Abstract
Rasopathies are a group of genetic disorders caused by germline mutations in multiple genes of the Extracellular signal-Regulated Kinases 1 and 2 (ERK1/2) pathway. The only previously identified missense mutation in SHOC2, a scaffold protein of the ERK1/2 pathway, led to Noonan-like syndrome with loose anagen hair. Here we report a novel mutation in SHOC2(c.519G>A; p.M173I) that leads to a Rasopathy with clinical features partially overlapping those occurring in Noonan and Cardio-Facio-Cutaneous syndromes. Studies to clarify the significance of this SHOC2 variant revealed that the mutant protein has impaired capacity to interact with protein phosphatase 1c (PP1c), leading to insufficient activation of RAF-1 kinase. This SHOC2 variant thus is unable to fully rescue ERK1/2 activity in cells depleted of endogenous SHOC2. We conclude that SHOC2 mutations can cause a spectrum of Rasopathy phenotypes in heterozygous individuals. Importantly, our work suggests that individuals with mild Rasopathy symptoms may be under-diagnosed.
Keywords: SHOC2, ERK1/2 pathway, Rasopathies, signaling
Rasopathies are phenotypically similar syndromes with overlapping features. These autosomal dominant disorders typically present with distinctive craniofacial features, a wide spectrum of congenital heart defects, short stature and variable neurocognitive impairments [Rauen, 2013]. Rasopathies are caused by mutations that deregulate the RAS-mediated Extracellular signal-Regulated Kinases 1 and 2 (ERK1/2) signal transduction pathway [Allanson and Roberts, 2011; Tartaglia, et al., 2011].
Noonan syndrome (NS; MIM# 163950) is the most common Rasopathy, affecting between 1 in 1,000 to 1 in 2,500 children worldwide. Individuals with NS typically have distinct craniofacial features that may include wide-spaced, and down-slanting palpebral fissures with ptosis and epicanthal folds, a short nose with a depressed nasal bridge and anteverted nares, lowset ears with prominent helices which may be posteriorly rotated and a high arched palate. Cardiac defects occur in 50-80% of individuals with pulmonic stenosis and hypertrophic cardiomyopathy (HCM) being the most common. Other findings include short stature, intellectual disability in up to 30% of patients, ophthalmologic, renal and musculoskeletal defects, lymphatic dysfunction, bleeding diathesis, cryptorchidism and predisposition to leukemia [Allanson, et al., 2010]. Mutations associated with NS are found in non-receptor protein tyrosine phosphatase PTPN11 (50%); the Ras guanine nucleotide exchange factor SOS1 (13%); small GTPases KRAS, NRAS, and RIT1; and the serine/threonine kinase RAF1 (3-17%). Additionally, the molecular cause for the disorder is not known for ~20% of patients with NS [Allanson and Roberts, 2011].
Cardio-Facio-Cutaneous syndrome (CFC; MIM# 115150) is a rare Rasopathy with craniofacial features which are similar to but often coarser than those in NS [Rauen, 2012]. Cardiac findings are similar to those in NS and include cardiac arrhythmias, heart defects and HCM in frequency similar to those of NS and Costello syndrome [Rauen, 2006; Siegel, et al., 2011; Yoon, et al., 2007]. Most individuals have short stature, a broad forehead, macrocephaly, bitemporal narrowing, shallow orbital ridges, cutaneous abnormalities (such as sparse hair with abnormal texture, dystrophic nails, ichthyosis, hyperpigmentation and hyperkeratosis) and cognitive delay [Allanson, et al., 2011; Yoon, et al., 2007]. Brain anomalies, such as crowding of the posterior fossa or Chiari I malformations, are not uncommon. At least 4 genes are associated with CFC: BRAF (~75%-80%), MAP2K1 and MAP2K2 (~10%-15%), and KRAS (<5%) [Allanson, et al., 2011; Rauen, 2012; Rauen, 2013].
SHOC2 (MIM# 602775) is a scaffold protein in the ERK1/2 pathway, which tethers RAS, RAF-1 and the catalytic subunit of protein phosphatase 1c (PP1c). Recruitment of PP1c enables dephosphorylation of the S259 residue of RAF-1 resulting in the stimulation of RAF-1 kinase activity by phosphorylation of RAF-1 at Ser338 [Rodriguez-Viciana, et al., 2006]. The only previously reported pathogenic SHOC2 variant (c.4A>G; p.S2G) is associated with Noonan-like syndrome with loose anagen hair (NS/LAH). Most reported individuals with NS/LAH have a prominent forehead, macrocephaly, growth hormone deficiency, sparse, loose and slow-growing anagen hair, hyperpigmented skin with eczema or ichthyosis, mild psychomotor delays, hypernasal voices and Attention Deficit Hyperactivity Disorder (ADHD) that improves with age [Cordeddu, et al., 2009; Mazzanti, et al., 2003]. Cardiac defects appear to include an over-representation of mitral valve dysplasia and septal defects in comparison with classic NS. Other reports have expanded the clinical phenotype to include structural brain anomalies and myelofibrosis [Gripp, et al., 2013]. The p.Ser2Gly mutation creates a new recognition site for N-terminal myristoylation, causing aberrant targeting of SHOC2 protein to the plasma membrane and impaired translocation to the nucleus upon stimulation with growth factor.
We report here the identification of a novel SHOC2 functional variant (c.519G>A; p.M173I) in two individuals who do not have classic symptoms of NS, CFC or NS/LAH, but display features overlapping all three conditions. In this study we have examined the EGF signal transduced through the SHOC2 p.M173I variant in order to demonstrate that it alters the ability of SHOC2 to accelerate ERK1/2 phosphorylation. We provide evidence that the M173I mutation leads to changes in the assembly of the SHOC2-RAS-RAF-1-PP1c complex.
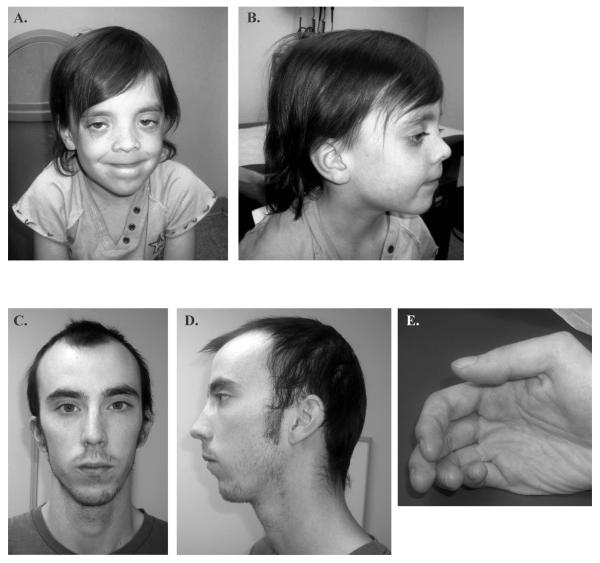
The first patient is a 5 year old female referred for evaluation of right optic nerve hypoplasia, bilateral nystagmus, speech delay, poor hair growth and hyperactive behavior. In addition, magnetic resonance imaging showed multiple irregularities of unknown significance in the midbrain, pons and medulla. She is the oldest child of non-consanguineous parents of European ancestry. She is the 6 pound 9 ounce product of a 34 weeks gestation, who was delivered by emergency C-section due to cephalopelvic disproportion. Milestones, including walking at 11-12 months of age and speech onset, were normal. The family history was significant, as both parents required special education classes in high school. She had average stature (47th percentile), macrocephaly (98th percentile), sparse and slow-growing hair which was not loose, normal skin pigmentation, bilateral ptosis and nystagmus, normal chest with wide-spaced nipples, and clubbed nails (Fig. 1A and B). A Simulconsult database query (http://www.simulconsult.com/run/index.html) using the keywords ptosis, macrocephaly, optic nerve hypoplasia and poor hair growth, suggested CFC as a possible diagnosis. A chromosome microarray was negative for copy number variants. DNA sequencing of a Rasopathy gene panel including BRAF, CBL, HRAS, KRAS, MAP2K1, MAP2K2, NRAS, PTPN11, RAF1 SHOC2 and SOS1 identified a novel heterozygous SHOC2 variant c.519G>A; p.M173I. Nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of ATG translation initiation codon in the reference sequence for human SHOC2 (GenBank: NM_007373.3), according to journal guidelines (http://www.hgvs.org/mutnomen/). The initiation codon is codon 1. This variant was not found in 6,500 asymptomatic individuals. Both parents were tested, and our proband’s father had a heterozygous copy of the p.M173I variant. He was 6′3″ tall, had intellectual disability, a high-arched palate, sparse hair and clubbed nails (Fig. 1C, D, and E). Echocardiograms and renal ultrasounds on both patients were negative (Supp. Table S1 provides a comparison of our patients with classic findings in NS, CFC and NS/LAH). This novel c.519G>A variant has been submitted to the NCBI ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar/) (SCV000147871.1).
Figure 1. Phenotypes of individuals with the p.M173I SHOC2 variant.
A and B, Proband face and profile, showing macrocephaly, ptosis, normal skin pigmentation and hair, which has never been cut. C, D & E: Face and, profile of proband’s father, showing sparse hair, normal skin pigmentation, and lack of ptosis and clubbed nails. Additional features of both individuals are described in the text.
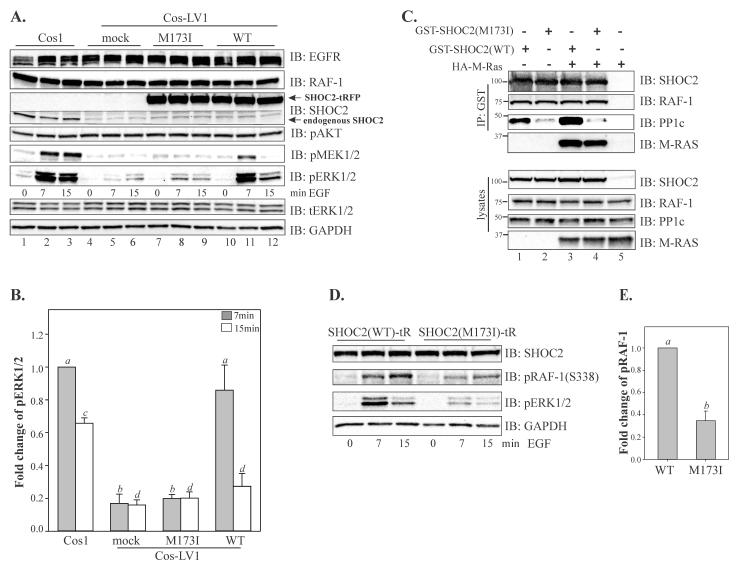
To evaluate the functional consequences of the SHOC2 p.M173I variant, we performed a series of experiments in cells lacking endogenous SHOC2 protein (see Supp. Materials and Methods). Cos1 cells with constitutive knock-down of SHOC2 (Cos1-LV1) described in our earlier studies were utilized [Galperin, et al., 2012]. In these cells, phosphorylation of MEK1/2 and ERK1/2 is severely impaired upon stimulation with physiological amounts of EGF. The single-base substitution (c.519G>A) identified in both patients was introduced into SHOC2 in the tagRFP-N1 vector. In addition to p.M173I, six “silent” variants were also introduced in the SHOC2 cDNA to render it resistant to shRNA without changing its amino acid sequence [Galperin, et al., 2012]. Cells were transfected with wild-type (WT) SHOC2-tRFP and the SHOC2 (M173I)-tRFP, and then stimulated with a low (physiological) concentration of EGF (0.2ng/ml). We observed that while the constitutive depletion of SHOC2 led to a dramatic decrease in the extent of phosphorylation of MEK1/2 and ERK1/2 upon EGFR activation (Fig. 2A, lanes 4-6), transient expression of the WT SHOC2-tRFP rescued EGF-induced ERK1/2 phosphorylation to the extent of the endogenous SHOC2 (Fig. 2A, lanes 10-12). As expected, the maximum increase in ERK1/2 phosphorylation was observed 7 min after stimulation of cells with EGF. Conversely, the SHOC2 (M173I)-tRFP mutant expressed to a similar level as WT SHOC2-tRFP was only able to restore ~10% above the basal level of EGF-induced ERK1/2 activity (Fig. 2A, lanes 7-9 and Fig. 2B). Overexpression of the SHOC2 (M173I)-tRFP in parental Cos1 cells did not lead to changes in ERK1/2 activity (Supp. Figure S1A and B). We also did not observed changes in AKT phosphorylation. These findings indicated that the M173I mutation altered the function of the SHOC2 protein.
Figure 2. Molecular evaluation of the SHOC2 p.M173I variant.
A, Cos-LV1 cells were transiently transfected with full-length SHOC2-tRFP or SHOC2 (M173)-tRFP truncated mutants. Cells were serum-starved for up to 10 hours and treated with 0.2ng/ml EGF for indicated times at 37°C. The lysates were probed by immuno-blotting for EGFR, RAF-1, SHOC2, activated MEK1/2 (pMEK1/2), activated ERK1/2 (pERK1/2), total ERK1/2 (tERK1/2) and GAPDH (loading control). Dotted line shows area of the blot that was cropped to conserve space. B, Multiple blots from the experiments exemplified in A were analyzed. Bars represent the mean values (±S.E., n = 3) of phosphorylated ERK1/2 activity normalized to total ERK1/2 in arbitrary units (pERK/ERK), (a vs. b, c vs. d, P < 0.05, one-way ANOVA followed by a Student’s t-test was used to determine differences in phosphorylated ERK1/2). C, 293FT cells transiently co-transfected with expression vectors encoding GST-SHOC2 and HA-M-RAS. GST-SHOC2 were immunoprecipitated (IP) with glutathione sepharose beads, and the immunoprecipitates were probed with GST antibodies to detect SHOC2, RAF-1, PP1c and HA antibodies to detect M-RAS. Cell lysates were immunoblotted with anti-SHOC2, anti-RAF-1, anti-PP1c and anti-HA antibody. D, Cos-LV1 cells transiently expressing SHOC2 (WT)-tRFP and SHOC2(M173I)-tRFP were serum-starved for up to 10 hours and treated with 0.2ng/ml EGF for indicated times at 37°C. The lysates were probed by immuno-blotting for SHOC2, phospho-(S338) RAF-1, activated ERK1/2 (pERK1/2), and GAPDH (loading control). Results in each panel are representative of three independent experiments. E, Multiple blots from the experiments exemplified in D were analyzed. Bars represent the mean values (±S.E., n = 3) of phosphorylated RAF-1 (7 min of EGF treatment) normalized to GAPDH in arbitrary units (pRAF-1/GAPDH) (a vs. b, P = 0.029).
The Met173 amino acid residue of SHOC2 is located in the fourth leucine-rich repeat (LRR4) of the SHOC2 LRR domain and is conserved among all SHOC2 vertebrate orthologues (Supp. Figure S2A). We speculated that the disparity in the capacity of the SHOC2-M173I mutant to enhance ERK1/2 signaling was due to alterations in protein stability. To assess the physical effect of p.M173I, we analyzed a molecular model of the full LRR domain of SHOC2 [Jeoung, et al., 2013]. From both the model and the alternating hydrophilic/hydrophobic pattern found in a beta-sheet, Met173 is predicted to be a solvent-facing residue located on the concave surface of the SHOC2 LRR domain. The M173I mutation can be readily accommodated without any perturbation to surrounding residues suggesting that the point mutation does not destabilize the fold of SHOC2 (Supp. Figure S2B). To experimentally test the stability of the SHOC2 mutant in comparison to that of WT SHOC2, we measured the half-life of WT and the SHOC2 (p.M173I) mutant in Cos-LV1 cells. To accomplish this, Cos-LV1 cells were transiently transfected with WT SHOC2-tRFP or the SHOC2 (M173I)-tRFP mutant. We found that the M173I substitution in SHOC2 did not affect the half-life of this long-lived protein (~ 12h) (Supp. Figure S2C), suggesting that protein stability was not affected by the M173I variant.
To examine whether the reduced ability of SHOC2-M173I to enhance activity of the ERK1/2 pathway is due to altered subcellular localization of SHOC2, we investigated the subcellular localization of SHOC2-M173I. Fluorescence microscopy analysis showed that SHOC2-tRFP and SHOC2 (M173I)-tRFP were distributed in both the cytosol and nucleus (Supp. Figure S2D). We next compared the cytosolic/nuclear distribution of the SHOC2-M173I mutant to WT SHOC2 by subcellular fractionation of Cos-LV1 cells transiently expressing either WT SHOC2-tRFP or SHOC2 (M173I)-tRFP proteins (Supp. Figure S2E). Our results suggest that the sub-cellular distribution of SHOC2 (M173I) variant was unaltered.
Next, we investigated whether the M173I mutation altered the capacity of SHOC2 to tether RAS and RAF-1 proteins to the scaffold complex. We expressed WT and the SHOC2 (p.M173I) variant in 293FT cells and tested their ability to interact with HA-M-RAS and RAF-1. The capacity of SHOC2-M173I to form a complex with M-RAS and RAF-1 was comparable to that of WT SHOC2 (Fig. 2C), showing that the p.M173I substitution does not affect SHOC2-RAS-RAF-1 complex assembly. These observations were not entirely surprising as we have previously determined that an N-terminal domain of SHOC2 mediates binding of RAS [Jeoung, et al., 2013].
Since SHOC2 has been shown to function as a regulatory subunit of PP1c resulting in activation of RAF-1 kinase [Rodriguez-Viciana, et al., 2006], we hypothesized that the M173I mutation might affect the SHOC2 interaction with PP1c. Although SHOC2 does not contain a canonical PP1c binding motif [R/K]-x(0,1)-[V/I]-x-F, the sequences in LRR4 of SHOC2 contained a hydrophilic motif 169KKLR172 that is found in several PP1c-binding proteins [Bennett, et al., 2006]. To determine whether p.M173I is part of a PP1c-binding motif and whether mutation affects SHOC2-PP1c binding affinity, we transfected 293FT cells with the WT or the M173I mutant of GST-SHOC2 and tested for their interaction with PP1c using immunoprecipitation. Results in Figure 2C (lanes 2 and 4) show that the GST-tagged M173I mutant of SHOC2 failed to precipitate endogenous PP1c effectively, suggesting that the M173I substitution alters the interaction of SHOC2 and PP1c. The function of PP1c in the SHOC2-RAS-RAF-1 complex is associated with activation of RAF1 kinase activity [Rodriguez-Viciana, et al., 2006]. Thus, we assessed whether phosphorylation of RAF-1 at Ser 338, a step that is required for efficient RAF-1 activation, and levels of phospho-ERK were altered concomitantly. In cells expressing WT SHOC2-tRFP, stimulation with EGF led to a dramatic increase in RAF-1-(S338) phosphorylation (Fig. 2D and E). Conversely, in cells expressing SHOC2 (M173I)-tRFP, only a mild increase in RAF-1-(S338) phosphorylation was observed, supporting the hypothesis that the M173I substitution in SHOC2 altered the interaction of SHOC2 with PP1c thus contributing to decreased RAF-1/ERK1/2 activity.
The c.519G>A; p.M173I substitution reported here is only the second SHOC2 mutation reported in patients with Rasopathy symptoms. Phenotypic features of patients with the M173I mutation are significantly different from those reported for patients with the c.4A>G; p.S2G SHOC2 mutation. While NS/LAH patients usually present with significant short stature, intellectual disability, cardiac anomalies and multiple cutaneous findings [Cordeddu, et al., 2009; Gripp, et al., 2013], our proband has craniofacial features suggestive of NS and sparse slow-growing hair that is more characteristic of CFC. She does not have short stature, a cardiac anomaly, hyperpigmentation, ichthyosis or loose anagen hair. Her developmental delays are relatively mild compared to many patients with CFC or NS/LAH, especially considering that both of her parents have mild intellectual disability. Her father has tall stature and very subtle clinical features that do not clearly denote a Rasopathy, other than his sparse hair and clubbed nails. We thus conclude that SHOC2 mutations can cause a spectrum of Rasopathy phenotypes of varying severity. This is not surprising, given that variable phenotypes have been reported for most other Rasopathy-associated genes. A recent study by Justino et al., 2014, re-emphasized the clinical heterogeneity of all neuro-cardio-facio-cutaneous syndromes, the absence of consensus on distinct diagnostic criteria and the necessity of multigene panels for molecular diagnosis. Clinical heterogeneity associated with the SHOC2 c.4A>G; p.S2G variant has also been reported [Cordeddu, et al., 2009; Hoban, et al., 2012; Justino, et al., 2014].
The p.M173I SHOC2 substitution causes loss of function in the ERK1/2 pathway. While the majority of mutations associated with Rasopathies are gain-of-function mutations, loss-of-function mutations in PTPN11 have been reported to cause Leopard syndrome, a Rasopathy with the added feature of lentigenes [Kontaridis et al., 2006]. CFC can be caused by both loss- and gain-of-function mutations in B-RAF. [Rodriguez-Viciana et al., 2006a]. This implies that the deregulation of the ERK1/2 pathway rather than up-regulation is the critical factor in the causation of Rasopathies.
In summary, our results agree with a substantial set of data suggesting that deregulation of the ERK1/2 pathway has a significant effect on development. Our report of patients with mild Rasopathy symptoms caused by a novel SHOC2 mutation should improve the diagnostic accuracy of patients with findings in the Rasopathy spectrum and provide new evidence for understanding the pathogenesis of these disorders. Our data show that p.M173I is a functional variant by demonstrating that it causes an alteration in ERK1/2 activity and that reduced binding of SHOC2 to the catalytic subunit of PP1c is the cause of the ERK1/2 pathway dysregulation. A recent study by Young et al. (2013) proposed two PP1c binding motifs that are closely localized on the exposed convex side of SHOC2, SLVK and KIPF [Young, et al., 2013]. An attractive hypothesis for our observations is that these motifs are alternatively utilized for efficient PP1c recruitment to the SHOC2 scaffolding complex. Future studies will be needed to confirm this conclusion, to decipher the physiological role of the p.M173I SHOC2 variant in development and to generate a comprehensive model of SHOC2 function.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by grant from the National Cancer Institute (R00CA126161 to EG), the National Institute of General Medical Sciences (P20GM103486) and by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director under Award Number(s) [1U01HG007674]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Drs. Matthew S. Gentry, Rebecca Dutch, Charles Waechter, Louis Hersh and Stacy Smith for critical reading of the manuscript, Dr. Craig Vander Kooi for help with SHOC2 modeling, the Viral Production Core at the Department of Molecular and Cellular Biochemistry (University of Kentucky) for assistance with production of lentiviruses. This core is supported in part by grant from the National Institute of General Medical Sciences (P20GM103486).
Contract grants sponsors: NIGMS award (P20GM103486); NCI award (R00CA126161).
Footnotes
Disclosure statement: The authors declare no conflict of interest.
REFERENCES
- Allanson JE, Anneren G, Aoki Y, Armour CM, Bondeson ML, Cave H, Gripp KW, Kerr B, Nystrom AM, Sol-Church K, Verloes A, Zenker M. Cardio-facio-cutaneous syndrome: does genotype predict phenotype? American journal of medical genetics. Part C, Seminars in medical genetics. 2011;157(2):129–135. doi: 10.1002/ajmg.c.30295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanson JE, Bohring A, Dorr HG, Dufke A, Gillessen-Kaesbach G, Horn D, Konig R, Kratz CP, Kutsche K, Pauli S, Raskin S, Rauch A, et al. The face of Noonan syndrome: Does phenotype predict genotype. American journal of medical genetics. Part A. 2010;152A(8):1960–1966. doi: 10.1002/ajmg.a.33518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanson JE, Roberts AE. Noonan Syndrome. In: Pagon RAAM, Bird TD, et al., editors. GeneReviews™. University of Washington, Seattle; Seattle (WA): 2011. 1993-2014. [Google Scholar]
- Bennett D, Lyulcheva E, Alphey L, Hawcroft G. Towards a comprehensive analysis of the protein phosphatase 1 interactome in Drosophila. J Mol Biol. 2006;364(2):196–212. doi: 10.1016/j.jmb.2006.08.094. [DOI] [PubMed] [Google Scholar]
- Cordeddu V, Di Schiavi E, Pennacchio LA, Ma’ayan A, Sarkozy A, Fodale V, Cecchetti S, Cardinale A, Martin J, Schackwitz W, Lipzen A, Zampino G, et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nature Genetics. 2009;41(9):1022–1026. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin E, Abdelmoti L, Sorkin A. Shoc2 is targeted to late endosomes and required for Erk1/2 activation in EGF-stimulated cells. PloS one. 2012;7(5):e36469. doi: 10.1371/journal.pone.0036469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Zand DJ, Demmer L, Anderson CE, Dobyns WB, Zackai EH, Denenberg E, Jenny K, Stabley DL, Sol-Church K. Expanding the SHOC2 mutation associated phenotype of Noonan syndrome with loose anagen hair: structural brain anomalies and myelofibrosis. Am J Med Genet A. 2013;161(10):2420–2430. doi: 10.1002/ajmg.a.36098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban R, Roberts AE, Demmer L, Jethva R, Shephard B. Noonan syndrome due to a SHOC2 mutation presenting with fetal distress and fatal hypertrophic cardiomyopathy in a premature infant. Am J Med Genet A. 2012;158A:1411–1413. doi: 10.1002/ajmg.a.35318. [DOI] [PubMed] [Google Scholar]
- Jeoung M, Abdelmoti L, Jang ER, Vander Kooi CW, Galperin E. Functional Integration of the Conserved Domains of Shoc2 Scaffold. PloS one. 2013;8(6):e66067. doi: 10.1371/journal.pone.0066067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justino A, Dias P, Pina MJ, Sousa S, Cirnes L, Sousa AB, Machado JC, Costa JL. Comprehensivc massive parallel DNA sequencing strategy for the genetic diagnosis of the neuro-cardio-facio-cutaneous syndromes. Eur J Hum Genet. 2014 doi: 10.1038/ejhg.2014.97. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating effects. J Biol Chem. 2006:6785–6792. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- Mazzanti L, Cacciari E, Cicognani A, Bergamaschi R, Scarano E, Forabosco A. Noonan-like syndrome with loose anagen hair: a new syndrome? Am J Med Genet A. 2003;118A(3):279–286. doi: 10.1002/ajmg.a.10923. [DOI] [PubMed] [Google Scholar]
- Rauen KA. Distinguishing Costello versus cardio-facio-cutaneous syndrome: BRAF mutations in patients with a Costello phenotype. Am J Med Genet A. 2006;140(15):1681–1683. doi: 10.1002/ajmg.a.31315. [DOI] [PubMed] [Google Scholar]
- Rauen KA. Cardiofaciocutaneous Syndrome. In: Pagon RAAM, Bird TD, et al., editors. GeneReviews™. University of Washington, Seattle; Seattle (WA): 2012. 1993-2014. [Google Scholar]
- Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet. 2013 doi: 10.1146/annurev-genom-091212-153523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS, McCormick F, Rauen KA. Germline mutations in genes within the MAPK pathway cause cardiofacio-cutaneous syndrome. Science. 2006a;311:1287–1290. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Oses-Prieto J, Burlingame A, Fried M, McCormick F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol Cell. 2006b;22(2):217–230. doi: 10.1016/j.molcel.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Siegel DH, McKenzie J, Frieden IJ, Rauen KA. Dermatological findings in 61 mutation-positive individuals with cardiofaciocutaneous syndrome. Br J Dermatol. 2011;164(3):521–529. doi: 10.1111/j.1365-2133.2010.10122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best practice & research. Clinical endocrinology & metabolism. 2011;25(1):161–179. doi: 10.1016/j.beem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G, Rosenberg J, Blaser S, Rauen KA. Neurological complications of cardio-facio-cutaneous syndrome. Dev Med Child Neurol. 2007;49(12):894–899. doi: 10.1111/j.1469-8749.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- Young LC, Hartig N, Munoz-Alegre M, Oses-Prieto JA, Durdu S, Bender S, Vijayakumar V, Vietri Rudan M, Gewinner C, Henderson S, Jathoul AP, Ghatrora R, et al. An MRAS, SHOC2, and SCRIB Complex Coordinates ERK Pathway Activation with Polarity and Tumorigenic Growth. Mol Cell. 2013;52(5):679–692. doi: 10.1016/j.molcel.2013.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.