Abstract
Background and Purpose
Carotid artery intima-media thickness (IMT) and plaque are non-invasive markers of subclinical arterial injury that predict incident cardiovascular disease. We evaluated predictors of longitudinal changes in IMT and new plaque over a decade in a longitudinal multiethnic cohort.
Methods
Carotid IMT and plaque were evaluated in Multi-Ethnic Study of Atherosclerosis participants at exams 1 and 5, a mean (standard deviation) of 9.4 (0.5) years later. Far wall carotid IMT was measured in both common (CCA) and internal carotid arteries. A plaque score was calculated from all carotid segments. Mixed effects longitudinal and multivariate regression models evaluated associations of baseline risk factors and time-updated medication use with IMT progression and plaque formation.
Results
The 3,441 MESA participants were 60.3 (9.4) years old (53% female; 26% African-American, 22% Hispanic, 13% Chinese); 1,620 (47%) had carotid plaque. Mean CCA IMT progression was 11.8 (12.8) μm/year. 1,923 (56%) of subjects developed new plaque. IMT progressed more slowly in Chinese (β=−2.89, p=0.001) and Hispanic participants (β=−1.81, p=0.02), and with higher baseline high-density lipoprotein cholesterol (per 5 mg/dL, β=−0.22, p=0.03), antihypertensive use (β=−2.06, p=0.0004), and time on antihypertensive medications (years) (β=−0.29, p<0.0001). Traditional risk factors were associated with new plaque formation, with strong associations for cigarette use (odds ratio 2.31, p<0.0001) and protection by African-American ethnicity (odds ratio 0.68, p<0.0001).
Conclusions
In a large, multi-ethnic cohort with a decade of follow-up, ethnicity is a strong, independent predictor of carotid IMT and plaque progression. Anti-hypertensive medication use was associated with less subclinical disease progression.
Keywords: Carotid arteries, Risk Factors, Epidemiology, Atherosclerosis
Background
Carotid artery intima-media thickness (IMT) and plaque presence are non-invasive markers of subclinical arterial injury that predict incident cardiovascular disease (CVD).1–3 Although clinical trials have used short-term change in carotid IMT as a surrogate endpoint to assess the impact of pharmacotherapeutic agents in homogenous populations with high levels of CVD risk factors, little is known about the predictors of carotid IMT progression in large, heterogeneous populations.4 Carotid plaque represents a later stage of arterial injury. Its presence and extent also are associated with incident CVD, yet little is known about the predictors of carotid plaque progression.1, 5–8 The predictors of carotid IMT and plaque progression in an ethnically diverse population are unknown. We hypothesized that CVD risk factors are the major predictors of longitudinal changes in carotid IMT and plaque progression, but that race/ethnicity and use of antihypertensive and lipid-lowering medications also would affect progression.
Methods
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort of 6,814 participants free of known CVD at baseline. The aim of MESA is to investigate risk factors and subclinical CVD progression in an ethnically diverse population.9 MESA enrolled participants from six different field centers located in: Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota. Details of MESA’s design have been published previously.9 The study was approved by the institutional review boards of all the MESA field centers, the University of Washington Data Coordinating Center and the University of Wisconsin Atherosclerosis Imaging Research Program (UW AIRP). All participants provided informed consent. Our analysis was restricted to those participants with both baseline (Exam 1, 7/2000–8/2002) and follow-up (Exam 5, 3/2010–2/2012) risk factor and carotid ultrasound data. Baseline laboratory samples were collected following a 12-hour fast.
Carotid Ultrasonography
At baseline, B-mode ultrasound images of the right and left common, bifurcation, and internal carotid artery segments were recorded on Super-VHS videotape with a Logiq 700 ultrasound system using the M12L transducer (General Electric Medical Systems, CCA frequency 13 MHz). Video images were digitized at high resolution and frame rates using a Medical Digital Recording device (PACSGEAR, Pleasanton, CA) and converted into DICOM compatible digital records. The same ultrasound system and digitizing equipment were used at Exam 5; however, the video output was directly digitized using the same recorder settings without videotape. Trained, certified sonographers used pre-selected reference images from Exam 1 to match the scanning conditions of the initial study, including display depth, angle of approach, internal landmarks, degree of jugular venous distension, and ultrasound system settings. Ultrasound images were reviewed and interpreted by the UW AIRP MESA Carotid Ultrasound Reading Center. Images were imported into syngo Ultrasound Workplace reading stations loaded with Arterial Health Package software (Siemens Medical, Malvern, PA) for IMT measurement and plaque scoring. Measurements of Exam 1 and Exam 5 carotid ultrasound images were performed simultaneously. Images were matched side by side on a video monitor and measured contemporaneously, however Exam 1 IMT measurements were not considered in choosing the Exam 5 site or making the Exam 5 measurements
This analysis primarily focused on CCA IMT and carotid plaque score. Internal carotid artery IMT data are presented in Data supplements I and II. The distal CCA was defined as the distal 10-mm of the vessel. IMT was defined as the intima-media thickness measured as the mean of the mean left and right mean far wall distal CCA wall thicknesses. Carotid plaque score (0–12) was defined as the number of carotid plaques in the internal, bifurcation, and common segments of both carotid arteries.10 Carotid plaque was defined as a discrete, focal wall thickening ≥1.5 cm or focal thickening at least 50% greater than the surrounding IMT.1
Ultrasound Quality Assurance
The intra-class correlation coefficients (ICC) for intra-reader reproducibility for mean CCA IMT was 0.99. The ICC for inter-reader CCA IMT reproducibility was 0.95. For mean ICA, intra-reader reproducibility was between 0.98–0.99 and inter-reader reproducibility was 0.93. To assess scan-rescan reproducibility, 44 scans were repeated by 3 sonographers. The Pearson correlation coefficient was 0.94. Mean (SD) differences were 0.006 (0.036–0.760) mm. There were no outliers noted on limit of agreement analysis for matched segments. For carotid plaque presence and score, intra-reader reproducibility was kappa=0.83 (95% confidence interval [CI] 0.70–0.96) and inter-reader reproducibility was kappa=0.89 (95% CI 0.72–1.00).
Statistical Methods
Descriptive statistics are reported as means (standard deviations) for continuous and percentages for categorical variables. Paired t-tests were used to compare Exam 1 and 5 continuous variables; chi-squared tests for categorical variables. Plaque score progression by ethnicity was compared using a Kruskal-Wallis test.
For IMT progression, two sets of complimentary models were created. First, a multivariate linear regression model with scaled change of carotid IMT (μm/year) as the outcome measure was created. Scaled change accounted for variability in participant follow-up times. Second, a mixed effects longitudinal change model with adjustment for estimated baseline with the outcome modeled as a continuous variable (μm) was created (Data Supplement III).11 This model was fit with random slopes and intercepts for each participant and contained three components: cross-sectional, longitudinal, and transient.11 The cross-sectional component analyzed the association of baseline CVD risk factors with estimated carotid IMT at baseline, whereas the longitudinal component analyzed this association with IMT progression (μm/year) over the observation period. When modeling associations of change, inclusion of the measured baseline as a covariate can result in measurement error, so measured baseline IMT was not included as a covariate in either model to avoid bias.12 In the mixed effects model, baseline IMT was accounted by the cross-sectional term to estimate the baseline IMT using both fixed and random effects. The mixed effects model permits improved statistical efficiency given subject-specific random effects and maximum use of available data even among those with missing data. To demonstrate the consistency between the two models, both sets of results are shown.
For new carotid plaque formation, a multivariate logistic regression model was created. Because we desired to construct the most informative model, all models for carotid IMT progression and plaque formation included the covariates listed in Data Supplement IV and also included baseline antihypertensive and statin pharmacotherapy or their time-varying use (years) Statistical significance was set at two sided p<0.05. Analyses were performed in SAS (Version 9.2, Cary, NC: SAS. Institute Inc.).
Results
Descriptive Characteristics
Participants were followed for a mean (standard deviation) of 9.4 (0.5) years. Their characteristics are described in Table 1.
Table 1.
Baseline and Follow-up Descriptive Statistics
| Variable | Exam 1 | Exam 5 | P value |
|---|---|---|---|
| N=3441 | N=3441 | ||
| Age (years) | 60.3 (9.4) | 69.8 (9.3) | <0.0001 |
| Male sex % (N) | 47.0% (1618) | ||
| Race/Ethnicity % (N) | |||
| Caucasian | 39.2% (1350) | ||
| African-American | 26.4% (909) | ||
| Hispanic | 21.5% (740) | ||
| Chinese | 12.9% (442) | ||
| Annual income % (N) | |||
| < $16,000 | 14.6 (490) | 15.3 (509) | <0.0001 |
| $16,000–$34,999 | 24.2 (812) | 25.0 (831) | |
| $35,000–$99,999 | 45.1 (1509) | 42.1 (1398) | |
| ≥ $100,000 | 16.0 (537) | 17.6 (586 | |
| Smoking % (N) | |||
| Current | 11.4% (390) | 7.31% (250) | <0.0001 |
| Former | 36.6% (1258) | 47.1% (1610) | |
| Never | 52.0% (1788) | 45.7% (1562) | |
| Systolic blood pressure (mmHg) | 124.4 (20.2) | 124.3(20.8) | 0.73 |
| High-density lipoprotein cholesterol (mg/dL) | 51.01 (14.9) | 55.93 (16.9) | <0.0001 |
| Total cholesterol (mg/dL) | 194.1 (35.1) | 182.7 (36.9) | <0.0001 |
| Glucose (mg/dL) | 95.1 (26.0) | 102.1 (27.8) | <0.0001 |
| Body-mass index (kg/m2) | 28.3 (5.3) | 28.5 (5.5) | <0.0001 |
| Diabetes mellitus, % (N) | 10 (345) | 19.7 (674) | <0.0001 |
| Family history of coronary heart disease, % (N) | 43.5 (1415) | N/A | |
| Use of antihypertensive medication, % (N) | 34.9 (1200) | 55.8 (1919) | <0.0001 |
| Use of statin medication, % (N) | 15.1 (519) | 37.4 (1287) | <0.0001 |
| Mean-mean CCA IMT (μm) | 754.6 (179.8) | 865.5 (198.2) | <0.0001 |
| Left CCA IMT (μm) | 756.4 (210.4) | 866.1 (233.8) | <0.0001 |
| Right CCA IMT(μm) | 753.6 (190.2) | 866.5 (215.2) | <0.0001 |
| Left CCA IMT absolute progression (μm) | 109 (160.2) | 0.17* | |
| Right CCA IMT absolute progression (μm) | 114.2 (141.8) | ||
| Carotid plaque presence, %(N) | 47.1% (1620) | 68.0% (2338) | <0.0001 |
| Carotid Plaque Score | 1.11 (1.64) | 2.29 (2.45) | <0.0001 |
p value represents the comparison between left CCA and right CCA absolute progression
CCA = common carotid artery, IMT= intima-media thickness. Values are mean (standard deviation) unless noted otherwise.
Carotid IMT Progression: Multivariate Model of Scaled Change
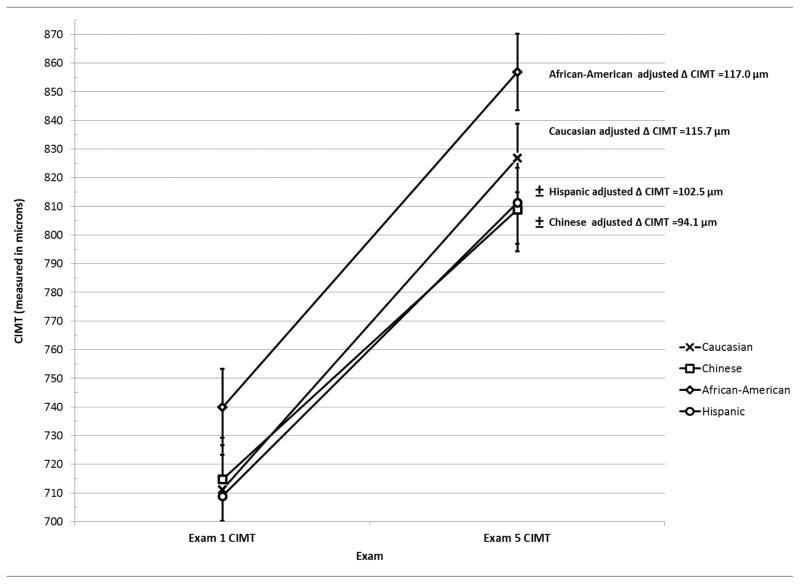
At Exam 1, several traditional CVD risk factors were associated with CCA IMT (Table 2). Predictors of IMT progression were similar in models using baseline antihypertensive and statin pharmacotherapy and time-varying use of these medications (Tables 3 and 4). Diabetes mellitus (β=2.85, p=0.02) and time on statin pharmacotherapy (β=−0.16, p=0.04) were additional predictors of IMT progression in the time-varying model. In both models, antihypertensive medication use was associated with slower IMT progression (p<0.0001) as were Chinese (p=0.001) and Hispanic ethnicities (p=0.02) (Figure 1). Predictors of ICA IMT progression were similar to CCA IMT progression (Data Supplements I and II).
Table 2.
Baseline Traditional Risk Factors and Baseline (Exam 1) Carotid Intima-Media Thickness
| Multivariate Linear Regression Model* (Model R2 = 0.29) | Mixed Effects Longitudinal Model* (Estimated Baseline) | |||
|---|---|---|---|---|
|
| ||||
| Predictor | Parameter estimate (SE) | P value | Parameter estimate (SE) | P value |
| Age (per decade) | 74.49 (3.49) | <0.0001 | 73.62 (3.34) | <0.0001 |
| Male sex | 39.35 (6.75) | <0.0001 | 39.03 (6.41) | <0.0001 |
| Body-mass index (per 5 kg/m2) | 17.45 (3.27) | <0.0001 | 14.59 (3.06) | <0.0001 |
| Systolic blood pressure (per 10 mmHg) | 14.21 (1.60) | <0.0001 | 14.80 (1.53) | <0.0001 |
| Fasting glucose (per 10 mg/dL) | 3.54 (1.59) | 0.03 | 3.12 (1.49) | 0.04 |
| High-density lipoprotein cholesterol (per 5 mg/dL) | −3.06 (1.14) | 0.007 | −3.33 (1.09) | 0.002 |
| Total cholesterol (per 10 mg/dL) | 4.12 (0.86) | <0.0001 | 4.12 (0.82) | <0.0001 |
| African-American ethnicity | 29.78 (7.80) | 0.0001 | 28.72 (7.46) | 0.0001 |
| Current cigarette use | 22.26 (9.50) | 0.02 | 23.59 (9.17) | 0.01 |
| Former cigarette use | 20.90 (6.40) | 0.001 | 20.20 (6.13) | 0.001 |
Outcome modeled in μm,
SE = standard error
Table 3.
Predictors of Carotid Intima-Media Thickness Progression: Baseline Use of Anti-Hypertensive and Statin Medications
| Multivariate Linear Regression Model* | Mixed Effects Longitudinal Model* | |||
|---|---|---|---|---|
|
| ||||
| Predictors | Parameter Estimates (SE) | P value | Parameter Estimates (SE) | P value |
| Chinese ethnicity | −2.89 (0.88) | 0.001 | −2.29 (0.90) | 0.01 |
| Hispanic ethnicity | −1.81 (0.76) | 0.02 | −1.40 (0.79) | 0.08 |
| High-density lipoprotein cholesterol (per 5 mg/dL) | −0.22 (0.10) | 0.03 | −0.18 (0.10) | 0.08 |
| Anti-hypertensive medication use (baseline) | −2.06 (0.58) | 0.0004 | −2.11 (0.60) | 0.0004 |
| Statin medication use (baseline) | 1.31 (0.73) | 0.07 (NS) | 1.59 (0.75) | 0.03 |
Outcome modeled in μm/year
SE = standard error
Table 4.
Predictors of Carotid Intima-Media Thickness Progression with Anti-hypertensive and Statin Medications Modeled as Time-Varying Covariates
| Multivariate Linear Regression Model* | Mixed Effects Longitudinal Model* | |||
|---|---|---|---|---|
|
| ||||
| Predictors | Parameter Estimates (SE) | P value | Parameter Estimates (SE) | P value |
| Chinese ethnicity | −2.93 (0.88) | 0.0009 | −2.34 (0.90) | 0.009 |
| Hispanic ethnicity | −2.09 (0.76) | 0.006 | −1.59 (0.79) | 0.04 |
| High-density lipoprotein cholesterol (per 5 mg/dL) | −0.24 (0.10) | 0.02 | −0.19 (0.10) | 0.06 |
| Anti-hypertensive medication use (years) | −0.29 (0.07) | <0.0001 | −2.04 (0.72)† | 0.005 |
| Statin medication use (years) | −0.16 (0.08) | 0.04 | −0.95 (0.74)† | 0.20 |
| Diabetes mellitus | 2.85 (1.23) | 0.02 | 1.89 (1.23) | 0.13 |
Outcome modeled in μm/year unless noted
Modeled in μm of change over observation period
SE = standard error
Figure 1.
Adjusted Carotid Intima-Media Thickness Progression by Ethnicity
± Adjusted IMT progression rate differs compared to Caucasian ethnicity (p<0.05)
Models adjusted for: age, sex body-mass index, systolic blood pressure, tobacco use, total cholesterol, high-density lipoprotein, glucose, income, education, family history, diabetes mellitus, antihypertensive medication use, statin medication use
Error bars represent estimated means +/− standard error of the mean
Carotid IMT Progression: Mixed Effects Longitudinal Model
Similar to the multivariate models, in the cross-sectional component of the mixed effects model, traditional CVD risk factors were associated with estimated baseline CCA IMT (Table 2). The estimated CCA IMT baseline in the mixed effects model yielded the same risk factor associations with similar parameter estimates when compared to a cross-sectional analysis using a multivariate linear model and Exam 1 IMT. Also, predictors of IMT progression were similar between models using baseline antihypertensive and statin pharmacotherapy, as well as time-varying use of these medications (Tables 3 and 4). The only slight difference was that baseline statin use was a statistically significant predictor in the mixed effects model (β=1.59, p=0.03) (Table 3). Antihypertensive medication use was strongly associated with slower IMT progression modeled as either baseline (p=0.0004) or time-varying (p=0.005) use, as was Chinese ethnicity (p=0.01).
Carotid Plaque Formation
There were 1,923 (56%) participants that formed new carotid plaque. Several traditional CVD risk factors were associated with carotid plaque formation. Caucasians had higher plaque score. Over 59% of Caucasians formed new plaque; Chinese had the lowest new plaque formation rate (49.8%; Data Supplement V). There only were slight differences between the baseline antihypertensive and statin pharmacotherapy models (Data Supplement VI) and the time-varying models (Table 5). Current cigarette smoking was a strong predictor of new plaque formation (OR 2.31, p<0.0001). Compared with Caucasian ethnicity, African-American, Hispanic and Chinese ethnicities were associated with less plaque formation.
Table 5.
Predictors of New Carotid Plaque: Anti-hypertensive and Statin Medications as Time-Varying Covariates
| Variable | Odds Ratio (95% CI) | p value |
|---|---|---|
| Age (per decade) | 1.63 (1.49–1.79) | <0.0001 |
| Current cigarettes | 2.31 (1.79–2.99) | <0.0001 |
| Total cholesterol (per 10 mg/dL) | 1.03 (1.01–1.06) | 0.005 |
| African-American | 0.68 (0.55–0.83) | 0.0002 |
| Chinese | 0.69 (0.53–0.91) | 0.008 |
| Hispanic | 0.75 (0.59–0.95) | 0.016 |
| Former cigarettes | 1.27 (1.08–1.51) | 0.004 |
| Systolic blood pressure (per 10 mmHg) | 1.1 (1.05–1.15) | <0.0001 |
| Fasting glucose (per 10 mg/dL) | 1.05 (1.01–1.1) | 0.022 |
| HDL-C (per 5 mg/dL) | 0.97 (0.94–1.0) | 0.021 |
CI = confidence interval;
Maximum rescaled R2 for new carotid plaque =0.15, AUC for new carotid plaque =0.70
Discussion
In this large, multi-ethnic cohort with nearly a decade of prospective observation, Hispanic and Chinese ethnicity as well as antihypertensive medication use at baseline and throughout the observation period consistently were associated with slower carotid IMT progression. Cigarette smoking at baseline was associated with an increase in new carotid plaque formation, whereas African-American ethnicity was associated with less carotid plaque formation.
Numerous clinical trials have evaluated the short-term effects of pharmacotherapeutic interventions on carotid IMT progression in homogenous populations with increased levels of a specific risk factor. However, very few studies examined longitudinal IMT progression in a heterogeneous population that is more representative of United States population and healthier individuals than clinical trials participants. Previous investigations have been limited by factors including: low inter- and intra-reader correlations (0.59–0.75), low inter-scan reproducibility measures, use of lower frequency ultrasound transducers, manual IMT measurement, restriction to one carotid artery, and very homogenous cohorts.6
Consistent associations between CVD risk factors and prevalent carotid IMT have been described; however, few studies investigated progression of carotid IMT and plaque. Our model of cross-sectional associations between CCA IMT and risk factors had one of the highest adjusted Radj2 values reported to date (0.29).5, 13–15 Despite relatively strong associations of traditional risk factors and baseline IMT, in the fully-adjusted model only Chinese and Hispanic ethnicities and use of anti-hypertensive medications were consistent, independent predictors of slower IMT progression. The scaled change and mixed effects models yielded similar results with similar parameter estimates. Inter- and intra-reader reliability and the scan reproducibility measures were among the highest reported with precision as high as those attained in clinical trials.6, 16, 17
In the fully-adjusted model, we observed a strong, independent protective effect of Chinese and Hispanic ethnicity on IMT progression. MESA is a unique cohort, as no previous cohort has had the ethnic diversity to demonstrate ethnic associations with IMT progression.5 We found a persistent and strong inverse effect of anti-hypertensive medications on progression of subclinical carotid disease. The strong protective effect of anti-hypertensive medication use is consistent with current knowledge of compensatory changes to arterial walls to elevated systemic blood pressures. In clinical trials, use of anti-hypertensive medications is associated with decreased IMT progression.18, 19 Based on these data, anti-hypertensive medication use may be the strongest modifiable predictor of slowing IMT progression over time. In contrast to anti-hypertensive medication use, statin medication use at baseline was weakly and positively associated with IMT progression, however when modeled as a time-updated covariate, time on statin medication was inversely associated with IMT progression. Statin medication use at baseline may be a marker of an increased antecedent burden of CVD risk factors prior to study enrollment. We did not find a significant effect on plaque progression and use of statin medications.
Traditional CVD risk factors are associated with carotid plaque presence and burden; however, few studies have analyzed the predictors of carotid plaque progression over time.20–22 In our study, current cigarette smoking was a strong predictor of new plaque across ethnicities. When risk of new plaque formation was adjusted for traditional CVD risk factors, Hispanic, African-American, and Chinese ethnicities had lower risks of new carotid plaque. These ethnic differences in carotid plaque progression are novel and clinically relevant findings since prior investigations were of Caucasians. Our carotid plaque findings are similar to those in other arterial territories. In the coronary arteries, African-American ethnicity is associated with less calcification and slower calcium progression, despite more adverse risk factor profiles.23, 24 In our study, African-American ethnicity was associated with thicker CCA IMT but with less carotid plaque formation than Caucasians. Because of pathophysiological differences between arterial wall thickening and plaque formation, it is not surprising that the predictors for progression of these markers differ.
Limitations
As an observational study, the described associations do not confirm causation. Our participants were a subset of the MESA who returned for Exam 5 and may be biased based on survival to this exam. Exam 5 participants were healthier and less likely to have a non-fatal CVD event than the original MESA cohort; which could have reduced the strength of the associations we identified. Carotid IMT and plaque progression are surrogate markers for CVD risk; however, they provide important insights into the pathophysiology of arterial injury, the substrate for cerebrovascular disease and cognitive decline. Carotid artery disease also is strongly associated with and reflective of changes in other arterial beds. This investigation focused on CCA IMT. Only half as many participants had ICA images available for analysis given the inability to match ICA segments across examinations, yet the results (Data supplements I and II) were similar. Although regression models were adjusted for measured risk factor and sociodemographic variables, unmeasured lifestyle exposures and risk factors that may be specific to each ethnicity cannot be accounted for. We cannot exclude a systematic error in measurement due to non-blinding, however the random error associated with measuring such a small quantity on one exam would be the most prominent source of bias, rather than any systematic measurement bias. Any systematic measurement error that did occur would be non-differential in nature and therefore would bias the results towards the null. Also, segments were traced with semi-automated border detection tool with very minimal edits by the reader, except when interface determination by the software was incorrect. Finally, MESA is a United States cohort so the generalizability of these findings to populations outside the United States may be limited.
Conclusions
Ethnicity is associated independently with progression of carotid wall thickening and plaque formation, subclinical markers of arterial injury and CVD risk. The most powerful pharmacologic and modifiable risk factors impacting progressive carotid wall injury are use of antihypertensive medications and cigarette smoking.
Supplementary Material
Acknowledgments
A full list of MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources
Supported by contracts HC95159-HC95169 and HL07936 from the National Heart, Lung and Blood Institute (NHLBI), grant ES015915 from the National Institute of Environmental Health Sciences, and grants RR024156 and RR025005 from the National Center for Research Resources. This publication was developed under Science to Achieve Results research assistance agreement RD831697 from the US Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors. The EPA does not endorse any products or commercial services mentioned in this publication. Drs. Tattersall and Gepner were supported by a Ruth L. Kirschstein National Research Service Award from the NHLBI to the University of Wisconsin Cardiovascular Research Center (T32 HL07936). Dr. Korcarz was supported by a K23 mentored patient oriented research career development award (K23) HL 094760.
Footnotes
Disclosures
Stein: Wisconsin Alumni Research Foundation
References
- 1.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the american society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 3.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A meta-analysis. Jama. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 4.Espeland MA, O’Leary DH, Terry JG, Morgan T, Evans G, Mudra H. Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of hmg-coa reductase inhibitors. Curr Control Trials Cardiovasc Med. 2005;6:3. doi: 10.1186/1468-6708-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herder M, Johnsen SH, Arntzen KA, Mathiesen EB. Risk factors for progression of carotid intima-media thickness and total plaque area: A 13-year follow-up study: The Tromsø study. Stroke. 2012;43:1818–1823. doi: 10.1161/STROKEAHA.111.646596. [DOI] [PubMed] [Google Scholar]
- 6.Bots ML, Baldassarre D, Simon A, de Groot E, O’Leary DH, Riley W, et al. Carotid intima-media thickness and coronary atherosclerosis: Weak or strong relations? Eur Heart J. 2007;28:398–406. doi: 10.1093/eurheartj/ehl482. [DOI] [PubMed] [Google Scholar]
- 7.Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, et al. Risk factors for progression of common carotid atherosclerosis: The atherosclerosis risk in communities study, 1987–1998. Am J Epidemiol. 2002;155:38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- 8.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, et al. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: The rotterdam study. Circulation. 2002;105:2872–2877. doi: 10.1161/01.cir.0000018650.58984.75. [DOI] [PubMed] [Google Scholar]
- 11.Adar SD, Sheppard L, Vedal S, Polak JF, Sampson PD, Diez Roux AV, et al. Fine particulate air pollution and the progression of carotid intima-medial thickness: A prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanez ND, 3rd, Kronmal RA, Shemanski LR, Psaty BM. A regression model for longitudinal change in the presence of measurement error. Ann Epidemiol. 2002;12:34–38. doi: 10.1016/s1047-2797(01)00280-0. [DOI] [PubMed] [Google Scholar]
- 13.Rundek T, Blanton SH, Bartels S, Dong C, Raval A, Demmer RT, et al. Traditional risk factors are not major contributors to the variance in carotid intima-media thickness. Stroke. 2013;44:2101–2108. doi: 10.1161/STROKEAHA.111.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, et al. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular health study collaborative research group. Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 15.Polak JF, Pencina MJ, Meisner A, Pencina KM, Brown LS, Wolf PA, et al. Associations of carotid artery intima-media thickness (imt) with risk factors and prevalent cardiovascular disease: Comparison of mean common carotid artery imt with maximum internal carotid artery imt. J Ultrasound Med. 2010;29:1759–1768. doi: 10.7863/jum.2010.29.12.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogan S, Duivenvoorden R, Grobbee DE, Kastelein JJ, Shear CL, Evans GW, et al. Ultrasound protocols to measure carotid intima-media thickness in trials; comparison of reproducibility, rate of progression, and effect of intervention in subjects with familial hypercholesterolemia and subjects with mixed dyslipidemia. Ann Med. 2010;42:447–464. doi: 10.3109/07853890.2010.499132. [DOI] [PubMed] [Google Scholar]
- 17.Dogan S, Plantinga Y, Crouse JR, 3rd, Evans GW, Raichlen JS, O’Leary DH, et al. Algorithms to measure carotid intima-media thickness in trials: A comparison of reproducibility, rate of progression and treatment effect. J Hypertens. 2011;29:2181–2193. doi: 10.1097/HJH.0b013e32834b0eba. [DOI] [PubMed] [Google Scholar]
- 18.Wang JG, Staessen JA, Li Y, Van Bortel LM, Nawrot T, Fagard R, et al. Carotid intima-media thickness and antihypertensive treatment: A meta-analysis of randomized controlled trials. Stroke. 2006;37:1933–1940. doi: 10.1161/01.STR.0000227223.90239.13. [DOI] [PubMed] [Google Scholar]
- 19.Tropeano AI, Saleh N, Hawajri N, Macquin-Mavier I, Maison P. Do all antihypertensive drugs improve carotid intima-media thickness? A network meta-analysis of randomized controlled trials. Fundam Clin Pharmacol. 2011;25:395–404. doi: 10.1111/j.1472-8206.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 20.Gardener H, Della Morte D, Elkind MS, Sacco RL, Rundek T. Lipids and carotid plaque in the northern manhattan study (nomas) BMC Cardiovasc Disord. 2009;9:55. doi: 10.1186/1471-2261-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herder M, Johnsen SH, Arntzen KA, Mathiesen EB. Risk factors for progression of carotid intima-media thickness and total plaque area: A 13-year follow-up study: The tromso study. Stroke. 2012 doi: 10.1161/STROKEAHA.111.646596. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer IM, Iglesias del Sol A, Hak AE, Bots ML, Hofman A, Witteman JC. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: The rotterdam study. Stroke. 2003;34:2374–2379. doi: 10.1161/01.STR.0000088643.07108.19. [DOI] [PubMed] [Google Scholar]
- 23.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: Results from the multi-ethnic study of atherosclerosis (mesa) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 24.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: The multi-ethnic study of atherosclerosis (mesa) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.