Abstract
Epigenetic mechanisms appear to play an important role in neurodevelopment. We investigated the effects of acute ethanol exposure on anxiety measures and function of histone deacetylases (HDAC) and DNA methyltransferases (DNMT) in the amygdala and bed nucleus of stria terminalis (BNST) of adolescent rats. One hour after ethanol exposure, rats were subjected to anxiety measures. A subset of adolescent rats was exposed to two doses (24 hrs apart) of ethanol (2 g/kg) to measure rapid ethanol tolerance to anxiolysis. The HDAC and DNMT activities and mRNA levels of DNMT isoforms were measured in the amygdala and BNST. The lower dose of ethanol (1 g/kg) produced neither anxiolysis, nor inhibited the HDAC and DNMT activities in the amygdala and BNST, except DNMT activity in BNST was attenuated. Anxiolysis by ethanol was observed at 2 and 2.25 g/kg, whereas higher doses (2.5 and 3 g/kg) were found to be sedative. DNMT activity in the amygdala and BNST, and nuclear HDAC activity in the amygdala, but not in the BNST were also inhibited by these doses of ethanol. A lack of tolerance was observed on ethanol-induced inhibition of DNMT activity in the amygdala and BNST, and nuclear HDAC activity in the amygdala, as well to anxiolysis produced by ethanol (2 g/kg). The DNMT1, DNMT3a, and DNMT3b mRNA expression in the amygdala was not affected by either one or two doses of 2 g/kg. However, DNMT1 and DNMT3a expression in the BNST was increased whereas DNMT3l mRNA was decreased in the amygdala after two doses of 2 g/kg ethanol. These results suggest that reduced sensitivity to anxiolysis and the lack of rapid tolerance to the anxiolytic effects of ethanol and inhibition of HDAC and DNMT functions may play a role in engaging adolescents in binge drinking patterns.
Keywords: Adolescence, Amygdala, Anxiolysis, Bed nucleus of stria terminalis, Histone deacetylases, DNA methyltransferases, Rapid ethanol tolerance
Introduction
Adolescent alcohol drinking is increasingly becoming a major health concern and appears to be responsible for the development of psychiatric disorders including substance abuse in adulthood (Grant et al., 2001; Miller et al., 2007; Brown et al., 2008; Squeglia et al., 2009). Several studies have shown differences in the effects of ethanol on neuronal function and behaviors between adolescents and adults (Little et al., 1996; Doremus et al., 2005; Maldonado-Devincci et al., 2010). Adolescent rats have been shown to be less sensitive to several behavioral effects of ethanol, such as sedation, motor-impairment, and anxiolysis (Little et al., 1996; Silveri and Spear, 1998; Varlinskaya and Spear, 2002; White et al., 2002) whereas they were found to be more sensitive to ethanol-disrupted spatial memory (Markwiese et al., 1998; Pyapali et al., 1999). In contrast, adult rats were found to be more sensitive to ethanol-induced spatial impairment as compared with adolescent rats (Rajendran and Spear, 2004). In addition, adolescent rats have been shown to be less sensitive to the anxiolytic effects of acute ethanol (0.25 g/kg to 1 g/kg) as compared to adult rats in the social behavior test (Varlinskaya and Spear, 2002). The pharmacologically-validated elevated plus maze (EPM) and light/dark box (LDB) exploration tests have traditionally been used for the measurements of anxiety-like behaviors in rodents (Gao and Cutler 1992; Kliethermes 2005; Langen et al., 2002; Lister 1987; Onaivi and Martin, 1989; Pellow et al., 1985; Sakharkar et al., 2012). We previously demonstrated that the 1g/kg dose of ethanol produces anxiolytic-like effects in adult rats, using the LDB and EPM exploration tests (Pandey et al., 2008; Sakharkar et al, 2012), however, it is unclear whether this or higher doses of acute ethanol produce anxiolysis in adolescents. In the present study, we investigated the effects of different doses of ethanol on the anxiolysis in adolescent rats using the LDB and EPM exploration tests.
Three types of ethanol tolerance (acute, chronic, and rapid tolerance) have been shown to develop in animal models (Hoffman and Tabakoff, 1989). While acute tolerance is explained by a reduced sensitivity during single ethanol exposure, chronic tolerance occurs after prolonged exposure to ethanol (Khanna et al., 1987; 1992). Rapid ethanol tolerance is demonstrated by the requirement of an increased dose of drug on the second exposure to observe a similar behavioral outcome as observed after the first exposure (Crabbe et al., 1979; Sakharkar et al., 2012). Acute tolerance to some of the effects of ethanol exposure such as motor-impairment and social interaction has been shown, however, rapid tolerance to the hypnotic effects of ethanol has not been observed in adolescent rats (Silveri and Spear, 1999; Morales et al., 2011; Ramirez et al., 2011). Ethanol possesses strong anxiolytic properties and is a major contributing factor leading to the development and maintenance of alcoholism (Cooper et al., 1995; Kushner et al., 2000; Pandey et al., 2008; Moberg and Curtin, 2009; Moonat et al., 2011). In our previous study, we found the development of rapid tolerance to the anxiolytic effects of ethanol in adult rats, when measured using the LDB and EPM tests of anxiety (Sakharkar et al., 2012). Currently, the development of rapid tolerance to the anxiolytic effects of ethanol in adolescent rats is not well understood. In the current study, using the two-exposure (24 hours apart) ethanol paradigm we examine whether adolescent rats develop rapid tolerance to the anxiolytic effects of ethanol.
Recent advances in the field of epigenetics show that early life experiences including alcohol exposure can alter gene expression patterns via impacting the chromatin altering machinery, culminating in abnormal neurodevelopment with impacts on both physical and mental health later in life (Witt 2010; Murgatroyd and Spengler, 2011; Szyf, 2013). Studies from our laboratory and others have recently implicated epigenetic mechanisms, such as histone deacetylase (HDAC)-mediated histone deacetylation and DNA methyltransferase (DNMT)-mediated DNA methylation in neuroadaptative processes resulting from ethanol exposure (Moonat et al., 2013; Pandey et al., 2008; Pascual et al., 2012; Sakharkar et al., 2012; Starkman et al., 2012). Recently, it was shown that histone acetylation is altered in the prefrontal cortex after the chronic intermittent ethanol exposure of adolescent rats, the effects of which are likely to be regulated via changes in histone acetyltransferase (HAT) and HDAC activities (Pascual et al., 2012). We have previously shown that acute ethanol exposure inhibits HDAC activity in the amygdala of adult rats, and the degree of this inhibition is reduced after a second dose of ethanol, which is associated with the development of rapid tolerance to the anxiolytic effects of ethanol. Conversely, rapid tolerance to the anxiolytic effects of ethanol is prevented by treatment with the HDAC inhibitor, trichostatin A, in adult rats (Sakharkar et al., 2012). The amygdala and bed nucleus of stria terminalis (BNST), are crucial nuclei of the extended amygdala within the emotional circuitry of the brain in mediating fear and anxiety responses and have been implicated in the negative affective state of alcoholism (Koob et al., 1998; Koob, 2003; Pandey, 2004). We therefore also examined the effects of different doses of acute ethanol exposure and ethanol tolerance on the HDAC and DNMT activities in the amygdala and BNST of adolescent rats. In addition, we have also measured the mRNA levels of different isoforms of DNMTs i.e. DNMT1, DNMT3a, DNMT3b, and DNMT3l in the amygdala and BNST after one and two exposures of ethanol.
Methods
Animals and ethanol treatment
Adolescent male Sprague Dawley (SD) rats (postnatal days 31–35) were used in the present study. All procedures were conducted in accordance with the NIH guidelines for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee. Ethanol was diluted in n-saline (20% w/v), and was intraperitoneally (IP) injected to achieve different doses of 1, 2, 2.25, 2.5, and 3 g/kg of body weight. One hour post-injection, rats were subjected to the measurements of anxiety-like behaviors during light phase of their light/dark cycle using the light/dark box exploration (LDB) test, as described below. To examine if the adolescents develop the rapid ethanol tolerance (RET) to the anxiolytic effects of acute ethanol exposure, as observed in the adult male SD rats in our previous studies (Sakharkar et al., 2012), we employed a similar two-dose acute ethanol exposure paradigm as previously described (Sakharkar et al., 2012). On the first day, rats were IP injected with either n-saline or ethanol (2 g/kg) and were not subjected to behavioral measurements. On the following day (24 hrs after the first injection), n-saline treated rats were injected with either n-saline (Control group) or ethanol (2g/kg) (Ethanol group), whereas ethanol-treated rats were injected with 2 g/kg doses of ethanol (Tolerance group). One hour post-injection, rats were subjected to the measurements of anxiety-like behaviors by LDB and EPM tests.
Immediately after the behavioral measurements, animals were anesthetized and decapitated. The brains were taken out to dissect the amygdala and BNST tissues, which were quickly frozen and stored at −80 °C until used to measure HDAC and DNMT activities, as described below. Blood was obtained from all the rats injected with ethanol at the time of brain collections for the measurement of ethanol levels using the Analox Alcohol Analyzer (Lunenburg, MA).
Light/dark box exploration test
The LDB exploration test for anxiety-like measurement was performed, as described previously by us (Pandey et al., 2008; Sakharkar et al., 2014). In brief, rats were acclimated to the test room and were placed into the dark compartment of the light/dark box. The activity of the rats in terms of time spent and the ambulations in each compartment was monitored by the computer by capturing the break of infrared beam sensors during the 5-min test session. The results are calculated as the mean percent time (± SEMs) spent in either the light or dark compartment. Total ambulations in the light and dark compartments were represented as the general activity of the rat.
Elevated plus maze test
The EPM test of anxiety measurement was employed as described previously by us and others (File 1993; Pandey et al., 2008; Sakharkar et al., 2014). Rats were acclimated to the test room and placed on the central platform of the elevated plus maze. The number of entries and the time spent on open and closed arms of the plus maze was recorded for 5-min test session. The results are calculated in terms of percent entries (± SEMs) and time (± SEMs) spent onto the open arms, whereas number of closed arm entries (± SEMs) is represented as general activity of rats.
DNMT activity in the amygdala and BNST
DNMT activity in amygdala and BNST tissues was measured using the EpiQuik™ DNA methyltransferases activity/inhibition assay kit (Epigentek; Brooklyn, NY), as described by us previously (Zhang et al., 2013). Nuclear fractions of the tissue lysates were prepared using a nuclear extraction kit (Sigma, St. Louis, MO). 10 μg of nuclear protein was used for measuring the DNMT activity according to the manufacturer’s protocol and activity was assayed in terms of optical density (O.D.) using an ELISA plate reader at 450 nm (Dynex Technologies, Chantilly, VA). The results were calculated as O.D. /mg protein and are represented as the mean ± SEM.
HDAC activity in the amygdala and BNST
HDAC activity in the amygdala and BNST tissues was measured, as described by us previously (Pandey et al., 2008; Sakharkar et al., 2012; 2014). In brief, cytosolic and nuclear protein fractions were prepared using a nuclear extraction kit (Sigma) and HDAC activity (HDAC class I and II) was assayed using the colorimetric HDAC activity assay kit (BioVision Research, Mountain View, CA). The enzymatic activity was calculated as O.D. /mg protein and then represented as the mean ± SEM.
Quantification of mRNA using Real-Time PCR
Total mRNA was isolated using TRIZOL reagent (Life Technologies, Grand Island, NY, USA) and amounts were determined by measuring optical densities. Total RNA (1 μg) was reverse transcribed using random hexamers and MMLV Reverse Transcriptase (Life Technologies, Grans Island, NY) in a final volume of 20μl, according to manufacturer’s instructions. Quantitative real-time PCR was performed using Mx3000P QPCR System (Agilent Technologies, Santa Clara, CA) with SYBR green master mix (Fermentas, Glen Burnie, MD). Data were analyzed with MxPro software. Primers corresponding to selected mRNAs are shown in Table 1. The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as an internal control for sample normalization. Target cDNAs (DNMT1, DNMT3a, DNMT3b, and DNMT3l) were analyzed in duplicate for each measurement and the amounts of G3PDH were measured in parallel. PCR conditions included a 10 min 95 °C hold followed by 40 cycles at 95 °C for 30 s, 60 °C for 1 min, and 72 °C for 1 min. Relative abundance was determined for each gene after normalization to G3PDH using the following equation: 2−[Ct (target gene)/Ct (control gene)]. Results are represented as fold change in the mRNA levels.
Table 1.
List of primers for the measurement of mRNA levels in the amygdala and BNST of the rat
| G3PDH | Forward: ACAAGATGGTGAAGGTCGGTGTGA Reverse: AGCTTCCCATTCTCAGCCTTGACT |
| DNMT1 | Forward: AAGCCAGCTATGCGACTTGGAAAC Reverse: ACA ACC GTTGGCTTTCTGAGTGAG |
| DNMT3a | Forward: CACCTACAACAAGCAGCCCATGTA Reverse: AGCCTTGCCAGTGTCACTTTCATC |
| DNMT3b | Forward: TGTGCAGAGTCCATTGCTGTAGGA Reverse: GCT TCCGCCAATCACCAAGTCAAA |
| DNMT3l | Forward: CGAGGATGGACACCAGAGCTACA Reverse: AAGGCAGGCACAGGAAGCAAA |
Statistical analysis
The group differences were evaluated for significance using a one way ANOVA test. Tukey’s test was applied for Post hoc multiple comparisons and the p<0.05 was considered to be significant.
Results
Low anxiolysis sensitivity and lack of rapid tolerance to ethanol-induced anxiolysis
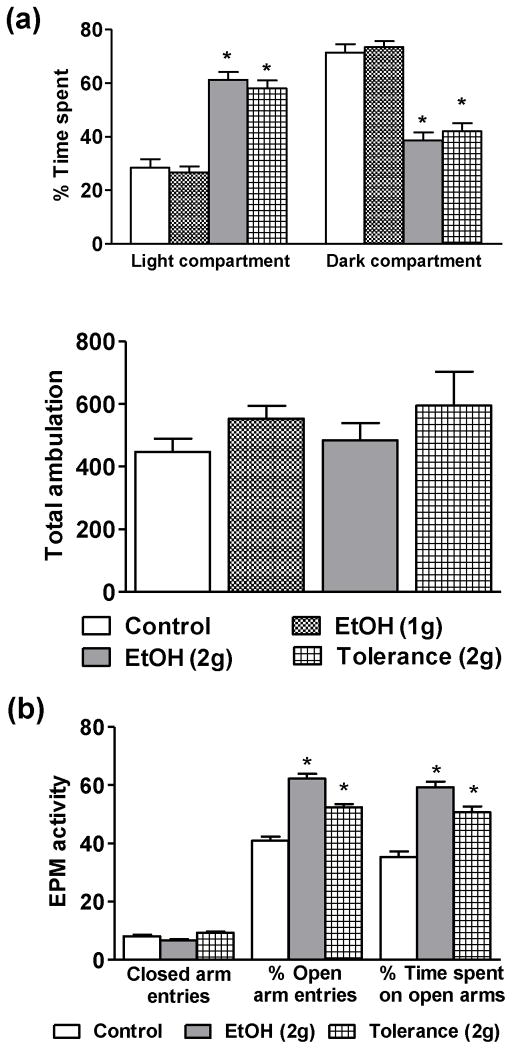
We first examined the effects of low doses of ethanol on anxiolysis in adolescent rats using LDB (Fig. 1a) and EPM exploration tests (Fig. 1b). In the LDB test, no significant differences in the time spent in light and dark compartments were observed between rats treated with n-saline (Control) or ethanol (1g/kg), suggesting that a lower dose of ethanol is not sufficient to produce anxiolysis in adolescent rats (Fig. 1a). However, 2.0 g/kg of ethanol produces anxiolytic-like effects, as seen by the significant (p<0.001) increases in the percent time spent in the light compartment, as compared to n-saline treated rats (Fig. 1a). We have further confirmed the anxiolytic-like effects of 2 g/kg dose of ethanol using EPM test. Similar to LDB test, the anxiolytic-like effects of 2g/kg dose of ethanol were observed in the EPM test (Fig. 1b). The percent open arm entries and the time spent onto the open arms by the ethanol treated rats was significantly (p<0.001) increased as compared to n-saline treated rats (Fig. 1b).
Figure 1.
The effects of low doses of ethanol (1 and 2 g/kg) and tolerance (2 g/kg twice 24 hrs apart) on the percent time spent in each compartment and total ambulation of the light/dark box (LDB) exploration (a) and on the closed arm entries, percent entries and time spent onto the open arms of the elevated plus maze (EPM) tests (b) of anxiety-like behaviors. Values are the mean ± SEM of 6–8 rats for the LDB test and 8–9 rats for the EPM. *Significantly different from the control group [p<0.001; ANOVA (F3, 26 = 43.2, p<0.001 for percent time spent in LDB and F2, 23 = 55.4, p<0.001 for percent open arm entries and F2, 23 = 38.9, p<0.001 for percent time spent in open arms of EPM) followed by Tukey’s test].
We have previously shown development of RET to the anxiolytic effects of acute ethanol exposure (1 g/kg) occur in adult male rats (Sakharkar et al., 2012). Therefore in this study we treated rats with two consecutive anxiolytic doses of ethanol (2 g/kg 24 hr apart) to examine if RET to the anxiolytic effects of ethanol develops during adolescence. We did not observe the development of RET to the anxiolytic effects of ethanol in the adolescents (Figs. 1a, b). Two doses of 2 g/kg ethanol (24 hr apart) produced anxiolysis, similar to that of the single ethanol dose (2 g/kg) without modulating total ambulations (Figs. 1a, b). Adolescent rats treated with 2 g/kg dose of ethanol for two days spent significantly more time in the light compartment and less time in the dark, as compared to n-saline controls, with no significant differences in the time spent in each compartment as compared to animals treated with single doses of ethanol (2 g/kg) (Fig. 1a). Similarly, in the EPM test, animals from the Tolerance (2g) group had a significantly higher percent open arm entries and also spent more time in the open arms as compared with n-saline controls (Fig. 1b). The closed arm entries in EPM and total ambulations in LDB of ethanol treated rats did not significantly differ from n-saline treated rats (Fig. 1a, b) showing no changes in the general activity of the rats. Blood ethanol levels (mg %) of the animals in various groups (mean± SEMs; n=8–17) were 88.0 ± 5.0 [EtOH (1g)], 184.1 ± 9.2 [EtOH (2g)], and 177.6 ± 5.5 [Tolerance (2g)]. These results suggest that a lower dose (1 g/kg) of ethanol was not able to produce anxiolysis in adolescence, while a moderate dose (2 g/kg) of ethanol that was able to produce anxiolysis but not RET to the anxiolytic effects of ethanol.
Effects of lower doses of ethanol on HDAC activity in amygdala and BNST
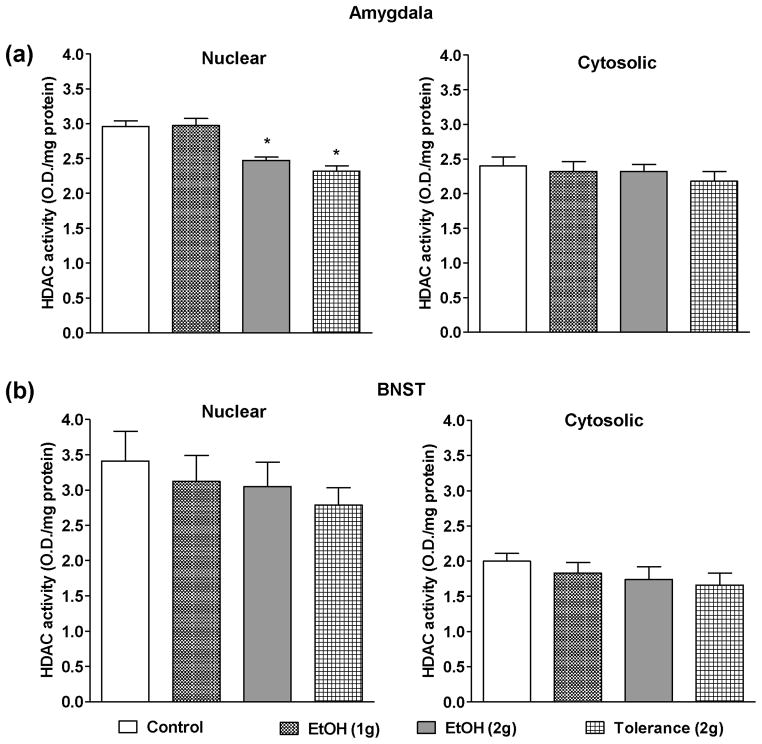
HDAC activity was measured in nuclear and cytosolic protein fractions of both the amygdala (Fig. 2a) and BNST (Fig. 2b) of the adolescent rats treated with n-saline and ethanol at different doses i.e. 1 g/kg, 2 g/kg, and also 2 g/kg for two days (24 hrs apart). Nuclear HDAC activity in the amygdala was not inhibited after acute ethanol exposure at the dose of 1 g/kg, but was significantly inhibited by 2g/kg ethanol exposure (Fig. 2a). In addition, the development of rapid tolerance at the ethanol-induced inhibition of nuclear HDAC activity was not observed with two doses of 2g/kg ethanol exposure. This paradigm also produced a significant reduction in nuclear HDAC activity in the amygdala (Fig. 2a). Interestingly, none of the ethanol treatment paradigms affected the cytosolic HDAC activity in the amygdala (Fig. 2a). Also, neither nuclear nor cytosolic HDAC activities in the BNST were affected by the ethanol exposure at different doses (1 or 2g/kg), as compared to controls suggesting that the effect of ethanol on HDAC activity in the amygdala may be brain region and isoform-specific (Fig. 2b). Taken together, these results indicate that a moderate, but not a lower, dose of ethanol was able to inhibit nuclear HDAC activity without producing cellular tolerance at nuclear HDACs in the amygdala.
Figure 2.
The effects of low doses of ethanol (1 and 2 g/kg) and tolerance (2 g/kg twice 24 hrs apart) on the nuclear and cytosolic HDAC activities in the amygdala (a) and BNST (b). Values are the mean ± SEM of 5–6 and 6–8 rats for amygdala and BNST respectively. *Significantly different from the control group [p<0.01–0.001, ANOVA (F3, 17 = 18.5, p<0.001 for nuclear HDAC activity in amygdala) followed by Tukey’s test].
Effects of lower doses of ethanol on DNMT activity in amygdala and BNST
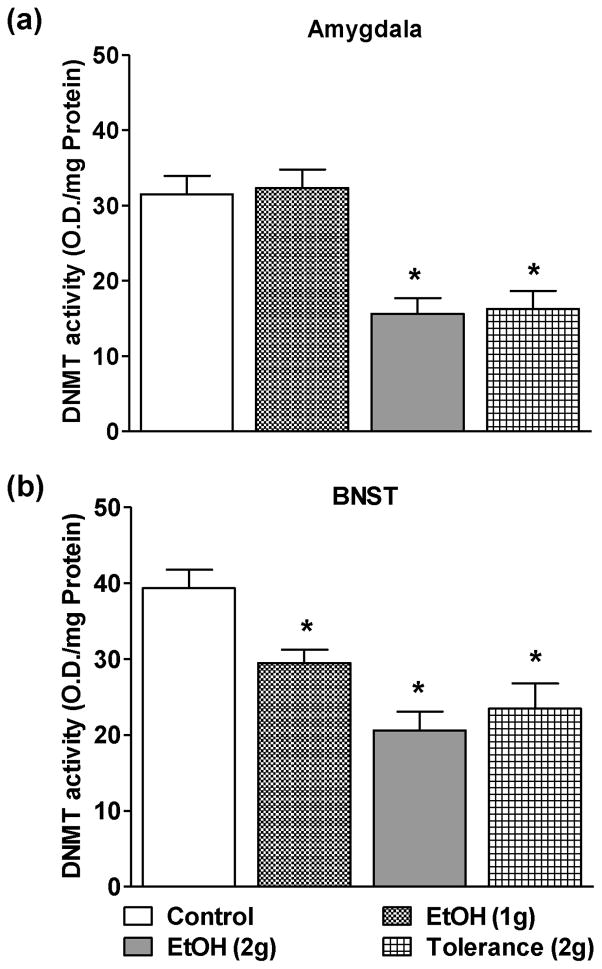
The effects of lower doses of ethanol on DNMT activity in the nuclear protein fractions of amygdala (Fig. 3a) and BNST (Fig. 3b) were also examined. Similar to the HDAC activity, DNMT activity was also not affected in the amygdala of adolescent rats treated with the 1g/kg ethanol (Fig. 3a), but significantly inhibited by the 2 g/kg dose of ethanol compared to n-saline treated adolescent rats (Fig. 3a). Interestingly, DNMT activity in the BNST was inhibited by both doses of ethanol (1 g/kg and 2 g/kg) as compared to n-saline controls (Fig. 3b). Moreover, two consecutive doses (24 hrs apart) of ethanol (2g/kg) to adolescent rats [Tolerance(2g)] significantly attenuated the DNMT activity in both, the amygdala and BNST, as compared to the n-saline controls (Figs. 3a,b) indicating lack of rapid tolerance to ethanol-induced inhibition of DNMT activity in these brain circuitries of adolescent rats.
Figure 3.
The effects of low doses of ethanol (1 and 2 g/kg) and tolerance (2 g/kg twice 24 hrs apart) on the DNMT activities in the amygdala (a) and BNST (b). Values are the mean ± SEM of 6–8 rats for both amygdala and BNST. *Significantly different from the control group [p<0.05–0.001; ANOVA (F3, 26 = 15.2, p<0.001 for amygdala and F3, 26 = 11.9, p<0.001 for BNST) followed by Tukey’s test].
Effects of acute ethanol exposure on DNMT mRNA levels in amygdala and BNST
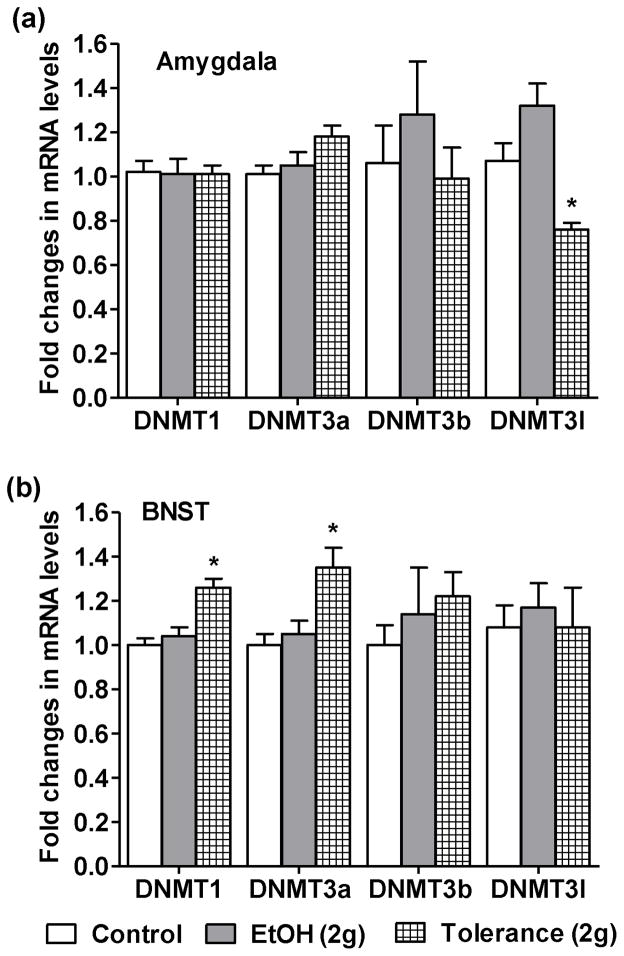
We have further examined the effects of one (EtOH 2 g/kg) and two doses of ethanol [Tolerance (2g)] on the expression of DNMT1, DNMT3a, DNMT3b and DNMT3l mRNA levels in the amygdala and BNST (Figs. 4a, b). Expression of DNMT1, 3a, and 3b was not affected by single or double consecutive (24 hrs apart) ethanol exposure in the amygdala (Fig. 4a), while DNMT3l expression was decreased after two doses of ethanol [Tolerance (2g)]. DNMT1 and DNMT3a mRNA levels were increased after the two doses of ethanol exposure (Fig. 4b) without producing any change in the mRNA levels of DNMT3b and DNMT3l in BNST. The mRNA levels of all DNMT isoforms were unaffected in BNST after single ethanol exposure.
Figure 4.
The effects of one [EtOH (2g)] and two [EtOH (2g) 24 hrs apart-tolerance group] doses of ethanol exposure on the mRNA expression of different isoforms of DNA methyltransferases (DNMTs), i.e. DNMT1, 3a, 3b and 3l in the amygdala (a) and BNST (b). Values are the mean ± SEM of 5 rats in each group. *Significantly different from the control group [p<0.05–0.001; ANOVA (F2, 12 = 12.6, p<0.001 for DNMT3l in amygdala; F2, 12=14.5, p<0.001 for DNMT1 in BNST; and F2, 12=6.8, p<0.01 for DNMT3a in BNST) followed by Tukey’s test].
Effects of higher doses of ethanol on anxiety measures
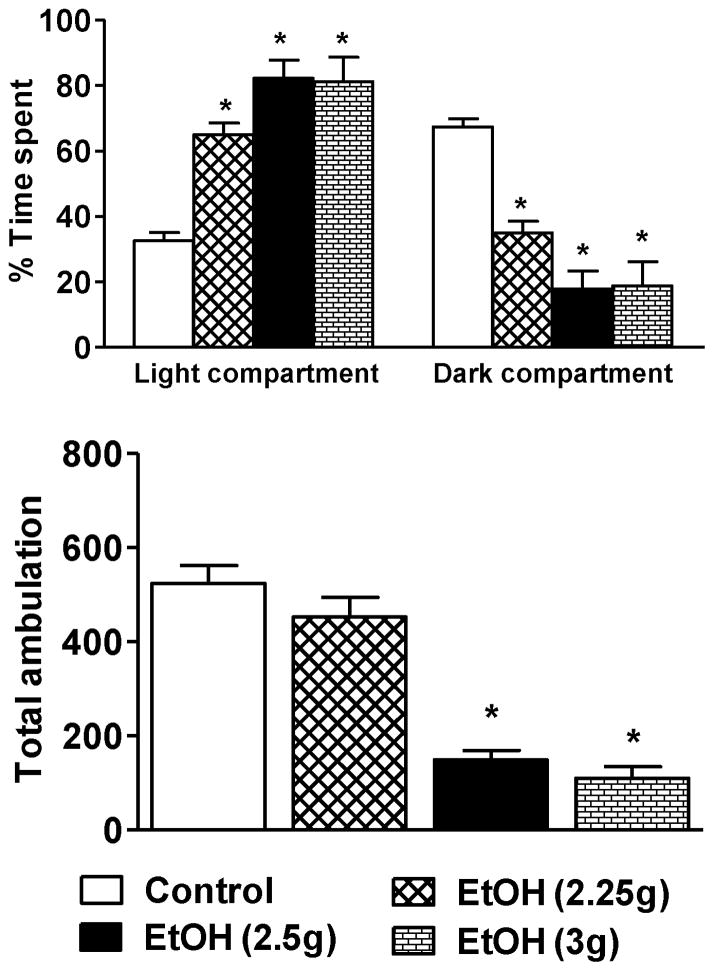
We next examined the effects of higher doses (2.25, 2.5, and 3.0 g/kg) of ethanol exposure on anxiolysis in adolescent rats using the LDB test. All higher doses of ethanol produced anxiolytic-like effects in adolescent rats (Fig. 5). No differences in the general activity of the rats treated with 2.25 g/kg of ethanol were observed in the LDB test as compared to n-saline controls (Fig. 5). However, an increase in the dose of ethanol to 2.5 g/kg and 3 g/kg was sedative in adolescent rats. The percent time spent in the light compartment of LDB was significantly increased in rats treated with 2.5 and 3 g/kg doses of ethanol as compared to n-saline-treated rats (Fig. 5). Concomitantly, total ambulations were also significantly attenuated (Fig. 5) in these animals, which is indicative of reductions in general activity, most likely due to the sedative effects of higher doses (2.5 g/kg and 3 g/kg) of ethanol (Fig. 5). Blood ethanol levels (mg %) of the animals in various groups (mean± SEMs; n=7–9) were 233.3 ± 12.7 [EtOH (2.25g)], 242.5 ± 11.0 [EtOH (2.5g)], and 333.6 ± 10.6 [EtOH (3g)].
Figure 5.
The effects of higher doses of ethanol exposure on the percent time spent in each compartment and total ambulation of the light/dark box exploration test of anxiety-like behaviors. Values are the mean ± SEM of 6–13 rats. *Significantly different from the control group [p<0.001; ANOVA (F3, 29 = 35.6, p<0.001 for percent time spent in LDB and F3, 29 = 30.1, p<0.001 for total ambulations) followed by Tukey’s test].
Effects of higher doses of ethanol on HDAC and DNMT activities in amygdala and BNST
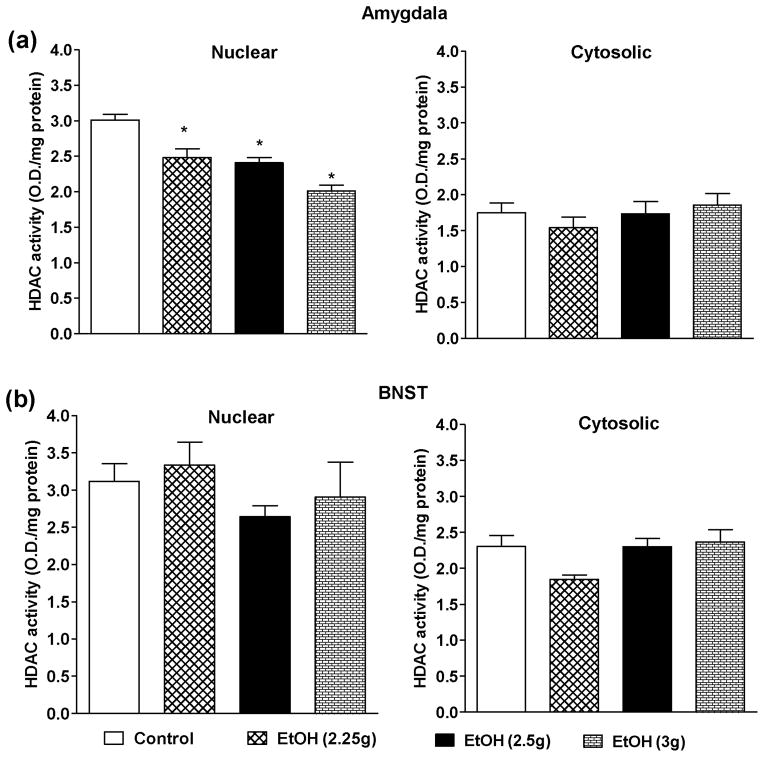
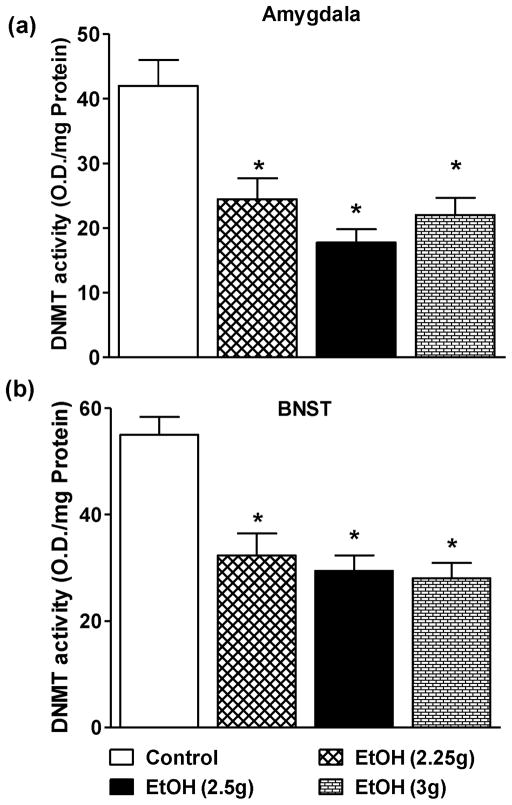
The effects of higher doses of ethanol on HDAC and DNMT activities in the amygdala and BNST were also examined. All higher doses of ethanol significantly inhibited the nuclear but not cytosolic HDAC activities in the amygdala of adolescent rats in a dose-dependent manner (Fig. 6a). Interestingly, none of ethanol doses were able to alter the nuclear or cytosolic HDAC activities in BNST (Fig. 6b). In contrast, DNMT activity was significantly inhibited in the amygdala and BNST by all higher doses of ethanol investigated as compared to n-saline treated adolescent rats (Figs. 7a, b). These results indicate that only nuclear HDACs in the amygdala and DNMTs in the amygdala and the BNST are sensitive to acute ethanol exposure in adolescent rats. Furthermore, HDACs in the BNST of adolescent rats are insensitive to acute ethanol exposure indicating differential effects of ethanol on epigenetic modifiers in the developing brain regions.
Figure 6.
The effects of higher doses of ethanol exposure on the nuclear and cytosolic HDAC activities in the amygdala (a) and BNST (b). Values are the mean ± SEM of 5–9 for both amygdala and BNST. *Significantly different from the control group [p<0.01–0.001, ANOVA (F3, 24 = 25.0, p<0.001 for nuclear HDAC activity in amygdala) followed by Tukey’s test]. Sakharkar et al., HDAC, DNMT and ethanol anxiolysis in Adolescent
Figure 7.
The effects of higher doses of ethanol exposure on the DNMT activities in the amygdala (a) and BNST (b). Values are the mean ± SEM of 5–9 rats in each group for both amygdala and BNST. *Significantly different from the control group [p<0.01–0.001; ANOVA (F3, 24 = 12.2, p<0.001 for amygdala and F3, 24 = 17.3, p<0.001 for BNST) followed by Tukey’s test].
Discussion
The data presented indicate that reduced sensitivity to anxiolysis and lack of development of rapid tolerance to anxiolysis correlated with low sensitivity to ethanol-induced inhibition of HDAC and DNMT activities and lack of tolerance to these epigenetic effects of ethanol in the amygdala and BNST brain regions of adolescent rats. In addition, we observed differential changes in DNMT isoform expression after acute ethanol treatment in the amygdala and BNST of adolescent rats. Also, BNST HDACs are insensitive to lower as well as higher doses of ethanol. Adolescent rats did not undergo anxiolysis after exposure to 1 g/kg dose of ethanol. On the contrary, our previous studies demonstrated that this low dose of ethanol was sufficient to produce anxiolytic effects in adult rats (Pandey et al., 2008; Sakharkar et al., 2012). Similar observations were made by Varlinskaya and Spear (2002), using the social behavior test of anxiety measurements. However, adolescent rats required higher doses of ethanol (2 g/kg and 2.25 g/kg) to exhibit the anxiolysis without modulation in general activity. The present study also indicates that anxiolytic-like effects produced by ethanol exposure at the 2.5 and 3 g/kg doses in adolescent rats. Total ambulations of these rats were decreased as compared to controls, which indicate the dampening of general activity. The decrease in general activity of rats at the higher doses (≥2.5 g/kg) may be attributed to the sedative effects of ethanol. Because, we have seen significant reductions in total ambulations, caution should be exerted in the interpretation of anxiolytic-like effects of these doses of ethanol in adolescent rats. We therefore suggest that the 2 g/kg and 2.25 g/kg doses of ethanol are anxiolytic and non-sedative in the adolescent rats. These results indicate that higher doses of ethanol are required to produce anxiolytic-like effects in adolescent rats.
The development of rapid tolerance to acute ethanol effects on several behaviors such as sedation, anxiolysis, and hypnotic effects in adults play an important role in the development of alcoholism (Hoffman and Tabakoff, 1989; Kalant, 1998; Sakharkar et al., 2012). It has been shown that adolescent rats are less sensitive to RET to the motor impairing effects of ethanol compared with adult rats (Silveri and Spear, 2001). Here, we examined the development of rapid tolerance to the anxiolytic-like effects of ethanol using a 2 g/kg dose and found that adolescent rats were not able to develop RET to anxiolysis produced by acute ethanol. On the other hand, development of rapid tolerance to the anxiolytic effects of acute ethanol was observed in adult rats (Sakharkar et al., 2012). Interestingly, adolescent rats also do not display anxiety-like behaviors during withdrawal after acute ethanol exposure but anxiety-like behaviors were observed in adult rats under a similar ethanol treatment paradigm (Doremus-Fitzwater and Spear, 2007). It has been shown that adolescent rats consume more ethanol than adult rats (Truxell et al., 2007; Vetter et al., 2007). Additionally, clinical and preclinical studies have shown that alcohol is able to facilitate social interactions in adolescent and this factor may promote drinking behaviors (Beck et al., 1993; Smith et al., 1995; Varlinskaya and Spear, 2002). The data presented here clearly suggest that failure to develop rapid tolerance to the anxiolytic effects of ethanol in conjunction with low sensitivity to anxiolysis in adolescent rats may play a permissive role in promoting heavy binge-drinking behavior in adolescents. Clinical studies support this notion as it has been shown that anxiety sensitivity is a crucial factor in promoting heavy drinking in college students (Stewart et al., 2001; Lawyer et al., 2002). In addition, low sensitivity to alcohol and increased hangover resistance may be involved in binge drinking and serve as predictor for the development of alcohol use disorders in adulthood (Schuckit, 1994; Piasecki et al., 2012).
In a series of studies we reported that HDACs serve as molecular targets within the epigenome in the amygdaloid circuitry to modulate anxiety-like behaviors in rats (Pandey et al., 2008; Moonat et al., 2013; Sakharkar et al., 2014; You et al., 2014). We have previously observed ethanol’s ability to inhibit HDAC activity within amygdala associated with its anxiolytic effects and with the development of rapid tolerance in adult rats (Sakharkar et al., 2012). Here, we investigated a similar phenomenon in the developing amygdala and BNST and found that nuclear, but not cytosolic, HDAC activity was inhibited in the amygdala of adolescent rats at the anxiolytic and higher doses of ethanol (≥2 g/kg). However, neither cytosolic nor nuclear HDAC activity was inhibited in the BNST of adolescent rats. These results indicate that the perturbations in histone acetylation in the amygdala via HDAC inhibition secondary to ethanol exposure may be involved in its anxiolytic actions. Also, it was found that only nuclear HDACs are sensitive to ethanol-induced inhibition in the amygdala of adolescent rats. The lower dose of ethanol (1g/kg) produces anxiolysis and inhibits HDAC activity in the amygdala of adult rats (Sakharkar et al., 2012); however this dose of ethanol is not effective in adolescent rats despite similar blood ethanol levels. Furthermore, HDAC activity was inhibited in the adolescent rats treated with single or double (24 hrs apart) exposure of ethanol (2g/kg), which was associated with the anxiolysis, indicating the lack of development of rapid tolerance in adolescent rats. We have previously shown that HDAC inhibition was able to increase histone(H3-K9 &H4-K8) acetylation in the amygdala of adults, thereby further regulating the gene transcription leading to the behavioral effects of ethanol, such as anxiolysis and development of tolerance (Pandey et al., 2008; Sakharkar et al., 2012; Moonat et al., 2013). In adolescent rats, ethanol-induced HDAC inhibition may also be playing a similar role in the regulation of gene transcription underlying its anxiolytic actions and appears to be specific to the amygdala, and not the BNST.
DNA methylation has emerged as an important epigenetic mechanism in the regulation of gene expression (Feng and Fan, 2009). Three different isoforms of DNMTs, viz. DNMT1, 3a, and 3b have been identified that regulate promoter DNA methylation and maintain the methylation status to either silence or facilitate gene expression (Goll and Bestor 2005; Turek-Plewa and Jagodziński 2005). Recently another DNMT isoform, DNMT3l, has been shown to be essential in the regulation of DNMT 3a and 3b catalytic activity in the brain and involved in the regulation of DNA methylation during development (Chédin 2011; Arand et al., 2012). Here, we observed the inhibition of global DNMT activity by ethanol in both the amygdala and BNST. Although DNMT activity was not inhibited at the 1 g/kg dose in the amygdala, a comparable dose did inhibit the DNMT activity in the BNST, indicating ethanol’s brain region-specific ability in the differential regulation of DNA methylation via DNMTs. The close association of DNMT inhibition with HDAC inhibition in the amygdala in tandem with the anxiolytic-like effects of ethanol suggests that interaction between DNA methylation and histone deacetylation may be dynamic and associated with the regulation of gene expression in the amygdala and that are implicated in the anxiolytic effects of acute ethanol. Interestingly, the first exposure of ethanol (2 g/kg) inhibits DNMT activity in the amygdala and BNST without modulating the expression of DNMT isoforms, suggesting ethanol-induced inhibition of catalytic activity of DNMTs. Although a down-regulation of DNMT3l levels was observed following two doses of ethanol, a single dose of 2 g/kg did not alter the expression of any of the DNMT isoforms in amygdala. Moreover, DNMT1 and 3a expression was increased in the BNST after the two ethanol doses, with no significant change after a single ethanol dose (2 g/kg). These conflicting effects on DNMT expression and DNMT activity inhibition by ethanol in the amygdala and BNST warrants further investigation as to what role specific isoforms play in ethanol’s actions in the adolescent brain. However, in another study we observed that the knockdown of one of the three DNMT isoforms i.e. DNMT1, DNMT3a and DNMT3b by siRNA treatment leads to increases in other two isoforms (Kundakovic et al., 2009). Recently, we reported that DNMT activity and DNMT3a protein expression in cultured astrocytes was attenuated by ethanol (Zhang et al., 2013). LaPlant et al. (2010) found that DNMT3a expression was up-regulated in the nucleus accumbens by chronic cocaine use and chronic social stress, suggesting an important role for DNMT3a in regulating emotional behavior and spine plasticity. More recently, perinatal protracted ethanol exposure in Long-Evans rats increased DNMT activity; while divergently decreasing the DNMT1 and DNMT3a expression in the hippocampus (Perkins et al., 2013). In our studies, we observed a similar incongruity in the ability of ethanol to inhibit DNMT activity, with a reciprocal increase in DNMT1 and DNMT3a expression in the BNST of adolescent rats.
In conclusion, the present study pinpoints the reduced sensitivity and lack of development of tolerance to the anxiolytic effects of acute ethanol exposure in adolescent rats. We also observed that a higher dose of ethanol is required to inhibit HDAC and DNMT activity in the amygdala without development of rapid tolerance to these effects of ethanol. These findings stand in contrast to our previous study indicating that lower doses of ethanol produce anxiolysis and rapid tolerance in adult rats with corresponding effects on HDAC activity inhibition in the amygdala (Sakharkar et al., 2012). Clinical studies suggest that anxiety sensitivity appears to be involved in promoting heavy drinking in adolescents (Stewart et al., 2001; Lawyer et al., 2002). The preclinical data collected here suggest the possibility that low sensitivity to ethanol-induced anxiolysis and inhibition of HDAC and DNMT activities in the amygdala in adolescence may be involved in promoting binge drinking. Further, detailed epigenetic mechanisms of gene regulation that involves the HDAC and DNMT in the amygdala and BNST are necessary during intermittent ethanol exposure in adolescent rats in order to fully understand this phenomenon.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA-019971(NADIA project), AA-010005, AA-013341, and AA-016690, and by the Department of Veterans Affairs (Merit Review Grant; Research Career Scientist award) to S.C.P.
Footnotes
Conflict of Interest
No biomedical financial interests or potential conflicts of interest were reported by authors
References
- Arand J, Spieler D, Karius T, Branco MR, Meilinger D, Meissner A, Jenuwein T, Xu G, Leonhardt H, Wolf V, Walter J. In Vivo control of CpG and Non-CpG DNA methylation by DNA methyltransferases. PLoS Genet. 2012;8:e1002750. doi: 10.1371/journal.pgen.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KH, Thombs DL, Summons TG. The social context of drinking scales: construct validation and relationship to indicants of abuse in an adolescent population. Addict Behav. 1993;18:159–169. doi: 10.1016/0306-4603(93)90046-c. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S. A development perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121:S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédin F. The DNMT3 family of mammalian de novo DNA methyltransferases. Prog Mol Biol Transl Sci. 2011;101:255–285. doi: 10.1016/B978-0-12-387685-0.00007-X. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Rigter H, Uijlen J, Strijbos C. Rapid development of tolerance to the hypothermic effect of ethanol in mice. J Pharmacol Exp Ther. 1979;208:128–133. [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2007;31:1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. Int Rev Neurobiol. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- Gao B, Cutler MG. Effects of acute administration of the 5-HT3 receptor antagonist, BRL 46470A, on the behaviour of mice in a two compartment light-dark box and during social interactions in their home cage and an unfamiliar neutral cage. Neuropharmacology. 1992;31:743–748. doi: 10.1016/0028-3908(92)90035-n. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Hartford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Tabakoff B. Mechanisms of alcohol tolerance. Alcohol Alcohol. 1989;24:251–252. [PubMed] [Google Scholar]
- Kalant H. Research on tolerance; what can we learn from history. Alcohol Clin Exp Res. 1998;22:67–76. doi: 10.1111/j.1530-0277.1998.tb03618.x. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Campanelli C, Lê AD, Kalant H. Effect of raphe lesions on the development of chronic tolerance to pentobarbital and cross-tolerance to ethanol. Psychopharmacology. 1987;91:473–478. doi: 10.1007/BF00216013. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Weiner J, Chau A, Shah G. Ketamine retards chronic but not acute tolerance to ethanol. Pharmacol Biochem Behav. 1992;42:347–350. doi: 10.1016/0091-3057(92)90538-q. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytiä P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol Pharmacol. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Langen B, Dietze S, Fink H. Acute effect of ethanol on anxiety and 5-HT in the prefrontal cortex of rats. Alcohol. 2002;27:135–141. doi: 10.1016/s0741-8329(02)00219-7. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolaños CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer SR, Karg RS, Murphy JG, McGlynn FD. Heavy drinking among college students is influenced by anxiety sensitivity, gender, and contexts for alcohol use. J Anxiety Disord. 2002;16:165–173. doi: 10.1016/s0887-6185(02)00092-0. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Badanich KA, Kirstein CL. Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol. 2010;44:57–66. doi: 10.1016/j.alcohol.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable vs. predictable threat. J Abnorm Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addic Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73:763–773. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Age differences in the expression of acute and chronic tolerance to ethanol in male and female rats. Alcohol Clin Exp Res. 2011;35:1614–1624. doi: 10.1111/j.1530-0277.2011.01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Spengler D. Epigenetics of early child development. Front Psychiatry. 2011;2:16. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Martin BR. Neuropharmacological and physiological validation of a computer-controlled two-compartment black and white box for the assessment of anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:963–976. doi: 10.1016/0278-5846(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Pandey SC. The gene transcription factor cyclic AMP-responsive element binding protein: role in positive and negative affective states of alcohol addiction. Pharmacol Ther. 2004;104:47–58. doi: 10.1016/j.pharmthera.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–2319. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perkins A, Lehmann C, Lawrence RC, Kelly SJ. Alcohol exposure during development: Impact on the epigenome. Int J Dev Neurosci. 2013;31:391–397. doi: 10.1016/j.ijdevneu.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Alley KJ, Slutske WS, Wood PK, Sher KL, Shiffman S, Heath AC. Low sensitivity to alcohol: relations with hangover occurrence and susceptibility in an ecological momentary assessment investigation. J Stud Alcohol Drugs. 2012;73:925–932. doi: 10.15288/jsad.2012.73.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci. 2004;1021:441–444. doi: 10.1196/annals.1308.060. [DOI] [PubMed] [Google Scholar]
- Ramirez RL, Varlinskaya EI, Spear LP. Effect of the selective NMDA NR2B antagonist, ifenprodil, on acute tolerance to ethanol-induced motor impairment in adolescent and adult rats. Alcohol Clin Exp Res. 2011;35:1149–1159. doi: 10.1111/j.1530-0277.2011.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC. Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol. 2014 Feb 17;:1–14. doi: 10.1017/S1461145714000054. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res. 2012;36:61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Smith GT, Goldman MS, Greenbaum PE, Christiansen BA. Expectancy for social facilitation from drinking: the divergent paths of high-expectancy adolescents. J Abnorm Psychol. 1995;104:32–40. doi: 10.1037//0021-843x.104.1.32. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman BG, Sakharkar AJ, Pandey SC. Epigenetics-beyond the genome in alcoholism. Alcohol Res. 2012;34:293–305. [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Zvolensky MJ, Eifert GH. Negative-reinforcement drinking motives mediate the relation between anxiety sensitivity and increased drinking behavior. Pers Individ Dif. 2001;31:157–171. [Google Scholar]
- Szyf M. DNA methylation, behavior and early life adversity. J Genet Genomics. 2013;40:331–338. doi: 10.1016/j.jgg.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Turek-Plewa J, Jagodziński PP. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell Mol Biol Lett. 2005;10:631–647. [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC. Reversal of deficits in dendritic spines, BDNF, and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol. 2014;17:313–322. doi: 10.1017/S1461145713001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kusumo H, Sakharkar AJ, Pandey SC, Guizzetti M. Regulation of DNA methylation by ethanol induces tissue plasminogen activator expression in astrocytes. J Neurochem. 2013;128:344–349. doi: 10.1111/jnc.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]