SUMMARY
Cytolysin-mediated translocation (CMT), performed by Streptococcus pyogenes, utilizes the cholesterol-dependent cytolysin Streptolysin O (SLO) to translocate the NAD+-glycohydrolase (SPN) into the host cell during infection. SLO is required for CMT and can accomplish this activity without pore formation, but the details of SLO's interaction with the membrane preceding SPN translocation are unknown. Analysis of binding domain mutants of SLO and binding domain swaps between SLO and homologous cholesterol-dependent cytolysins revealed that membrane binding by SLO is necessary but not sufficient for CMT, demonstrating a specific requirement for SLO in this process. Despite being the only known receptor for SLO, this membrane interaction does not require cholesterol. Depletion of cholesterol from host membranes and mutation of SLO's cholesterol recognition motif abolished pore formation but did not inhibit membrane binding or CMT. Surprisingly, SLO requires the co-expression and membrane localization of SPN to achieve cholesterol-insensitive membrane binding; in the absence of SPN, SLO's binding is characteristically cholesterol-dependent. SPN's membrane localization also requires SLO, suggesting a co-dependent, cholesterol-insensitive mechanism of membrane binding occurs, resulting in SPN translocation.
Keywords: cholesterol-dependent cytolysin, Streptococcus pyogenes, Streptolysin O
INTRODUCTION
The translocation of toxic effector proteins into the host cell during infection is a common strategy used by bacteria to promote pathogenesis. Delivery of bacterial toxins requires a secretion system with a dedicated translocator. Many Gram-negative bacteria direct toxins across the host cell membrane into the cytosol through the specialized needle apparatus of Type III secretion systems (Izore et al., 2011). Other toxins, including anthrax and diphtheria toxins, gain access to the host cytosol through the endocytic pathway after the translocator binds to the plasma membrane, allowing endocytosis of the translocator-toxin complex (Collier, 2001; Sandvig & van Deurs, 2005; Young & Collier, 2007). The Gram-positive bacterium Streptococcus pyogenes uses a different method of effector delivery termed cytolysin-mediated translocation (CMT) to transport an effector into the host cell during infection (Madden et al., 2001).
The process of CMT requires the cholesterol-dependent cytolysin (CDC) Streptolysin O (SLO) for the directed translocation of the S. pyogenes NAD+-glycohydrolase (SPN) across the host cell membrane (Madden et al., 2001; Bricker et al., 2002). After gaining access to the host cell cytosol, SPN cleaves β-NAD+ to produce nicotinamide and ADP-ribose (Ghosh et al., 2010). SPN and SLO are members of the large repertoire of virulence factors produced by S. pyogenes, which can result in varied clinical manifestations of disease including impetigo, pharyngitis, and necrotizing fasciitis (Cunningham, 2000). Previous work has shown that S. pyogenes mutants deficient in either SPN or SLO exhibit decreased cytotoxicity, implicating CMT in pathogenesis (Bricker et al., 2005; Bricker et al., 2002; Madden et al., 2001; Meehl & Caparon, 2004). Although it has been shown that SLO is required for the translocation of SPN, the mechanism by which this process occurs is unknown.
The original model of CMT predicted that SPN diffuses through pores in the host membrane created by SLO. However, several subsequent findings suggest the mechanism is more complex. First, the highly homologous CDC Perfringolysin O (PFO) is not CMT competent when expressed from a SLO− strain of S. pyogenes, despite having robust pore-forming activity (Meehl & Caparon, 2004). Second, SLO has an additional 66 residues at its amino terminus (N-terminal extension) not found in related CDCs, and removal of this sequence abrogates the ability of SLO to translocate SPN without inhibiting pore formation (Meehl & Caparon, 2004). Third, a recent study of SLO mutants unable to form pores led to the discovery that pore formation by SLO is not required for CMT (Magassa et al., 2010), suggesting that pore formation and SPN translocation may be independent processes performed by SLO. The ability to translocate SPN in the absence of a pore indicates that specific contacts between SLO and the host cell membrane may be essential for CMT.
SLO, PFO, and intermedilysin (ILY) are representatives of the CDC family of proteins, which are oligomeric pore-forming proteins secreted by several species of pathogenic Gram-positive bacteria (Tweten, 2005; Heuck et al., 2010), and have also recently been discovered in two Gram-negative species (Hotze et al., 2013). These proteins share 40% to 70% identity, and previous analysis of resolved structures illustrates that all members of this family likely have an analogous structure and a similar pore-forming mechanism (Hotze & Tweten, 2012; Bourdeau et al., 2009; Gilbert et al., 1999; Polekhina et al., 2005; Rossjohn et al., 1997). Each cytolysin monomer initially contacts the host cell membrane via Domain 4 of the protein, which contains three small hydrophobic loops (the first of which constitutes the cholesterol recognition motif) and the undecapeptide, which is a stretch of eleven amino acids that are important for stabilizing cytolysins in the host cell membrane upon binding, coordinating membrane binding with monomer-monomer interactions in most CDCs (Dowd & Tweten, 2012; Hotze & Tweten, 2012), and facilitating structural changes required for pore formation (Farrand et al., 2010; Ramachandran et al., 2002; Soltani et al., 2007a; Soltani et al., 2007b; Weis & Palmer, 2001).
As their name suggests, cholesterol plays an important role in the function of cholesterol-dependent cytolysins. It is absolutely required by all CDCs for pore formation, as recognition of cholesterol triggers the insertion of the hydrophobic loops—a prerequisite for insertion of the β-barrel pore (Hotze & Tweten, 2012). However, the role of cholesterol in initial membrane binding by these cytolysins is not universal. Many studies have shown that cholesterol is the sole receptor for PFO, and both PFO membrane binding and pore formation are inhibited by cholesterol depletion (Flanagan et al., 2009; Giddings et al., 2003; Heuck et al., 2000; Soltani et al., 2007b; Heuck et al., 2007). However, the primary receptor for ILY (and a small subset of CDCs similar to ILY) is CD59, a human-specific membrane-anchored regulatory protein that inhibits the membrane attack complex of the complement pathway (Giddings et al., 2004; Hotze & Tweten, 2012; Gelber et al., 2008). Consequently, depletion of cholesterol from the membrane prevents pore formation by ILY but does not inhibit membrane binding (Giddings et al., 2003).
To date, cholesterol remains the only identified receptor for SLO, and specific mutations in the cholesterol recognition motif of SLO significantly impair its ability to bind to cholesterol (Farrand et al., 2010). In this study we explore the role for cholesterol binding by SLO in CMT by extracting it from the membrane and by mutating SLO's cholesterol recognition motif. The results demonstrate that the efficiency of CMT is unaffected by the concentration of membrane cholesterol, and altering SLO's cholesterol recognition motif does not inhibit CMT. These manipulations also do not significantly affect SLO's localization to the host cell membrane, revealing a novel cholesterol-insensitive mode of SLO membrane binding that occurs during infection. Surprisingly, this cholesterol-insensitive binding requires the co-expression and membrane localization of SPN, suggesting that CMT may be a cholesterol-insensitive process that occurs following an interaction between SPN and SLO at the host cell membrane.
RESULTS
Membrane binding by SLO is necessary but not sufficient for CMT
To evaluate the role of SLO's membrane-binding Domain 4 in CMT, an internal deletion in slo was generated to create a mutant protein lacking this domain (SLOΔD4, Table S1). Immunoblot analysis of cell-free culture supernatants demonstrated that the truncated protein is secreted and stable (Figure S1). To verify that the deletion of this domain abrogates membrane binding, total membranes from A549 cells (human lung fibroblasts) were harvested following infection with strains encoding wild-type SLO, SLO− (Ruiz et al., 1998), or SLOΔD4. Wild-type SLO localized to the host cell membrane fraction, whereas, as expected, SLOΔD4 was not detected (Figure 1). Membrane binding is also necessary for SLO's cytolytic activity, as cell-free culture supernatant from wild-type bacteria lysed rabbit erythrocytes while no lysis was detected with culture supernatant containing SLOΔD4 (Figure 1). To determine if membrane binding by SLO is necessary for its CMT activity, the cytosolic fractions of A549 cells infected with wild-type, SLO−, or the SLOΔD4-expressing strain were analyzed post-infection for the presence of SPN. The wild-type strain is CMT competent as shown by the presence of SPN in the host cell cytosol, but SLOΔD4 is deficient for CMT despite equivalent production and secretion of SLOΔD4 as well as SPN (Figure S1), indicating that membrane binding by SLO is required for CMT. To determine if membrane binding is sufficient for SLO to translocate SPN, SLO chimeras were generated that encode the membrane-binding Domain 4 sequences from the homologous CDCs ILY and PFO (SLO/ILYD4 and SLO/PFOD4, Table S1). Based on an alignment of Domain 4 primary sequences, SLO and ILY are 34.7% identical and 49.6% similar, while SLO and PFO are 64.5% identical and 74.5% similar (Figure 2). The SLO/ILYD4 and SLO/PFOD4 chimeras are expressed and secreted (Figure S1), and are localized to the host cell membrane fraction following infection, although binding of SLO/ILYD4 is significantly reduced compared to wild-type SLO (Figure 1). As expected, SLO/ILYD4 from culture supernatants was unable to lyse rabbit erythrocytes, which lack ILY's CD59 receptor, but retained hemolytic activity against human erythrocytes, indicating that this chimera adopted the cell binding specificity of ILY. Similarly, SLO/PFOD4 exhibited more robust lytic activity characteristic of PFO (Meehl & Caparon, 2004) (Figure 1). To determine if membrane recognition by these chimeras is sufficient to promote CMT, the cytosolic fractions of A549 cells infected with the SLO/ILYD4- and SLO/PFOD4-expressing strains were analyzed post-infection for the presence of SPN. Despite retaining membrane-binding ability, SLO/ILYD4 and SLO/PFOD4 were unable to translocate SPN into the host cell cytosol (Figure 1), although SPN is expressed in these strains (Figure S1). This defect for SLO/ILYD4 was not due to the variant undecapeptide of ILY, which has three amino acid differences in the undecapeptide compared to other CDCs (Nagamune et al., 2004), since a SLO/ILYD4 chimera with a canonical undecapeptide sequence (SLO/ILYD4-ECW, Table S1) also could not translocate SPN despite more efficient binding to host cell membranes than wild-type SLO (Figure 1). Thus, even though the SLO/ILYD4, SLO/ILYD4-ECW, and SLO/PFOD4 proteins maintain the ability to bind to host cells, it is apparent that solely binding the membrane is not sufficient for CMT.
Figure 1. Membrane binding by SLO is necessary but not sufficient for CMT.
Immunoblot analyses of total membrane and cytosol prepared from A549 cells following 3 hrs of infection by the various S. pyogenes strains indicated at the top of the Figure that were developed with antisera against the proteins indicated on the right. Analyses of E-cadherin (membrane) and GAPDH (cytosol) were included as loading controls and the migration of several molecular weight standards (in kDa) are indicated at the left. Shown at the bottom are hemolytic titers of overnight culture supernatant for rabbit (Rb) and human (Hu) erythrocytes. ND, titer was below the limit of detection (sample undiluted); -, sample not tested. Immunoblots and rabbit titers are representative of a minimum of three independent experiments; human titers were performed once. Strains are: wild-type (SLO), a SLO-deficient mutant (SLO−), and mutants expressing: SLO lacking D4 (SLOΔD4), the SLO/ILY D4 chimera (SLO/ILYD4), the SLO/ILY D4 chimera with canonical undecapeptide sequence (SLO/ILYD4-ECW), and the SLO/PFO D4 chimera (SLO/PFOD4).
Figure 2. Domain 4 sequences of SLO and related CDCs.

ClustalW alignment of the Domain 4 amino acid sequences of SLO, PFO, and ILY. An asterisk, colon, or period below the alignment denotes identical, highly similar, and similar residues, respectively. Also shown is the undecapeptide sequence (dark bar above) and the atypical undecapeptide residues of ILY (dark bars below). Residues swapped between SLO D4 and PFO in various SLO/ PFOD4 chimeras are shown by the shaded (Swap 1 and 3) and dashed-line (Swap 2) boxes. Variant residues are shown by bars (Swap 1, Swap 3) and by filled circles (Swap 2). Boxes outlined by solid lines indicate the three hydrophobic loops (loops 1–3), with the critical Loop 1 threonine and leucine of the cholesterol recognition motif highlighted. The numbering of residues (shown at the right and left enclosed by parentheses) is based on the sequence of each respective protein (Genbank AAK33267.1, AAA23270.1, BAA89790.1; for SLO, PFO, ILY; respectively).
Membrane cholesterol reduction does not inhibit SLO binding or CMT
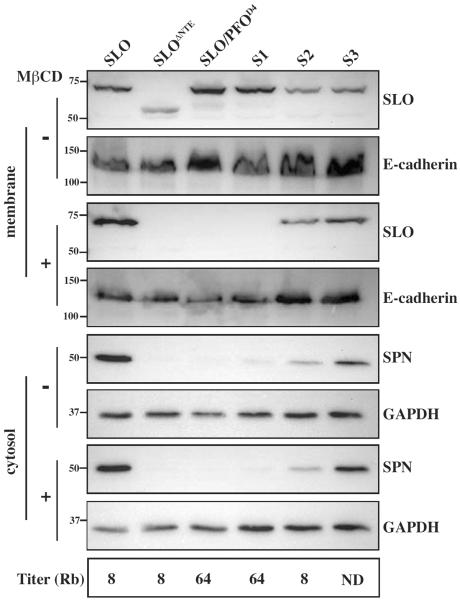
The only known receptor for SLO is cholesterol, yet PFO's membrane binding domain, which binds solely to cholesterol (Soltani et al., 2007b; Flanagan et al., 2009; Giddings et al., 2003; Heuck et al., 2000; Heuck et al., 2007), cannot substitute for SLO's Domain 4 to perform CMT. To evaluate the role for membrane cholesterol in SLO binding during an infection and its ability to conduct CMT, A549 cells were treated prior to infection with 5mM MβCD, a standard concentration used to remove cholesterol from A549 cells (Lee et al., 2009) and to study the role of cholesterol in CDC-membrane interactions (Johnson et al., 2012). Quantifying the level of cholesterol remaining after this treatment demonstrated that 5mM MβCD consistently removes ~60–70% of cholesterol from these cells, yet cells maintain full membrane integrity and viability (Figure S2). To determine if SLO binding and CMT efficiency correlate with the level of membrane cholesterol, untreated or MβCD-treated cells were infected with strains expressing wild-type SLO or SLO/PFOD4, and total host membranes and cytosolic fractions were harvested post-infection. As expected, SLO/PFOD4 did not bind to host membranes that had been treated with MβCD (Figure 3A). However, membrane binding of wild-type SLO was not significantly affected by cholesterol depletion (Figure 3A). Additionally, SPN was translocated to wild-type levels into MβCD-treated cells (Figure 3A), indicating that CMT is not sensitive to the amount of cholesterol available for binding by SLO.
Figure 3. SLO membrane binding and CMT are not sensitive to the level of membrane cholesterol.
(A) A549 cells were untreated (−) or treated (+) with 5mM MβCD to deplete membrane cholesterol for 30 minutes prior to infection with wild-type (SLO) or a strain expressing SLO/PFOD4. Following a 3h infection, total membranes and cytosol were harvested and subjected to immunoblot analyses as indicated, developed with antisera against the proteins specified to the right of the Figure. Analyses of E-cadherin (membrane) and GAPDH (cytosol) are included as loading controls and the migration of several molecular weight standards (in kDa) are shown at the left. Immunoblots shown are representative of at least three independent experiments. (B) Pore formation in A549 cells infected and treated as in (A) was quantitated by assessment of membrane permeability by staining with a fluorescent vital dye (Live/Dead™). Data are presented as the percentage of cells that were membrane-permeable following infection and represent the mean and the standard deviation derived from three independent experiments. Differences between mean values were tested for significance by the Tukey-Kramer multiple comparisons test. The asterisks indicate p≤0.0005.
To verify that MβCD treatment removed a sufficient amount of cholesterol from these cells to prohibit pore formation, membrane integrity of untreated cells or cells pretreated with MβCD was assessed post-infection with strains expressing wild-type SLO or SLO/PFOD4 using a membrane-impermeable fluorescent probe (Figure 3B). MβCD treatment prevented the cytolytic activity of both wild-type SLO and SLO/PFOD4 as demonstrated by the retention of membrane integrity. These data, corroborating the findings from experiments with SLO mutants deficient for pore formation (Magassa et al., 2010), indicate that pore formation is not necessary for SLO's CMT activity.
To further investigate a role for cholesterol in CMT, point mutations in Domain 4 of wild-type SLO and SLO/PFOD4 were introduced that have been shown to alter the concentration of cholesterol required for binding. A recent study generated a PFO derivative with aspartate 434 mutated to serine and found that this mutation decreased the concentration of cholesterol in model membranes required for binding by this purified PFO variant (Johnson et al., 2012). Since SLO's native residue at the equivalent position (508) is a serine, it was mutated to aspartate (SLOS508D, Table S1) to determine if this mutation would inhibit the ability of SLO to bind host membranes or conduct CMT. The reciprocal mutation was also made in SLO/PFOD4 (SLO/PFOD4 D508S, Table S1) to see if membrane binding under cholesterol-limiting conditions or CMT would be enhanced. However, analyzing membrane binding by these mutants to untreated or MβCD-treated cells revealed no difference in membrane binding from their parent strains, and the SLOS508D mutation did not affect the level of SPN that was translocated or confer CMT activity to SLO/PFOD4 (Figure S3). Together these data indicate that the CMT activity of SLO is not sensitive to the concentration of cholesterol in the host cell membrane.
An intact cholesterol recognition motif is not required for SLO membrane binding or CMT
Treating A549 cells with MβCD only removes up to 70% of cellular cholesterol. Therefore, it remains a possibility that cholesterol is required for SLO to perform CMT, and the level remaining after MβCD treatment is sufficient for this process. To further investigate a potential role for SLO-cholesterol binding in CMT, mutations were generated in the cholesterol-recognition motif (CRM), comprised of a threonine-leucine pair within loop 1 of the membrane-binding domain that is critical for cholesterol binding (Farrand et al., 2010) (Figure 2). Previous work has demonstrated that mutation of this leucine residue in PFO (PFOL491G) nearly abolishes binding of purified PFO to erythrocytes and decreases its hemolytic activity to 0.4%, indicating that this mutation results in nearly a complete loss of association with cholesterol (Soltani et al., 2007b). Similarly, it has been shown that mutation of both the threonine and leucine residues in SLO (SLOT564G L565G) inhibits binding of this purified variant to erythrocytes, cholesterol-containing liposomes, and purified cholesterol (Farrand et al., 2010). Single and/or double point mutations were generated in SLO (SLOL565G, SLOT564G L565G, Table S1) and the SLO/PFOD4 chimera (SLO/PFOD4 L565G, Table S1) to assess a requirement for cholesterol in binding and CMT. As expected, SLOL565G and SLOT564G L565G are completely non-lytic, and SLO/PFOD4 L565G has severely impaired activity, suggesting they cannot interact well with cholesterol to create the transmembrane pore (Figure 4). To determine if these mutations in SLO result in a loss of membrane binding and CMT activity, host cell membranes were harvested post-infection with strains expressing SLO, SLOL565G, SLOT564G L565G, SLO/PFOD4, and SLO/PFOD4 L565G, and membrane association was detected by immunoblotting. As expected from previous work (Soltani et al., 2007b), SLO/PFOD4 bearing this CRM mutation does not bind to host cell membranes (Figure 4). However, SLOL565G binds to the membrane to the same extent as wild-type SLO, and binding of SLOT564G L565G is impaired but not completely inhibited, confirming the results using MβCD that SLO binding to A549 cells during infection is not solely dependent on cholesterol. Additionally, the CMT activity of SLOL565G and SLOT564G L565G is identical to wild-type SLO (Figure 4). These data indicate that a direct interaction with cholesterol is not required for SLO membrane binding during infection or the translocation of SPN, and that the low level of membrane binding exhibited by SLOT564G L565G is sufficient for CMT activity.
Figure 4. A direct interaction with cholesterol is not required for SLO binding or CMT.
Immunoblot analyses of total membrane and cytosol prepared from A549 cells following 3 hrs of infection by the various strains indicated at the top of the Figure that were developed with antisera against the proteins indicated on the right. Analyses of E-cadherin (membrane) and GAPDH (cytosol) were included as loading controls and the migration of several molecular weight standards (in kDa) are indicated at the left. Shown at the bottom are hemolytic titers of overnight culture supernatant for rabbit (Rb) erythrocytes. ND, titer was below the limit of detection (sample undiluted). Immunoblots and titers are representative of at least three independent experiments.
SLO's N-terminal extension and residues in Domain 4 are required for cholesterol-insensitive binding and CMT
The sequences of SLO and PFO were compared to identify potential regions of SLO responsible for its cholesterol-insensitive mode of binding. The most obvious difference between these two CDCs is the N-terminal extension of SLO—the extra 66 amino acids at the amino terminus of SLO that have previously shown to be essential for the translocation of SPN, but not pore formation by SLO (Meehl & Caparon, 2004). Additionally, inspection of the Domain 4 sequences of SLO and PFO revealed that there are clusters of variable residues within this region between the second and third Domain 4 loops, and near the first loop, which contains the cholesterol recognition motif (Figure 2). To determine if these unique regions of SLO influence its cholesterol-insensitive mode of binding and CMT, we analyzed the membrane binding ability of SLO lacking its N-terminal extension (SLOΔNTE, Table S1) as well as three mutants generated by swapping clusters of PFO sequence in the SLO/PFOD4 chimera with the corresponding SLO residues (SLO/PFOD4-swap1 (S1), SLO/PFOD4-swap2 (S2), and SLO/PFOD4-swap3 (S3), Table S1). Immunoblot analysis of overnight culture supernatants demonstrated that the N-terminal deletion and all swap chimeras are produced and secreted like wild-type SLO and SLO/PFOD4 (Figure S1).
To evaluate a potential contribution of these regions to SLO's cholesterol-insensitive mode of binding, membranes from untreated or MβCD-treated cells were harvested post-infection with strains expressing SLO, SLOΔNTE, SLO/PFOD4, or the SLO/PFOD4 swap chimeras. SLOΔNTE and the chimeric swap proteins localized to the total membrane fraction post-infection in the absence of MβCD treatment, albeit to varying extents (Figure 5 and Table S3). However, treatment with MβCD revealed that these mutants exhibit varying levels of cholesterol dependency. SLOΔNTE and SLO/PFOD4-swap1 (S1) were completely unable to bind to host cells with low levels of cholesterol following MβCD treatment (Figure 5). Conversely, the chimeras encoding more native SLO residues between the second and third hydrophobic loops (SLO/PFOD4-swap2 (S2) and SLO/PFOD4-swap3 (S3)) exhibit binding to cell membranes treated with MβCD (Figure 5).
Figure 5. SLO's N-terminal extension and residues in Domain 4 are required for cholesterol-insensitive binding and CMT.
A549 cells were untreated (−) or treated (+) with 5mM MβCD to deplete membrane cholesterol for 30 minutes prior to infection with wild-type (SLO) and mutants expressing: SLO lacking its N-terminal extension (SLOΔNTE), SLO/PFOD4, SLO/PFOD4-swap1 (S1), SLO/PFOD4-swap2 (S2), and SLO/PFOD4-swap3 (S3). Following a 3h infection, total membranes and cytosol were harvested and subjected to immunoblot analyses as indicated, developed with the antisera against the proteins specified to the right of the Figure. Analyses of E-cadherin (membrane) and GAPDH (cytosol) were included as loading controls and the migration of several molecular weight standards (in kDa) are indicated at the left. Shown at the bottom hemolytic titers for rabbit erythrocytes (Rb). ND, titer is below the limit of detection (sample undiluted). Immunoblots and titers are representative of at least three independent experiments.
Analysis of host cell cytosolic fractions post-infection with the indicated strains confirms that SLOΔNTE lacks CMT activity and demonstrates that the cholesterol-insensitive binding of the SLO/PFOD4-swap 2 (S2) and SLO/PFOD4-swap3 (S3) mutants correlates with CMT activity. Similar to SLO− and SLO/PFOD4, SLO/PFOD4-swap1 (S1) was unable to translocate SPN. In contrast, the SLO residues introduced in the SLO/PFOD4-swap2 (S2) and SLO/PFOD4-swap3 (S3) chimeras confer CMT activity to these proteins (Figure 5). This activity correlates with the extent of membrane binding to cholesterol-depleted membranes, with the SLO/PFOD4-swap3 (S3) mutant exhibiting more robust CMT activity. Further supporting a pore-independent pathway for SPN uptake, cholesterol-insensitive binding and CMT activity exhibited by these chimeric swap proteins are inversely correlated with hemolytic activity. SLO/PFOD4-swap1 (S1) is the most hemolytic but is completely defective for SPN translocation, whereas SLO/PFOD4-swap3 (S3) has no pore forming activity but is competent for CMT. Together, these data demonstrate that SLO's cholesterol-insensitive mode of membrane binding and CMT activity require both its N-terminal extension as well as specific residues within the C-terminal membrane-binding Domain 4, and that cholesterol-insensitive membrane binding correlates with CMT competence.
SLO's N-terminal extension and residues in Domain 4 are required for SPN-membrane association
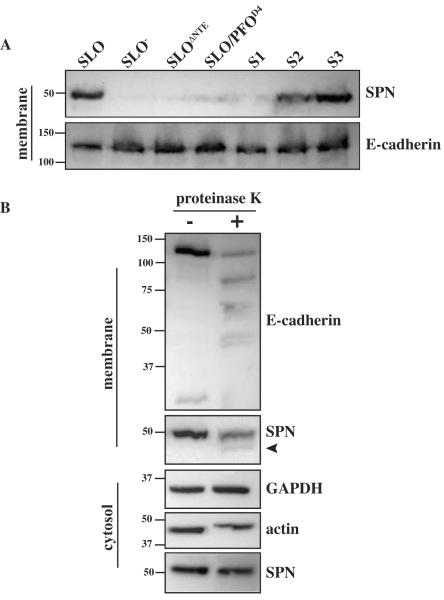
Since SLO membrane binding is necessary for the translocation of SPN into host cells, it seemed likely that SPN and SLO co-associate at the host cell surface prior to the translocation of SPN. To ascertain whether SPN localizes to the host cell membrane during infection, total membrane fractions were harvested following a 3h infection with wild-type bacteria and probed for the presence of SPN. Like SLO, SPN localized to the membrane fraction (Figure 6A). However, this localization is SLO-dependent, as SPN was not detected at host membranes post-infection with the SLO− strain (Figure 6A). Similarly, SPN did not localize to the host cell membrane during infection with strains expressing SLOΔNTE, SLO/PFOD4, or SLO/PFOD4-swap1 (S1)—all of which are versions of SLO that are defective for CMT and require wild-type cholesterol levels for membrane binding. In contrast, SPN was detected in the total membrane fraction post-infection with strains expressing the SLO/PFOD4-swap2 (S2) and SLO/PFOD4-swap3 (S3) chimeras, which exhibit intermediate levels of CMT activity and binding to cholesterol-depleted membranes (Figure 6A).
Figure 6. SLO's N-terminal extension and residues in Domain 4 are required for SPN-membrane localization.
(A) A549 total cell membranes were harvested following a 3h infection with wild-type (SLO), a mutant that does not express SLO (SLO−), and mutants expressing: SLOΔNTE, SLO/PFOD4, SLO/PFOD4-swap1 (S1), SLO/PFOD4-swap2 (S2), and SLO/PFOD4-swap3 (S3) and subjected to immunoblot analyses as indicated, developed with the antisera specified to the right of the Figure. Analysis of E-cadherin is included as a loading control and the migration of several molecular weight standards (in kDa) are indicated at the left. Immunoblots are representative of at least three independent experiments. (B) Cells were treated with 4 ug ml−1 proteinase K to cleave surface-exposed proteins following a 3h infection with wild-type bacteria. After incubation the protease was removed, and total cell membranes and cytosolic fractions were harvested and subjected to immunoblot analyses as indicated, developed with the antisera specified to the right of the Figure. The migration of several molecular weight standards (in kDa) are indicated at the left. Immunoblots are representative of at least three independent experiments.
The total membrane fraction is comprised of both plasma membrane and intracellular vesicles, so SPN's localization was further assessed by a protease protection assay conducted on intact infected cells just prior to preparation of the total membrane fraction. Following infection, cells were treated with proteinase K, which was then removed and cells lysed and fractionated to obtain the total membrane fraction. Immunoblot analysis was then used to determine if membrane-associated SPN was protected by a membrane-bound vesicle or was susceptible to protease cleavage due to localization at the cell surface. As expected, cytosolic (translocated) SPN, actin, and GAPDH were protected from degradation (cytosol, Figure 6B), while the exposed membrane protein E-cadherin was efficiently degraded by proteinase K, as demonstrated by the decrease in the amount of full-length protein and the appearance of multiple degradation products (membrane, Figure 6B). Similarly, the amount of SPN in the total membrane fraction was decreased and a prominent degradation product was observed following proteinase K treatment (arrow, Figure 6B). This result indicates that SPN in the total membrane fraction is exposed at the plasma membrane, although it cannot be ruled out that it subsequently enters into an endocytic vesicle prior to its entry into the cytosol. Together, these data demonstrate that SPN interacts with the host cell membrane, but only when it is co-secreted with CMT-competent SLO that is capable of cholesterol-insensitive membrane binding.
SLO's cholesterol-insensitive mode of membrane binding requires SPN
SPN was not detected at cell membranes when secreted with mutants of SLO that are restricted to cholesterol-dependent membrane binding, leading to the hypothesis that a co-dependent interaction between SPN and SLO at the host cell surface is necessary for SLO's cholesterol-insensitive mode of binding. To evaluate a role for SPN in SLO's cholesterol-insensitive mode of membrane binding, untreated or MβCD-treated cells were infected with wild-type bacteria expressing both SPN and SLO, or with a SPN deletion strain (Madden et al., 2001) (Table S1). Total host membranes were harvested post-infection, and membrane-localized SLO was detected by immunoblotting. As demonstrated in Figure 3, SLO is able to bind to cholesterol-depleted host cell membranes when expressed from the wild-type strain that also produces SPN. However, SLO loses the ability to bind cholesterol-depleted host cell membranes in the absence of SPN (Figure 7). These data demonstrate that SPN is essential for SLO's atypical cholesterol-insensitive interaction with the host membrane that occurs during infection.
Figure 7. SPN is required for the cholesterol-insensitive binding of SLO.

A549 cells were untreated (−) or treated (+) with 5mM MβCD to deplete membrane cholesterol for 30 minutes prior to infection with wild-type (WT) or mutant lacking SPN (SPN−). Following a 3h infection, total membranes were harvested and subjected to immunoblot analyses as indicated, developed with the antisera against the proteins specified to the right of the Figure. Analysis of E-cadherin is included as a loading control and the migration of several molecular weight standards (in kDa) are indicated at the left. Immunoblots are representative of at least three independent experiments.
DISCUSSION
The data presented in this study contribute to an emerging literature demonstrating that the CMT activity of SLO is dependent on a unique interaction with the host cell membrane, one that extends beyond the characteristic cholesterol-dependent cytolytic properties of the CDC family. This study has revealed a novel second mode of membrane binding for SLO that is insensitive to cholesterol but dependent on the co-expression of SPN. Furthermore, the ability of SPN to bind the host membrane is dependent on SLO, and co-dependent binding is associated with competence for CMT. Thus, despite being an archetypal CDC, SLO is uniquely adapted to perform CMT.
As an archetypical CDC, SLO shares the signature characteristics of this large and widely distributed family of bacterial toxins, identified in seven different genera of Gram-positive bacteria and two species of Gram-negative bacteria (Hotze et al., 2013; Hotze & Tweten, 2012). These toxins share a similar domain architecture, cholesterol-recognition motif, and a conserved cholesterol-dependent mechanism for triggering pore formation on host cells (Hotze & Tweten, 2012). However, SLO's adaptation for CMT has involved several gain-of-function adaptations that are neither required for pore formation nor inhibit its ability to form a pore. Analysis of these multiple adaptations has shown that they are related by their ability to promote the cholesterol-insensitive mode of binding, which itself is directly associated with competence for CMT. In prior work, it had been shown that SLO possesses a unique N-terminal extension that is required for CMT, but dispensable for classical cholesterol-dependent membrane binding (Meehl & Caparon, 2004). However, the function of this N-terminal extension in CMT was not established. In this study, it is revealed to be crucial for promoting cholesterol-insensitive membrane binding. Furthermore, the N-terminal extension was required for the ability of SPN to associate with the membrane, which was also important for SLO's cholesterol-insensitive membrane binding. Competence for CMT also required adaptation of residues in SLO's Domain 4, and these were shown to promote cholesterol-insensitive binding, thus altering SLO's membrane binding characteristics relative to other CDCs. Remarkably, none of these adaptations interfere with SLO's canonical CDC pore-forming function, revealing SLO to be a bifunctional toxin.
The dual nature of SLO was most dramatically illustrated by the analysis of various CDC chimeric proteins. In contrast to SLO, which possesses both cytolytic and CMT activities, most chimeric proteins were only able to accomplish a single function. Among the various SLO/PFO chimeras, SLO/PFOD4 and SLO/PFOD4-swap1 exhibited potent hemolytic activity but no CMT activity, whereas the SLO/PFOD4-swap3 chimera regained CMT activity but lost hemolytic activity. Similarly, mutation of SLO's cholesterol-recognition motif abolished pore formation and impaired membrane binding but had no effect on the level of SPN translocated. Since CMT does require Domain 4, these data highlight its important role in both pore formation and CMT, but reveal that each of these processes involves a unique interaction with the host cell membrane. For pore formation, receptor engagement mediated by Domain 4 initiates a complex series of significant structural changes between multiple domains (reviewed in (Tweten, 2005)). For example, following the initial binding event mediated by the cholesterol-recognition motif, the short hydrophobic loops 2 and 3 insert into the membrane to anchor the monomer in a perpendicular orientation for the subsequent insertion of the undecapeptide. This event is then conformationally coupled to structural changes in Domain 3 that promote oligomerization and pore formation (Dowd & Tweten, 2012). The failure of various chimeras to preserve both CMT and pore-forming activities suggest that SLO's adaptation for CMT required additional modifications in Domain 4 to maintain conformational coupling. Based on the location of the swapped residues, the failure of SLO/PFOD4-swap3 to function in pore formation may have resulted from an incompatibility between the swapped region and residues within or near the short hydrophobic loops 2 and 3. Taken together, the observation that CMT and pore-forming activities can be separated among the various chimeras supports the idea that wild-type SLO has evolved to participate in two distinct modes of membrane binding: the conventional recognition of cholesterol leading to pore formation, and a unique alternative interaction that promotes CMT. In addition, the fact that SLO's adaptation for CMT was accompanied by extensive alterations to maintain pore-forming activity indicates that both activities are critical for pathogenesis.
Since SLO's cholesterol-insensitive mode of binding also involves the membrane association of SPN, it seems likely that these two proteins co-associate at the host cell surface to form a complex capable of recognizing an alternative receptor(s). While numerous models of interaction are possible, the behavior of the various chimeras suggests that SLO may make an initial low-affinity contact with the host cell membrane analogous to the non-specific binding of the Staphylococcus aureus alpha toxin that is observed at high concentrations (Hildebrand et al., 1991). In the absence of SPN, SLO would be free to engage cholesterol by the canonical mechanism. However, in the presence of SPN, Domain 4 may undergo a different structural rearrangement that promotes a more stable interaction with the alternative receptor. This model of alternative conformational changes would explain why various chimeras were proficient at either CMT or pore formation, but not both, depending on which sub-domain of SLO's Domain 4 they possess. It would also explain why SLO does not sustain binding to cholesterol-depleted membranes in the absence of SPN, why SLO does not require cholesterol to bind to membranes in the presence of SPN, and why non-membrane associated SLO and SPN do not interact. It would also predict that SLO's CMT-adapted Domain 4 residues conformationally couple SPN binding and recognition of the alternative receptor.
While SLO is capable of both pore-forming and CMT activities, it is unlikely that an individual molecule can engage in both activities simultaneously. Thus, events that occur immediately following the initial binding event will be critical for driving SLO into one of these pathways. Since it seems likely that the default pathway will be the recognition of cholesterol leading to pore formation, a key node in directing the fate of SLO will be the status of SPN. One model would predict that CMT is dependent on the efficient co-delivery of SPN to SLO prior to its recognition of cholesterol in order to initiate the events that result in CMT. In support of this model, it has been shown that CMT requires delivery of both toxins from the same bacterial cell, as a co-infection with SLO− and SPN− mutants does not result in translocation of SPN (Madden et al., 2001). There is also considerable evidence that S. pyogenes has evolved multiple mechanisms to optimize the co-delivery of SLO and SPN to the membrane. First, expression of SLO and SPN is under tight co-ordinate control, as the genes that encode both these proteins are organized in an operon under the control of a single promoter (Kimoto et al., 2005). Second, unusual for non-membrane secretory proteins in bacteria, secretion of both SPN and SLO occurs via the signal recognition particle (SRP) arm of the general secretory (Sec) pathway (Rosch et al., 2008), which tightly couples transcription and translation with secretion (van Wely et al., 2001). Finally, secretion of extracellular toxins in S. pyogenes proceeds through the ExPortal, a dedicated microdomain of the bacterial cellular membrane that is dedicated to protein secretion by clustering the Sec translocons and accessory biogenesis factors into a single highly organized membrane microdomain (Rosch & Caparon, 2005). Together, these indicate that the transcription, translation, secretion, and post-secretion targeting of SPN and SLO are highly coordinated. This level of organization provides additional support to the idea that S. pyogenes is highly adapted to perform CMT.
Also consistent with high level of adaptation required for CMT is that it has not been possible to reconstitute CMT using purified components in vitro. This could reflect a strict requirement for temporal and spatial coordination of toxin delivery to the cell surface. Alternatively, CMT may require additional post-secretion processing of SLO and/or SPN, or involve bacterial components that have not yet been identified. In this regard, it has been noted that SLO can be isolated from the bacterial cell wall (Bensi et al., 2012), and we have found that SPN also may bind to the streptococcal cell wall (unpublished), raising the possibility that interaction at the cell wall is a necessary precursor to reorientation for interaction at the host cell membrane. Similar to SLO, SPN itself is highly adapted for CMT and possesses a dedicated N-terminal domain that is dispensable for its enzymatic NADase activity but required for its translocation (Ghosh & Caparon, 2006). Structural modeling predicts that this domain resembles a carbohydrate-binding domain (Kelley & Sternberg, 2009), not unlike the N-terminal lectin domain of the CDC lectinolysin, suggesting that it may directly participate in recognition of a cell surface receptor. SPN's association with the membrane is presumably transient, as it is rapidly introduced into the host cell cytosol by an unknown mechanism while SLO remains associated with the cell membrane. In examining the accessibility of SPN at the membrane to protease treatment, the degradation of SPN was not as extensive as cleavage of E-cadherin, suggesting that SPN in this total membrane fraction may represent two populations, plasma membrane-bound, surface-exposed SPN that is susceptible to proteinase K, and SPN that is protected within an intracellular vesicle. This suggests that following its association with the cell membrane, SPN may be internalized in a membrane-bound vesicle subsequent to its release into the cytosol.
Recognition of an alternative receptor by a CDC is not without precedent. ILY was the first CDC characterized that makes initial contact with the host membrane through a non-cholesterol receptor by recognizing the human-specific protein CD59 (Giddings et al., 2004). Another CD59-binding CDC, lectinolysin (LLY), also exhibits a cholesterol-independent mode of binding by recognizing difucosylated Lewis b and Lewis y antigens through its unique lectin domain, which similar to SLO's N-terminal extension is located distal to Domain 4 (Farrand et al., 2008; Feil et al., 2012). Glycan recognition by LLY has been shown to augment the hemolytic activity of this protein, likely by clustering LLY monomers to enhance oligomerization and subsequent pore formation (Farrand et al., 2008). However, in contrast to the glycan-binding function of LLY that supports lytic activity, recognition of a second receptor by SLO for CMT appears to be a separate event from cholesterol binding and pore formation.
In the present study, we have shown that SLO is an example of a growing number of toxins that bind to multiple receptors, which may allow these toxins to target different cell types, maintain activity under varied conditions, or exert disparate effects on the target cell. Among these are the potent vacuolating cytotoxin VacA of Helicobacter pylori, which engages different receptors to elicit varied cellular responses (Czajkowsky et al., 1999; Fujikawa et al., 2003; Seto et al., 1998; Utt et al., 2001; Yahiro et al., 2003). Additionally, anthrax toxin (Young & Collier, 2007), diphtheria toxin (Iwamoto et al., 1994), and botulinum neurotoxin B (Dong et al., 2003; Kitamura et al., 2005) require the simultaneous engagement of co-receptors for binding and/or internalization. It remains to be determined whether similar mechanisms are responsible for the interactions between SLO, SPN and the host cell membrane. However, future studies into the details of membrane binding and identification of the alternative membrane receptor will provide important insight into the mechanism of CMT.
EXPERIMENTAL PROCEDURES
Bacterial Strains
Escherichia coli TOP10 cultured in Luria-Bertani broth at 37°C was used for molecular cloning experiments. The S. pyogenes strain used was JRS4 (M serotype 6) (Scott et al., 1986). Todd-Hewitt broth supplemented with 0.2% autolysed yeast extract was used for the routine culture of S. pyogenes. Where appropriate, erythromycin was added to media to final concentrations of 750 μg ml−1 for E. coli and 1 μg ml−1 for S. pyogenes.
Manipulation and computational analyses of DNA
Plasmid DNA was isolated and used to transform E. coli using standard techniques. Electroporation was used to transform S. pyogenes as previously described (Caparon & Scott, 1989). Restriction endonucleases, ligases, and polymerases were used according to the manufacturers' recommendations. The alignment of Domain 4 sequences was generated using ClustalW.
Construction of SLO mutants
The nucleotide coding sequence of the SLO/PFOD4 (SLO residues 1-463, PFO residues 390–500) and SLO/ILYD4 (SLO residues 1-463, ILY residues 417–532) mutants were created by custom gene synthesis in the pUC57 vector (Genscript; Piscataway, NJ, USA). The nucleotide sequences were then removed from pUC57 and inserted into the pJRS233 temperature-sensitive shuttle vector containing an erythromycin resistance cassette using the restriction endonucleases XhoI and ClaI (Perez-Casal et al., 1993). Inverse PCR was used to generate the SLOΔD4 and SLO/ILYD4-ECW mutants from the SLO/ILYD4 nucleotide sequence, the SLO/PFOD4-swap1 and SLO/PFOD4-swap2 mutants from the SLO/PFOD4 nucleotide sequence, and the SLOL565G mutant from the wild-type SLO nucleotide sequence present in pJRS233. The SLO/PFOD4 L565G and SLO/PFOD4 D508S mutants were made from the SLO/PFOD4 sequence in pJRS233 and the SLOS508D and SLOT564G L565G mutants were made from the wild-type SLO sequence in pJRS233 using the Quikchange XL II mutagenesis kit (Agilent Technologies). Sequence overlap extension mutagenesis (Horton, 1997) was used to make the SLO/PFOD4-swap3 mutant from the SLO/PFOD4 nucleotide sequence in pJRS233. The product of the second round of PCR was cleaved with XhoI and ClaI and inserted into pJRS233. For the strain expressing SLOΔNTE, the sequence from (Meehl & Caparon, 2004) was cloned into pJRS233 at the XhoI and ClaI sites. The JRS4 (WT) wild-type slo allele was replaced with the mutants as previously described (Ji et al., 1996). All primer sequences are listed in Table S2 with restriction endonuclease sites underlined. The fidelity of all DNA sequences generated by PCR was validated by DNA sequencing analyses performed by a commercial vendor (Genewiz; South Plainfield, NJ, USA).
Analysis of hemolytic activity
Cell-free overnight S. pyogenes culture supernatants were used to measure the ability of various SLO mutants to lyse rabbit defibrinated erythrocytes (Hemostat Laboratories) or human red blood cells obtained from a healthy human volunteer (Madden et al., 2001). Hemolytic titer is defined as the reciprocal of the dilution that produced 50% lysis (Madden et al., 2001; Meehl & Caparon, 2004). If no detectable lysis was observed in the presence of undiluted supernatant, it was concluded that the hemolytic titer was below the limit of detection and was reported as “not detected” (ND). Data presented are representative of at least three independent experiments, with the exception of hemolysis experiments using human red blood cells, which were performed once.
Analysis of CMT
A549 cells (ATCC CCL-185) were grown in 75 cm2 tissue culture flasks in the presence of Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 50 mM HEPES, 8 mM L-glutamine, and 10% fetal bovine serum. Confluent cells were infected with various streptococcal strains as mentioned in the text. Streptococcal cultures were grown overnight in ThyB, back-diluted in fresh ThyB the morning of the infection to achieve an OD600 of 0.08 in 30mL media, and were allowed to double twice before washing the cells with PBS and resuspending the pellet in medium to an OD600 of 0.3. The A549 cells were incubated with 1mL of the resuspended streptococcal strains plus 14mLs of fresh medium for 3 hours at 37°C in the presence of 5% CO2. Total membranes and cytosolic fractions were harvested (see below), and the translocation of SPN into the host cytosol was detected by immunoblotting using commercial anti-SPN (Cosmo Bio Co.). GAPDH antibody (Biovision) and actin AC-40 (Sigma) were used as loading controls for the cytosolic fraction. Images shown are representative of at least three independent experiments.
A549 cell membrane extraction and cytosolic fractionation
Total membrane fractions were harvested as described previously, with some modifications (Trejo et al., 2010). Briefly, the cells were washed twice with cold PBS, scraped into 1.4mL homogenization buffer (10mM Tris-HCl pH 7.5, 5mM EDTA, protease inhibitors (Roche complete mini)), and lysed by 30 passages through a 22-gauge needle. The homogenates were spun to remove unlysed cells and cellular debris (600 × g, 15 minutes, 4°C) and the resulting supernatants were spun to pellet total membranes (100,000 × g, 1h, 4°C). Pellets were resuspended in 1X SDS sample buffer and analyzed by immunoblotting with anti-SLO antiserum (generated by Sigma-Genosys), and the supernatant was saved for analysis of CMT using anti-SPN as described above. E-cadherin antibody (Cell Signaling Technology) was used as a loading control for the total membrane fraction. Where indicated, 5mM methyl-β-cyclodextrin (MβCD, Sigma) was added to the media 30 minutes prior to infection and remained in the media over the course of the infection. Immunoblots were developed by chemiluminescence (SuperSignal® West Dura Extended Duration Substrate, Thermo Scientific) and images captured using a CCD camera-based system (ChemiDoc™, BioRad). Protein levels were quantitated by densitometry of captured images using Quantity One analysis software (version 4.6.9, BioRad) and data presented represent the mean and standard deviation derived from at least 3 independent experiments, presented in Table S3. For each experiment, a representative image is presented in the text, prepared for publication using Adobe Illustrator CS6.
Proteinase K protection assay
Following a 3h infection of A549 cells in T75 flasks as described above, the media was removed and cells were washed once with 5mL DMEM and incubated in either 5mL DMEM alone or 5mL DMEM with a final concentration of 4ug ml−1 proteinase K (Sigma). Cells were incubated at room temperature for 10 minutes. Following incubation, the media was removed and cells were gently washed twice with 5mL PBS before harvesting total membrane and cytosolic fractions as described above.
Assessing A549 cell membrane integrity
Following infection A549 cell membrane integrity was assessed by visualizing the extent of exclusion of the fluorescent membrane-impermeable dye EthD-1, a component of the LIVE/DEAD viability/cytotoxicity kit for mammalian cells (Molecular Probes). The cells were incubated with the staining solution for 15 minutes at 37°C prior to visualization by fluorescence microscopy.
Measuring membrane cholesterol concentration
A549 cells grown to near confluency in 75cm2 flasks were left untreated or treated with 5mM MβCD for 3.5h at 37°C. Following this incubation cells were washed with PBS, resuspended in 1.4mL homogenization buffer (10mM Tris-HCl pH 7.5, 5mM EDTA, protease inhibitors (Roche complete mini)), and spun at 500 × g for 10 minutes at 4°C. The pellet was resuspended in 1X reaction buffer from the Amplex Red cholesterol assay kit (Molecular Probes), and the level of membrane cholesterol was quantified by comparing samples to a cholesterol standard curve according to the manufacturer's instructions.
Statistical Analysis
Differences in mean values of the effect of MβCD treatment on pore formation and cell permeability were tested for significance by the Tukey-Kramer multiple comparisons test. The null hypothesis was rejected for p values less than 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Rod Tweten, Eileen Hotze, and Joe Vogel for their interest and many helpful discussions. This study was supported by Public Health Service Grant AI064721 from the National Institutes of Health and by an award from the American Heart Association awarded to C.C.M.
REFERENCES
- Bensi G, Mora M, Tuscano G, Biagini M, Chiarot E, Bombaci M, et al. Multi high-throughput approach for highly selective identification of vaccine candidates: the Group A Streptococcus case. Molecular & cellular proteomics : MCP. 2012;11:M111 015693. doi: 10.1074/mcp.M111.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau RW, Malito E, Chenal A, Bishop BL, Musch MW, Villereal ML, et al. Cellular functions and X-ray structure of anthrolysin O, a cholesterol-dependent cytolysin secreted by Bacillus anthracis. The Journal of biological chemistry. 2009;284:14645–14656. doi: 10.1074/jbc.M807631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker AL, Carey VJ, Wessels MR. Role of NADase in virulence in experimental invasive group A streptococcal infection. Infection and immunity. 2005;73:6562–6566. doi: 10.1128/IAI.73.10.6562-6566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker AL, Cywes C, Ashbaugh CD, Wessels MR. NAD+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Molecular microbiology. 2002;44:257–269. doi: 10.1046/j.1365-2958.2002.02876.x. [DOI] [PubMed] [Google Scholar]
- Caparon MG, Scott JR. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell. 1989;59:1027–1034. doi: 10.1016/0092-8674(89)90759-9. [DOI] [PubMed] [Google Scholar]
- Collier RJ. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA, Chapman ER. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. The Journal of cell biology. 2003;162:1293–1303. doi: 10.1083/jcb.200305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KJ, Tweten RK. The cholesterol-dependent cytolysin signature motif: a critical element in the allosteric pathway that couples membrane binding to pore assembly. PLoS Pathog. 2012;8:e1002787. doi: 10.1371/journal.ppat.1002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc Natl Acad Sci U S A. 2010;107:4341–4346. doi: 10.1073/pnas.0911581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S, Hotze E, Friese P, Hollingshead SK, Smith DF, Cummings RD, et al. Characterization of a streptococcal cholesterol-dependent cytolysin with a lewis y and b specific lectin domain. Biochemistry. 2008;47:7097–7107. doi: 10.1021/bi8005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil SC, Lawrence S, Mulhern TD, Holien JK, Hotze EM, Farrand S, et al. Structure of the lectin regulatory domain of the cholesterol-dependent cytolysin lectinolysin reveals the basis for its lewis antigen specificity. Structure. 2012;20:248–258. doi: 10.1016/j.str.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JJ, Tweten RK, Johnson AE, Heuck AP. Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry. 2009;48:3977–3987. doi: 10.1021/bi9002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber SE, Aguilar JL, Lewis KL, Ratner AJ. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol. 2008;190:3896–3903. doi: 10.1128/JB.01965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J, Anderson PJ, Chandrasekaran S, Caparon MG. Characterization of Streptococcus pyogenes beta-NAD+ glycohydrolase: re-evaluation of enzymatic properties associated with pathogenesis. The Journal of biological chemistry. 2010;285:5683–5694. doi: 10.1074/jbc.M109.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J, Caparon MG. Specificity of Streptococcus pyogenes NAD(+) glycohydrolase in cytolysin-mediated translocation. Molecular microbiology. 2006;62:1203–1214. doi: 10.1111/j.1365-2958.2006.05430.x. [DOI] [PubMed] [Google Scholar]
- Giddings KS, Johnson AE, Tweten RK. Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins. Proc Natl Acad Sci U S A. 2003;100:11315–11320. doi: 10.1073/pnas.2033520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings KS, Zhao J, Sims PJ, Tweten RK. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat Struct Mol Biol. 2004;11:1173–1178. doi: 10.1038/nsmb862. [DOI] [PubMed] [Google Scholar]
- Gilbert RJ, Jimenez JL, Chen S, Tickle IJ, Rossjohn J, Parker M, et al. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell. 1999;97:647–655. doi: 10.1016/s0092-8674(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Heuck AP, Hotze EM, Tweten RK, Johnson AE. Mechanism of membrane insertion of a multimeric beta-barrel protein: perfringolysin O creates a pore using ordered and coupled conformational changes. Mol Cell. 2000;6:1233–1242. doi: 10.1016/s1097-2765(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Heuck AP, Moe PC, Johnson BB. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Subcell Biochem. 2010;51:551–577. doi: 10.1007/978-90-481-8622-8_20. [DOI] [PubMed] [Google Scholar]
- Heuck AP, Savva CG, Holzenburg A, Johnson AE. Conformational changes that effect oligomerization and initiate pore formation are triggered throughout perfringolysin O upon binding to cholesterol. The Journal of biological chemistry. 2007;282:22629–22637. doi: 10.1074/jbc.M703207200. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Pohl M, Bhakdi S. Staphylococcus aureus alpha-toxin. Dual mechanism of binding to target cells. The Journal of biological chemistry. 1991;266:17195–17200. [PubMed] [Google Scholar]
- Horton RM. In vitro recombination and mutagenesis of DNA. SOEing together tailor-made genes. Methods Mol Biol. 1997;67:141–149. doi: 10.1385/0-89603-483-6:141. [DOI] [PubMed] [Google Scholar]
- Hotze EM, Le HM, Sieber JR, Bruxvoort C, McInerney MJ, Tweten RK. Identification and characterization of the first cholesterol-dependent cytolysins from Gram-negative bacteria. Infection and immunity. 2013;81:216–225. doi: 10.1128/IAI.00927-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotze EM, Tweten RK. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochimica et biophysica acta. 2012;1818:1028–1038. doi: 10.1016/j.bbamem.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto R, Higashiyama S, Mitamura T, Taniguchi N, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994;13:2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izore T, Job V, Dessen A. Biogenesis, regulation, and targeting of the type III secretion system. Structure. 2011;19:603–612. doi: 10.1016/j.str.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Ji Y, McLandsborough L, Kondagunta A, Cleary PP. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infection and immunity. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BB, Moe PC, Wang D, Rossi K, Trigatti BL, Heuck AP. Modifications in Perfringolysin O domain 4 alter the cholesterol concentration threshold required for binding. Biochemistry. 2012;51:3373–3382. doi: 10.1021/bi3003132. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nature protocols. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Fujii Y, Yokota Y, Taketo A. Molecular characterization of NADase-streptolysin O operon of hemolytic streptococci. Biochimica et biophysica acta. 2005;1681:134–149. doi: 10.1016/j.bbaexp.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Igimi S, Furukawa K, Furukawa K. Different response of the knockout mice lacking b-series gangliosides against botulinum and tetanus toxins. Biochimica et biophysica acta. 2005;1741:1–3. doi: 10.1016/j.bbadis.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Lee SH, Koo KH, Park JW, Kim HJ, Ye SK, Park JB, et al. HIF-1 is induced via EGFR activation and mediates resistance to anoikis-like cell death under lipid rafts/caveolae-disrupting stress. Carcinogenesis. 2009;30:1997–2004. doi: 10.1093/carcin/bgp233. [DOI] [PubMed] [Google Scholar]
- Madden JC, Ruiz N, Caparon M. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell. 2001;104:143–152. doi: 10.1016/s0092-8674(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Magassa N, Chandrasekaran S, Caparon MG. Streptococcus pyogenes cytolysin-mediated translocation does not require pore formation by streptolysin O. EMBO Rep. 2010;11:400–405. doi: 10.1038/embor.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl MA, Caparon MG. Specificity of streptolysin O in cytolysin-mediated translocation. Molecular microbiology. 2004;52:1665–1676. doi: 10.1111/j.1365-2958.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- Nagamune H, Ohkura K, Sukeno A, Cowan G, Mitchell TJ, Ito W, et al. The human-specific action of intermedilysin, a homolog of streptolysin O, is dictated by domain 4 of the protein. Microbiol Immunol. 2004;48:677–692. doi: 10.1111/j.1348-0421.2004.tb03479.x. [DOI] [PubMed] [Google Scholar]
- Perez-Casal J, Price JA, Maguin E, Scott JR. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Molecular microbiology. 1993;8:809–819. doi: 10.1111/j.1365-2958.1993.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Giddings KS, Tweten RK, Parker MW. Insights into the action of the superfamily of cholesterol-dependent cytolysins from studies of intermedilysin. Proc Natl Acad Sci U S A. 2005;102:600–605. doi: 10.1073/pnas.0403229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Heuck AP, Tweten RK, Johnson AE. Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin. Nat Struct Biol. 2002;9:823–827. doi: 10.1038/nsb855. [DOI] [PubMed] [Google Scholar]
- Rosch JW, Caparon MG. The ExPortal: an organelle dedicated to the biogenesis of secreted proteins in Streptococcus pyogenes. Molecular microbiology. 2005;58:959–968. doi: 10.1111/j.1365-2958.2005.04887.x. [DOI] [PubMed] [Google Scholar]
- Rosch JW, Vega LA, Beyer JM, Lin A, Caparon MG. The signal recognition particle pathway is required for virulence in Streptococcus pyogenes. Infection and immunity. 2008;76:2612–2619. doi: 10.1128/IAI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89:685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Ruiz N, Wang B, Pentland A, Caparon M. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Molecular microbiology. 1998;27:337–346. doi: 10.1046/j.1365-2958.1998.00681.x. [DOI] [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Delivery into cells: lessons learned from plant and bacterial toxins. Gene Ther. 2005;12:865–872. doi: 10.1038/sj.gt.3302525. [DOI] [PubMed] [Google Scholar]
- Scott JR, Guenthner PC, Malone LM, Fischetti VA. Conversion of an M− group A streptococcus to M+ by transfer of a plasmid containing an M6 gene. J Exp Med. 1986;164:1641–1651. doi: 10.1084/jem.164.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani CE, Hotze EM, Johnson AE, Tweten RK. Specific protein-membrane contacts are required for prepore and pore assembly by a cholesterol-dependent cytolysin. The Journal of biological chemistry. 2007a;282:15709–15716. doi: 10.1074/jbc.M701173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani CE, Hotze EM, Johnson AE, Tweten RK. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc Natl Acad Sci U S A. 2007b;104:20226–20231. doi: 10.1073/pnas.0708104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo HE, Lecuona E, Grillo D, Szleifer I, Nekrasova OE, Gelfand VI, Sznajder JI. Role of kinesin light chain-2 of kinesin-1 in the traffic of Na,K-ATPase-containing vesicles in alveolar epithelial cells. FASEB J. 2010;24:374–382. doi: 10.1096/fj.09-137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infection and immunity. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wely KH, Swaving J, Freudl R, Driessen AJ. Translocation of proteins across the cell envelope of Gram-positive bacteria. FEMS microbiology reviews. 2001;25:437–454. doi: 10.1111/j.1574-6976.2001.tb00586.x. [DOI] [PubMed] [Google Scholar]
- Weis S, Palmer M. Streptolysin O: the C-terminal, tryptophan-rich domain carries functional sites for both membrane binding and self-interaction but not for stable oligomerization. Biochimica et biophysica acta. 2001;1510:292–299. doi: 10.1016/s0005-2736(00)00360-6. [DOI] [PubMed] [Google Scholar]
- Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.