Abstract
Primary cultures from embryonic mouse ventral mesencephalon are widely used for investigating the mechanisms of dopaminergic neuronal death in Parkinson's disease models. Specifically, single mouse or embryo cultures from littermates can be very useful for comparative studies involving transgenic mice when the neuron cultures are to be prepared before genotyping. However, preparing single mouse embryo culture is technically challenging because of the small number of cells present in the mesencephalon of each embryo (150,000-300,000), of which only 0.5-5% are tyrosine hydroxylase (TH) -positive, dopaminergic neurons. In this study, we optimized the procedure for preparing primary mesencephalic neuron cultures from individual mouse embryos. Mesencephalic neurons that are dissociated delicately, plated on Aclar film coverslips, and incubated in DMEM supplemented with FBS for 5 days and then N2 supplement for 1 day resulted in the best survival of dopaminergic neurons from each embryo. Using this optimized method, we prepared mesencephalic neuron cultures from single Ndufs4+/+ or Ndufs4-/- embryos, and investigated the role of mitochondrial complex I in maneb-induced dopamine neuron death. Our results suggest that maneb toxicity to dopamine neurons is not affected by loss of mitochondrial complex I activity in Ndufs4-/- cultures.
Keywords: Maneb, Parkinson's disease, Mitochondria complex I, Dopaminergic neuron, Mesencephalon, Knockout mice
Introduction
Parkinson's disease is a neurodegenerative disorder that is characterized by selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the brain [1]. Although the mechanisms underlying selective dopaminergic neuronal death are not well defined, inhibition of mitochondrial complex I activity has been a leading theory [2] [3]. Recently, exposure to pesticides has been implicated in Parkinson's disease etiology, and paraquat and maneb are often used in combination to model Parkinson's disease in mice [4] [5]. Chronic administration of paraquat and maneb to rodents in vivo induces many key features of Parkinson's disease, including motor deficits, loss of dopaminergic neurons, and increased expression of α-synuclein [6-8] [9]. The role of inhibition of mitochondrial complex I activity in paraquat-induced dopaminergic neuronal death has been investigated, and a complex I-independent mechanism has been revealed [10-19]. However, the role of mitochondrial complex I inhibition in maneb-induced dopaminergic neuronal degeneration has not been examined.
Primary cultured mesencephalic dopaminergic neurons have been utilized to study the differentiation, physiology, and degeneration/regeneration of dopaminergic neurons. The standard protocol for culturing primary mesencephalic neurons consists of pooling dissected ventral mesencephalic tissue from multiple E13-E14 embryos, dissociating the tissue, and seeding cells on culture medium. However, primary mesencephalic cultures from mating homozygotes are problematic for many knockout animals, because the homozygotes may not survive to reproductive age or may be infertile [20]. Alternatively, primary neurons could be cultured from each embryo generated from mating heterozygotes. Currently, only a few studies have compared primary dopaminergic neurons cultured from single littermate embryos produced by heterozygote matings, due to the low survival rate of dopaminergic neurons cultured from single embryonic mesencephalon.
In this study, we describe an optimized protocol to culture mesencephalic dopaminergic neurons isolated from individual embryos. We applied this protocol to investigate the role of mitochondrial complex I activity in maneb-induced dopaminergic neuronal death.
Materials and methods
Generation of Ndufs4-null (Ndufs4-/-) or wild-type (Ndufs4+/+) embryos
The generation of Ndufs4-/- mice was described previously [20]. The Ndufs4+/+, Ndufs4+/-, and Ndufs4-/- littermate embryos were generated by breeding Ndufs4 heterozygotes (Ndufs4+/-). The genotype of each embryo was identified by PCR analysis and matched to each single embryo culture at the end of the experiment. All procedures were approved by the Institutional Animal Care and Use Committees of Chonnam National University and the University of Washington.
Primary mesencephalic neuronal cultures and drug treatments
Primary dopaminergic neurons were prepared from E14 mouse embryos as single-embryo cultures. The embryos were carefully separated and placed individually into a 35-mm dish containing phosphate-buffered saline (PBS, pH 7.2, Invitrogen, Carlsbad, CA) on ice. The mesencephalon of each embryo was dissected and separately transferred into a well of a 24-well plate containing PBS on ice. The forceps were dipped in 70% ethanol and wiped between handling each embryo. Each mesencephalon was washed with Dulbecco's modified Eagle medium (DMEM, Sigma, St Louis, MO) or minimum essential medium (MEM, Invitrogen), incubated at 37°C for 10 min, and then transferred to culture medium consisting of DMEM with 4 mM glutamine, 30 mM glucose, 10 mM HEPES buffer (pH 7.4), 100 IU/ml penicillin, 0.1 mg/ml streptomycin, and 10% fetal bovine serum (FBS, Invitrogen, Fig. 1–4), or in MEM containing B27 (Invitrogen, Fig. 1E). The tissue was then triturated with a narrow pipet tip (Cat # P-3207, ISC BioExpress, Kaysville, UT) for the indicated number of times (Fig. 1A) or for six times for the remaining figures. Then, 100 μl of dissociated cells were plated onto the culture bases, including Aclar film, glass coverslips, and chamber slides, as indicated in Fig. 1. Aclar embedding film (Electron Microscopy Sciences, Fort Washington, PA) was cut into 9-mm diameter disks. The Aclar or glass disks were sterilized in 70% ethanol, washed five times in autoclaved double-distilled H2O, and placed in 24-well plates. The Aclar disks, glass coverslips, and chamber slides (Nalge Nunc International, Naperville, IL) were precoated with 100 μg/ml poly-D-lysine and 4 μg/ml laminin (BD Bioscience, Bedford, MA). The dissociated cells (3-5 × 104/100 μl) were plated on each of the culture bases. The cultured cells were maintained at 37°C in a humidified 7% CO2 atmosphere. At 24 and 72 h after plating, 300 μl of prewarmed culture medium was added to each well. One-half volume of medium was removed and replenished with fresh culture medium every 48 h when needed. Pharmaceutical chemicals were from Sigma unless otherwise specified. Drug treatments were performed in defined serum-free N2 medium (Invitrogen). One-half volume of the medium was replaced with N2 medium on the day before drug treatment, and then again at the time of drug treatment. Cells treated with vehicle alone were used as controls. Cultures were treated with maneb at 5-6 days in vitro (DIV).
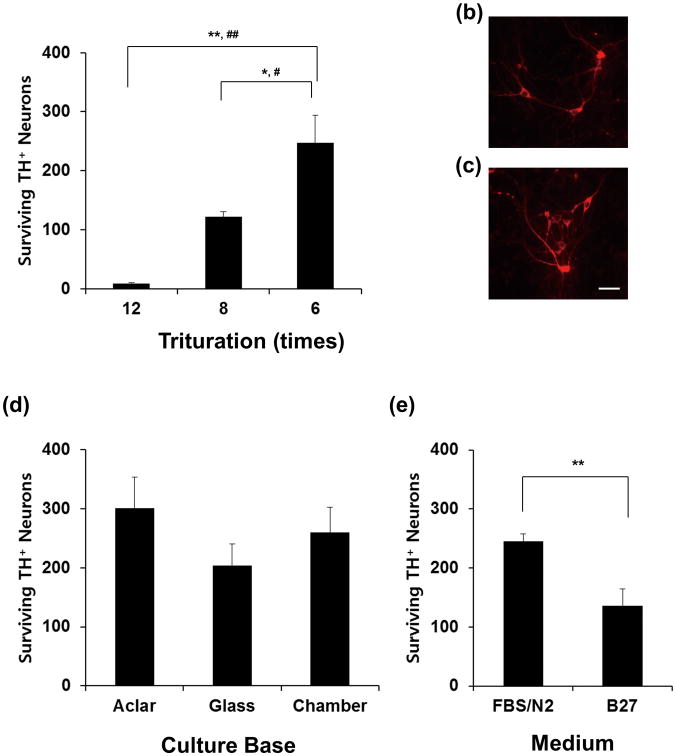
Fig. 1.
Optimization of the method for culturing mesencephalic primary neurons from individual embryos. (a) Mesencephalon from each E14 embryo was dissected and triturated by the indicated number of strokes using a narrow pipette tip, and 3-5 × 104 cells were plated on each of Aclar coverslips. (b, c) Representative immunofluorescence staining for tyrosine hydroxylase (TH) of cells dissociated 8 strokes (b) or 6 strokes (c), and plated on Aclar coverslip. (d) Cells were plated on Aclar coverslips, glass coverslips, or chamber slide, and the effect of the supporting culture base was compared. (e) The effect of culture medium on TH neuron survival. Values represent means; error bars represent s.e.m. *, p < 0.05; **, p < 0.01; #, Bonferroni adjusted p<0.05; ##, Bonferroni adjusted p<0.01.
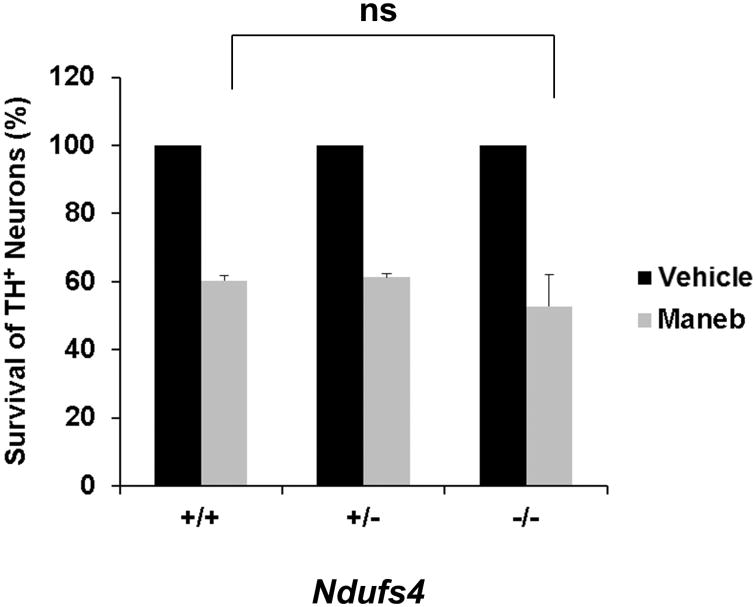
Fig. 4.
The maneb sensitivity of dopaminergic neurons derived from Ndufs4-/- or Ndufs4+/- was not significantly different from that of dopaminergic neurons derived from the wild-type littermates. Primary cells were treated with 10 μg/ml maneb or vehicle control on DIV 5, and the number of TH+ dopaminergic neurons was quantified at 24 h after treatment. Values represent means (n=3); error bars represent s.e.m. ns, not significant.
Immunocytochemistry and quantification of TH+ or NeuN+ neurons
Neuronal cultures were fixed with a solution of 4% paraformaldehyde and 4% sucrose, and blocked with a solution containing 5% BSA, 5% normal goat serum, and 0.1% Triton X-100 in PBS. Primary antibodies included mouse monoclonal antibody against tyrosine hydroxylase (TH; 1:500; Sigma), rabbit polyclonal antibody against TH (1:50,000; Pel-Freez, Rogers, AR), and rabbit polyclonal antibody against NeuN (1:2000; Sigma). Secondary antibodies were Alexa Fluor 488 (or 568) goat anti-rabbit IgG and Alexa Fluor 568 (or 488) goat anti-mouse IgG (1:200; Molecular Probes). Cells that immunostained positive for TH and had neurites that were twice the length of the soma were scored as TH+ neurons. Total TH+ neurons were counted on each culture base (an Aclar, a cover glass or a well of chamber slides).
HO-1 staining and quantification
Immunocytochemistry using rabbit polyclonal antibody against heme oxygenase 1 (HO-1; 1:1000; Enzo Life Sciences, Plymouth Meeting, PA) was done as described above. The intensity of HO-1 staining within TH positive cells was quantified using NIH ImageJ program (http://rsb.info.nih.gov/ij/) after taking photomicrographs.
Mitochondrial complex I activity assay
Complex I activity was measured using the NADH dehydrogenase activity assay as published [5]. Briefly, cells were incubated with tetrazolium (Sigma) with or without complex I inhibitor (10 μM rotenone) for 1 h. After incubation, the optical density (OD) of the more than 30 cells per treatment group was measured using NIH Image J after taking photomicrograph, and mitochondrial complex I (CI) activity (+ rotenone) among total NADH dehydrogenase activity (NDA) (- rotenone) was calculated.
Statistical analysis
Data were collected from at least three independent experiments, each with at least duplicate or triplicate determinations. Statistical analyses of data were performed using one-way ANOVA and post-hoc Student's t test. Bonferroni correction was applied for the multiple t-tests on repeated measurement.
Results
To optimize the protocol for culturing primary neurons from individual mesencephalon, we first tested several dissociation methods. The results were analyzed by immunostaining cells with antibody against tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis that is commonly used as a marker for dopaminergic neurons. Generally, enzymatic and/or mechanical dissociation have been used to maximize the dissociation and survival of dopaminergic neurons in primary culture of mesencephalon. However, for the small amount of tissue in a single ventral mesencephalon, we found that less than 75% of cells survived after enzymatic trypsin treatment compared to simple mechanical dissociation (data not shown). This suggests that tissues with small volumes are very sensitive to stress; therefore, the strength of trituration also was adjusted. As shown in Fig. 1A–C, a maximum of six homogenization strokes was optimal for the survival of dopaminergic neurons, whereas tissues subjected to fewer than six homogenization strokes were not fully separated and the cells grew as clumps (data not shown).
We tested several culture bases (supports) including glass, Aclar polymer, and chamber slides that have been used for the primary culture of dopaminergic neurons. Aclar polymer base resulted in the maximum number of surviving dopaminergic neurons (Fig. 1D). The plastic base gave the second-best result, but many cells were detached from the glass base, although all bases were coated with an equal amount of poly-D-lysine and laminin. Dissociated cells were incubated in MEM/B27 for 6 days, or in DMEM/FBS for 5 days and then in serum-free N2 medium for the 6th day (Fig. 1E). The results showed that 80.4% more TH+ neurons survived in N2 medium than in B27 medium (Fig. 1E). These data suggest that optimum conditions for culture of mesencephalic dopaminergic neurons isolated from single embryos include dissociation using six homogenization strokes, seeding cells onto Aclar polymer base, incubation in DMEM/FBS medium, and then replacing with serum-free N2 medium. We applied this method for the remaining studies.
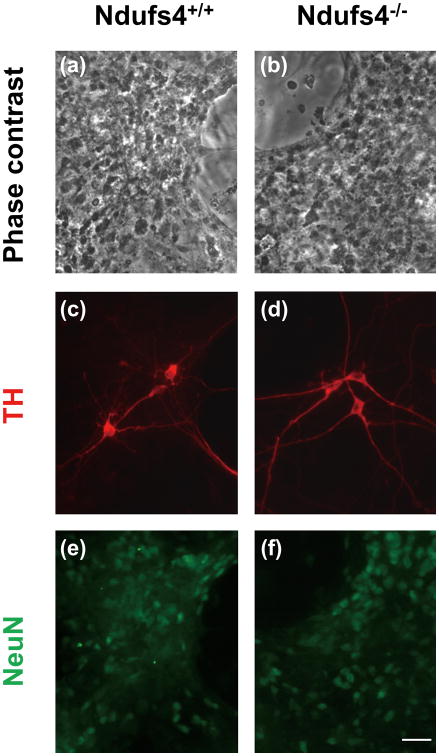
Using this optimized culture method, we investigated the role of mitochondrial complex I activity in maneb-induced dopaminergic cell death. Ndufs4 encodes a subunit that is required for complete assembly and function of mitochondrial complex I [21-25]. We obtained littermate embryos by breeding Ndufs4+/- mice, and cultured mesencephalic dopaminergic neurons from each embryo. The morphology of the cells from Ndufs4+/+ or Ndufs-/- embryos was indistinguishable (Fig. 2, A and B). The number and morphology of TH+ and NeuN+ neurons were not altered by Ndufs4 deletion (Fig. 2C-F) [19], although Ndufs4-/- cells do not have detectable mitochondrial complex I activity (Fig. 3A and [19]).
Fig. 2.
Morphology of primary neurons cultured from individual embryos. Ndufs4 heterozygotes (Ndufs4+/-) were bred to generate littermates of Ndufs4+/+ (a, c, e) and Ndufs4-/- (b, d, f) embryos. Primary mesencephalic neurons were cultured separately from each mouse E14 fetus. Representative photomicrographs of total cells (a, b), neurons immunostained for TH (c, d) or neurons immunostained for NeuN (e, f). Scale bar=30 μm.
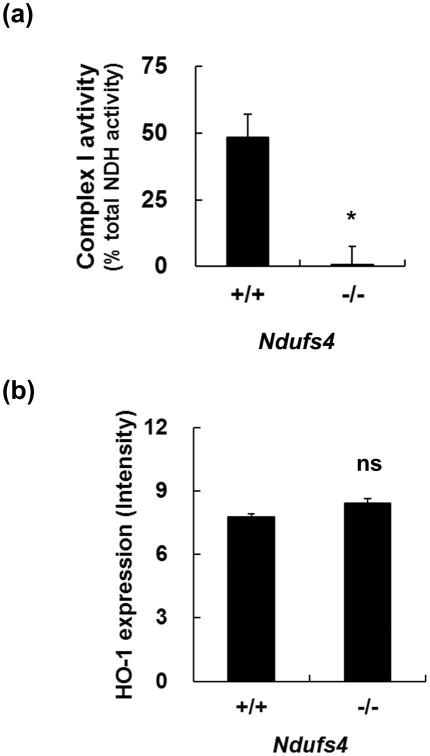
Fig. 3.
Characterization of the primary mesencephalic cells cultured from individual embryos. (a) Mesencephalic neurons from Ndufs4-/- embryos lack mitochondrial complex I activity. (b) Quantitation of heme oxygenase 1 (HO-1) immunoreactivity of primary mesencephalic cells. Values represent means (n=3); error bars represent s.e.m. *, p < 0.05. n.s., not significant.
To monitor the basal level of reactive oxygen species (ROS), TH+ dopaminergic neurons were co-stained for heme oxygenase-1 (HO-1), which is a ROS indicator [26]. The number of HO-1+ cells was not increased in the TH+ neurons cultured from Ndufs4-/- embryos compared to that from Ndufs4+/+ embryos (Fig. 3B). These results indicate that loss of complex I activity does not induce dopaminergic neuronal death or oxidative stress.
Mesencephalic neurons cultured from Ndufs4+/+, Ndufs4+/-, and Ndufs4-/- embryos were treated with 10 μg/ml maneb for 24 h. In all genotypes, approximately 55-60% of dopaminergic neurons survived the maneb treatment (Fig. 4). These data indicate that maneb-induced dopaminergic neuronal death is not affected by inhibition of mitochondrial complex I activity.
Discussion
We tested various culture conditions and established a procedure for culturing dopaminergic neurons from individual embryonic mesencephalon. This could be a useful technique for studies using genetically modified and knockout mice. In many cases, knockout of genes that are crucial for development results in embryonic or juvenile lethality. This restricts experiments using primary dopaminergic cultures derived from the breeding of those knockout animals. Our method is applicable as long as the heterozygotes survive to reproductive age and are fertile.
DMEM-based medium gave the best results in our study, whereas survival rates were much lower in MEM-based medium. Nevertheless, further improvement of our method might be needed, because improved media with new formulas are developed continuously. We found that serum-free N2 medium is effective to maintain healthy dopaminergic cell cultures, and prevents non-specific serum effects from occurring during culture and treatment. However, any drug treatment should be done within 3 days after switching to N2 medium. The optimum culture conditions persist for approximately 3 days in N2 medium; subsequently, glial overgrowth has a negative effect on neuronal survival (data not shown). If prolonged culture is needed, anti-glial agents could be applied to prevent glial growth and may maintain the neuron-enriched conditions in N2-based medium [27].
Maneb has been used in combination with paraquat to induce an in vivo Parkinson's disease model in mice. However, the mechanism of maneb toxicity was not understood. For example, it is not clear if maneb toxicity is influenced by inhibition or loss of mitochondrial complex I activity. Our data suggest that maneb toxicity is not significantly altered by loss of mitochondria complex I activity in primary cultured dopamine neurons.
Our study established a method for optimizing primary dopaminergic neuronal culture from individual embryonic mesencephalon. These results will enable further studies on dopaminergic neurons using genetically engineered mouse models.
Conclusion
We optimized the culture conditions for preparing dopaminergic neurons from individual mouse embryos. We investigated the role of mitochondrial complex I in dopamine neuron death using the optimized method. Loss of mitochondrial complex I activity was not sufficient to cause neuron death or ROS increase in Ndufs4-/- cells. Furthermore, maneb-induced dopamine neuron death was not affected by loss of mitochondrial complex I activity.
Acknowledgments
This work was financially supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2013R1A1A1059258, WSC), Chonnam National University, 2012 (WSC) and by NIH grant ES012215 (ZX).
Abbreviations
- PD
Parkinson's disease
- TH
tyrosine hydroxylase
- E
embryonic day
- DIV
days in vitro
Footnotes
Conflicts of interest: There are no conflicts of interest.
References
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 3.McNaught KS, Altomare C, Cellamare S, Carotti A, Thull U, Carrupt PA, et al. Inhibition of alpha-ketoglutarate dehydrogenase by isoquinoline derivatives structurally related to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neuroreport. 1995;6:1105–1108. doi: 10.1097/00001756-199505300-00008. [DOI] [PubMed] [Google Scholar]
- 4.Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson's disease--is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, et al. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- 7.Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chorfa A, Betemps D, Morignat E, Lazizzera C, Hogeveen K, Andrieu T, et al. Specific pesticide-dependent increases in alpha-synuclein levels in human neuroblastoma (SH-SY5Y) and melanoma (SK-MEL-2) cell lines. Toxicol Sci. 2013;133:289–297. doi: 10.1093/toxsci/kft076. [DOI] [PubMed] [Google Scholar]
- 9.Yang YX, Wood NW, Latchman DS. Molecular basis of Parkinson's disease. Neuroreport. 2009;20:150–156. doi: 10.1097/WNR.0b013e32831c50df. [DOI] [PubMed] [Google Scholar]
- 10.Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J Toxicol Clin Toxicol. 2000;38:123–128. doi: 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- 12.Yumino K, Kawakami I, Tamura M, Hayashi T, Nakamura M. Paraquat- and diquat-induced oxygen radical generation and lipid peroxidation in rat brain microsomes. J Biochem (Tokyo) 2002;131:565–570. doi: 10.1093/oxfordjournals.jbchem.a003135. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Matsubara K, Ohtaki K, Shiono H. Paraquat leads to dopaminergic neural vulnerability in organotypic midbrain culture. Neurosci Res. 2003;46:523–532. doi: 10.1016/s0168-0102(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K, Matsubara K, Ohtaki K, Fujimaru S, Saito O, Shiono H. Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res. 2003;976:243–252. doi: 10.1016/s0006-8993(03)02750-1. [DOI] [PubMed] [Google Scholar]
- 15.Dauer W, Przedborski S. Parkinson's Disease: Mechanisms and Models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 16.Uversky VN. Neurotoxicant-induced animal models of Parkinson's disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- 17.Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134:52–56. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat Neurotoxicity is Distinct from that of MPTP and Rotenone. Toxicol Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 19.Choi WS, Kruse SE, Palmiter RD, Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metabolism. 2008;7:312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scacco S, Petruzzella V, Budde S, Vergari R, Tamborra R, Panelli D, et al. Pathological mutations of the human NDUFS4 gene of the 18-kDa (AQDQ) subunit of complex I affect the expression of the protein and the assembly and function of the complex. J Biol Chem. 2003;278:44161–44167. doi: 10.1074/jbc.M307615200. [DOI] [PubMed] [Google Scholar]
- 22.van den Heuvel L, Ruitenbeek W, Smeets R, Gelman-Kohan Z, Elpeleg O, Loeffen J, et al. Demonstration of a new pathogenic mutation in human complex I deficiency: a 5-bp duplication in the nuclear gene encoding the 18-kD (AQDQ) subunit. Am J Hum Genet. 1998;62:262–268. doi: 10.1086/301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budde SM, van den Heuvel LP, Janssen AJ, Smeets RJ, Buskens CA, DeMeirleir L, et al. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochem Biophys Res Commun. 2000;275:63–68. doi: 10.1006/bbrc.2000.3257. [DOI] [PubMed] [Google Scholar]
- 24.Petruzzella V, Papa S. Mutations in human nuclear genes encoding for subunits of mitochondrial respiratory complex I: the NDUFS4 gene. Gene. 2002;286:149–154. doi: 10.1016/s0378-1119(01)00810-1. [DOI] [PubMed] [Google Scholar]
- 25.Vogel RO, van den Brand MA, Rodenburg RJ, van den Heuvel LP, Tsuneoka M, Smeitink JA, et al. Investigation of the complex I assembly chaperones B17.2L and NDUFAF1 in a cohort of CI deficient patients. Mol Genet Metab. 2007;91:176–182. doi: 10.1016/j.ymgme.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 27.Murer MG, Raisman-Vozari R, Yan Q, Ruberg M, Agid Y, Michel PP. Survival factors promote BDNF protein expression in mesencephalic dopaminergic neurons. Neuroreport. 1999;10:801–805. doi: 10.1097/00001756-199903170-00025. [DOI] [PubMed] [Google Scholar]