SUMMARY
Human CMV infections are a serious source of morbidity and mortality for immunocompromised patients and for the developing fetus. Because of this, the development of new strategies to prevent CMV acquisition and transmission is a top priority. Myeloid dendritic cells (DC) residing in the oral and nasal mucosae are among the first immune cells to encounter CMV during entry, and greatly contribute to virus dissemination, reactivation from latency, and horizontal spread. Albeit affected by the immunoevasive tactics of CMV, mucosal DC remain potent inducers of cellular and humoral immune responses against this virus. Their natural functions could thus be exploited to generate long-lasting protective immunity against CMV by vaccination via the oro-nasal mucosae. Although related, epithelial Langerhans-type DC (LC) and dermal monocyte-derived DC (MDDC) interact with CMV in dramatically different ways. While immature MDDC are fully permissive to infection, for instance, immature LC are completely resistant. Understanding these differences is essential to design innovative vaccines and new antiviral compounds to protect these cells from CMV infection in vivo.
Keywords: human cytomegalovirus, dendritic cell, tropism, mucosae
INTRODUCTION
Human CMV is a ubiquitous herpesvirus that causes asymptomatic primary infections in immunocompetent hosts, followed by the establishment of lifelong latency. Virus reactivation in healthy individuals is followed by virion shedding in body fluids such as urine and saliva, and by virus transmission to new hosts. The immune system, however, normally brings infection under control with no serious long-term consequences. Prolonged immunosuppression, by contrast, dramatically alters the delicate balance between CMV and the immune system, leading to the onset of severe disease [1, 2].
The oral cavity is likely to be a major site of CMV acquisition and spread to new hosts. Limited replication within oral mucosal cells at the time of initial infection promotes virus transfer to the circulation and, henceforth, to the bone marrow, where latency is established. Low-level virus amplification in the salivary glands after their seeding with reactivated CMV carried by myeloid cells is then thought to be the source of infectious virus in the saliva [1, 3, 4]. Although shedding from the oral cavity is highest during the first few months after primary infection, intermittent release of virions continues for the entire life of the host, substantially contributing to horizontal CMV transmission [1]. Up to 7.5% of normal people carry viral DNA in their saliva [5], with dramatic increases in AIDS (12%–31%) [5–7], periodontitis (44%–89%) [8] and transplant patients (25%–45%) [9]. The nasal cavity is also an important route of CMV acquisition and shedding. The presence of this virus was indeed detected in the nasal mucosae of healthy individuals [10], infants [11] and AIDS patients [12, 13].
Both the oral and nasal cavities are constantly patrolled by myeloid dendritic cells (DC), which effectively control the magnitude and type of the response raised against mucosal antigens by modulating the intensity and modality of T and B cell activation. Myeloid DC residing in peripheral tissues are conventionally considered "immature", based on their relatively low T-cell stimulatory ability and large antigen uptake capacity. Once activated by danger signals, immature DC undergo maturation by up-regulating cell surface expression of MHC class I and II molecules, and of co-stimulatory molecules and chemokine receptors, which guide their migration to the draining lymph nodes. Here, mature DC present pathogen-derived antigens to naïve T and B cells, stimulating their proliferation and differentiation into CD8+ or CD4+ T cells, and into antibody-producing plasma cells, respectively [14]. Mucosal tissues also contain small numbers of plasmacytoid DC (pDC) of lymphoid origin, whose primary task is to produce type I interferon in response to viral and bacterial infections [15].
The development of an anti-CMV vaccine to protect naïve individuals from primary infection and to prevent virus transmission is a top priority [16–18]. All live-attenuated vaccines tested so far were inoculated subcutaneously or intramuscularly [19, 20], and although specific responses were elicited, the level of protection conferred was not comparable to that achieved during natural infection [20, 21]. While over-attenuation of the CMV strain used in some of these trials was considered to be a reason for the partial failure of the vaccine [22], the route of inoculation may also have been suboptimal, as only circulating DC are recruited after subcutaneous or intramuscular injection of antigens [23]. Numerous non-CMV vaccine trials have shown that the direct targeting of mucosal DC is highly effective at inducing long-lasting responses [24–26]. Because T and B cells acquire mucosal homing properties only when stimulated by DC migrated out of mucosal tissues [26, 27], delivery of a CMV vaccine via the oral or nasal route might prove superior at preventing virus acquisition and transmission by stimulating the production of antibodies acting directly at these entry sites.
Live-attenuated CMV strains that closely mimic natural infection are arguably better than other vaccine stimuli at eliciting effective and long-lasting protective responses [20, 28, 29]. Targeting of such strains to mucosal DC, instead of to muscular tissues, may enhance their immunogenicity, particularly after deletion of the viral immunomodulatory genes from their genomes. A profound understanding of the viral and cellular determinants of CMV tropism for mucosal myeloid DC is however required to this end, as well as to design effective therapeutics to protect DC from infection in vivo, to properly manipulate DC responses before their use in DC vaccination protocols [30], and to produce potent CMV-specific T cells in vitro for use in adoptive immunotherapy regimens [31].
DC POPULATIONS OF ORAL AND NASAL MUCOSAE
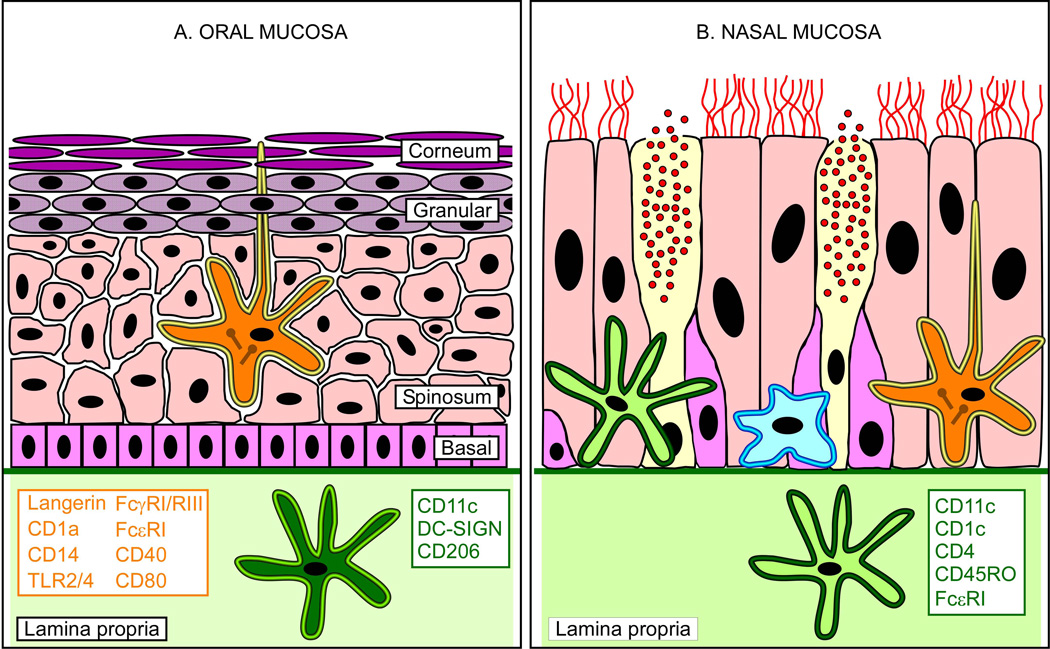
The human oral mucosa consists of the corneum, granular, spinosum and basal layers attached via the basement membrane to the underlying lamina propria (Table 1 and Figure 1A), and is mainly composed of keratinocytes, in regions directly exposed to strong shear forces, and of epithelial cells in softer areas [32, 33]. The epithelial layers are predominantly, if not exclusively, colonized by immature Langerhans-type DC (iLC), which are, therefore, the first professional antigen-presenting cells to encounter pathogens entering via the oral cavity [33–39]. Easily distinguished from other types of DC by the co-expression of CD1a and Langerin/CD207, and by the presence of the characteristic Birbeck granules, iLC are found in all areas of the mouth, but in particularly high density in the vestibulum, bucca, hard palatum and lingua [37, 40]. Albeit related to skin iLC, oral iLC possess distinctive features. Because mouth surfaces are usually moist and covered in mucus, the dendrites of oral iLC extend from the stratum corneum to the epithelial surface, where they scan the luminal space for the presence of potentially harmful microorganisms [41, 42]. Oral iLC also express the lipopolysaccharide (LPS) receptors CD14 and Toll-like receptor 2 and 4 (TLR2, TLR4) [36], the IgE and IgG receptors FcεRI, FcγRIII/CD16 and FcγRI/CD64, plus higher levels of MHC class I and II, and of the costimulatory molecules CD40, CD80, and CD86 [34]. In addition to iLC, the lamina propria of healthy oral mucosae also contains immature myeloid DC displaying the CD11c, DC-SIGN/CD209 and mannose receptor/CD206 markers, while pDC are virtually absent [35]. In the presence of inflammation, however, such as during chronic periodontitis, lichen planus and oral ulcers, large numbers of mature CD11c+ CD83+ DC and mature Langerhans cells (mLC) have been detected in the lamina propria and in the epithelium, respectively [34, 39, 43], indicating that under these conditions, maturation is not accompanied by DC migration out of the periphery and into the draining lymph nodes as usual [44, 45].
Table 1.
Main features of human oral and nasal mucosae
| ORAL MUCOSA | NASAL MUCOSA | |
|---|---|---|
| Type of tissue | Stratified squamous epithelium | Pseudostratified columnar epithelium |
| Layers | Corneum, granular, spinosum and basal layers attached to the lamina propria via the basement membrane | Epithelial layer attached to the lamina propria via the basement membrane |
| Non-DC cell types | Epithelial cells, keratinocytes | Epithelial cells, goblet cells (mucus) |
| Epithelial layer DC | iLC | iLC, macrophages, CD11c+ DC, pDC |
| Lamina propria DC | Macrophages, CD11c+ DC | Macrophages, CD11c+ DC, pDC |
| Lymphoid tissue | Palatine and lingual tonsil | Pharyngeal (adenoid) and tubal tonsil |
Figure 1. Myeloid dendritic cells in oral and nasal mucosae.
(A) The oral mucosa is comprised of a stratified epithelium attached to the lamina propria (light green area) via the basement membrane (green thick line). The proliferating stem cells of the basal layer (pink-colored rectangles) give rise to the keratinocytes of the stratum spinosum (rose-colored polyhedrons), which abundantly express different types of keratin. These cells subsequently evolve into keratohyalin granule containing granular cells (purple-dotted ovals) before losing their nuclei and becoming the corneocytes (purple lanceolated ovals) of the stratum corneum. LC (yellow rimmed, orange star) are the only DC type found in the epithelial layers of oral mucosae, and can be easily recognized by the presence of the cytoplasmic Birbeck granules (brown drumsticks), and by the expression of the surface markers listed in orange font. Contrary to skin LC, oral LC extend their dendrites to reach the surface of the epithelium, thus coming into direct contact with external microorganisms. LC are thus the first type of innate immune cells to encounter those pathogens that, like CMV, can use the oral cavity to enter into their hosts. Myeloid CD11c+ DC (dark green star), by contrast, are found deeper within the lamina propria, and are characterized by surface expression of the molecules listed in green font. (B) The nasal mucosa consists of a single layer of ciliated epithelial cells (rose-colored cylinders), basal cells (pink-colored polygons) and mucus (red dots)-secreting goblet cells (yellow colored cylinders) attached to the lamina propria via the basement membrane. Myeloid CD11c+ DC (light green star) expressing the surface markers listed in green font, and macrophages (light blue star) are found interspersed within the cells of the epithelial layer and the lamina propria, while LC reside exclusively in the epithelium. Drawings of epithelia are not to scale.
Simpler than the oral epithelium, the nasal mucosa consists of a single layer of ciliated and non-ciliated epithelial cells attached to the lamina propria via the basement membrane (Table 1 and Figure 1B). Interspersed within both compartments is a dense network of HLA-DR+ cells, consisting predominantly of macrophages and, secondarily, of DC expressing the CD1c, CD11c, CD4, CD45RO, and FcεRI markers, and bearing a strong resemblance to circulating DC [46]. As in oral tissues, iLC exist exclusively in the epithelium, where they represent approximately 40% of resident DC [35]. Contrary to oral mucosae, nasal epithelia also contain pDC [35].
The DC content of human and rodent mucosae is substantially different, a feature that must be considered when using rodents to test vaccines intended for mucosal delivery in humans. In oral tissues, iLC dominate in humans but are replaced by CD11b+ myeloid DC and by pDC in mice [47], while in nasal tissues, immature DC are prevalent in rats [48], but are replaced by a majority of macrophages in humans [46]. Additionally, although the mucosae of both species contain nasopharyngeal-associated lymphoreticular tissues (NALT) at birth, only mice retain these for life, while in humans older than two, NALT is replaced by the Waldeyer’s ring [49].
CMV TROPISM DETERMINANTS
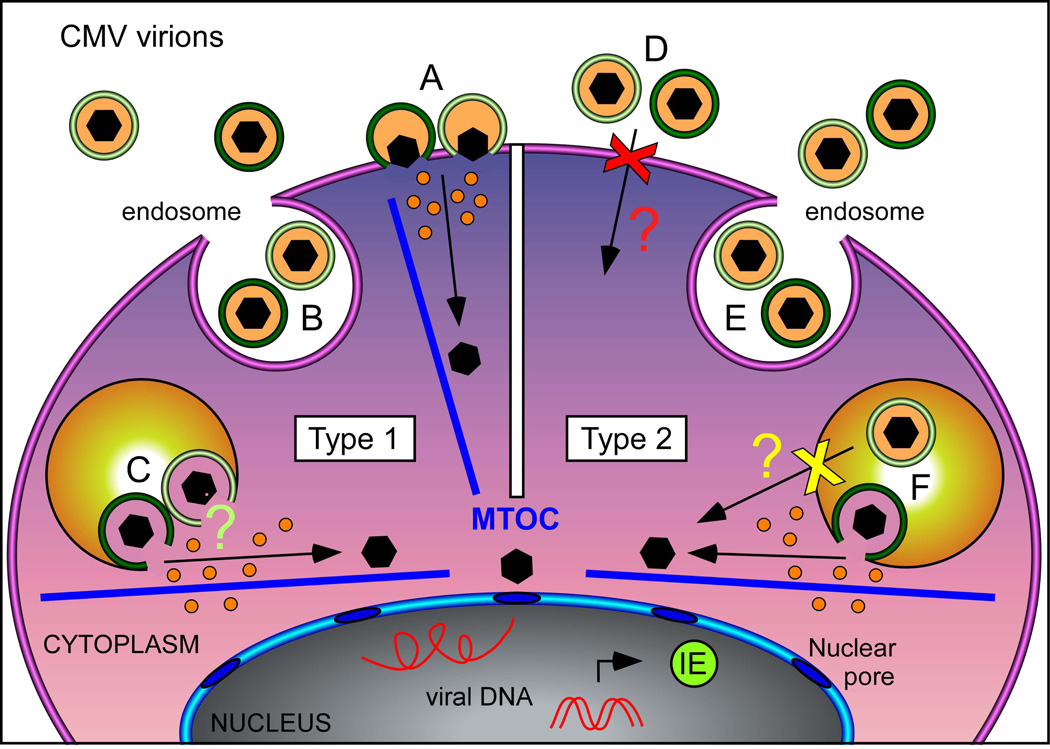
The unique ability of CMV to infect an astonishingly wide variety of cell types depends upon a finely tuned interplay between viral and cellular functions, whose activities are particularly important at the beginning of infection. On the one hand, the host cell largely determines which routes are available to CMV for entry, with some cell types such as fibroblasts allowing penetration by both fusion at the plasma membrane and macropinocytosis (designated Type 1 in Figure 2), and others, like epithelial and endothelial cells, permitting entry exclusively by macropinocytosis (designated Type 2 in Figure 2). On the other hand, CMV virions are equipped with numerous tegument and envelope proteins that facilitate entry into both cell types, and that endow this virus with its extremely broad tissue tropism [50]. Virion entry into Type 1 cells requires the trimeric complex gH/gL/gO to support fusion of the envelope with the plasma membrane [51–53], while penetration into Type 2 cells can proceed only in the presence of a functional gH/gL/UL128–131A complex [54–56], which promotes fusion of the envelope with endosomal membranes in a mechanism reported to require [57, 58] or not [59, 60] endosomal acidification, depending on the virus strain. While both complexes are present on the envelope of clinical-like CMV strains [55, 61, 62], attenuated strains lack the gH/gL/UL128–131A complex due to mutations in the UL128–131A locus [54], and are consequently unable to access Type 2 cells. Precisely why fusion at the cell surface does not occur, or if it does, why it is not followed by productive infection in Type 2 cells, as well as whether clinical-like strains use both routes to enter into Type 1 cells, or if one pathway is favored over the other remains unknown. Fusion of the envelope with cellular membranes is followed by the intracellular release of tegument proteins and naked capsids, which are transported along cellular microtubules [63] toward the nucleus, where viral genomes are deposited. A highly regulated series of viral gene transcription events then unfolds, with the immediate-early (IE), early and late genes being expressed in strict sequence. IE genes encode mostly trans-activators, like the very well known transcription factors IE1 and IE2, which cooperate in regulating the expression levels of numerous viral and cellular genes. Production of these essential proteins marks the beginning of the productive phase of infection, which usually proceeds in a virtually unrestricted manner [1, 50].
Figure 2. CMV entry routes.
CMV virions can enter host cells by fusion of the envelope with either the cell membrane or with cellular endosomes. Both pathways are accessible in fibroblasts, iLC and mLC (designated here as Type 1), while only entry by macropinocytosis is available in iMDDC, epithelial and endothelial cells (designated here as Type 2). Entry by fusion at the cell surface is mediated by the trimeric gH/gL/gO complex, while fusion with endosomes requires the presence of the pentameric gH/gL/UL128-131A complex. Clinical-like CMV strains such as TB40/E display both complexes on their envelopes (dark green toroids), while attenuated CMV strains such as AD169 only contain the trimeric complex (light green toroids). Virions from both pentamer + and pentamer− strains can enter Type 1 cells by fusion at the cell surface (A), and were observed within intracellular endosomes (B). Whether fusion of pentamer− envelopes with the endosomal membranes of Type 1 cells then occurs is currently unknown (C, light green question mark). Neither pentamer+ nor pentamer− virions can access Type 2 cells by fusion at the surface (D, red X mark), but the nature of the block has not been identified (red question mark). After macropinocytosis (E), only the envelope of pentamer+ virions can fuse with endosomal membranes (F), while pentamer− envelopes are unable to do so (yellow X mark) for as yet unknown reasons (yellow question mark). Upon membrane fusion, naked capsids (black hexagons) and tegument proteins (orange dots) are released into the host cell. Capsids are subsequently transported along cellular microtubules (blue bars) towards the nucleus, where viral genomes are inserted through the nuclear pores. Genome deposition is then followed by expression of the viral immediate-early proteins (IE), whose presence is absolutely required for the progress of lytic infection. MTOC, microtubule organizing center.
CMV TROPISM FOR LANGERHANS-TYPE DC
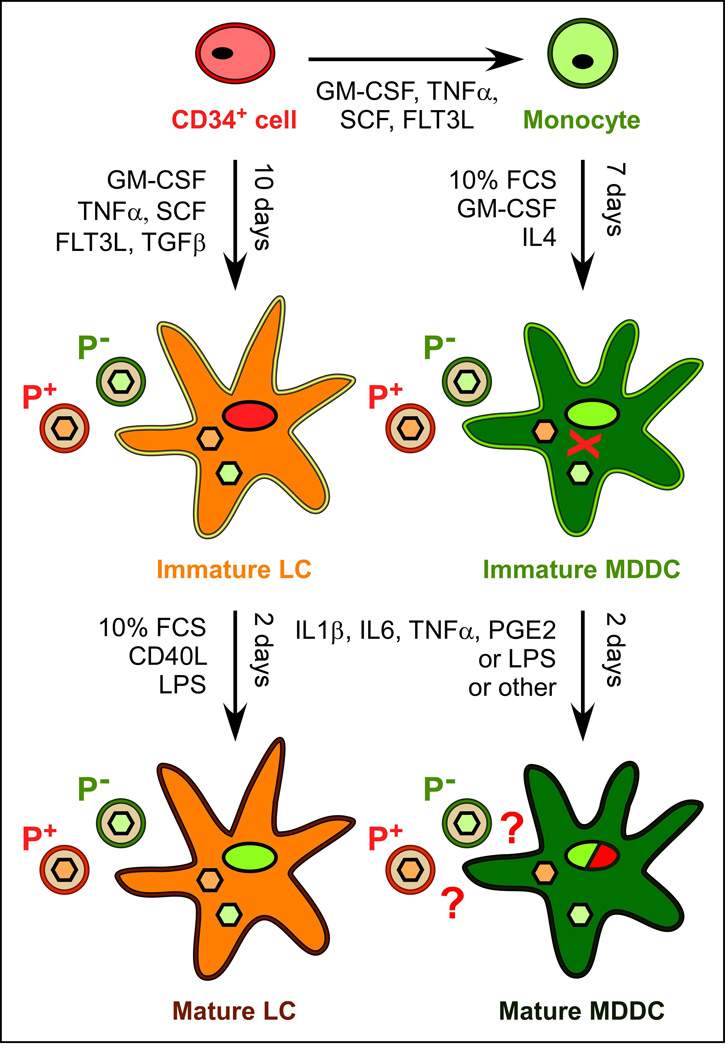
CMV interactions with oro-nasal iLC have not been studied yet. LC differentiated in vitro from blood CD34+ progenitors (Figure 3) have however been used as proxy [64–67], since these cells express not only CD1a and Langerin/CD207, but also some of the markers of oral LC, including TLR2, TLR4, FcγRIII/CD16, FcγRI/CD64, CD40, CD80 and CD86 [64, 68, 69].
Figure 3. LC and MDDC generation in vitro and their permissiveness to CMV infection.
Upon culture with the indicated cytokines, CD34+ progenitor cells can differentiate into CD14+ monocytes or iLC, while monocytes can give rise to iMDDC. CMV strains containing (P+) or not (P−) the gH/gL/UL128-131A pentameric complex on their envelopes (red and green toroids) penetrate into iLC and mLC equally well, and their capsids (pale green and pale red filled hexagons) reach the nucleus. Efficient transcription of the UL122/123 genes encoding the essential IE1 and IE2 proteins, however, occurs only in mLC (green nucleus) but not in iLC (red nucleus). Immature MDDC can be accessed by pentamer+ and pentamer− virions, but the latter do not subsequently reach the nucleus (red X symbol). The degree of mMDDC susceptibility to infection by pentamer+ and pentamer− is still unclear (red question marks and green/red nucleus) and may depend on the nature of the maturation stimulus. GM-CSF, granulocyte macrophage-colony stimulating factor; TNFα, tumor necrosis factor α; SCF, stem cell factor; FLT3L, Flt3 ligand; TGFβ, transforming growth factor β1; FCS, fetal calf serum; CD40L, CD40 ligand; LPS, lipopolysaccharide; IL4, IL6 and IL1β, interleukin 4, 6 and 1β; PGE2, prostaglandin E2.
Quite interestingly, while iLC are highly resistant to infection, mLC support replication and progeny production of both clinical-like and attenuated strains, albeit to reduced levels as compared to other cell types such as fibroblasts (Figure 3 and Table 2) [64, 65]. No difference was detected in the content of cell-associated viral genomes after exposure of iLC or mLC to pentamer+ or pentamer− strains, indicating that LC behave like Type 1 cells, and that presence of the gH/gL/UL128–131A complex is not essential for entry (Figure 3) [65]. Pentamer+ strains, however, initiate infection in about twice as many mLC as pentamer− strains (Table 2), suggesting that the ability to access both entry pathways is advantageous [65].
Table 2.
Susceptibility of Langerhans-type (grey shaded rows) and monocyte-derived dendritic cells to CMV infection
| DC GENERATION METHOD | CMV STRAIN AND % CMV+ CELLS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Progenitors | Medium | Serum | Cytokines | Maturation | Strain | Immature | Mature | Ag | ||
| MOI | % | MOI | % | ||||||||
| [64] | CD34+ cells Magnetic selection |
X-VIVO 10 days |
NO | GM-CSF, TNFα, SCF, FLT3L, TGFβ |
10% FCS, GM- CSF, CD40L Day 11, 48h |
P+ = TB40/E, VHL/E P− = AD169 P− = Towne P− = Toledo |
100 - - - |
3 − - - |
100 100 100 100 |
48–74 12–17 2–5 0 |
IE1/2 Day 2 |
| [65] | CD34+ cells Magnetic selection |
X-VIVO 10 days |
NO | GM-CSF, TNFα, SCF, FLT3L, TGFβ |
10% FCS, GM- CSF, CD40L Day 11, 48h |
P+ = BAC4 P+ = BADr P+ = TR-GFP P− = UL128mut P− = AD169 P− = Towne |
10 10 10 10 10 10 |
1.7 3.6 0.7 2.6 3.4 2 |
10 10 10 10 10 10 |
12 17 2.6 7.7 12 5.6 |
IE1/2 Day 2 |
| [108] | Monocytes PBMC adherence |
RPMI 7 days |
10% FCS |
GM-CSF, IL-4 |
NT | P+ = TB40/E, VHL/E P− = AD169, VHL/F |
1, 50 1, 50 |
10–90 < 1 |
- | - | IE1/2 Day 1 |
| [109] | Monocytes PBMC adherence TCR/CD19/CD56 removal |
RPMI 7 days |
10% FCS |
GM-CSF, IL-4 |
33% MCM Day 7, 72h |
P+ = NEWT, MOLD | - | - | 5 | 25 | IE1/2 Day 5 |
| [110] | Monocytes PBMC adherence |
RPMI 4–7 days |
10% FCS |
GM-CSF, IL-4 |
10% FCS, LPS, IFNγ,TNFα Day 7, 24h |
P+ = TB40/E | 10 5 1 |
30–70 30 8 |
50, 10 5 1 |
1.4 0.9 0.4 |
UL44 Day 2 |
| [111] | Monocytes PBMC adherence TCR/CD19/CD56 removal |
RPMI 7 days |
10% FCS |
GM-CSF, IL-4 |
NT | P+ = TB40/E | 10, 50 | 59–78 | - | - | IE1/2 Day 1 |
| [112] | Monocytes PBMC adherence CD2/CD19 removal |
RPMI 7 days |
10% FCS |
GM-CSF, IL-4 |
IL1β, IL6, TNFα, PGE2 Day 7, 24h |
P+ = Mix (4 strains P− = Mix (8 strains) |
1 50 1 |
10–20 50–80 <1 |
1 50 1 |
10–20 30–50 <1 |
UL83 UL32 Day 1 |
| [113] | Monocytes PBMC adherence |
RPMI 5–6 days |
1% Human Serum |
GM-CSF, IL-4 |
IL1β, IL6, TNFα, PGE2 Day 7, 48h |
P+ = BobU/E, BobB/E P− = BobU/F, BobB/F P− = AD169 |
1 1 1 |
35–50 0.3 0.4 |
1 1 1 |
19 15 20 |
IE1/2 Day 1 |
| [116] | Monocytes PBMC adherence |
RPMI 5–7 days |
10% FCS |
GM-CSF, IL-4 |
LPS Day 5, 48h |
P+ = TB40/E P− = AD169 P− = Towne |
5 5 5 |
10–15 1 1 |
5 5 5 |
1 1 1 |
IE1/2 Day 1 |
The fact that iLC are resistant to infection is particularly intriguing considering the exceptionally broad tissue tropism of CMV. Despite the fact that viral entry is usually heavily targeted by host antiviral mechanisms, virion penetration, capsid trafficking toward the nucleus, and nuclear deposition of viral genomes are not impaired in iLC. By contrast, transcription of the UL122/123 genes, encoding the essential IE1/IE2 proteins, is severely blunted [65]. Expression of the UL122/123 genes is under the control of the major immediate-early promoter (MIEP), which contains several binding sites for activating transcription factors such as NF-kB [70], Sp1 [71] and CREB1 [72], and for repressing ones such as the modulator-binding factors 1, 2 and 3 [73, 74], the methylated DNA-binding protein [75], the modulator recognition factor [76], Gfi1 [77], YY1 [78], and ERF [79]. The lack of IE1/IE2 expression in infected iLC may thus be ascribed to the absence of specific transcriptional activators, and/or to the activity of transcriptional repressors. Epigenetically acting factors, such as histone acetyltransferases, deacetylases, and methyltransferases may also participate in reducing the accessibility of the MIEP to the transcriptional machinery. As susceptibility to infection is acquired with maturation, expression of some of these “permissiveness modulators” may be stimulated by signaling events ensuing iLC exposure to LPS and/or CD40L. Maturation of infected iLC can indeed trigger viral replication and progeny production (L. Hertel, unpublished data).
Mature LC susceptibility to infection was proposed, but not compellingly demonstrated, to be regulated by the activity of nuclear domain 10 (ND10) bodies [80], consisting of focal accumulations of the transcriptional repressors PML, hDaxx, Sp100 and ATRX within the interchromosomal space of the nucleus [81–83]. These proteins were shown to block expression of the UL122/123 genes at very early times post-nuclear deposition of CMV genomes, by enfolding the MIEP in transcriptionally inactive chromatin [84–86]. This initial block is subsequently removed by the nuclear delivery of the viral tegument protein pp71, which stimulates hDaxx degradation and ATRX displacement from the MIEP [85–88], allowing for expression of the IE1/IE2 proteins [89–94]. If incoming viral genomes are sequestered within the ND10 bodies of iLC, and if pp71 is subsequently prevented from reaching the nucleus, then infection onset may be stalled [95]. So far, however, viral genomes have not been shown to localize within the ND10 bodies of infected iLC, mLC or CD34+ cells, and imaging data reporting the presence of pp71 within the nucleus of permissive mLC have not been quantified [80], providing scant evidence in support of a role for ND10 bodies and/or pp71 in controlling CMV tropism for LC.
Quite interestingly, mLC not only support lytic infection, but also viral reactivation from latency [96, 97]. In latently-infected CD34+ cells, viral genomes are maintained as nuclear episomes in the absence of lytic gene expression [98–100], and the transcriptionally silent MIEP is associated with deacetylated H4 histones and with the transcriptional repressor heterochromatin protein 1 [96, 97, 101]. CD34+ cell differentiation and maturation into mLC stimulates the acetylation of H4 histones and the release of heterochromatin protein 1 from the MIEP, enabling expression of the IE1/IE2 proteins and, ultimately, the release of viral progeny [96, 97, 101]. Intriguingly, maturation appears to be absolutely required for reactivation to occur, as no expression of the UL122/123 genes is detected in iLC differentiated from latently-infected CD34+ cells [96]. This implies that the acquisition of an intranuclear environment conducive to viral gene transcription, and characterized by the presence of select transcription factors, the absence of others, and the occurrence of specific epigenetic modifications of the viral genome, is intimately linked to the process of iLC maturation [102, 103].
CMV TROPISM FOR MONOCYTE-DERIVED DC
CMV interactions with non-LC DC isolated directly from the oro-nasal mucosae have also not been examined, but immature MDDC (iMDDC, Figure 3) [104] have been used as surrogates in numerous studies. Although these cells display some of the markers found on oro-nasal DC, including CD11c, CD1c, DC-SIGN/CD209 and CD206, and are considered by some to be analogous to dermal DC [105–107], the extent of iMDDC’s similarity to mucosal DC remains unclear.
In striking contrast to iLC, iMDDC are fully susceptible to CMV infection, but exclusively by clinical-like strains [108–114], although both pentamer+ and pentamer− virions can access these cells equally well (Figure 3 and Table 2) [112]. Direct fusion of the virion envelope with the plasma membrane was not detected in transmission electron micrographs of iMDDC exposed to the clinical-like strain VHL/E. Instead, virion internalization was shown to occur by macropinocytosis, involving cholesterol-enriched microdomains and requiring actin remodeling but not endosomal acidification [115]. This suggests that iMDDC behave like Type 2 cells. While the envelope composition is likely irrelevant for virion accumulation within macropinosomes, presence of the gH/gL/UL128–131A complex is essential for capsid translocation into the cytoplasm, and for infection onset [114]. Due to the milder and delayed acidification of iMDDC endosomes as compared to mMDDC, internalized VHL/E virions are not rapidly degraded. Instead, they retain their infectious properties, and can be transmitted to uninfected cells [115]. Although this was reported to occur with pentamer+ virions, transfer of pentamer− particles may be even more efficient, as these virions cannot reach the cytoplasm, and accumulate within cellular endosomes instead. Because of this, infected iMDDC were proposed to function as “Trojan horses”, spreading CMV to other cell types way before the production of viral progeny.
Lysis of infected iMDDC was reported to occur in all studies [108, 109, 113, 114] but one [112], and to be associated [108, 113, 114] or not [109] with the release of viral progeny, leading to the current consensus that CMV infection of iMDDC by clinical-like strains is productive, cytopathic, and lytic.
Entry of pentamer+ and pentamer− virions into mature MDDC (mMDDC) was also shown to take place with similar efficacies [112, 113], and to be followed [109, 112, 113] or not [110, 116] by the onset of productive infection (Figure 3 and Table 2). Interestingly, the highest proportions of infected mMDDC were obtained when a mix of pro-inflammatory cytokines was used as maturation stimulus [112, 113], while exposure to LPS alone or in combination with other cytokines yielded highly resistant cells [110, 116]. This suggests that the intracellular environment generated in response to endogenous activation signals is substantially more conducive to viral replication than that produced in response to exogenous, pathogen-produced molecules, such as LPS.
In contrast to iMDDC, mMDDC viability is not affected by infection [109, 112, 113], whether viral progeny is produced [109] or not [113]. Due to the ambiguity in literature data, however, it remains unclear whether mMDDC are Type 1 or 2 cells, if presence of the gH/gL/UL128–131A complex is absolutely required for infection, and at what exact step, if any, is infection blocked.
The C-type lectin receptor DC specific ICAM-grabbing non-integrin (DC-SIGN), abundantly expressed on the surface of iMDDC, was shown to bind gB on the envelope of virions from clinical-like and attenuated strains, and to promote the efficient transfer of bound virions to uninfected cells [117]. Pre-incubation of iMDDC with anti-DC-SIGN antibodies before infection also reduced the number of infected cells by four-fold, suggesting that this molecule may be involved in CMV tropism for MDDC. Several questions however remain: does DC-SIGN act as an entry receptor, stimulating virion internalization, or does it simply enhance virion attachment, as suggested by the fact that soluble DC-SIGN can increase iMDDC infection rates but only at specific concentrations [118]? Why are iMDDC resistant to infection by attenuated strains despite the presence of gB on their envelopes? Does DC-SIGN perhaps synergize with the gH/gL/UL128–131A complex to promote internalization of pentamer+ but not pentamer− strains? Why are LPS-matured mMDDC completely resistant to infection [110, 116], despite still expressing sizable levels of DC-SIGN [119, 120]? More work thus needs to be completed to fully elucidate the role of this molecule during CMV infection of MDDC.
Naturally-infected monocytes circulating in the blood of healthy seropositive individuals, as well as experimentally-infected CD14+ cells, carry viral genomes in the absence of lytic gene expression [97, 101, 121, 122]. Akin to LC, monocyte differentiation into iMDDC does not strongly induce viral gene expression, a status reversed by maturation [97, 122, 123]. Immature MDDC generated from the monocytes of patients with active CMV infections also show no [109] or minimal [124] evidence of active infection, and their maturation was reported to enhance [109] or inhibit [124] viral reactivation. Interestingly, thus, iMDDC are more permissive to direct CMV infection than LPS-matured mMDDC (Table 2), but do not efficiently support viral reactivation, an event for which maturation seems to be strictly required.
CMV INTERACTIONS WITH LC AND MDDC: DC ARE NOT ALL EQUAL
Despite being related and sharing many features and functions, LC and MDDC interactions with CMV are remarkably different (Figure 3 and Table 3), an interesting finding in view of the specific anatomical location occupied by each of these DC in vivo. While iMDDC reside deep within skin and mucosae, iLC are uniquely located in the outermost layers of these tissues (Figure 1), and are particularly adept at resisting pathogen invasion, especially as it comes to viruses [125, 126].
Table 3.
Interactions of Langerhans-type and monocyte-derived dendritic cells with CMV: a comparison
| iLC | mLC | iMDDC | mMDDC | |
|---|---|---|---|---|
| CMV entry | Pentamer+ Pentamer− |
Pentamer+ Pentamer− |
Pentamer+ Pentamer− |
Pentamer+ Pentamer− |
| Entry routes | ? | ? | Endocytosis Pentamer+ |
? |
| Pentamer required for lytic cycle |
− | − | + | − Data conflict |
| DC-SIGN involved | − | − | + Pentamer+ |
? |
| Internalized particles infectious |
? | ? | + | ? |
| IE1/IE2 expression | − | ++ | +++ | ++ Data conflict |
| Early and late gene expression |
− | + | + | + |
| Replication cycle completed |
− | + | + | + |
| Progeny released | − | + | + | + |
| Progeny cell-associated | ? | ? | − | + |
| DC viability maintained | + | − | − | + |
| Carriage of latent viral genomes |
+ | + | + | + |
| Reactivation – IE1/IE2 transcription |
− | + | − | + |
HIV-1 virions, for instance, are effectively captured by iLC via surface Langerin before being degraded within the Birbeck granules [127], specific to these cells [128]. By contrast, iMDDC bind HIV-1 virions via surface DC-SIGN. Instead of being routed for destruction, however, particles are subsequently maintained in infectious form on and within iMDDC [129, 130], before being transferred to T cells via the so-called “infectious synapse” [131–134]. Whereas iMDDC promote HIV-1 dissemination and transmission to T cells, thus, iLC effectively prevent it. Fully infectious, enveloped CMV particles have also been observed within the endosomes of iMDDC at 48 hours post-infection, where they constitute a “cell-protected viral pool” capable of transmitting infection to other cell types before viral replication onset. The intracellular accumulation of CMV virions is again fostered by their binding to cell surface DC-SIGN [115]. Whether such gatherings also occur in LC, and whether these DC internalize particles by macropinocytosis is currently unknown. The facts that LC do not express DC-SIGN [135, 136], behave like Type 1 cells, and do not contain large numbers of virions at very early (4 hours) or later (30 hours) times post-infection [65], strongly argue against these events also occurring in LC. Interestingly, although a portion of penetrated CMV virions may be degraded within LC’s Birbeck granules akin to HIV-1 particles, CMV replication is mostly blocked at the step of viral gene transcription. Intranuclear antiviral factors are thus likely to be the ones involved in blocking CMV replication in iLC, more than cytoplasmic effectors.
Entry of pentamer+ strains into iMDDC is consistently followed by viral gene expression and genome replication [108–113]. This leads first to the severe blunting of iMDDC’s immune functions because of the activity of CMV-encoded immunoevasive proteins [137–139], and later to cell death ensuing the release of viral progeny. Viral gene expression after CMV entry into iLC, by contrast, is minimal and insufficient to promote the onset of viral replication [64], leaving iLC viability and functionality fully intact. Blocking infection at the level of viral gene transcription may thus represent a particularly useful strategy for iLC to protect epithelial tissues from viral attack by actively removing virions from the extracellular environment without, however, succumbing to infection. Granting viral particles access to the cytoplasm while remaining viable may also provide enough time and opportunity for viral antigens to be selected and loaded onto MHC molecules for later presentation to T and B cells, thus fostering the generation of strong adaptive immune responses against CMV.
Although iLC and iMDDC show opposite degrees of susceptibility to direct CMV infection, both DC types do not support viral reactivation after differentiation from latently-infected progenitor cells. This implies that the intracellular conditions supporting the successful establishment of infection after exposure of iMDDC to viral particles are not sufficient to trigger viral gene expression from latent genomes. LPS-induced maturation or activation by inflammatory cytokines can however stimulate viral reactivation in both DC types [97, 122, 123], suggesting that the mechanisms controlling this event may be similar in mLC and mMDDC.
Finally, onset of productive CMV infection has been associated with profound changes in the immune functions of both mLC and iMDDC, including reductions in surface levels of MHC class I and II, costimulatory and adhesion molecules [64, 109–113, 140, 141], impaired migration in response to chemotactic stimuli [66, 116, 140], alterations in the profile of secreted cytokines and chemokines [110, 112, 116, 142–144] and, in the case of iMDDC, maturation impairments [110, 112, 143], changes that, combined, prevent these DC from effectively stimulating T cell activation and proliferation [64, 109–113, 145]. As this review focuses on viral tropism for DC, however, I refer the reader to the following recent literature [138, 139, 146].
CONCLUSIONS AND FUTURE DIRECTIONS
Despite sharing the same origin as myeloid cells, LC and MDDC respond to CMV in ways as diverse as those of more unrelated cell types such as fibroblasts and epithelial cells. If these differences observed in vitro are also found in vivo, epidermal LC and dermal DC are likely to play dramatically different roles in the development of CMV pathogenesis. Comparing the susceptibility and responses to CMV infection of in vitro-derived DC to those of DC directly isolated from oral and nasal mucosae is thus an important task for the future.
As mucosal DC functions can be strongly influenced by the activity of neighboring cells, and by the presence of pathogenic or commensal microorganisms, assessing DC responses to CMV infection within the context of surrounding tissues should also reveal essential information about viral pathogenesis and the generation of antiviral immune responses. Both simpler epithelial cell-DC or keratinocyte-DC co-culture systems, and more complex three-dimensional models of oral mucosae reconstructed in vitro [147] may be used to this end, in addition to animal models. The oral mucosa of rabbits, dogs and rhesus macaques was reported to be more similar to the human one than that of rodents, at least in terms of thickness, cell composition, keratinization and permeability [148], and would thus be a good choice for this type of study. Nothing is currently known, however, about rabbit, canine and rhesus CMV tropism for DC, or about the permissiveness of rabbit, dog and rhesus DC to human CMV infection. Establishing these models would be instrumental to test the effectiveness of new anti-CMV vaccines specifically developed for delivery to mucosal DC.
The recent discovery of macropinocytosis and of the pentameric complex as key mediators of CMV entry into Type 2 cells highlighted the importance of understanding viral tropism for different cell types to improve current vaccination strategies, and to formulate new antiviral compounds blocking viral entry. Improving our knowledge of the pathways granting CMV access to LC and MDDC will help find new ways to protect these cells from infection in vivo, which, in turn, should expedite the acquisition of protective immunity in infected individuals. The identification of the viral determinants of tropism for DC may also support the production of improved vaccination inocula, such as dense bodies with envelopes tailored to preferentially access mucosal DC [149, 150], and live-attenuated viruses capable of entry but not of replication in DC. Immature LC’s remarkable resistance to infection is particularly promising to this end, making these cells potential sources of new antiviral molecules.
Finally, the role played by maturation in creating an intracellular environment conducive to both lytic replication and reactivation deserves more attention. Understanding which specific maturation signals promote susceptibility to infection and which, by contrast, lead to resistance is a priority. This will help reduce CMV horizontal transmission rates by preventing not only the onset of viral replication after direct infection, but also viral reactivation from latent reservoirs.
ACKNOWLEDGMENTS
I would like to thank Dr. E. S. Mocarski, in whose laboratory I initiated my studies on CMV interactions with LC, and all those colleagues who contributed important data to the field of CMV tropism for DC. I apologize to those authors whose work could not be cited due to space limitations. I am also grateful to Dr. A.H. Lucas for critical reading of this paper, and to the National Institute of Health (grant AI099372) and the Canadian Institutes of Health Research for providing financial support.
ABBREVIATIONS
- DC
dendritic cell
- DC-SIGN
DC specific ICAM-grabbing non-integrin
- IE
immediate-early
- iLC
immature Langerhans-type DC
- LC
Langerhans-type DC
- LPS
lipopolysaccharide
- MDDC
monocyte-derived DC
- MIEP
major immediate-early promoter
- mLC
mature Langerhans cells
- NALT
nasopharyngeal-associated lymphoreticular tissues
- ND10
nuclear domain 10
- TLR
toll-like receptor
BIBLIOGRAPHY
- 1.Mocarski ES, Shenk T, Pass RF. In: Cytomegaloviruses. Virology Fields DM, Knipe aPMH, editors. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2701–2772. [Google Scholar]
- 2.Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417–470. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- 3.Britt W. In: HCMV entry into host, establishment of infection, spread in host, mechanisms of tissue damage. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Arvin A, Campadelli-Fiume G, Mocarski E, et al., editors. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 4.Pass RF. In: Cytomegalovirus. Fields virology, Howley DKaP, editor. Philadelphia: Lippincott/The Williams & Wilkins Co; 2001. pp. 2675–2705. [Google Scholar]
- 5.de Franca TR, de Albuquerque Tavares Carvalho A, Gomes VB, Gueiros LA, Porter SR, Leao JC. Salivary shedding of Epstein-Barr virus and cytomegalovirus in people infected or not by human immunodeficiency virus 1. Clin Oral Investig. 2012;16:659–664. doi: 10.1007/s00784-011-0548-5. [DOI] [PubMed] [Google Scholar]
- 6.Griffin E, Krantz E, Selke S, Huang ML, Wald A. Oral mucosal reactivation rates of herpesviruses among HIV-1 seropositive persons. J Med Virol. 2008;80:1153–1159. doi: 10.1002/jmv.21214. [DOI] [PubMed] [Google Scholar]
- 7.Miller CS, Berger JR, Mootoor Y, Avdiushko SA, Zhu H, Kryscio RJ. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J Clin Microbiol. 2006;44:2409–2415. doi: 10.1128/JCM.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuyns I, Gugerli P, Mombelli A. Viruses in periodontal disease - a review. Oral Dis. 2005;11:219–229. doi: 10.1111/j.1601-0825.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 9.Correia-Silva Jde F, Victoria JM, Guimaraes AL, et al. Cytomegalovirus shedding in the oral cavity of allogeneic haematopoietic stem cell transplant patients. Oral Dis. 2007;13:163–169. doi: 10.1111/j.1601-0825.2006.01240.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan BW, Woo JK, Liew CT. Cytomegalovirus infection of the nasopharynx. J Clin Pathol. 2002;55:970–972. doi: 10.1136/jcp.55.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wejse C, Birkebaek NH, Nielsen LP, Andersen HM. Respiratory tract infections in cytomegalovirus-excreting and nonexcreting infants. Pediatr Infect Dis J. 2001;20:256–259. doi: 10.1097/00006454-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Jutte A, Fatkenheuer G, Hell K, Salzberger B. CMV sinusitis as the initial manifestation of AIDS. HIV Med. 2000;1:123–124. doi: 10.1046/j.1468-1293.2000.00015.x. [DOI] [PubMed] [Google Scholar]
- 13.Marks SC, Upadhyay S, Crane L. Cytomegalovirus sinusitis A new manifestation of AIDS. Arch Otolaryngol Head Neck Surg. 1996;122:789–791. doi: 10.1001/archotol.1996.01890190085019. [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st century: a tool for decisionmaking, Committee to Study Priorities for Vaccine Development. Washington, DC: Division of Health Promotion and Disease Prevention, Institute of Medicine; 1999. [Google Scholar]
- 17.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths P, Plotkin S, Mocarski E, et al. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine. 2013;31(Suppl 2):B197–B203. doi: 10.1016/j.vaccine.2012.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schleiss MR, Heineman TC. Progress toward an elusive goal: current status of cytomegalovirus vaccines. Expert Rev Vaccines. 2005;4:381–406. doi: 10.1586/14760584.4.3.381. [DOI] [PubMed] [Google Scholar]
- 20.Sung H, Schleiss MR. Update on the current status of cytomegalovirus vaccines. Expert Rev Vaccines. 2010;9:1303–1314. doi: 10.1586/erv.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schleiss MR. Cytomegalovirus vaccine development. Curr Top Microbiol Immunol. 2008;325:361–382. doi: 10.1007/978-3-540-77349-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heineman TC, Schleiss M, Bernstein DI, et al. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. J Infect Dis. 2006;193:1350–1360. doi: 10.1086/503365. [DOI] [PubMed] [Google Scholar]
- 23.Guy B. Strategies to improve the effect of vaccination in the elderly: the vaccine producer’s perspective. J Comp Pathol. 2010;142(Suppl 1):S133–S137. doi: 10.1016/j.jcpa.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 25.Cattamanchi A, Posavad CM, Wald A, et al. Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clin Vaccine Immunol. 2008;15:1638–1643. doi: 10.1128/CVI.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmgren J, Svennerholm AM. Vaccines against mucosal infections. Curr Opin Immunol. 2012;24:343–353. doi: 10.1016/j.coi.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 28.Skenderi F, Jonjic S. Viral vaccines and vectors - some lessons from cytomegaloviruses. Periodicum Biologorum. 2012;114:201–210. [Google Scholar]
- 29.McVoy MA. Cytomegalovirus vaccines. Clin Infect Dis. 2013;57(Suppl 4):S196–S199. doi: 10.1093/cid/cit587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feuchtinger T, Opherk K, Bicanic O, et al. Dendritic cell vaccination in an allogeneic stem cell recipient receiving a transplant from a human cytomegalovirus (HCMV)-seronegative donor: induction of a HCMV-specific T(helper) cell response. Cytotherapy. 2010;12:945–950. doi: 10.3109/14653241003587645. [DOI] [PubMed] [Google Scholar]
- 31.Kapp M, Tan SM, Einsele H, Grigoleit G. Adoptive immunotherapy of HCMV infection. Cytotherapy. 2007;9:699–711. doi: 10.1080/14653240701656046. [DOI] [PubMed] [Google Scholar]
- 32.Moharamzadeh K, Brook IM, Van Noort R, Scutt AM, Thornhill MH. Tissue-engineered oral mucosa: a review of the scientific literature. J Dent Res. 2007;86:115–124. doi: 10.1177/154405910708600203. [DOI] [PubMed] [Google Scholar]
- 33.Novak N, Haberstok J, Bieber T, Allam JP. The immune privilege of the oral mucosa. Trends Mol Med. 2008;14:191–198. doi: 10.1016/j.molmed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Allam JP, Novak N, Fuchs C, et al. Characterization of dendritic cells from human oral mucosa: a new Langerhans’ cell type with high constitutive FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:141–148. doi: 10.1067/mai.2003.1607. [DOI] [PubMed] [Google Scholar]
- 35.Allam JP, Niederhagen B, Bucheler M, et al. Comparative analysis of nasal and oral mucosa dendritic cells. Allergy. 2006;61:166–172. doi: 10.1111/j.1398-9995.2005.00965.x. [DOI] [PubMed] [Google Scholar]
- 36.Allam JP, Peng WM, Appel T, et al. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008;121:368–374. doi: 10.1016/j.jaci.2007.09.045. e361. [DOI] [PubMed] [Google Scholar]
- 37.Allam JP, Stojanovski G, Friedrichs N, et al. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63:720–727. doi: 10.1111/j.1398-9995.2007.01611.x. [DOI] [PubMed] [Google Scholar]
- 38.Barrett AW, Cruchley AT, Williams DM. Oral mucosal Langerhans’ cells. Crit Rev Oral Biol Med. 1996;7:36–58. doi: 10.1177/10454411960070010301. [DOI] [PubMed] [Google Scholar]
- 39.Cutler CW, Jotwani R. Dendritic cells at the oral mucosal interface. J Dent Res. 2006;85:678–689. doi: 10.1177/154405910608500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruchley AT, Williams DM, Farthing PM, Lesch CA, Squier CA. Regional variation in Langerhans cell distribution and density in normal human oral mucosa determined using monoclonal antibodies against CD1, HLADR, HLADQ and HLADP. J Oral Pathol Med. 1989;18:510–516. doi: 10.1111/j.1600-0714.1989.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 41.Jaitley S, Saraswathi T. Pathophysiology of Langerhans cells. J Oral Maxillofac Pathol. 2012;16:239–244. doi: 10.4103/0973-029X.99077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito H, Takekoshi T, Miyauchi M, et al. Three-dimensional appearance of Langerhans cells in human gingival epithelium as revealed by confocal laser scanning microscopy. Arch Oral Biol. 1998;43:741–744. doi: 10.1016/s0003-9969(98)00066-1. [DOI] [PubMed] [Google Scholar]
- 43.Feng P, Yee KK, Rawson NE, Feldman LM, Feldman RS, Breslin PA. Immune cells of the human peripheral taste system: dominant dendritic cells and CD4 T cells. Brain Behav Immun. 2009;23:760–766. doi: 10.1016/j.bbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jotwani R, Palucka AK, Al-Quotub M, et al. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J Immunol. 2001;167:4693–4700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farthing PM, Matear P, Cruchley AT. The activation of Langerhans cells in oral lichen planus. J Oral Pathol Med. 1990;19:81–85. doi: 10.1111/j.1600-0714.1990.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 46.Jahnsen FL, Gran E, Haye R, Brandtzaeg P. Human nasal mucosa contains antigen-presenting cells of strikingly different functional phenotypes. Am J Respir Cell Mol Biol. 2004;30:31–37. doi: 10.1165/rcmb.2002-0230OC. [DOI] [PubMed] [Google Scholar]
- 47.Mascarell L, Lombardi V, Louise A, et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122:603–609. doi: 10.1016/j.jaci.2008.06.034. e605. [DOI] [PubMed] [Google Scholar]
- 48.Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991;173:1345–1356. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Debertin AS, Tschernig T, Tonjes H, Kleemann WJ, Troger HD, Pabst R. Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin Exp Immunol. 2003;134:503–507. doi: 10.1111/j.1365-2249.2003.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 51.Hobom U, Brune W, Messerle M, Hahn G, Koszinowski UH. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J Virol. 2000;74:7720–7729. doi: 10.1128/jvi.74.17.7720-7729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanarsdall AL, Chase MC, Johnson DC. Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells. J Virol. 2011;85:11638–11645. doi: 10.1128/JVI.05659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isaacson MK, Compton T. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J Virol. 2009;83:3891–3903. doi: 10.1128/JVI.01251-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hahn G, Revello MG, Patrone M, et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A. 2005;102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol. 2005;79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80:710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryckman BJ, Rainish BL, Chase MC, et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol. 2008;82:60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinzger C. Entry route of HCMV into endothelial cells. J Clin Virol. 2008;41:174–179. doi: 10.1016/j.jcv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Patrone M, Secchi M, Bonaparte E, Milanesi G, Gallina A. Cytomegalovirus UL131-128 products promote gB conformational transition and gB-gH interaction during entry into endothelial cells. J Virol. 2007;81:11479–11488. doi: 10.1128/JVI.00788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akter P, Cunningham C, McSharry BP, et al. Two novel spliced genes in human cytomegalovirus. J Gen Virol. 2003;84:1117–1122. doi: 10.1099/vir.0.18952-0. [DOI] [PubMed] [Google Scholar]
- 62.Adler B, Scrivano L, Ruzcics Z, Rupp B, Sinzger C, Koszinowski U. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J Gen Virol. 2006;87:2451–2460. doi: 10.1099/vir.0.81921-0. [DOI] [PubMed] [Google Scholar]
- 63.Ogawa-Goto K, Tanaka K, Gibson W, et al. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J Virol. 2003;77:8541–8547. doi: 10.1128/JVI.77.15.8541-8547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hertel L, Lacaille VG, Strobl H, Mellins ED, Mocarski ES. Susceptibility of immature and mature Langerhans cell-type dendritic cells to infection and immunomodulation by human cytomegalovirus. J Virol. 2003;77:7563–7574. doi: 10.1128/JVI.77.13.7563-7574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lauron EJ, Yu D, Fehr AR, Hertel L. Human cytomegalovirus infection of langerhans-type dendritic cells does not require the presence of the gH/gL/UL128-131A complex and is blocked after nuclear deposition of viral genomes in immature cells. J Virol. 2014;88:403–416. doi: 10.1128/JVI.03062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee AW, Hertel L, Louie RK, et al. Human cytomegalovirus alters localization of MHC class II and dendrite morphology in mature Langerhans cells. J Immunol. 2006;177:3960–3971. doi: 10.4049/jimmunol.177.6.3960. [DOI] [PubMed] [Google Scholar]
- 67.Lee AW, Wang N, Hornell TM, et al. Human cytomegalovirus decreases constitutive transcription of MHC class II genes in mature Langerhans cells by reducing CIITA transcript levels. Mol Immunol. 2011;48:1160–1167. doi: 10.1016/j.molimm.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strunk D, Rappersberger K, Egger C, et al. Generation of human dendritic cells/Langerhans cells from circulating CD34+ hematopoietic progenitor cells. Blood. 1996;87:1292–1302. [PubMed] [Google Scholar]
- 69.Rozis G, Benlahrech A, Duraisingham S, Gotch F, Patterson S. Human Langerhans’ cells and dermal-type dendritic cells generated from CD34 stem cells express different toll-like receptors and secrete different cytokines in response to toll-like receptor ligands. Immunology. 2008;124:329–338. doi: 10.1111/j.1365-2567.2007.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sambucetti LC, Cherrington JM, Wilkinson GW, Mocarski ES. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. Embo J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang D, Fickenscher H, Stamminger T. Analysis of proteins binding to the proximal promoter region of the human cytomegalovirus IE-1/2 enhancer/promoter reveals both consensus and aberrant recognition sequences for transcription factors Sp1 and CREB. Nucleic Acids Res. 1992;20:3287–3295. doi: 10.1093/nar/20.13.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunninghake GW, Monick MM, Liu B, Stinski MF. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. J Virol. 1989;63:3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kothari S, Baillie J, Sissons JG, Sinclair JH. The 21bp repeat element of the human cytomegalovirus major immediate early enhancer is a negative regulator of gene expression in undifferentiated cells. Nucleic Acids Res. 1991;19:1767–1771. doi: 10.1093/nar/19.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shelbourn SL, Kothari SK, Sissons JG, Sinclair JH. Repression of human cytomegalovirus gene expression associated with a novel immediate early regulatory region binding factor. Nucleic Acids Res. 1989;17:9165–9171. doi: 10.1093/nar/17.22.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang XY, Ni YS, Saifudeen Z, Asiedu CK, Supakar PC, Ehrlich M. Increasing binding of a transcription factor immediately downstream of the cap site of a cytomegalovirus gene represses expression. Nucleic Acids Res. 1995;23:3026–3033. doi: 10.1093/nar/23.15.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang TH, Oka T, Asai T, et al. Repression by a differentiation-specific factor of the human cytomegalovirus enhancer. Nucleic Acids Res. 1996;24:1695–1701. doi: 10.1093/nar/24.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zweidler-Mckay PA, Grimes HL, Flubacher MM, Tsichlis PN. Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol Cell Biol. 1996;16:4024–4034. doi: 10.1128/mcb.16.8.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu R, Baillie J, Sissons JG, Sinclair JH. The transcription factor YY1 binds to negative regulatory elements in the human cytomegalovirus major immediate early enhancer/promoter and mediates repression in non-permissive cells. Nucleic Acids Res. 1994;22:2453–2459. doi: 10.1093/nar/22.13.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bain M, Mendelson M, Sinclair J. Ets-2 Repressor Factor (ERF) mediates repression of the human cytomegalovirus major immediate-early promoter in undifferentiated non-permissive cells. J Gen Virol. 2003;84:41–49. doi: 10.1099/vir.0.18633-0. [DOI] [PubMed] [Google Scholar]
- 80.Saffert RT, Penkert RR, Kalejta RF. Cellular and viral control over the initial events of human cytomegalovirus experimental latency in CD34+ cells. J Virol. 2010;84:5594–5604. doi: 10.1128/JVI.00348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Negorev D, Maul GG. Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene. 2001;20:7234–7242. doi: 10.1038/sj.onc.1204764. [DOI] [PubMed] [Google Scholar]
- 82.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 83.Lallemand-Breitenbach V, de The H. PML nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2:a000661. doi: 10.1101/cshperspect.a000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woodhall DL, Groves IJ, Reeves MB, Wilkinson G, Sinclair JH. Human Daxx-mediated repression of human cytomegalovirus gene expression correlates with a repressive chromatin structure around the major immediate early promoter. J Biol Chem. 2006;281:37652–37660. doi: 10.1074/jbc.M604273200. [DOI] [PubMed] [Google Scholar]
- 85.Lukashchuk V, McFarlane S, Everett RD, Preston CM. Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection. J Virol. 2008;82:12543–12554. doi: 10.1128/JVI.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hwang J, Kalejta RF. Proteasome-dependent, ubiquitin-independent degradation of Daxx by the viral pp71 protein in human cytomegalovirus-infected cells. Virology. 2007;367:334–338. doi: 10.1016/j.virol.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 87.Saffert RT, Kalejta RF. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J Virol. 2006;80:3863–3871. doi: 10.1128/JVI.80.8.3863-3871.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tavalai N, Papior P, Rechter S, Stamminger T. Nuclear domain 10 components promyelocytic leukemia protein and hDaxx independently contribute to an intrinsic antiviral defense against human cytomegalovirus infection. J Virol. 2008;82:126–137. doi: 10.1128/JVI.01685-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tavalai N, Stamminger T. Intrinsic cellular defense mechanisms targeting human cytomegalovirus. Virus Res. 2011;157:128–133. doi: 10.1016/j.virusres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Ahn JH, Hayward GS. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J Virol. 1997;71:4599–4613. doi: 10.1128/jvi.71.6.4599-4613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Korioth F, Maul GG, Plachter B, Stamminger T, Frey J. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp Cell Res. 1996;229:155–158. doi: 10.1006/excr.1996.0353. [DOI] [PubMed] [Google Scholar]
- 92.Wilkinson GW, Kelly C, Sinclair JH, Rickards C. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate early gene product. J Gen Virol. 1998;79(Pt 5):1233–1245. doi: 10.1099/0022-1317-79-5-1233. [DOI] [PubMed] [Google Scholar]
- 93.Nevels M, Paulus C, Shenk T. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc Natl Acad Sci U S A. 2004;101:17234–17239. doi: 10.1073/pnas.0407933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park JJ, Kim YE, Pham HT, Kim ET, Chung YH, Ahn JH. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J Gen Virol. 2007;88:3214–3223. doi: 10.1099/vir.0.83171-0. [DOI] [PubMed] [Google Scholar]
- 95.Saffert RT, Kalejta RF. Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J Virol. 2007;81:9109–9120. doi: 10.1128/JVI.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reeves MB, Lehner PJ, Sissons JG, Sinclair JH. An in vitro model for the regulation of human cytomegalovirus latency and reactivation in dendritic cells by chromatin remodelling. J Gen Virol. 2005;86:2949–2954. doi: 10.1099/vir.0.81161-0. [DOI] [PubMed] [Google Scholar]
- 97.Reeves MB, MacAry PA, Lehner PJ, Sissons JG, Sinclair JH. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci U S A. 2005;102:4140–4145. doi: 10.1073/pnas.0408994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mendelson M, Monard S, Sissons P, Sinclair J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol. 1996;77(Pt 12):3099–3102. doi: 10.1099/0022-1317-77-12-3099. [DOI] [PubMed] [Google Scholar]
- 99.Bolovan-Fritts CA, Mocarski ES, Wiedeman JA. Peripheral blood CD14(+) cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood. 1999;93:394–398. [PubMed] [Google Scholar]
- 100.Khaiboullina SF, Maciejewski JP, Crapnell K, et al. Human cytomegalovirus persists in myeloid progenitors and is passed to the myeloid progeny in a latent form. Br J Haematol. 2004;126:410–417. doi: 10.1111/j.1365-2141.2004.05056.x. [DOI] [PubMed] [Google Scholar]
- 101.Reeves MB, Sinclair JH. Analysis of latent viral gene expression in natural and experimental latency models of human cytomegalovirus and its correlation with histone modifications at a latent promoter. J Gen Virol. 2010;91:599–604. doi: 10.1099/vir.0.015602-0. [DOI] [PubMed] [Google Scholar]
- 102.Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:297–313. doi: 10.1007/978-3-540-77349-8_17. [DOI] [PubMed] [Google Scholar]
- 103.Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol. 2006;87:1763–1779. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- 104.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 106.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 107.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 108.Riegler S, Hebart H, Einsele H, Brossart P, Jahn G, Sinzger C. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J Gen Virol. 2000;81:393–399. doi: 10.1099/0022-1317-81-2-393. [DOI] [PubMed] [Google Scholar]
- 109.Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schonrich G. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity. 2001;15:997–1009. doi: 10.1016/s1074-7613(01)00239-4. [DOI] [PubMed] [Google Scholar]
- 110.Moutaftsi M, Mehl AM, Borysiewicz LK, Tabi Z. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood. 2002;99:2913–2921. doi: 10.1182/blood.v99.8.2913. [DOI] [PubMed] [Google Scholar]
- 111.Grigoleit U, Riegler S, Einsele H, et al. Human cytomegalovirus induces a direct inhibitory effect on antigen presentation by monocyte-derived immature dendritic cells. Br J Haematol. 2002;119:189–198. doi: 10.1046/j.1365-2141.2002.03798.x. [DOI] [PubMed] [Google Scholar]
- 112.Beck K, Meyer-Konig U, Weidmann M, Nern C, Hufert FT. Human cytomegalovirus impairs dendritic cell function: a novel mechanism of human cytomegalovirus immune escape. Eur J Immunol. 2003;33:1528–1538. doi: 10.1002/eji.200323612. [DOI] [PubMed] [Google Scholar]
- 113.Senechal B, Boruchov AM, Reagan JL, Hart DN, Young JW. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood. 2004;103:4207–4215. doi: 10.1182/blood-2003-12-4350. [DOI] [PubMed] [Google Scholar]
- 114.Gerna G, Percivalle E, Lilleri D, et al. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J Gen Virol. 2005;86:275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- 115.Haspot F, Lavault A, Sinzger C, et al. Human cytomegalovirus entry into dendritic cells occurs via a macropinocytosis-like pathway in a pH-independent and cholesterol-dependent manner. PLoS One. 2012;7:e34795. doi: 10.1371/journal.pone.0034795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varani S, Frascaroli G, Homman-Loudiyi M, Feld S, Landini MP, Soderberg-Naucler C. Human cytomegalovirus inhibits the migration of immature dendritic cells by down-regulating cell-surface CCR1 and CCR5. J Leukoc Biol. 2005;77:219–228. doi: 10.1189/jlb.0504301. [DOI] [PubMed] [Google Scholar]
- 117.Halary F, Amara A, Lortat-Jacob H, et al. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653–664. doi: 10.1016/s1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- 118.Plazolles N, Humbert JM, Vachot L, Verrier B, Hocke C, Halary F. Pivotal advance: The promotion of soluble DC-SIGN release by inflammatory signals and its enhancement of cytomegalovirus-mediated cis-infection of myeloid dendritic cells. J Leukoc Biol. 2011;89:329–342. doi: 10.1189/jlb.0710386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Relloso M, Puig-Kroger A, Pello OM, et al. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168:2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 121.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 122.Reeves MB, Compton T. Inhibition of inflammatory interleukin-6 activity via extracellular signal-regulated kinase-mitogen-activated protein kinase signaling antagonizes human cytomegalovirus reactivation from dendritic cells. J Virol. 2011;85:12750–12758. doi: 10.1128/JVI.05878-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reeves MB, Sinclair JH. Circulating dendritic cells isolated from healthy seropositive donors are sites of human cytomegalovirus reactivation in vivo. J Virol. 2013;87:10660–10667. doi: 10.1128/JVI.01539-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Varani S, Frascaroli G, Gibellini D, et al. Impaired dendritic cell immunophenotype and function in heart transplant patients undergoing active cytomegalovirus infection. Transplantation. 2005;79:219–227. doi: 10.1097/01.tp.0000147359.63158.29. [DOI] [PubMed] [Google Scholar]
- 125.de Jong MA, Geijtenbeek TB. Langerhans cells in innate defense against pathogens. Trends Immunol. 2010;31:452–459. doi: 10.1016/j.it.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 126.Cunningham AL, Abendroth A, Jones C, Nasr N, Turville S. Viruses and Langerhans cells. Immunol Cell Biol. 2010;88:416–423. doi: 10.1038/icb.2010.42. [DOI] [PubMed] [Google Scholar]
- 127.de Witte L, Nabatov A, Pion M, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 128.Mc Dermott R, Ziylan U, Spehner D, et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol Biol Cell. 2002;13:317–335. doi: 10.1091/mbc.01-06-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Neumann AK, Thompson NL, Jacobson K. Distribution and lateral mobility of DC-SIGN on immature dendritic cells--implications for pathogen uptake. J Cell Sci. 2008;121:634–643. doi: 10.1242/jcs.022418. [DOI] [PubMed] [Google Scholar]
- 130.Soilleux EJ, Morris LS, Leslie G, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 131.Arrighi JF, Pion M, Garcia E, et al. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Freer G, Matteucci D. Influence of dendritic cells on viral pathogenicity. PLoS Pathog. 2009;5:e1000384. doi: 10.1371/journal.ppat.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cavrois M, Neidleman J, Greene WC. The achilles heel of the trojan horse model of HIV-1 trans-infection. PLoS Pathog. 2008;4:e1000051. doi: 10.1371/journal.ppat.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Geijtenbeek TB, van Kooyk Y. DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Curr Top Microbiol Immunol. 2003;276:31–54. doi: 10.1007/978-3-662-06508-2_2. [DOI] [PubMed] [Google Scholar]
- 135.de Witte L, Nabatov A, Geijtenbeek TB. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol Med. 2008;14:12–19. doi: 10.1016/j.molmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 136.Soilleux EJ, Coleman N. Langerhans cells and the cells of Langerhans cell histiocytosis do not express DC-SIGN. Blood. 2001;98:1987–1988. doi: 10.1182/blood.v98.6.1987. [DOI] [PubMed] [Google Scholar]
- 137.Powers C, DeFilippis V, Malouli D, Fruh K. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol. 2008;325:333–359. doi: 10.1007/978-3-540-77349-8_19. [DOI] [PubMed] [Google Scholar]
- 138.Rolle A, Olweus J. Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasures. Apmis. 2009;117:413–426. doi: 10.1111/j.1600-0463.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 139.Gredmark-Russ S, Soderberg-Naucler C. Dendritic cell biology in human cytomegalovirus infection and the clinical consequences for host immunity and pathology. Virulence. 2012;3 doi: 10.4161/viru.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moutaftsi M, Brennan P, Spector SA, Tabi Z. Impaired lymphoid chemokine-mediated migration due to a block on the chemokine receptor switch in human cytomegalovirus-infected dendritic cells. J Virol. 2004;78:3046–3054. doi: 10.1128/JVI.78.6.3046-3054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kessler T, Reich M, Jahn G, et al. Human cytomegalovirus infection interferes with major histocompatibility complex type II maturation and endocytic proteases in dendritic cells at multiple levels. J Gen Virol. 2008;89:2427–2436. doi: 10.1099/vir.0.2008/001610-0. [DOI] [PubMed] [Google Scholar]
- 142.Frascaroli G, Varani S, Mastroianni A, et al. Dendritic cell function in cytomegalovirus-infected patients with mononucleosis. J Leukoc Biol. 2006;79:932–940. doi: 10.1189/jlb.0905499. [DOI] [PubMed] [Google Scholar]
- 143.Wagner CS, Walther-Jallow L, Buentke E, Ljunggren HG, Achour A, Chambers BJ. Human cytomegalovirus-derived protein UL18 alters the phenotype and function of monocyte-derived dendritic cells. J Leukoc Biol. 2008;83:56–63. doi: 10.1189/jlb.0307181. [DOI] [PubMed] [Google Scholar]
- 144.Chang WL, Baumgarth N, Yu D, Barry PA. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J Virol. 2004;78:8720–8731. doi: 10.1128/JVI.78.16.8720-8731.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raftery MJ, Wieland D, Gronewald S, Kraus AA, Giese T, Schonrich G. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J Immunol. 2004;173:3383–3391. doi: 10.4049/jimmunol.173.5.3383. [DOI] [PubMed] [Google Scholar]
- 146.Sinclair J. Manipulation of dendritic cell functions by human cytomegalovirus. Expert Rev Mol Med. 2008;10:e35. doi: 10.1017/S1462399408000872. [DOI] [PubMed] [Google Scholar]
- 147.Sivard P, Dezutter-Dambuyant C, Kanitakis J, et al. In vitro reconstructed mucosa-integrating Langerhans’ cells. Exp Dermatol. 2003;12:346–355. doi: 10.1034/j.1600-0625.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 148.Harris D, Robinson JR. Drug delivery via the mucous membranes of the oral cavity. J Pharm Sci. 1992;81:1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- 149.Sauer C, Klobuch S, Herr W, Thomas S, Plachter B. Subviral dense bodies of human cytomegalovirus stimulate maturation and activation of monocyte-derived immature dendritic cells. J Virol. 2013;87:11287–11291. doi: 10.1128/JVI.01429-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cayatte C, Schneider-Ohrum K, Wang Z, et al. Cytomegalovirus vaccine strain towne-derived dense bodies induce broad cellular immune responses and neutralizing antibodies that prevent infection of fibroblasts and epithelial cells. J Virol. 2013;87:11107–11120. doi: 10.1128/JVI.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]