|---|
| BP-I versus MDD | BP-I versus HC | MDD versus HC | ||||
| Age, years, mean (SD) | 30 (8) | 27 (7) | 26 (7) | 0.336a | 0.156a | 0.896a |
| Ethnicity, non- white, % | 28 | 48 | 40 | 0.230b | 0.540b | 0.830b |
| Sex, female, % | 68 | 68 | 67 | 1.000b | 0.560b | 0.560b |
| Education, years, mean (SD) | 12 (2) | 12 (2) | 15(2) | 0.966a | 0.000a | 0.000a |
| YMRS score, mean (SD) | 8(6) | 7(5) | 1 (1) | 0.446a | - | - |
| HAM-D score, mean (SD) | 32 (7) | 38 (8) | 1 (1) | 0.011a | - | - |
| Reaction time, msec, average (SD) | 855 (162) | 847 (205) | 745 (128) | 0.970a | 0.002a | 0.004a |
| Accuracy, average (SD) | 97 (5)% | 93 (17)% | 98 (2)% | 0.043a | 0.550a | 0.002a |
| d’, average (SD) | 0.989 (0.013) | 0.977 (0.040) | 0.993 (0.008) | 0.191a | 0.842a | 0.060a |
BP-I = bipolar disorder; MDD = major depressive disorder; HC = healthy comparison; SD = standard deviation; YMRS = Young Mania Rating Scale; HAM-D = Hamilton Rating Scale for Depression.
ANOVA.
Chi-square.
The diagnosis of BP-I or MDD was made using the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient version (SCID-I/P) (25). Additionally, manic and depressive symptoms were assessed using the Young Mania Rating Scale (YMRS) (26) and Hamilton Rating Scale for Depression (HAM-D) (27), respectively. All participants were 18 to 45-years-old, were physically and neurologically healthy, and if female, had a negative urine pregnancy test. Potential participants were excluded by medical or neurological illnesses that might influence brain function including a history of seizures (other than infantile febrile seizures) or serious neurological disease such as dementia or Parkinson's disease, any contraindications to receiving an MRI, and an IQ < 80. Participants with BP-I and MDD were unmedicated and had been so for at least 14 days prior to scan except for one participant with BP-I who was started on lithium the night before the scan and another participant with BP-I who was briefly treated with quetiapine (300 mg/day for the three days leading up to the scan). No medications were discontinued for the purpose of the study; instead subjects were recruited off medication. See Table 2 for the rates of comorbid psychiatric illness among the BP-I and MDD participants.
Table 2.
Rates of comorbid psychiatric disorders in the bipolar I disorder (BP-I) and major depressive disorder (MDD) groups
| No. of participants in BP-I group with: | No. of participants in MDD group with: | |
|---|---|---|
| Comorbid anxiety disordera | 2 | 4 |
| Alcohol abuse or dependence in full remission | 3 | 1 |
| Cannabis dependence in full remission | 1 | 0 |
| Alcohol and cannabis dependence in full remission | 3 | 5 |
| Current alcohol abuse | 0 | 4 |
| Current alcohol abuse and cannabis dependence | 3 | 1 |
| Alcohol abuse in full remission and current cannabis abuse | 0 | 2 |
Panic disorder, posttraumatic stress disorder, generalized anxiety disorder,obsessive compulsive disorder, or social anxiety disorder.
fMRI task
All participants received an fMRI scan while performing a modified continuous performance task with emotional and neutral distracters (CPT-END) (24). The CPT-END task used in our lab is similar to the task developed by Yamasaki et al. (24), and was written using E-Prime version 1.0 [Psychology Software Tools, Inc., 2002 (University of Pittsburgh, Pittsburgh PA, USA)] on a personal computer. The CPT-END task involves a visual oddball paradigm. Each imaging session consisted of 10 runs of 132 stimuli presented at 3,000 msec intervals for 2,000 msec each. A fixation cross was presented between images. Successive targets and distracters were separated by at least 12 secs. Participants responded to targets (circles) by pressing a button with the right index finger and press on another button with the right middle finger for all other stimuli. Seventy percent of the visual cues were simple colored squares, 10% were simple colored circles, 10% were emotionally neutral pictures, and 10% were emotionally unpleasant pictures. The emotionally neutral and unpleasant pictures were images of scenes taken from the International Affective Picture System (IAPS) (University of Florida, Gainesville, FL, USA) (28). These images have been well-studied to generate normalized ratings of emotional valence (28). Responding to circles requires sustained attention (without any emotional element) and viewing the emotional scenes generates emotional responses (with the attentional element removed by subtracting activation from the neutral scenes). Two participants in the BP-I group were excluded from the analysis for poor performance (less than 50% accuracy) on the CPT-END (these two participants were not included in the total number of subjects given above).
Image acquisition and analysis
All fMRI scans were performed at the University of Cincinnati's Center for Imaging Research using a 4.0 Tesla Varian Unity INOVA Whole Body MRI/MRS system (Varian Inc., Palo Alto, CA, USA). Non-ferromagnetic high-resolution visual goggles (Resonance Technologies, Inc., Northridge, CA, USA) were used to present the video stimuli in the MRI scanner. Anatomical localization was obtained using a high-resolution, T1-weighted, 3-D brain scan (29). To encompass the entire brain a mid-sagittal localizer scan was acquired to place 35 contiguous 5-mm axial slices. Next, to correct for ghost and geometric distortions, a multi-echo reference scan was obtained (30). During the CPT-END task, whole-brain images (volumes) were acquired every 3 seconds using a T2*-weighted gradient-echo echoplanar imaging (EPI) pulse sequence [repetition time (TR)/echo time (TE) = 3000/30 msec, field of view = 20.8 × 20.8 cm, matrix 64 × 64 pixels, slice thickness = 5 mm, flip angle = 75°].
All analyses of the fMRI data were conducted using Analysis of Functional NeuroImages [(AFNI) http://afni.nimh.nih.gov/afni]. Before the analysis the raw MRI images were reconstructed in order to convert the raw scanner data into AFNI format. Preprocessing steps performed in AFNI included co-registration based upon scanner coordinates for both structural and EPI (functional) images and motion correction. Motion for each subject was determined for the six directions of rotation and translation and was corrected using a six-parameter rigid body transformation (31). Participants were excluded from analysis if the maximum motion was > 5 mm. The average total displacement for all subjects was < 1 mm and the average displacement between any successive TR pair was < 0.1 mm. In addition to standard motion correction, each volume was inspected for signal artifacts using a semi-automated algorithm in AFNI and excluded from further analysis if visual inspection indicated uncorrectable head movement. Less than 16 volumes (10%) on average were removed from each run. One participant in the HC and two participants each in the BP-I and MDD groups could not be used because of excessive motion during one of the fMRI scans (these participants were not included in the total number of subjects given above). Finally, anatomical and functional maps were transformed into stereotactic Talairach space using the ICBM452 template (Laboratory of Neuroimaging, University of California at Los Angeles, Los Angeles, CA, USA). A voxelwise analysis was performed in AFNI using the groupana command. Activation during emotional images, neutral images, and circles were compared against activation during square trials. A group (MDD, BP-I, HC) by cue (emotional, neutral, and circle) ANOVA was then performed using groupana. Post-hoc contrasts were then performed between each group. A separate analysis was done to control for depression severity in the MDD versus BP-I patient groups. HAM-D scores were used as a covariate in an ANOVA performed using 3dttest++ in the BP-I versus MDD group. Based on Monte Carlo simulation using 10,000 iterations, significant activation differences between groups were defined as p = 005 with a cluster of 37 voxels that resulted in a corrected threshold of p = 0.05 (32-34).
Results
Demographic, clinical, and performance variables
As seen in Table 1, there were no significant differences in age, race, or sex between the three groups. Level of education did not differ between MDD and BP-I participants while the HC group had significantly higher education levels than the BP-I and MDD groups [F(2,75) = 13, p < 0.01]. There were no significant differences in the YMRS score between the MDD and BP-I groups. Participants in the MDD group had significantly higher HAM-D scores compared to the BP-I group [F(1,50) = 7.0, p < 0.01].
Regarding performance on the CPT-END, there was a significant main effect of reaction time (F(2,152)=7.7, p < 0.01) (Table 1). Post-hoc comparisons using the Tukey Honestly Significant Difference (HSD) test indicated that HC participants had significantly faster reaction times compared to the BP-I (p = 0.002) and MDD (p = 0.004) groups. There was also a significant main effect of accuracy [F(2,152) = 6.5, p < 0.01) (Table 1). Post-hoc comparisons using the Tukey HSD test showed that participants with MDD had significantly lower accuracy compared to the BP-I (p = 0.04) and HC (p = 0.002) groups. There were no differences in response bias (d′) between the groups [F(2,152) = 3.0, p = 0.058) (Table 1).
fMRI analysis
Emotional versus cognitive processing across all groups
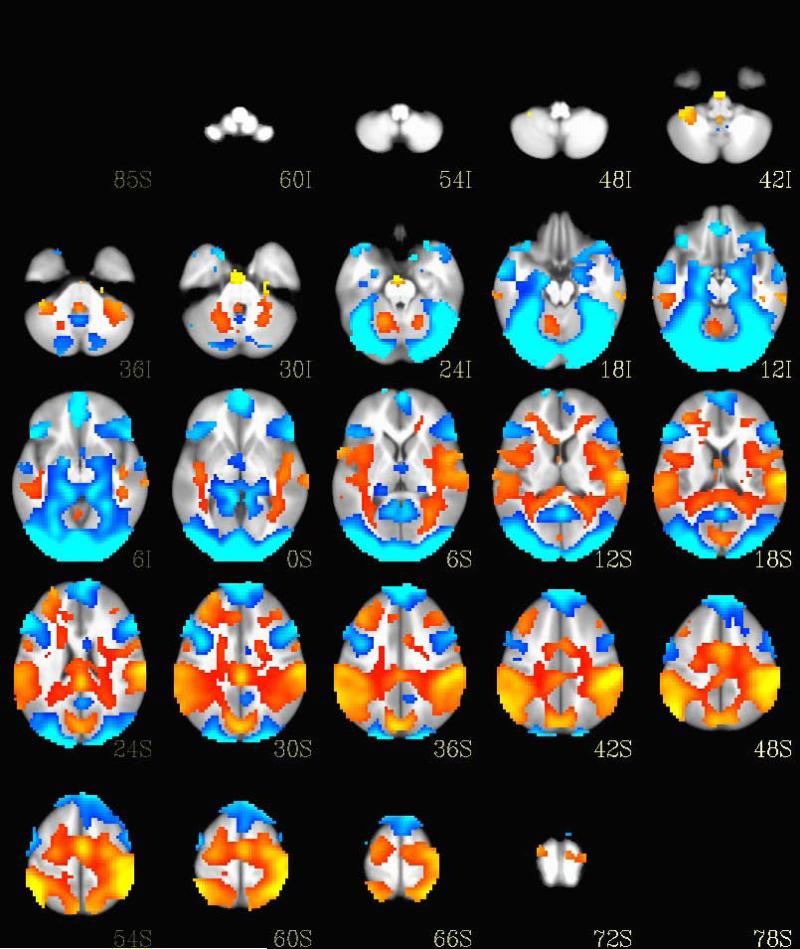
As depicted in Figure 1, the task was able to discriminate between ventral emotional and dorsal cognitive brain networks similar to the activation seen in Yamasaki et al. (24).
Fig. 1.
Brain activation differences between the attentional (circle trials) and emotional trials across all three participant groups. Regions activated by the circle trials are shown in orange while the regions activated by the emotional images are shown in blue.
Emotional processing in MDD versus BP-I
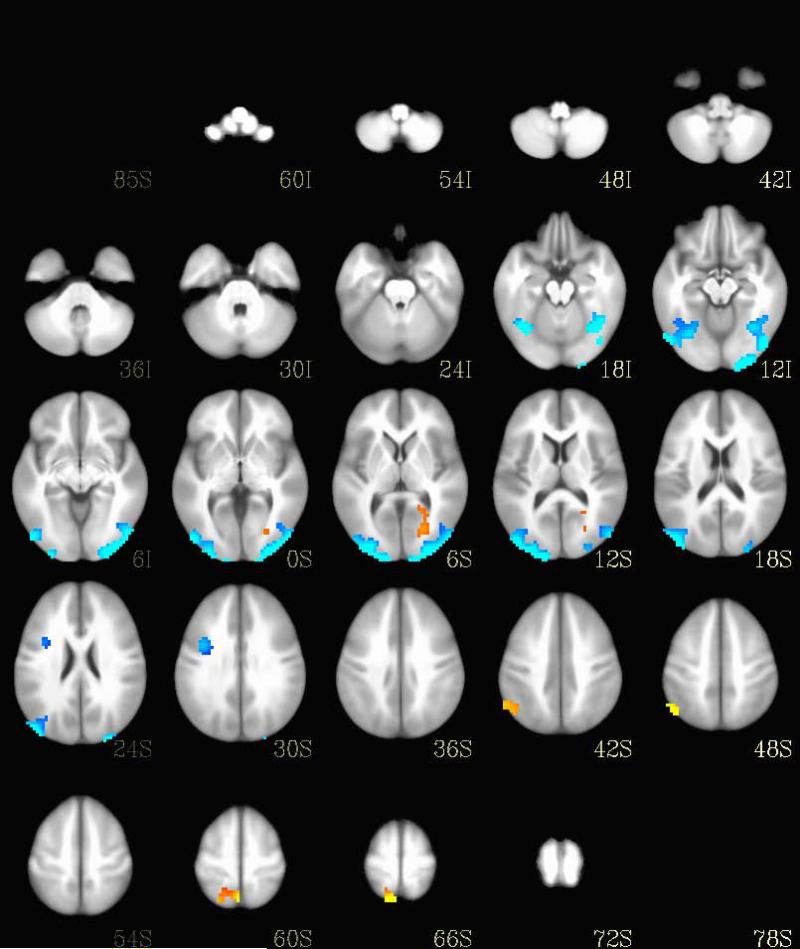
As depicted in Figure 2, seven regions showed significant differences in brain activation between MDD and BP-I during the emotional images (Table 2). Participants with MDD showed relatively increased activation bilaterally in the middle occipital gyrus, cuneus, and middle temporal gyrus, and in the left frontal gyrus. Participants with BP-I showed relatively increased activation in the left lingual gyrus, middle occipital gyrus, posterior cingulate gyrus, and parahippocampal gyrus, and in the right precuneus and inferior parietal lobule.
Fig. 2.
Brain activation differences during emotional trials between the bipolar I disorder and major depressive disorder groups. Regions with increased activation in bipolar I disorder are shown in orange and regions with increased activation in major depressive disorder are shown in blue.
Attentional processing in MDD versus BP-I
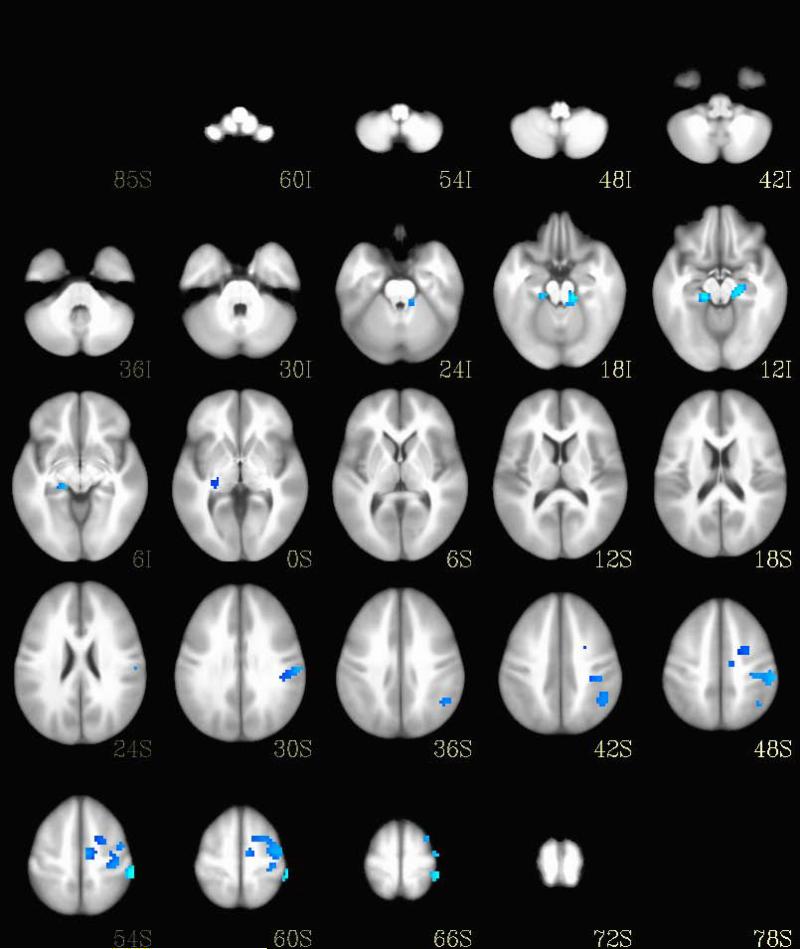
As depicted in Figure 3, six regions showed significant differences in brain activation between MDD and BP-I during the circle images (Table 3). Participants with MDD showed relatively increased activation in the left inferior parietal lobule, medial frontal gyrus, parahippocampal gyrus, and cerebellum, and in the right parahippocampal gyrus and culmen.
Fig. 3.
Brain activation differences during circle trials between the bipolar I disorder and major depressive disorder groups. Regions with increased activation in bipolar I disorder are shown in orange and regions with increased activation in major depressive disorder are shown in blue.
Table 3.
Clusters showing significant differences in participants with bipolar I disorder (BP-I) versus participants with major depressive disorder (MDD) during emotional images (emotional) and circles (attentional)
| Region of interest | Laterality | Center of mass Talairach coordinates |
Volume (3-mm3 voxels) | Greater activation in | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| BP-I versus MDD emotional | ||||||
| Lingual gyrus, cuneus, middle occipital gyrus, middle temporal gyrus | L | +22.5 | +97.5 | –15.5 | 616 | MDD |
| Cuneus, middle occipital gyrus, middle temporal gyrus | R | –19.5 | +100.5 | +8.5 | 603 | MDD |
| Lingual gyrus, middle occipital gyrus, posterior cingulate gyrus, parahippocampal gyrus | L | +25.5 | +97.5 | +5.5 | 69 | BP-I |
| Precuneus | R | –4.5 | +61.5 | +65.5 | 66 | BP-I |
| Inferior frontal gyrus, precentral gyrus | R | –37.5 | –4.5 | +29.5 | 60 | MDD |
| Inferior parietal lobule | R | –49.5 | +61.5 | +47.5 | 50 | BP-I |
| BP-I versus MDD attentional | ||||||
| Inferior parietal lobule | L | +52.5 | +34.5 | +53.5 | 295 | MDD |
| Parahippocampal gyrus, culmen | R | –13.5 | +28.5 | –12.5 | 65 | MDD |
| Parahippocampal gyrus, cerebellum | L | +13.5 | +28.5 | –18.5 | 61 | MDD |
| Inferior parietal lobule | L | +40.5 | +55.5 | +47.5 | 57 | MDD |
| Medial frontal gyrus | L | +7.5 | +13.5 | +53.5 | 48 | MDD |
| Postcentral gyrus, inferior parietal lobule | L | +58.5 | +19.5 | +29.5 | 40 | MDD |
Significant at p = 0.05 at threshold p = 0.005 at cluster threshold of 37. L = left; R = right.
HAM-D as a covariate in MDD versus BP-I
Differences in HAM-D scores between the MDD and BP-I groups accounted for activation differences in the left cuneus and right inferior frontal gyrus and right precentral gyrus during the emotional task. There was no overlap in regions during the attentional part of the task.
Emotional and attentional processing in BP-I versus HC
Sixteen regions showed significant differences in brain activation between BP-I and HC participants during emotional images and three regions showed significant differences in activation during the circle images (Table 4).
Table 4.
Clusters showing significant differences in participants with bipolar I disorder (BP-I) versus healthy comparison (HC) participants during emotional images
| Region of interest | Laterality | Center of mass Talairach coordinates |
Volume (3-mm3 voxels) | Greater activation in | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Culmen, declive, | R | −37.5 | +55.5 | −21.5 | 1040 | HC |
| parahippocampal gyrus | ||||||
| Transverse temporal gyrus | L | +64.5 | +10.5 | +11.5 | 689 | BP-I |
| Middle and inferior occipital gyrus, lingual gyrus, cuneus | Bilateral | +16.5 | +100.5 | +5.5 | 612 | HC |
| Precuneus | R | −22.5 | +46.5 | +17.5 | 225 | BP-I |
| Middle frontal gyrus | R | −37.5 | −7.5 | +32.5 | 129 | HC |
| Culman | R | −7.5 | +37.5 | −24.5 | 111 | BP-I |
| Middle temporal gyrus | R | −52.5 | −4.5 | −15.5 | 103 | HC |
| Postcentral gyrus | R | −52.5 | +19.5 | +14.5 | 56 | BP-I |
| Precuneus | L | +4.5 | +67.5 | +47.5 | 56 | BP-I |
| Superior frontal gyrus | R | −31.5 | −55.5 | +32.5 | 44 | BP-I |
| Middle temporal gyrus | R | −43.5 | +37.5 | −0.5 | 43 | BP-I |
| Cuneus | L | +1.5 | +91.5 | +32.5 | 40 | BP-I |
| Uncus | R | −16.5 | −1.5 | −18.5 | 39 | HC |
| Parahippocmpal gyrus | L | +19.5 | +22.5 | −21.5 | 38 | BP-I |
| Insula | L | +37.5 | −1.5 | +17.5 | 38 | BP-I |
| Lentiform nucleus | R | −25.5 | −7.5 | +8.5 | 37 | BP-I |
Significant at p = 0.05 at threshold p = 0.005 at cluster threshold of 37. R = right; L = left.
Emotional and attentional processing in MDD versus HC
Eleven regions showed significant differences in brain activation between MDD and HC participants during emotional images (Table 5) and four regions showed significant differences in activation during the circle images (see Table 6).
Table 5.
Clusters showing significant differences in participants with major depressive disorder (MDD) versus healthy comparison (HC) participants during emotional images
| Region of interest | Laterality | Center of mass Talairach coordinates |
Volume (3-mm3 voxels) | Greater activation in | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Declive | R | −40.5 | +61.5 | −21.5 | 200 | HC |
| Superior temporal gyrus | L | +58.5 | +7.5 | +2.5 | 196 | MDD |
| Insula | R | −37.5 | −13.5 | −0.5 | 185 | MDD |
| Cingulate gyrus | R | −1.5 | +22.5 | +20.5 | 157 | MDD |
| Middle temporal gyrus | R | −61.5 | −1.5 | −9.5 | 132 | HC |
| Lingual gyrus | L | +22.5 | +97.5 | −15.5 | 76 | MDD |
| Parahippocampal gyrus | L | +16.5 | +19.5 | −24.5 | 66 | MDD |
| Middle temporal gyrus | R | −43.5 | +82.5 | +20.5 | 62 | MDD |
| Middle frontal gyrus | R | −25.5 | −31.5 | −15.5 | 42 | HC |
| Superior frontal gyrus | L | +1.5 | −7.5 | +50.5 | 42 | MDD |
| Culmen | R | −25.5 | +49.5 | −24.5 | 40 | MDD |
Significant at p = 0.05 at threshold p = 0.005 at cluster threshold of 37. R = right; L = left.
Table 6.
Clusters showing significant differences in participants with bipolar I disorder (BP-I) versus healthy comparison (HC) participants and participants with major depressive disorder (MDD) versus HC participants during circle images (attentional)
| Region of interest | Laterality | Center of mass Talairach coordinates |
Volume (3-mm3 voxels) | Greater activation in | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| BP-I versus HC attentional | ||||||
| Superior frontal gyrus | L | +28.5 | +1.5 | +65.5 | 61 | HC |
| Culmen | R | −13.5 | +25.5 | −24.5 | 52 | HC |
| Cingulate gyrus | L | +1.5 | +31.5 | +29.5 | 47 | HC |
| MDD versus HC attentional | ||||||
| Insula | L | +37.5 | −13.5 | −3.5 | 88 | MDD |
| Postcentral gyrus | L | +31.5 | +34.5 | +65.5 | 82 | MDD |
| Middle frontal gyrus | R | −28.5 | −58.5 | +8.5 | 45 | HC |
| Inferior temporal gyrus | R | −55.5 | +1.5 | −33.5 | 37 | HC |
Significant at p = 0.05 at threshold p = 0.005 at cluster threshold of 37. L = left; R = right.
Discussion
This study evaluated both attentional and emotional processing in patients with MDD or BP-I during a depressive episode. We observed common and distinct patterns of brain activation in participants with BP-I and MDD. During the viewing of emotional images, the major differences between the two patient groups were found in primary and secondary visual processing regions with BP-I participants showing decreased activation in these regions compared to MDD participants. Comparing the MDD and BP-I groups with HC participants showed that the BP-I group was driving most of the differences seen in the BP-I versus MDD comparison (see Tables 3–6 and Figure 3). Participants with BP-I showed decreased activation in the middle occipital gyrus, lingual gyrus, and middle temporal gyrus compared to both MDD and HC participants. During attentional processing, participants with MDD had increased activation in the parahippocampus, parietal lobe, and postcentral gyrus. However, among these regions, only the postcentral gyrus also showed differences between MDD and HC participants (Table 5).
Although there were no difference between the two patients groups in our a prior defined cortico-limbic network, activation differences were found in several regions relevant for to the emotional and attentional processing required for the task. During the emotional task activation differences were found in several regions that may be involved in emotional processing, self-referential imagery, and in processing emotional facial expressions including the parahippocampus, posterior cingulate cortex, and middle temporal gyrus (35-43). The posterior cingulate cortex is a key node in the default mode network (DMN) (39, 40). Therefore, it is possible there may be differences in the DMN in these two mood disorders. The direction of differences suggests that the DMN is over active in MDD participants and a prior research study found altered connectivity in this region in participants with MDD (44).
These findings are contrary to our hypothesis that there would be differences in the two patient groups in the major cortico-limbic regions responsible for the regulation of emotions. The lack of differences seen in depression between the MDD and BP-I groups suggests a similar mechanism at work in these two groups. It has been proposed that depression may be a result of a nonspecific response to brain injury and thus depression seen in BP-I may be secondary to the insults caused by manic episodes (45). Instead, differences in visual processing regions predominated, and these differences appear to be driven by an abnormal decrease in activation seen in the BP-I participants. In fact, posterior activation changes are a very common finding in studies across mood states in BP-I participants (46-51). Differences in these visual processing regions suggest perceptual changes in BP-I that cut across mood states. In their meta-analysis Goodman and Jamison (52) found deficits in visual skill measures across mood states in bipolar disorder. It is not clear whether alterations in occipital brain regions and visual skill deficits result from or are part of the underlying pathology in bipolar disorder. If these changes are part of the underlying pathology in bipolar disorder it may alter the way we conceptualize the disorder.
Prior research has also suggested the occipital cortex may be relevant in MDD (53, 54). Bhagwager et al. (53) found that GABA neurotransmission was altered in the occipital cortex in MDD. Furey et al. (54) found that increased baseline activation in the occipital cortex predicted antidepressant response. Regardless of the role they play in the pathology of bipolar disorder and MDD, changes in visual regions may serve as useful targets for biomarkers in diagnosis and treatment response.
Several prior studies directly compared participants with MDD and BP-I during depression using fMRI. In this regard, Lawrence et al. (55) found that participants with BP-I had increased subcortical and ventral prefrontal activation compared to MDD participants. However, this study was not as highly powered with only 11 participants in the BP-I group and nine in the MDD group. Almeida and colleagues (56) examined 15 participants with BP-I and 16 participants with MDD during depression and noted differences in effective connectivity between amygdala and orbitomedial prefrontal cortex (during the viewing of happy faces) in patients with MDD compared to those with BP-I. Later, this group examined differences in amygdala activation between 15 participants with BP-I during a depressive episode, 15 participants with BP-I during remission, 15 participants with MDD during a depressive episode, and 15 HC participants (57). They found that the BP-I depressed group had increased activation in the left amygdala when viewing sad faces compared to all the other groups. In addition, the most common emotion evoked in the CPT-END task was disgust which may also explain the lack of amygdala activation.
Despite this study being the largest to evaluate emotional and attentional processing in patients with MDD and patients with BP-I during a depressive episode, there are several important limitations. Participants in the MDD group had more depressive symptoms as reflected in significantly higher HAM-D scores than the BP-I group. However, differences in HAM-D scores were used as a covariate in a separate analysis and did not explain the majority of activation differences found. Regarding the performance of the task, the HC group had superior performance on the task as measured by quicker reaction times compared to the BP-I and MDD groups and greater accuracy compared to the MDD group. Thus, the HC participants may have been better able to engage the task which may limit the interpretation of the comparisons of the patient and HC groups. However, this is less a concern as few differences were found in the main comparison of BP-I versus MDD participants.
Acknowledgements
This study was supported by National Institute of Mental Health grants P50MH077138 and K23MH081214 (MAC).
Footnotes
Disclosures
MAC has received research support from NIMH. DEF has received research support from NIMH, Elsevier, and the USAMRMC. JRS has received research support from Eli Lilly & Co., Shire, Forest Research Laboratories, and the American Academy of Child and Adolescent Psychiatry. MPD has received research support from AstraZeneca, Eli Lilly & Co., Johnson & Johnson, Janssen, Pfizer, Otsuka, Sumitomo, NIDA, NIMH, NIAAA, NARSAD, GlaxoSmithKline, Merck, Novartis, Lundbeck, Somerset, and Shire; has served on the lecture bureau for Bristol-Myers Squibb, Merck, and Otsuka; and has received honoraria/travel for consulting from Merck, Schering-Plough, Pfizer, Eli Lilly & Co., Dey, Lundbeck, and Sunovian. CMA has received research support from AstraZeneca, Eli Lilly & Co., Pfizer, Otsuka, Forest, Sunovion, Novartis, GlaxoSmithKline, Amylin, Lundbeck, Takeda, Roche, and Merck; and is on the lecture bureau for Merck and Sunovion. SMS is a consultant to WebMD, Procter & Gamble, and Sunovion. JCE, CTS, EBN, and ML do not have any conflicts of interest to report.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Arlington: 2013. [Google Scholar]
- 2.Strakowski S. Differential brain mechanisms in bipolar and unipolar disorders considerations from brain imaging. In: Soares J, editor. Brain Imaging in Affective Disorders. Marcel Dekker; New York: 2003. [Google Scholar]
- 3.Strakowski S, DelBello M, Adler C. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 4.Adler C, DelBello M, Strakowski S. Brain network dysfunction in bipolar disorder. CNS Spectrums. 2006;11:312–320. doi: 10.1017/s1092852900020800. [DOI] [PubMed] [Google Scholar]
- 5.Strakowski S, DelBello M, Sax K, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 6.Strakowski SM, DelBello MP, Adler C, Cecil DM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 7.Bearden CE, Hoffmann KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- 8.Cerullo MA, Adler CM, DelBello MP, Strakowski SM. The functional neuroanatomy of bipolar disorder. Int Rev Psychiatry. 2009;21:314–322. doi: 10.1080/09540260902962107. [DOI] [PubMed] [Google Scholar]
- 9.Mayberg H, Liotti M, Brannan S, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;165:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 10.Videbech P, Raynkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 11.Sheline Y, Sanghavi M, Mintum M, Gado M. Depression duration but not age predicts hippocampul volume. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheline Y, Gado M, Kraemer H. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 13.Mayberg H, Brannan S, Tekell J, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 14.Mayberg H, Silva A, Brannan S, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 15.Mayberg H. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 16.Ketter T, Wang P, Dieckman N, Lembke A, Becker O, Camilleri C. Brain anatomic circuits and the pathophysiology of affective disorder. In: Soares J, editor. Brain Imaging in Affective Disorders. Marcel Decker; New York: 2003. [Google Scholar]
- 17.Goldapple K, Segal Z, Garson C, et al. Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 18.Husaain M, McDonald W, Doraiswamy P, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–99. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan K, McDonald W, Escalona P, et al. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Arch Gen Psychiatry. 1992;49:553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan K, McDonald W, Doraiswamy P, et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Clin Neurosci. 1993;243:41–46. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- 21.Greenwald B, Kramer-Ginsberg E, Bogerts B, et al. Qualitative magnetic resonance imaging findings in geriatric depression. Possible link between later-onset depression and Alzheimer's disease? Psycholog Med. 1997;27:421–431. doi: 10.1017/s0033291796004576. [DOI] [PubMed] [Google Scholar]
- 22.Parashos I, Tupler L, Blitchington T, Krishnan K. Magnetic-resonance morphometry in patients with major depression. Psychiatry Res. 1998;84:7–15. doi: 10.1016/s0925-4927(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 23.Pillay S, Renshaw P, Bonello C, Lafter B, Fava M, Yurgelun-Todd D. A quantitative magnetic resonance imaging study of caudate and lenticular nucleus gray matter volume in primary unipolar major depression: relationship to treatment response and clinical severity. Psychiatry Res. 1998;84:61–74. doi: 10.1016/s0925-4927(98)00048-1. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki H, LaBar K, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. PNAS. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID-I/P, Version 2.0, 4/97 revision) Biometrics Research, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- 26.Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang P, Bradely M, Cuthbert B. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-6. University of Florida; Gainesville: 2005. [Google Scholar]
- 29.Lee JH, Garwood M, Menon R, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- 30.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox R, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 33.Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:257–314. [Google Scholar]
- 34.Friston KJ, Worsley KJ, Frackowiak RSJ, et al. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 35.Surgulaze S, Brammer M, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Ameida J, Mechelli A, Hassel S, et al. Abnormally increased effective connectivity between parahippocampal gyrus and ventromedial prefrontal regions during emotion labeling in bipolar disorder. Psychiatric Res. 2009;174:195–201. doi: 10.1016/j.pscychresns.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maddock RF, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frodl T, Bokde A, Scheuerecker J, et al. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry. 2010;67:161–167. doi: 10.1016/j.biopsych.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Bucker RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 40.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura M, Shah NJ, Zilles K, Fink GR. Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. J Cogn Neurosci. 2005;17:183–198. doi: 10.1162/0898929053124956. [DOI] [PubMed] [Google Scholar]
- 42.Maddock RJ, Buonocore MH. Activation of left posterior cingulate gyrus by the auditory presentation of threat-related words: an fMRI study. Psychiatric Res. 1997;75:1–14. doi: 10.1016/s0925-4927(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 43.Vandenberghe R, Price C, Wise R, et al. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- 44.Bluhm R, Williamson P, Lanius R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 45.Strakowski SM, Adler CM, DelBello MP. Is depression simply a nonspecific response to brain injury? Curr Psychiatry Rep. 2013;15:386–395. doi: 10.1007/s11920-013-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C-H, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen C-H, Lennox B, Jacob R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Marchand WR, Lee JN, Thatcher J, et al. A preliminary longitudinal fMRI study of frontal-subcortical circuits in bipolar disorder using a paced motor activation paradigm. J Affect Disord. 2007;103:237–241. doi: 10.1016/j.jad.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter T. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disord. 2007;9:345–357. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 50.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fleck DE, Eliassen JC, Durling M, et al. Functional MRI of sustained attention in bipolar mania. Mol Psychiatry. 2012;17:325–336. doi: 10.1038/mp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2nd Edition. Oxford University Press; New York: 2007. [Google Scholar]
- 53.Bhagwager Z, Wylezinska M, Jezzard P. Reduction in occipital cortex γ-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 54.Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, Zarate CA., Jr Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry. 2013;70:280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 56.Almeida JR, Versace A, Mechelli A, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almeida JRC, Versace A, Hassel S, et al. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]