Abstract
Background
The presence of advanced adenomas in younger individuals is a criterion for Lynch syndrome (LS). However, the utility of screening advanced adenomas for loss of mismatch repair (MMR) protein expression to identify suspected LS remains unclear.
Aims
Determine the prevalence of MMR defects to understand whether these patients harbor a defined genetic risk for CRC.
Methods
The study cohort included adult patients ≤45 years of age with advanced adenomas (villous histology, ≥1 cm in diameter, ≥3 polyps of any size) endoscopically removed between 2001 and 2011. Clinical records were reviewed along with detailed pathological review and immunohistochemical MMR analysis.
Results
A total of 76 (40.1 % male, age 40.6 ± 5.4 years) patients met inclusion and exclusion criteria. Indications for colonoscopy were gastrointestinal (GI) bleeding 39 (51.3 %), CRC in a first-degree relative 17 (22.4 %) and somatic GI symptoms 20 (26.3 %). Index colonoscopy revealed a median of 1 adenoma (range 1–4), mean diameter of 12.9 ±7.1 mm, 40 (52.6 %) with villous histology. The mean follow-up duration was 3.3 ± 2 years. Recurrent adenomas developed in 24 (31.6 %), of which 8 (10.5 %) were advanced adenomas; none of these patients developed CRC. One of 66 (1.5 %) adenomas available for immunohistochemical (IHC) testing revealed loss of MLH1 and PMS2.
Conclusions
IHC screening of advanced adenomas from patients younger than 45 years of age identified potential LS in one of 64 patients. The low yield of IHC screening in this population suggests that universal IHC screening of advanced adenomas from patients younger than 45 years of age for MMR defects is not an efficient strategy for identifying LS subjects.
Keywords: Colonoscopy, Colonic polyps, Colorectal Neoplasms, Hereditary Nonpolyposis, Microsatellite Instability, Immunohistochemistry
Introduction
Colorectal cancer (CRC) is the third most common cancer in the USA and the second leading cause of cancer deaths [1, 2]. It is widely accepted that adenomatous colon polyps serve as precursor lesions to at least a subset of CRC, with advanced adenomas (adenomas with villous histology, size ≥10 mm, ≥3 synchronous adenomas) representing a key step in progression to CRC [3–6]. In view of the observation that the prevalence of both adenomas and CRC increases with age, the recommendations from multi-society guidelines advocate screening for CRC be started at age 45–50 years, depending on risk factors, most notably family history [7–9]. The issue of what age routine screening should begin for colorectal cancer is in large part guided by prospective studies showing that the prevalence of adenomas in average-risk patients aged 40–49 is <9 % [10, 11]. However, the epidemiology and natural history of adenomas among younger individuals is incompletely understood, because these younger subjects do not routinely undergo colonoscopy in the absence of either a family history of CRC or of symptoms [12]. This dilemma is highlighted because individuals with young age of onset of adenomatous colon polyps are presumably at increased risk for the subsequent development of CRC and may also represent unrecognized familial CRC (either polyposis or non-polyposis) syndromes [12–14].
Among the inherited familial CRC syndromes, hereditary non-polyposis colon cancer or Lynch syndrome (HNPCC/LS) accounts for 3–5 % of all CRC [15]. Individuals affected by LS typically develop CRC with a mean age of 45 years and affected individuals harbor an 80 % life-time risk of CRC. [16] Affected individuals are also at high risk for extracolonic malignancy, a feature that further emphasizes the importance of identifying these subjects [15]. While the number of adenomatous polyps in individuals with LS is similar to that seen in the general population, the polyp dwell time (duration for transformation of normal colon mucosa to CRC) is significantly shorter in LS [17]. Accordingly, a major goal of screening for LS is to promote timely intervention in affected patients and their first-degree relatives. Such interventions have been shown to improve mortality in affected families [18, 19].
The genetic basis for classical LS resides in one or more germline mutations in DNA mismatch repair (MMR) genes. Most of the known mutations involve MLH1, PMS2, MSH2, MSH6 and EPCAM [15, 20]. Causative mutations result in defective or absent expression of the cognate protein, and this feature has led to the use of immunohistochemical (IHC) analysis of tumor tissue to demonstrate loss of staining as a surrogate for loss of MMR expression [21]. However, defective MMR can also arise from somatic inactivation (via methylation-induced transcriptional silencing) of the MLH1 gene, a feature noted in ~15 % of sporadic colon cancers and typically associated with BRAF (V600E) mutation [20].
The current national guidelines recommend IHC and/or microsatellite instability (MSI) testing as first line screening tools for LS from tumor tissue [16, 22]. An emerging opinion, however, suggests using these tools for reflex LS screening of all colorectal cancers [16]. Nevertheless, while there is general consensus on the role of IHC examination of CRC samples to detect MMR protein expression, there remains uncertainty regarding the value of such screening in colorectal adenomas, even in LS families [12, 23]. Some studies demonstrated concordant loss of MMR staining in both carcinomas and adenomas of LS subjects, suggesting that loss of MMR expression is both an early event and a reliable predictor of increased susceptibility to carcinogenesis [24, 25]. Other studies, however, reported the yield of screening all adenomas from younger patients to be low [23, 26].
Based upon the limited data regarding the clinical utility of MMR screening in individuals with young age of onset of advanced adenomas, we undertook to describe the clinical course of younger patients (age ≤ 45 years) with advanced adenomas and to determine the prevalence of MMR defects with the goal of understanding whether these patients represent a distinct category with defined genetic risk for early-onset CRC.
Methods
Patients
Consecutive patients were identified from an institutional database of subjects undergoing index colonoscopy at Washing ton University School of Medicine between 2001 and 2009, who were aged 18–45 at time of procedure. Patients in whom all polyps were removed during the index colonoscopy were included in the study. Advanced adenomas were defined by the presence of one or more of the following endoscopic or histologic findings: (1) villous or tubulovillous adenoma, (2) presence of high-grade dysplasia (3) size ≥ 10 mm and (4) ≥3 adenomas of any size. Patients with concurrent or prior CRC, inflammatory bowel disease, known inherited cancer syndrome (including familial adenomatous polyposis and LS), incomplete colonoscopy or incomplete records were excluded. Additionally, patients with <12 months of endoscopic follow-up after initial colonoscopy were excluded. This study was approved by the Institutional Review Board of Washington University School of Medicine (#201105072).
Data Collection
A manual chart review was performed and data was extracted from each patient’s electronic medical record. Patient demographics (age, gender, race, weight and height), clinical data (past medical and surgical history, indication for colonoscopy and gastrointestinal symptoms at the time of colonoscopy), family history and colonoscopy details (quality of colon preparation, sedation used, endoscopic findings, final diagnosis, post procedure recommendations and pathology reports) were abstracted.
MMR Immunohistochemistry (IHC) and Histologic Examination
All polyps were histologically examined by two study pathologists (IN and RFW), and the diagnosis of adenoma was confirmed. Twelve cases had insufficient tissue for IHC within the paraffin block from the initial polypectomy. This was due to the sample being exhausted, either because of small original sample size or additional levels being obtained from the specimen block during the initial pathologic examination. In cases with multiple polyps, the largest polyp was selected for IHC, which was performed on 4-µm sections from formalin-fixed, paraffin-embedded tissue blocks on a Ventana Benchmark XT automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) using standard protocols. Monoclonal antibodies were used at the indicated dilutions to MLH1 (Ventana; clone M1; monoclonal; prediluted), PMS2 (Cell Marque; clone EPR3947; monoclonal; prediluted), MSH2 (Cell Marque; clone G219–1129; monoclonal; prediluted) and MSH6 (Ventana; clone 44; monoclonal; prediluted). Ventana’s ultraView Universal DAB Detection Kit was utilized and staining-visualized using hydrogen peroxide substrate and a 3,3’-diaminodenzidine tetrahydrochloride (DAB) chro-mogen. Manufacturer-recommended conditions for antigen retrieval were used (Ventana CC1, EDTA-Tris, pH 8.0 solution). Nuclear staining within epithelium of adenomas for MLH1, PMS2, MSH2 and MSH6 was considered positive. Lymphocytes and uninvolved colonic epithelium within the surrounding tissue were used as an internal positive control. All immunostains were reviewed by both study pathologists and discrepancies resolved by consensus review. The loss of nuclear staining was recorded in biopsy samples. Paired loss of MSH2 and MSH6, or MSH6 and PMS2 antibodies individually, was considered to be indicative of Lynch syndrome. In the case of MHL1/PMS2 paired loss, BRAF testing for the V600E mutation was performed.
BRAF V600E Mutational Analysis
Testing for the BRAF V600E mutation was performed at the Washington University Anatomic and Molecular Pathology core laboratory. Tissue sections from formalin-fixed and paraffin-embedded blocks were used for DNA extraction following the manufacturer’s instructions (Puregene DNA Purification kit, Qiagen). PCR amplification for exon 15 of BRAF was carried out using the following primer pairs: forward primer sequence: 5′-TCATA ATGCTTGCTCTGATAGGA-3′ and reverse primer sequence: 5′-GGCCAAAAATTTAATCAGTGGA-3′ to give an amplicon size of 215 base pairs. PCRs contained 100 ng of DNA, 50 mmol/L potassium chloride, 10 mmol/ L TRIS–HCl, pH 8.3, 4.0 mmol/L magnesium chloride, 200 µmol/L of each dNTP, 0.2 µmol/L of primers and 1.0 U of Platinum TaqDNA polymerase (Life Technologies, Carlsbad, CA). Following an initial denaturation step, 35 cycles that included 30 s of denaturation at 94 °C, 1 min of annealing at 51 °C and followed by 30 s of extension at 72 °C were performed. The final extension step was lengthened to 7 min. To determine the presence of the mutation, restriction length polymorphism analysis was performed using TspRI (New England Biolabs, Ipswich, MA) digestion, which results in two fragments (120 and 95 bp) in the wild-type sequence and a single uncut fragment in the mutated sequence.
Statistical Analysis
The Shapiro–Wilk test was used to assess normality of data. Normally distributed data are reported as mean ± standard error of the mean, skewed data as median and range. Grouped continuous variable data were compared using two-tailed Student’s t test and Mann–Whitney U test where appropriate. Intergroup and categorical comparisons were made using the chi-square and Fisher’s exact tests. A p value of < 0.05 was required for statistical significance. Time to adenoma recurrence was estimated using Kaplan–Meier algorithms, and the log rank test was used for intergroup comparisons [27]. All statistical analyses were performed using PASW 19.0 (SPSS, Inc., Chicago, IL).
Results
Patient Characteristics
Ninety-three patients age 18–45 years had ≥1 advanced adenomas identified on colonoscopy. Of these, 17 patients were excluded due to <12 months of endoscopic follow-up. The baseline demographic and clinical characteristics of the 76 patients who met all the inclusion and exclusion criteria are summarized in Table 1. The majority of patients were female (59.2 %) and Caucasian (71.1 %). The median patient age was 42 years (range 19–45); 52 (68.4 %) patients were aged 40–45, 19 (25 %) aged 31–40 and 5 (6.6 %) aged 18–30. Three patients had a personal history of malignancy including breast cancer (1), renal cell carcinoma (1) and gastric cancer (1). Family history was positive for CRC in a first-degree relative in 17 (22.4 % of patients) and colon adenomas in a first-degree relative in 2 (2.6 %). Seventeen (22.4 %) patients had a family history of extracolonic malignancy including breast (6), prostate (5), pancreatic (2), lung (2) and melanoma (2).
Table 1.
Baseline characteristics
| n = 76 | |
|---|---|
| Age, year (mean ± SEM) | 40.6 ± 5.4 |
| Female gender | 45 (59.2 %) |
| BMI (mean ± SEM) | 30.7 ± 8.1 |
| Tobacco use | 9 (11.8 %) |
| Race | |
| African American | 22 (28.9 %) |
| Caucasian | 54 (71.1 %) |
| Personal history of cancer | 2 (2.3 %) |
| First-degree relative with CRC | 17 (22.4 %) |
| Indications for colonoscopy | |
| Gastrointestinal bleeding | 39 (51.3 %) |
| Family history of colorectal cancer | 17 (22.4 %) |
| Other gastrointestinal symptoms | 20 (26.3 %) |
Index Colonoscopy and Histological Findings
The indications for index colonoscopy included lower gastrointestinal bleeding in 39 (51.3 %) patients, change in bowel habits in 20 (26.3 %) and history of CRC in a first-degree relative in 17 (22.4 %). A total of 135 adenomas were removed with a median of 1 (range 1–4) adenoma per patient. The average adenoma size was 12.8 ± 6.9 mm (range 3–45 mm). Villous histological features were identified in ≥1 polyps in 43 (51.8 %) patients. Adenomas were located proximal to the splenic flexure in 47 (34.8 %) patients. The diagnosis of advanced adenomas was based on adenoma size ≥10 mm in 62 (81.6 %) cases, villous histology in seven (9.2 %), and >2 synchronous tubular adenomas <10 mm in size in seven (9.2 %). All adenomas were removed endoscopically with no immediate or delayed complications. There were no differences in adenoma characteristics between patients with and without a family history of colon cancer (Table 2).
Table 2.
Adenoma characteristics
| Family history of colorectal cancer |
|||
|---|---|---|---|
| Yes (n = 19) |
No (n = 57) |
p value | |
| Age (years) | 38.8 ± 6.4 | 41.1 ± 4.9 | 0.1 |
| Adenoma size (mm) | 11.9 ± 6.2 | 13.2 ± 7.4 | 0.5 |
| Median number adenomas (range) | 1 (1–3) | 1 (1–4) | 0.4 |
| Histology | |||
| Tubular adenoma | 26 | 7 | 0.3 |
| Tubulovillous adenoma | 12 | 27 | |
| Villous adenoma | 0 | 4 | |
Immunohistochemical Analysis (IHC)
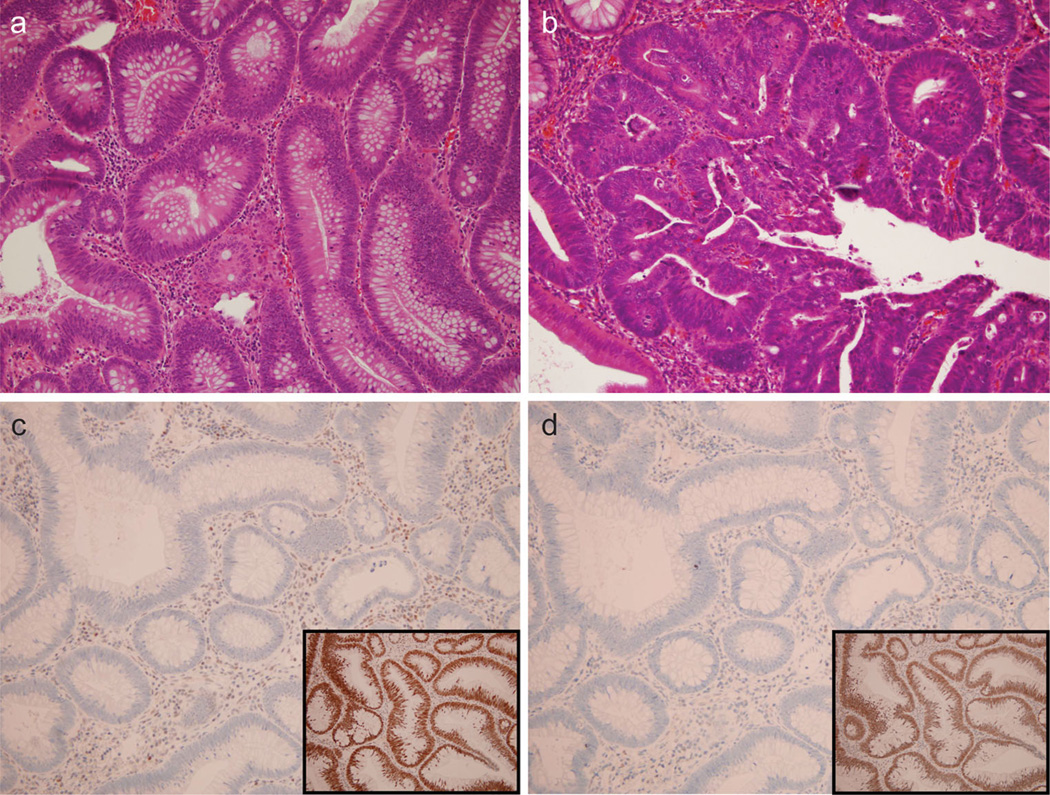
Sixty-four out of 76 (82 %) cases had sufficient paraffin-embedded tissue for immunohistochemistry analysis. However, there was no difference in the mean adenoma size (10.9 ± 1.9 vs. 12.8 ± 0.9 mm) in cases when IHC could not be performed due to insufficient tissue, as compared to in cases where IHC was performed (p = 0.3). All adenomas showed typical histologic features including increased nuclear size with nuclear pseudostratification (Fig. 1a). Villous architecture was seen in 34 cases while high-grade dysplasia was identified in 6 cases (Fig. 1b). No invasive carcinoma was identified in any sample.
Fig. 1.
Histologic features of advanced adenomas and MMR immu-nohistochemistry. a Typical adenoma (2009 magnification, H&E stain). b Adenoma in another patient with high-grade dysplasia displaying loss of nuclear polarity and cribriform gland architecture (400× magnification, H&E stain). c Loss of MLH1 nuclear positivity (200× magnification, IHC) in the 19-year-old female subject from this study. Note the positive control example of retained MLH1 (inset) in a different subject. d Loss of PMS2 nuclear positivity in the same 19-year-old female subject as panel C (200× magnification, IHC). Note the positive control example of retained PMS2 in a different subject (inset)
IHC for MLH1, PMS2, MSH2 and MSH6 on these 64 cases revealed retained nuclear staining for all proteins in 63/64 cases (98.4 %) and no polyps showed even partial loss of MMR proteins. One case showed loss of nuclear staining of both MLH1 and PMS2 (Fig. 1c, d). Due to the concurrent loss of both MLH1 and PMS2, analysis for BRAF V600E mutation was undertaken. The result was negative for the mutation and thus highly suggestive of Lynch syndrome. This polyp was removed from a 19-year-old female, with a family history of colorectal cancer, in whom a 2-cm left-sided adenoma was identified at index colonoscopy. Interval examination at 1 year was normal; this patient was subsequently lost to follow-up despite multiple attempts, and no further family history was available.
Of the 12 cases where there was insufficient tissue for IHC from the initial polypectomy specimen, advanced adenomas developed during follow-up in five patients. Two of these subjects had sufficient paraffin-embedded tissue from the subsequent polypectomy to perform IHC analysis. Samples from both of these patients showed retained nuclear expression of all four mismatch-repair proteins. Overall, IHC analysis in 66 subjects revealed one instance of loss of MMR staining, leading to a yield of presumptive LS of 1.5 %.
Follow-up Colonoscopy and Histology Findings
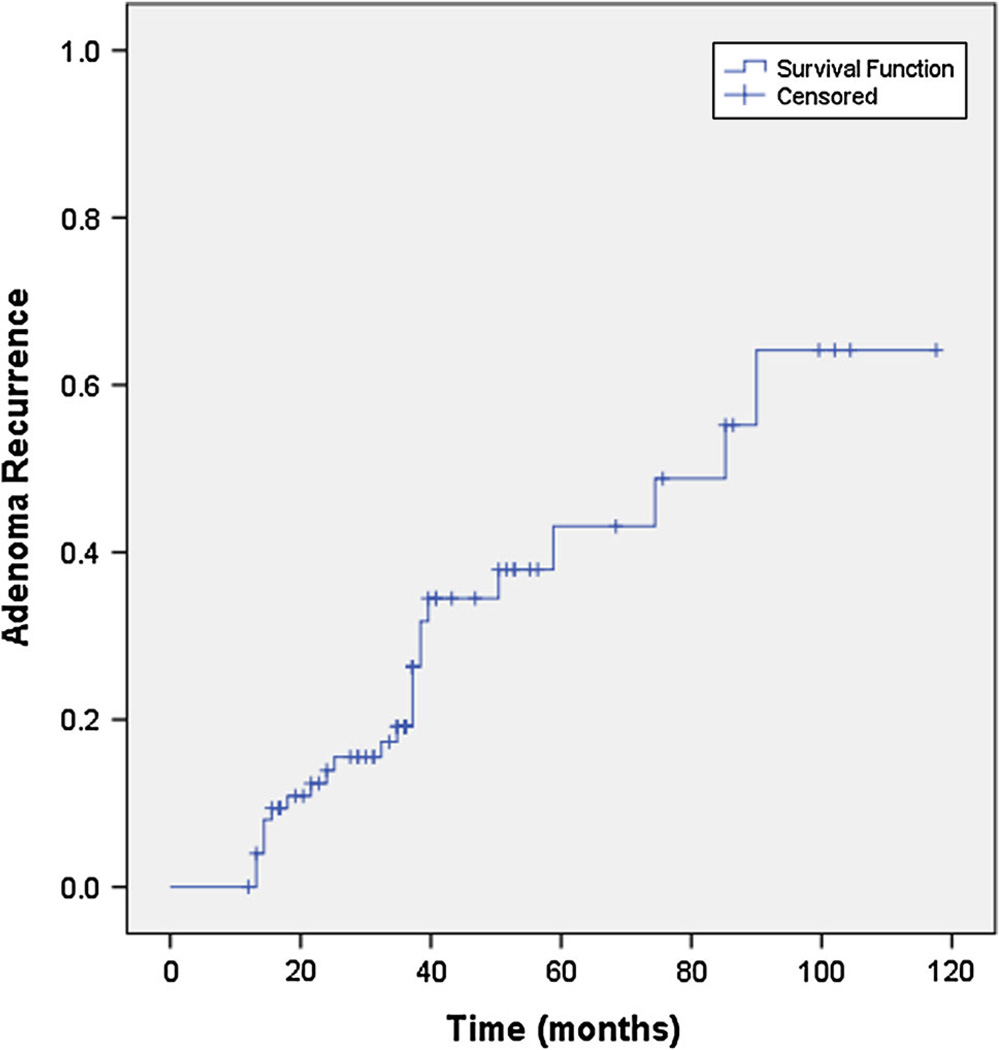
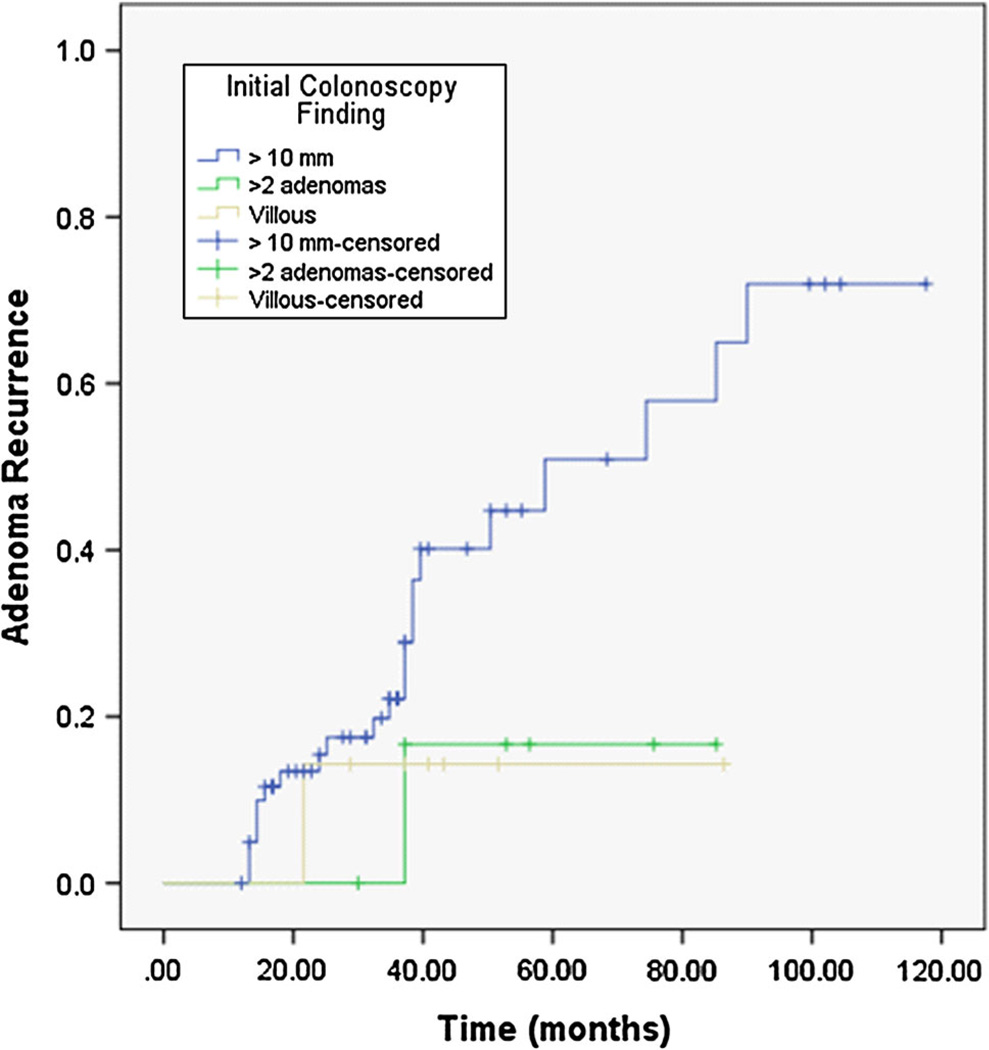
The average duration of endoscopic follow-up was 37 ± 25 (range 12–118) months. Recurrent adenomas were found in 24 (31.6 %) patients, of which eight (10.5 %) revealed advanced adenomas (5/12 cases without IHC and 19/45 with IHC on initially discovered adenoma, p = 0.51). The Kaplan–Meier estimate of median time to adenoma recurrence (Fig. 2) was 85.2 months (95 % CI 42–128 months). There was no significant difference in median time to adenoma recurrence when patients were stratified based on initial colonoscopy findings (adenoma ≥10 mm vs. villous histology vs. >2 synchronous adenomas),p = 0.18 (Fig. 3). The mean size of recurrent adenomas was 7.0 ± 0.9 (range 3–20) mm. None of the patients developed CRC during follow-up. In patients who developed recurrent adenomas, the average time interval for development of was 26.4 ± 10.3 (range 14–90) months. On univariant analysis, only older age at time of index colonoscopy was associated with increased risk of adenoma recurrence on follow-up colonoscopy (Table 3). Over the period of this study, six (16.6 %) patients were referred for genetic counseling; two of these patients had a family history significant for CRC in a first-degree relative and one had a first-degree relative with adenomatous polyps.
Fig. 2.
Kaplan–Meier estimate of time to adenoma recurrence
Fig. 3.
Kaplan–Meier estimate of time to adenoma recurrence, stratified by findings on initial colonoscopy (Log rank p = 0.18)
Table 3.
Risk factors for adenoma recurrence
| Recurrent adenoma |
|||
|---|---|---|---|
| Yes (n = 24) |
No (n = 52) |
p value | |
| Age (years) | 42.5 ± 3.3 | 39.7 ± 5.9 | 0.036 |
| Female gender | 11 | 32 | 0.5 |
| Non-caucasian race | 7 | 15 | 0.98 |
| Body mass index (kg/M2) | 32.8 ± 9.3 | 29.8 ± 7.5 | 0.18 |
| Family history of CRC | 5 | 14 | 0.57 |
| Adenoma size (mm) | 13.8 ± 6.7 | 12.4 ± 7.3 | 0.42 |
| Median number adenomas (range) | 1 (1–2) | 1 (1–3) | |
| Histology | |||
| Tubular adenoma | 8 | 25 | 0.12 |
| Tubulovillous adenoma | 16 | 23 | |
| Villous adenoma | 0 | 4 | |
Discussion
The central objectives of this study were to describe the prevalence of polyp recurrence among younger patients (age ≤ 45 years) with advanced adenomas and to determine the utility of IHC staining to detect MMR expression as a means of identifying a potential high-risk population for early-onset CRC. The key findings demonstrate loss of MMR staining in only one of sixty-six cases (1.5 %). Recurrent adenomas developed in 30 % of patients during 3 years of follow-up, with no CRC found during surveillance. While the results of this retrospective study have to be viewed with caution, our data suggest that universal IHC screening of advanced adenomas from patients younger than 45 years of age may not be an effective strategy for detecting LS and further suggest that the overwhelming majority of these cases most likely represent sporadic adenomas.
Early identification of LS patients is critical, as intensive endoscopic surveillance has been shown to decrease the incidence of CRC in this population [18, 28, 29]. To that end, the original Bethesda Guidelines recommended screening for MSI in all adenomas resected from patients younger than 40 years, regardless of patient or adenoma characteristics [30]. This recommendation was, however, removed from the subsequent version of the guidelines, due to lack of evidence. Indeed, only one prior study has evaluated the utility of MSI and IHC screening of adenomas removed from younger patients without known LS [23]. In that study, Velayos et al. evaluated 40 adenomas from 34 patients <40 years of age using MSI testing as well as IHC detection for loss of MSH2 or MLH1. In addition, none of the adenomas demonstrated MSI or loss of MMR staining [23]. However, the generalizability of those findings is somewhat limited by virtue of the study design. Specifically, the vast majority of adenomas in that study were low risk (<5 mm in size with no dysplasia or villous histology) [31]. The current study extends those findings to high-risk adenomas, but our central findings and conclusions reinforce those of Velayos et al. In the current study, we identified only one potential case of LS in a 19-year-old patient with a family history of CRC. The current findings are also in accord with other studies examining the prevalence of LS, in which MSI screening of adenomas identified MSI in six of 378 patients (1.6 %) [32].
We recognize that cases of LS may have been missed in our cohort due to the limitations of MMR IHC testing of adenomas. Several prior studies have evaluated the diagnostic utility of IHC in adenomas from patients with known LS and found loss of staining by IHC in 60–80 % of adenomas [31, 33, 34]. Moreover, the presence of MMR deficiency is more prevalent in large adenomas (>8 mm) or those with advanced histologic features (high-grade dysplasia, villous histology); and over 90 % of polyps evaluated in our study met these criteria. In those settings, loss of MMR IHC expression exhibits a sensitivity of >90 % in identifying MMR deficiency [25, 31, 33, 34]. That said, the current findings should be interpreted with caution. In the first place, we recognize that MSI and LS cannot be completely excluded even in a setting of retained IHC [25]. Secondly, no germline testing was performed in our cohort. Nevertheless, the current findings strongly imply confidence in assigning a category of sporadic polyps in the overwhelming majority of large polyps, in keeping with earlier findings demonstrating 100 % concordance of MSI and MMR staining in polyps 10 mm or larger although MMR expression may be retained in polyps <8 mm despite underling MSI [34]. Finally, our findings are also in line with those of Goel et al. [35] who found that Lynch syndrome was infrequent in young patients with non-familial colorectal cancer.
The further observation that no cases of CRC were detected among this population during 3 years of endoscopic surveillance also bears discussion. The polyp dwell time, defined as duration of transition from normal colon mucosa to formation of a CRC is estimated to be ~10 years in the average-risk subjects [36]. By contrast, polyp dwell time is estimated to be <3 years in LS subjects [17]. The observation that none of the patients in our cohort developed CRC over this period of surveillance suggests that these polyps are most likely sporadic adenomas and cases of LS are unlikely to have been missed. Indeed, 31.6 % of our cohort developed recurrent adenomatous polyps, with 10.4 % developing advanced adenomas during surveillance. These rates of adenoma recurrence are similar to those seen in average-risk individuals over age 50 with sporadic adenomatous polyps [37, 38]. These observations together suggest that the majority of younger patients with advanced adenomas have a phenotype similar to that of older individuals with sporadic adenomas. By corollary, these patients exhibit a markedly lower risk of developing CRC compared to LS patients. Extending these conclusions, we suggest that traditional surveillance guidelines, with repeat colonoscopy at 3 years following removal of an advanced adenoma, even in subjects <45 years of age appears appropriate.
There are several limitations to the findings of this retrospective study. Foremost are the sample size and the single center experience. Additionally, as noted above, germline testing for LS was not performed in any subject, and records from the six patients who were referred for genetic counseling were not available. IHC analysis of adenoma tissue from 10 patients was not possible due to insufficient tissue available for analysis; however, there was no significant difference in the adenoma recurrence rate between the cohort as a whole and patients in whom IHC could not be performed. Finally, it is important to acknowledge that surveillance in our cohort was only 3 years, which may account for the failure to find CRC. It remains possible that this cohort is at increased risk for CRC at further intervals and thus the current findings have to be viewed as preliminary.
In conclusion, MMR IHC screening of advanced adenomas from patients younger than 45 years of age resulted in the identification of potential LS in only one of sixty-six patients. The low yield of MMR IHC screening in this population suggests that the overwhelming majority of these patients have sporadic adenomas, a conclusion supported by the observation that adenoma recurrence rate is similar to that seen in older individuals with sporadic colon polyps. Accordingly, universal MMR IHC screening of advanced adenomas from patients younger than 45 years of age for MMR defects does not appear to be warranted.
Acknowledgments
Supported in part by (NIHMS320979) (VMK) and Grants DK-52574, DK-56260 and HL-38180 to (NOD). This work was also supported by funding from the Buehrle Family fund and the Gale Family fund for colorectal cancer research.
Footnotes
Presented in preliminary form at the Annual Meeting of the American Gastroenterological Association, 2012, San Diego.
Conflict of interests None.
Contributor Information
Vladimir M. Kushnir, Email: vkushnir@wustl.edu, Division of Gastroenterology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8124, St Louis, MO 63110, USA.
ILKe Nalbantoglu, Department of Pathology, Washington University School of Medicine, St Louis, MO, USA.
Rao Watson, Department of Pathology, Washington University School of Medicine, St Louis, MO, USA.
Jonathan Goodwin, Division of Gastroenterology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8124, St Louis, MO 63110, USA.
Elyas Safar, Division of Gastroenterology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8124, St Louis, MO 63110, USA.
Reena V. Chokshi, Division of Gastroenterology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8124, St Louis, MO 63110, USA
Riad R. Azar, Division of Gastroenterology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8124, St Louis, MO 63110, USA
Nicholas O. Davidson, Email: NOD@dom.wustl.edu, Division of Gastroenterology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8124, St Louis, MO 63110, USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 3.Michor F, Iwasa Y, Lengauer C, et al. Dynamics of colorectal cancer. Semin Cancer Biol. 2005;15:484–493. doi: 10.1016/j.semcancer.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Huang CS, Farraye FA, Yang S, et al. The clinical significance of serrated polyps. Am J Gastroenterol. 2011;106:229–240. doi: 10.1038/ajg.2010.429. quiz 241. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 6.Ahnen DJ. The American College of Gastroenterology Emily Couric Lecture-the adenoma-carcinoma sequence revisited: has the era of genetic tailoring finally arrived? Am J Gastroenterol. 2011;106:190–198. doi: 10.1038/ajg.2010.423. [DOI] [PubMed] [Google Scholar]
- 7.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 8.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 10.Thoma MN, Castro F, Golawala M, et al. Detection of colorectal neoplasia by colonoscopy in average-risk patients age 40–49 versus 50–59 years. Dig Dis Sci. 2011;56:1503–1508. doi: 10.1007/s10620-011-1565-6. [DOI] [PubMed] [Google Scholar]
- 11.Imperiale TF, Wagner DR, Lin CY, et al. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346:1781–1785. doi: 10.1056/NEJM200206063462304. [DOI] [PubMed] [Google Scholar]
- 12.Stoffel EM, Syngal S. Adenomas in young patients: what is the optimal evaluation? Am J Gastroenterol. 2005;100:1150–1153. doi: 10.1111/j.1572-0241.2005.41967.x. [DOI] [PubMed] [Google Scholar]
- 13.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148:419–426. doi: 10.7326/0003-4819-148-6-200803180-00004. [DOI] [PubMed] [Google Scholar]
- 14.Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006;64:614–626. doi: 10.1016/j.gie.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 15.Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelstein DL, Axilbund J, Baxter M, et al. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin Gastroenterol Hepatol. 2011;9:340–343. doi: 10.1016/j.cgh.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 19.de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130:665–671. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Geiersbach KB, Samowitz WS. Microsatellite instability and colorectal cancer. Arch Pathol Lab Med. 2011;135:1269–1277. doi: 10.5858/arpa.2011-0035-RA. [DOI] [PubMed] [Google Scholar]
- 21.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison J, Bronner M, Leach BH, et al. Lynch syndrome screening in newly diagnosed colorectal cancer in general pathology practice: from the revised Bethesda guidelines to a universal approach. Scand J Gastroenterol. 2011;46:1340–1348. doi: 10.3109/00365521.2011.610003. [DOI] [PubMed] [Google Scholar]
- 23.Velayos FS, Allen BA, Conrad PG, et al. Low rate of microsat-ellite instability in young patients with adenomas: reassessing the Bethesda guidelines. Am J Gastroenterol. 2005;100:1143–1149. doi: 10.1111/j.1572-0241.2005.40862.x. [DOI] [PubMed] [Google Scholar]
- 24.Shia J, Klimstra DS, Nafa K, et al. Value of immunohisto-chemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005;29:96–104. doi: 10.1097/01.pas.0000146009.85309.3b. [DOI] [PubMed] [Google Scholar]
- 25.Halvarsson B, Lindblom A, Johansson L, et al. Loss of mismatch repair protein immunostaining in colorectal adenomas from patients with hereditary nonpolyposis colorectal cancer. Mod Pathol. 2005;18:1095–1101. doi: 10.1038/modpathol.3800392. [DOI] [PubMed] [Google Scholar]
- 26.Kang KJ, Min BH, Ryu K, et al. Clinical usefulness of micro-satellite instability test in Korean young patients with high-risk features associated with adenoma. Clin Res Hepatol Gastroen-terol. 2012;36:378–383. doi: 10.1016/j.clinre.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Fine JP. Survival analysis. Methods Mol Biol. 2007;404:303–318. doi: 10.1007/978-1-59745-530-5_15. [DOI] [PubMed] [Google Scholar]
- 28.Jarvinen HJ, Renkonen-Sinisalo L, Aktan-Collan K, et al. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009;27:4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 29.Stupart DA, Goldberg PA, Algar U, et al. Surveillance colonos-copy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Colorectal Dis. 2009;11:126–130. doi: 10.1111/j.1463-1318.2008.01702.x. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 31.De Jong AE, Morreau H, Van Puijenbroek M, et al. The role of mismatch repair gene defects in the development of adenomas in patients with HNPCC. Gastroenterology. 2004;126:42–48. doi: 10.1053/j.gastro.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Loukola A, Salovaara R, Kristo P, et al. Microsatellite instability in adenomas as a marker for hereditary nonpolyposis colorectal cancer. Am J Pathol. 1999;155:1849–1853. doi: 10.1016/S0002-9440(10)65503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh MD, Buchanan DD, Pearson SA, et al. Immunohisto-chemical testing of conventional adenomas for loss of expression of mismatch repair proteins in Lynch syndrome mutation carriers: a case series from the Australasian site of the colon cancer family registry. Mod Pathol. 2012;25:722–730. doi: 10.1038/modpathol.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yurgelun MB, Goel A, Hornick JL, et al. Microsatellite instability and DNA mismatch repair protein deficiency in Lynch syndrome colorectal polyps. Cancer Prev Res. 2012;5:574–582. doi: 10.1158/1940-6207.CAPR-11-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goel A, Nagasaka T, Spiegel J, et al. Low frequency of Lynch syndrome among young patients with non-familial colorectal cancer. Clin Gastroenterol Hepatol. 2010;8:966–971. doi: 10.1016/j.cgh.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 37.van Heijningen EMB, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology. 2013;144:1410–1418. doi: 10.1053/j.gastro.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. New Engl J Med. 2000;342:1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]