Abstract
Hepatic ischemia-reperfusion (IRP) injury is a significant clinical problem during tumor resection surgery (Pringle maneuver), and liver transplantation. However, the relative contribution of necrotic and apoptotic cell death to the overall liver injury is still controversial. In order to address this important issue in a standard murine model of hepatic IRP injury, plasma biomarkers of necrotic cell death such as micro-RNA-122, full-length cytokeratin-18 (FK18) and high mobility group box-1 (HMGB1) protein, and apoptosis including plasma caspase-3 activity and caspase-cleaved cytokeratin-18 (CK18), coupled with markers of inflammation (hyper-acetylated HMGB1) were compared with histological features in H&E- and TUNEL-stained liver sections. After 45 min of hepatic ischemia and 1–24h of reperfusion, all necrosis markers increased dramatically in plasma by 40-to->10,000-fold over baseline with a time course similar to ALT. These data correlated well with histological characteristics of necrosis. Within the area of necrosis, most cells were TUNEL-positive; initially (≤ 3h of RP) the staining was restricted to nuclei but later spread to the cytosol characteristic for karyorrhexis during necrotic cell death. In contrast, the lack of morphological evidence of apoptotic cell death and relevant caspase-3 activity in the postischemic liver correlated well with the absence of caspase-3 activity and CK18 (except a minor increase at 3h RP) in plasma. The quantitative comparison of FK18 (necrosis) and CK18 (apoptosis) release indicated the dominant cell death by necrosis during IRP and only a temporary and very minor degree of apoptosis. These data suggest that the focus of future research should be on the elucidation of necrotic signaling mechanisms to identify relevant targets, which may be used to attenuate hepatic IRP injury.
Keywords: Inflammation, Cytokeratin-18, Caspases, High mobility group box 1, miR-122, neutrophils
INTRODUCTION
Hepatic ischemia-reperfusion injury is a significant clinical problem during tumor resection surgery using the Pringle maneuver, during liver transplantation and during hemorrhagic shock. This clinical condition has been recognized for more than 30 years and is extensively investigated using various animal models. The initial hypothesis that ischemia-reperfusion injury is caused by an intracellular oxidant stress induced by xanthine oxidase1 was rapidly disputed.2,3 Subsequently, it was recognized that the postischemic oxidant stress was largely derived from resident Kupffer cells4 and neutrophils,5,6 which are responsible for an early and late injury phase, respectively. Although pro-inflammatory mediators such as TNF-α,7 complement factors8 and chemokines9,10 as activators of neutrophils have been identified relatively early, the concept of sterile inflammation emerged more recently.11,12 Currently, a major focus of research is the identification of damage associated molecular patterns such as high mobility group box-1 (HMGB1) protein and DNA fragments13 released by damaged cells and the involvement of toll-like receptors in triggering the formation of cytokines in macrophages.14
The views on the mechanisms of cell death during hepatic ischemia-reperfusion injury have changed over time. Initially, it was thought that inflammatory cells kill hepatocytes by necrosis, a conclusion supported by extensive liver enzyme release.15 However, with the emerging recognition of apoptotic cell death during the late 1990s, a major shift occurred.16 Using the TUNEL assay as the test for apoptosis, it was concluded that up to 80% of hepatocytes and endothelial cells die by apoptosis during the first 3h of reperfusion.17,18 Also, reports emerged that caspase inhibitors protected against hepatic ischemia-reperfusion injury.19,20 Most of these studies were performed in rats. However, a detailed morphological study, which considered the hallmarks of apoptosis such as cell shrinkage, chromatin condensation and apoptotic bodies together with hepatic caspase-3 activity could not find any relevant increase in apoptotic cell death in the postischemic liver tissue.21 In this study it was concluded that >95% of all cell death occurred by necrosis. Importantly, it was also demonstrated that most necrotic cells stained positive with the TUNEL assay.21 These findings supported the previously raised concern that this assay, which detects DNA strand breaks,22 may not be specific for apoptosis.23 Taken together, the preponderance of the experimental evidence suggested that oncotic necrosis was the dominant mode of cell death during hepatic ischemia-reperfusion injury in rats.24
During the last decade more and more ischemia-reperfusion studies were performed in mice. Using the TUNEL assay and occasionally additional parameters such as cleavage of procaspase-3 or Bax protein expression, many reports still argue for a substantial role of apoptosis over necrosis.25–27 However, most of these studies do not directly compare apoptotic and necrotic cell death. In addition, the fact that apoptotic cells, in contrast to necrotic cells, are only visible for a limited time in tissue sections can make a quantitative comparison difficult. Thus, the question remains: how relevant is apoptosis for the overall postischemic cell injury? To address this important question, plasma biomarkers for necrotic and apoptotic cell death were used because these parameters are more reflective of the process in the entire liver. Previous studies have shown the utility of plasma levels of total HMGB1 protein, microRNA-122 (miR-122) and full-length cytokeratin 18 as indicator for cell necrosis, hyper-acetylated HMGB1 as a marker of inflammation and caspase-cleaved fragments of cytokeratin 18 and caspase-3 activities as measure of apoptotic cell death in acetaminophen hepatotoxicity and bile duct ligation.28–33
MATERIALS AND METHODS
Animals and experimental procedures
C57BL/6J mice (20–25 g bodyweight) were purchased from Jackson Laboratories (Bar Harbor, ME). All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985). All experimental protocols were approved by the Animal Use Committees of the University of Kansas Medical Center. Under ketamine anesthesia (mixture of 75 mg/kg ketamine, 5.1 mg/kg xylazine, and 0.75 mg/kg acepromazine given intramuscularly) a laparotomy was performed, and the blood supply to the median and left hepatic lobes was occluded with an atraumatic vascular clamp for 45 min as described.34 Reperfusion was initiated by removal of the clamp. The body temperature was maintained in all animals with an infrared heating lamp. After reperfusion was initiated, 4 ml/kg of physiological saline was injected intraperitoneally, the abdominal incision was closed with 4-0 silk and wound clips, and the animals were allowed to recover. Sham-operation included all procedures except clamping of the blood supply. Groups of animals were sacrificed at the end of ischemia, or after 1, 3, 6, 12 and 24h of reperfusion. Blood was drawn from the vena cava into a heparinized syringe. The liver was rapidly removed, rinsed in saline and sections were placed in phosphate-buffered 10% (vol/vol) formalin solution and embedded in paraffin for histological evaluations or snap-frozen in liquid nitrogen.
In addition to the time course experiments, groups of animals were treated with the pan-caspase inhibitor Z-VD-fmk (10 mg/kg i.p.) 30 min before ischemia and at the time of reperfusion or vehicle (50 mM Tris buffer (pH 7.0) (10 ml/kg).35 Furthermore, as positive control for apoptosis, some animals were treated intraperitoneally with 700 mg/kg D-galactosamine (Sigma, St. Louis, MO) and 100 µg/kg Salmonella abortus equi endotoxin (Sigma) (Gal/ET) dissolved in sterile phosphate-buffered saline (pH 7.0).35
ALT activities and caspase activation
Plasma alanine aminotransferase (ALT) activities were determined by using the Pointe Scientific ALT Kit (Pointe Scientific Canton, MI) according to the manufacturer's instructions. Caspase-3 activity in the liver or plasma was determined by measuring the z-VAD-FMK-inhibitable cleavage of the fluorescent caspase-3 substrate Ac-DEVD-AMC (Sigma Aldrich St. Louis, MO) as described.32 In addition, caspase-3 processing in liver and plasma was evaluated by western blotting using a caspase-3 antibody (Cell Signaling, Danvers, MA) as described in detail.33
Measurement of mechanism-based biomarkers of liver injury in plasma
Full-length and caspase-cleaved cytokeratin-18 were identified and quantified by mass spectrometry as previously described in detail.28–30 MicroRNA-122 (miR-122) was measured by qRT-PCR;31 let-7d provided biological standardization and lin-4 served as an internal standard for the assay. Total HMGB1 was quantified by immunoassay and mass spectrometry.28–30 The absolute quantification of hyper-acetylated HMGB1 was determined by mass spectrometry as previously described as a biomarker of immune cell activation.28 The investigators measuring the plasma biomarkers were blinded to the treatment groups of the animals.
Histology
Sections of formalin-fixed paraffin-embedded liver samples were stained with hematoxylin and eosin (H&E) for evaluation of liver cell death by a Board Certified Pathologist (AF) as described in detail.21,36 Additional liver sections were stained with the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) in situ cell death assay (Roche) for visualization of DNA strand breaks as previously described.36 The quantitation of TUNEL-positive cells was performed by manually counting the number of TUNEL-positive cells per randomly selected 10 high power fields (HPF) per section. The percentage of cells showing TUNEL-positive nuclei and cytoplasm was similarly quantified. All TUNEL-positive cells were included in the results but hepatocytes and non-parenchymal cells were individually identified.
Statistics
Data are given as means ± SE. Statistical analysis was performed with Sigmaplot 11.0 (Systat Software, Inc., Chicago, IL). Data were assessed using one way ANOVA followed by Student-Newman-Keul’s post-hoc test for comparisons between means or Dunnett’s post-hoc test for comparisons to a control. For data not normally distributed, we used the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. P < 0.05 was considered significant.
RESULTS
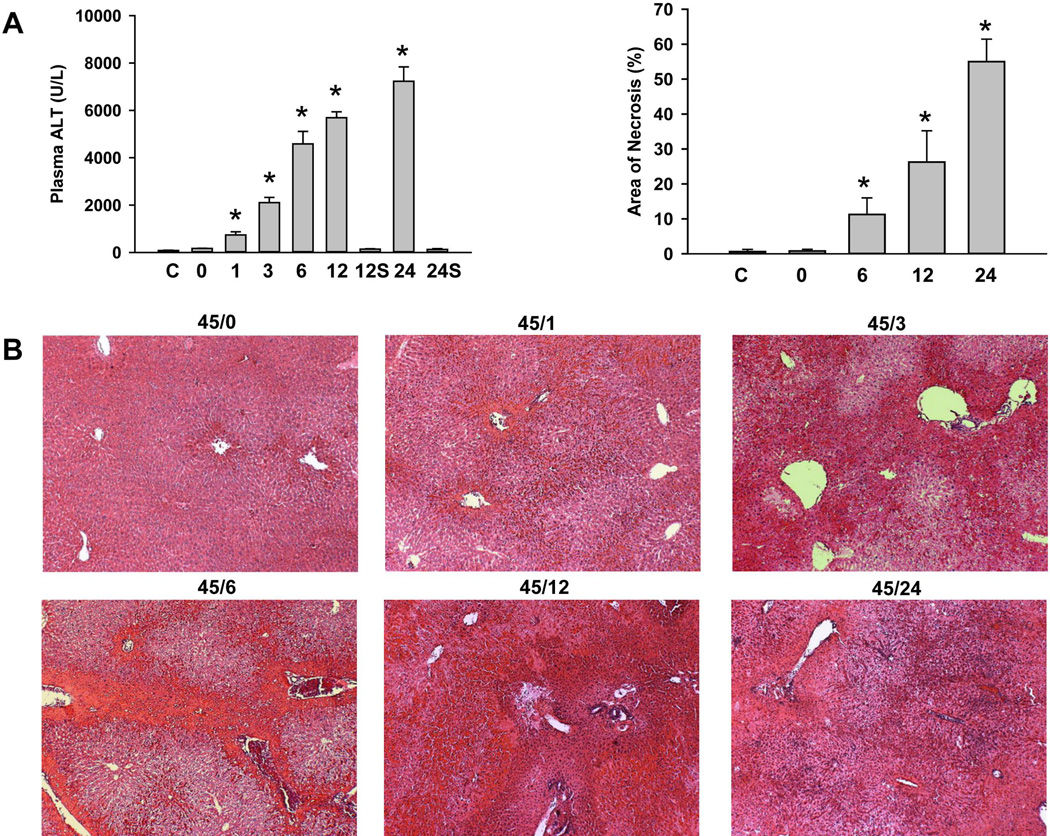
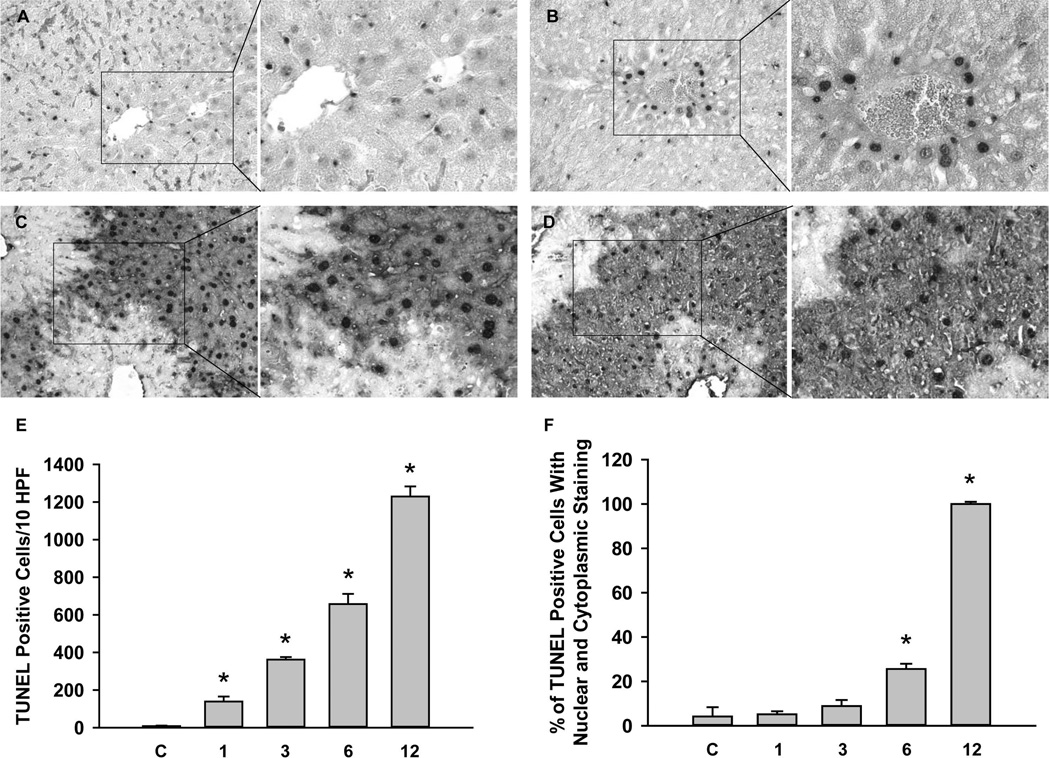
C57Bl/6 mice were subjected to 45 min of hepatic ischemia followed by up to 24h of reperfusion. Progressive liver injury, as indicated by the increase of plasma ALT activities, was observed during reperfusion between 1h and 24h (Figure 1A). No change in plasma ALT activities was observed in sham-operated animals. H&E-stained tissue sections showed tissue damage consistent with extensive necrosis (Figure 1B). The area of necrosis was about 10% at 6h and progressed to >50% at 24h of reperfusion (Figure 1A). No morphological evidence of apoptosis (apoptotic bodies) was detectable at any time during reperfusion. Staining sections with the TUNEL assay, which detects DNA strand breaks, showed very few individual positive cells (< 1 positive cell per HPF) in controls or after ischemia (data not shown). However, during reperfusion the number of positive cells substantially increased (Figure 2). During the first hour of reperfusion the staining occurred mainly in the nuclei of non-parenchymal cells (Figure 2A). At 3h, the nuclei of individual hepatocytes stained positive (Figure 2B). At 6h of reperfusion, the number of TUNEL-positive cells increased dramatically, however, staining was observed in nuclei and the cytosol (Figure 2C). By 12h, many nuclei were disintegrated and the cytosol was intensely stained owing to the release of DNA fragments into the cytosol (Figure 2D) and by 24h, when most nuclei in the necrotic areas disappeared, only the cytosol of hepatocytes was stained (not shown). After 3h, 6h, 12h, and 24h (not shown) reperfusion, greater than 95% of all TUNEL-positive cells observed were hepatocytes. The progression of necrosis based on TUNEL staining is further illustrated following quantitation of both total TUNEL-positive cells (Figure 2E) as well as the percent of those exhibiting nuclear and cytoplasmic TUNEL staining (Figure 2F). Although the number of total TUNEL-positive cells increased steadily during reperfusion, the staining of the cytosol developed between 3 and 6h and increased steeply afterwards (Figure 2F). These data are consistent with initial nuclear DNA fragmentation, which progressed to karyorrhexis and karyolysis. This TUNEL staining pattern during ischemia-reperfusion was fundamentally different compared to a positive control for apoptosis. In Gal/ET-treated animals numerous individual TUNEL-positive cells or apoptotic bodies are readily observed where nuclei were stained but not the cytosol.33,36,37
Figure 1. Liver injury caused by ischemia/reperfusion.
Mice were either untreated controls (C) or subjected to either sham (S) operation or 45 min liver ischemia followed by reperfusion for various times (0, 1, 3, 6, 12 or 24h). Liver injury was assessed by plasma alanine aminotransferase (ALT) activities or quantitation of the areas of necrosis (Panel A). Data are expressed as mean ± SE of n= 3–6 animals per time point. *P<0.05 (compared to control, C). Representative H&E stained sections (Panels B) are shown for ischemia (45/0) and various times of reperfusion (45/xx). Magnification: ×50
Figure 2. TUNEL staining following ischemia/reperfusion injury.
Liver sections obtained from mice subjected to 45 min ischemia and 1h, 3h, 6h and 12h reperfusion (Panel A–D). For each section a low (×50) and a high (×200) magnification of the area in the box is shown. TUNEL positive cells for untreated controls (C), and animals subjected to 45 min ischemia and 1–12h of reperfusion were quantified. Panel E shows the total TUNEL-positive cells per 10 HPF and Panel F shows the percent of TUNEL-positive cells with both nuclear and cytoplasmic staining. Data are expressed as mean ± SEM of n= 3 animals per time point. *P<0.05 (compared to control, C)
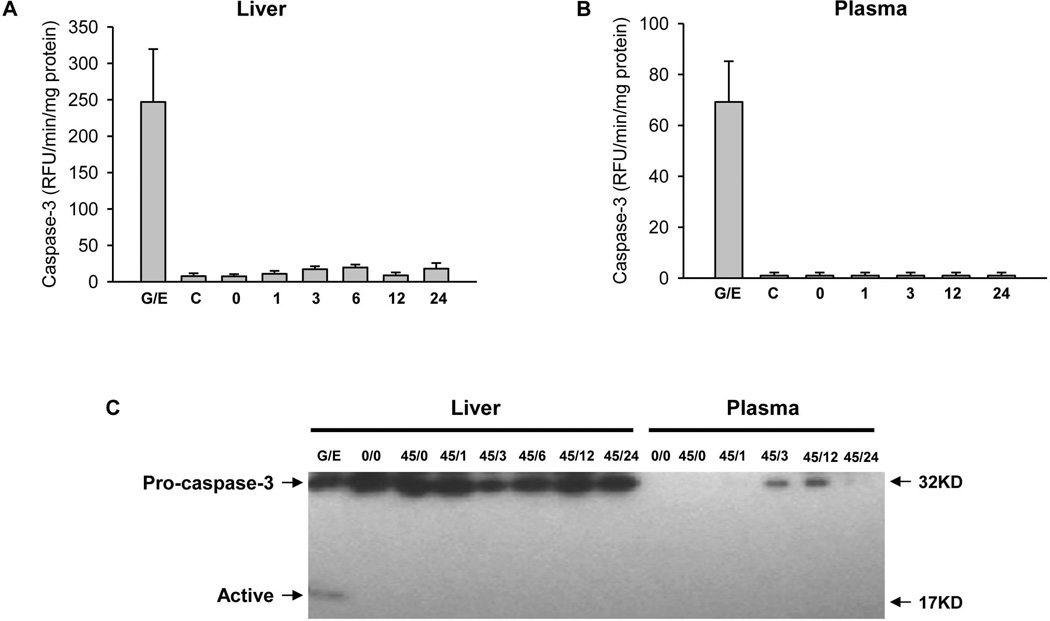
It is well established that caspase-3 is an executioner caspase, which is critical for apoptotic cell death in all liver cells.38 The activation of caspase-3 can be measured with an enzyme activity assay and with the documentation of the formation of an active, e.g. 17 kD, fragment by Western blotting. Compared to very low levels of caspase-3 activities in controls, Gal/ET treatment induced an extensive increase in enzyme activity in the liver and in plasma (Figures 3A,B). This was confirmed by the reduction of pro-caspase-3 and the appearance of the active 17 kD fragment in the liver (Figure 3C). In striking contrast to these observations in a liver with extensive apoptosis, livers from postischemic animals did not show any relevant increase of caspase-3 activity in the liver or plasma (Figures 3A,B) or evidence for caspase-3 processing (Figure 3C) at any time point between 1 and 24h of reperfusion. The fact that no caspase-3 activity and no active caspase-3 fragments were detectable in the postischemic liver and in plasma suggest that this is a primary necrotic process and not secondary necrosis.39
Figure 3. Caspase activation during hepatic ischemia/reperfusion injury.
Caspase-3 enzyme activity was measured in mice treated with galactosamine/endotoxin (G/E), untreated controls (C), and mice subjected to either sham operation or 45 min liver ischemia followed by reperfusion for various times (0, 1, 3, 6, 12 or 24 h). (Panel A) Caspase-3 activity in liver. (Panel B) Caspase-3 activity in plasma. Data are expressed as mean ± SE of n= 3–6 animals per time point. (Panel C) Western blot showing procaspase-3 and the active caspase-3 fragment in liver and plasma following G/E treatment or ischemia/reperfusion injury.
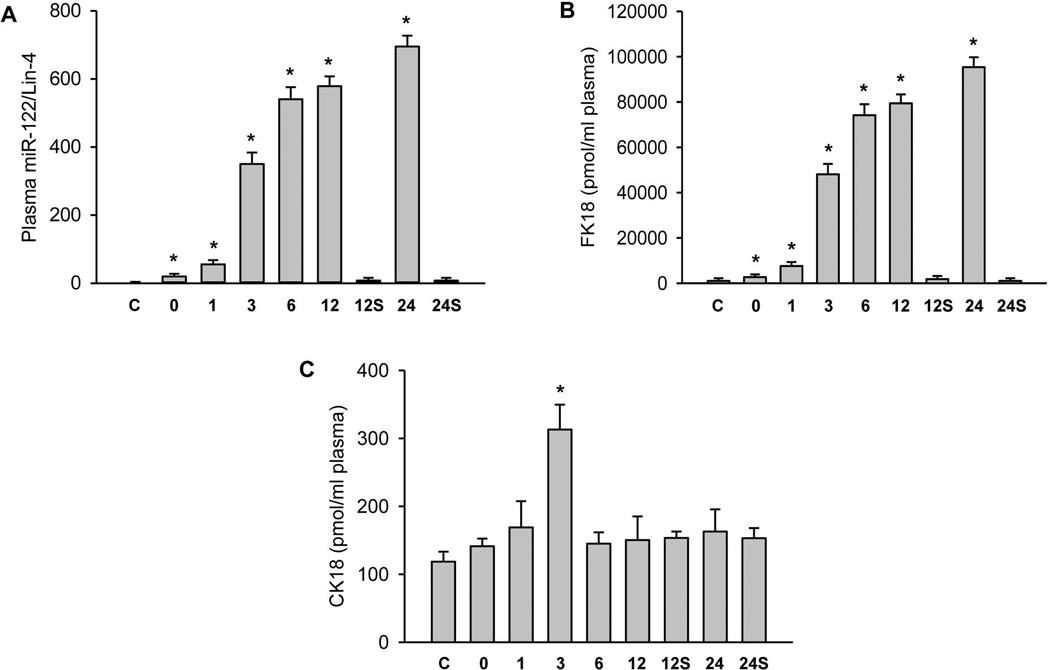
miR-122 has been shown to be a highly sensitive marker for necrotic cell death.31,40 Although there was no relevant increase in sham-operated animals compared to untreated controls, miR-122 levels increased dramatically during ischemia-reperfusion (Figure 4A) following the enhanced plasma ALT activities during reperfusion (Figure 1A). However, peak miR-122 levels at 12–24h of reperfusion were 36,000-to-43,500-fold above baseline compared to ALT levels, which were elevated 70-to-90-fold at this time.
Figure 4. Plasma biomarkers of cell necrosis and apoptosis during ischemia/reperfusion injury.
Mice were either untreated controls (C) or_subjected to sham operation for 12 or 24h (S) or 45 min liver ischemia followed by reperfusion for various times (0, 1, 3, 6, 12 or 24h). (Panel A) Plasma levels of microRNA-122. (Panel B) Plasma levels of full-length keratin-18 (FK18). (Panel C) Plasma levels of the caspase-cleaved keratin-18 fragment (CK18). Data are expressed as mean ± SE of n= 3–6 animals per time point. *P<0.05 (compared to control).
Full-length cytokeratin-18 (FK18) is a filamentous protein, which can be passively released by necrotic cells. In contrast, a caspase-cleaved fragment of cytokeratin-18 (CK18) reflects apoptotic cell death.41 During ischemia-reperfusion, a time-dependent, massive release of FK18 was observed with peak levels at 12–24h (Figure 4B). Compared to baseline levels in controls (218 ± 42 pmol/ml), FK18 concentrations were 220-to-440-fold higher between 3 and 24h of reperfusion, respectively. In contrast, the CK18 levels did not change significantly except a less than 3-fold increase at 3h of reperfusion (Figure 4C). While this temporary increase of CK18 was unexpected, it is important to note that apoptosis and necrosis are not mutually exclusive events in the liver and it is possible that there is a slight increase in apoptosis at the 3h time point. However, when taking into account the overall magnitude of increase of CK18 compared to that of FK18, this slight increase in CK18 appears biologically insignificant.
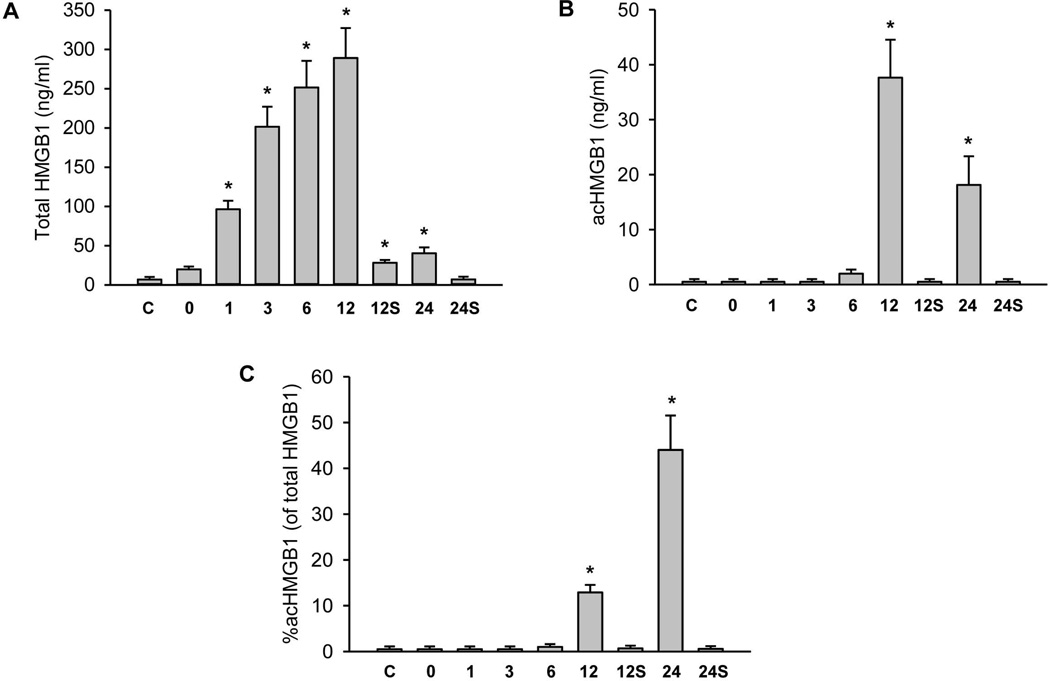
HMGB1 protein is another protein that can be passively released from necrotic cells;42 however, an acetylated form (acHMGB1) can also be secreted by activated macrophages.43 Total HMGB1 levels in plasma increased extensively during reperfusion reaching peak levels of 41-fold over controls at 12h of reperfusion (Figure 5A). Interestingly, a sharp decline was observed at 24h. In contrast, acHMGB1 levels were only significantly increased at 12 and 24h suggesting a later activation of macrophages (Figure 5B). The percentage of acHMGB1 compared to total HMGB1 was less than 1% in controls, sham-operated animals and during ischemia-reperfusion up to 6h. However, acHMGB1 levels represented 13% at 12h and even 45% at 24h of reperfusion (Figure 5C) indicating that the early increase of plasma HMGB1 concentrations during reperfusion was exclusively derived from necrotic cells but activated macrophages significantly contributed at later time points.
Figure 5. HMGB1 levels following ischemia/reperfusion.
Mice were untreated controls (C) or subjected to either sham operation for 12 or 24h (S) or 45 min liver ischemia followed by reperfusion for various times (0, 1, 3, 6, 12 or 24h). (Panel A) Plasma levels of total HMGB1. (Panel B) Acetylated HMGB1 plasma levels. (Panel C) Percent of acHMGB1 of the total HMGB1. Data are expressed as mean ± SE of n= 3–6 animals per time point. *P<0.05 (compared to control).
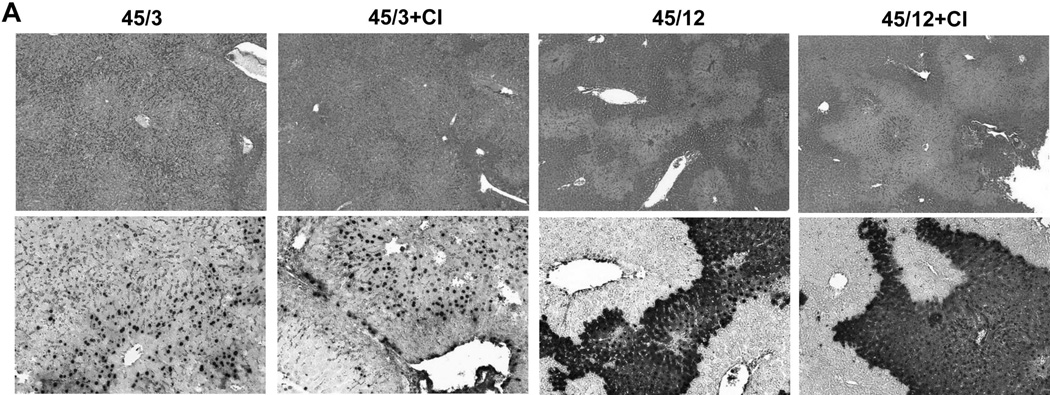
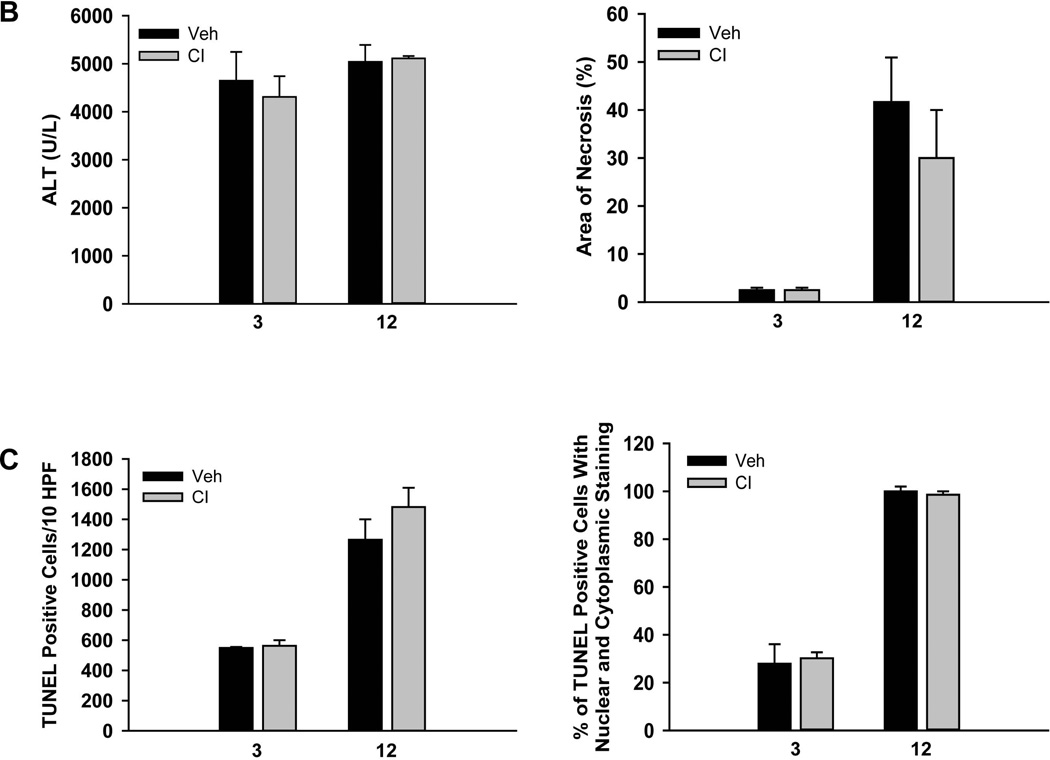
The plasma biomarkers suggested no relevant involvement of apoptotic cell death in the mechanism of hepatic ischemia-reperfusion injury. To further test this hypothesis, animals were treated with a pancaspase inhibitor, which has been shown to be highly effective in a model of hepatic apoptosis.35 However, compared with the vehicle-treated control, pretreatment with the pancaspase inhibitor had no significant effect on liver injury assessed by plasma ALT activities and the area of necrosis at 3h and at 12h of reperfusion (Figure 6A,B). In addition, the number of TUNEL-positive cells or the pattern of TUNEL staining was the same at both time points (Figures 6A,C). Furthermore, there was no change in the percentage of nuclear and cytoplasmic stained TUNEL-positive cells at either time point (Figures 6C). These findings confirmed the results obtained with the circulating biomarkers.
Figure 6. A pancaspase inhibitor against does not protect against ischemia/reperfusion injury.
Mice were subjected to 45 min liver ischemia followed by reperfusion for either 3 or 12h with administration of 10 mg/kg of a pancaspase inhibitor (CI) or the respective vehicle (Veh) as described in the methods. (Panel A) Representative liver sections stained with H&E (top row) or the TUNEL assay (bottom row) showing lack of protection of a caspase inhibitor against ischemia/reperfusion injury. (Panel B) Plasma ALT levels and area of necrosis. (Panel C) Quantitation of either the total TUNEL-positive cells per 10 HPF or the percent of TUNEL-positive cells with both nuclear and cytoplasmic staining at 3 and 12h of reperfusion. Data are expressed as mean ± SEM of n= 4 animals per time point.
DISCUSSION
The objective of the present investigation was to assess the utility of plasma biomarkers in evaluating the overall degree of apoptotic versus necrotic cell death in hepatic ischemia-reperfusion injury. Our study using established markers of necrosis (ALT, FK18, miR-122, HMGB1), inflammation (acHMGB1), and apoptosis (caspase activity, caspase-cleaved cytokeratin-18) demonstrated overwhelming evidence for necrosis and consistently negligible levels of apoptosis.
In the last decade it became common practice to report both necrotic (histology, ALT) and apoptotic (TUNEL assay) parameters in studies of hepatic ischemia-reperfusion injury. Numerous original papers and reviews still claim a substantial role of apoptotic cell death although rarely a direct quantitative comparison to necrosis is used. This is not a trivial issue because apoptotic cell death can be controlled effectively by pancaspase inhibitors as has been demonstrated in models of apoptosis such as galactosamine/endotoxin35,44,45 or the agonistic anti-Fas antibody.36,45,46 Thus, if apoptosis would be pathophysiologically relevant in the postischemic liver, effective therapeutic interventions would be available and could be used. However, the only clinical study using a pancaspase inhibitor showed only a moderate beneficial effect during liver transplantation when the inhibitor was added to the storage solution.47 However, this protection could have been caused by affecting other proteases, which can be inhibited by high concentrations of a caspase inhibitor.48 This clinical study also revealed a problem. Treating the organ recipient with the caspase inhibitor was actually detrimental most likely due to inhibiting apoptosis of neutrophils.47 Since neutrophils are key cells aggravating reperfusion injury,5 the caspase inhibitor can delay the injury resolution by prolonging the inflammatory response. Nevertheless, several clinical studies have shown a dominant role of necrosis during hepatic liver transplantation.49,50
The gold standard for apoptosis assessment is generally morphology (e.g., cell shrinkage, chromatin condensation and margination, and formation of apoptotic bodies). These characteristic morphological features are easily detectable in models of apoptotic cell death such as Fas- or TNF-receptor apoptosis models24,37,51 These models also show an extensive increase in caspase-3 activity and have readily detectable caspase-3 processing.36,46 As a consequence of the dominant apoptotic cell death, pancaspase inhibitors are almost 100% effective in reducing liver injury.35,36,46 Furthermore, apoptosis is also characterized by DNA damage as visualized by the TUNEL assay. However, the TUNEL-positive cells only show a distinct nuclear staining during apoptosis.24,36,37 In striking contrast to these apoptosis models, hepatic ischemia-reperfusion in mice showed morphological characteristics of necrosis including cell swelling, vacuolation, karyorrhexis and karyolysis. Blinded assessment of the tissue by the pathologist did not reveal any evidence for apoptotic bodies suggesting limited apoptotic cell death. However, ischemia-reperfusion injury involved massive ALT release, no increase of caspase activity and no evidence of caspase-3 processing. In addition, the extensive TUNEL staining of cells was typical for a necrotic process, i.e. it included extensive staining of the cytosol due to the formation of large DNA fragments during karyorrhexis which are released into the cytosol upon nuclear disintegration.24 This is in contrast to apoptosis during which chromatin condensation, rather than nuclear disintegration, occurs, confining the TUNEL stain to the nucleus.21,36,37 In addition, a caspase inhibitor had no effect on reperfusion injury as indicated by ALT activities and histological quantitation of the area of necrosis. In addition, the number of TUNEL-positive cells and the staining pattern (nucleus and cytosol) was not affected by a pancaspase inhibitor.
Although most of our data support the hypothesis of mainly necrosis as mode of cell death during hepatic ischemia-reperfusion, there is the possibility of secondary necrosis. In general, if apoptotic cell death cannot be completed, i.e., if any time during the apoptotic process the ATP levels drop substantially, the cell membrane may become permeable and many characteristics of necrosis surface including ALT release.24,39 However, because secondary necrosis starts as an apoptotic process, caspase-3 activation is still present during cell contents release.46 Consequently, apoptosis and the resulting secondary necrosis can be completely inhibited by a caspase inhibitor.46 The fact that no caspase activation was detectable during the entire reperfusion period and that a pancaspase inhibitor, which has been shown to eliminate TNF-induced apoptotic liver injury,35 did not protect strongly suggest that this is not an apoptotic process which deteriorated into secondary necrosis.
Despite this overwhelming evidence for necrotic cell death during reperfusion injury, one has to consider some limitations of apoptosis quantitation in tissue, which makes a direct comparison of apoptosis and necrosis difficult. Whereas necrotic cells are visible in tissue sections for several days, apoptotic cells, in general, disappear within a few hours, which may lead to underestimation of apoptotic cell death. One way to overcome this disadvantage would be to use sensitive plasma biomarkers, with the release of such biomarkers into the circulation grounded in a solid mechanistic, cell death mode-dependent, basis.52,53
The full-length form of cytokeratin-18 (FK18), miR-122 and HMGB1 are passively released by necrotic cells, which has been demonstrated in various liver disease processes in mice and humans including acetaminophen hepatotoxicity,28–30,40,42 obstructive cholestasis33 and human liver transplantation.49,50 In principle, the release of these molecules from necrotic cells is similar to ALT, however, these compounds, especially miR-122 and FK18, can be detected earlier than ALT, can be measured after a lower stress and the relative change from baseline in response to an insult is much higher than ALT in both preclinical models and in the clinical context of drug-induced liver injury.29,54 Given the fact that each of these 3 parameters were dramatically increased in plasma during reperfusion and the time course of each followed closely the changes in plasma ALT activities and histopathological changes strongly supports the conclusion of extensive necrotic cell death during the reperfusion phase after hepatic ischemia.
The caspase-cleaved fragment of cytokeratin-18 (CK18) is widely considered an indicator of caspase activation and apoptosis.41 With the exception of the 3h reperfusion time point, plasma CK18 levels did not change during ischemia or reperfusion suggesting no evidence for caspase activation or apoptotic cell death. At the 3h time point, CK18 levels increased 3-fold over baseline compared to the more than 200-fold increase for FK18 at this time point, which results in a ratio of CK18-to-FK18 of 1:150. In contrast, in the apoptosis model of galactosamine/endotoxin-induced liver injury (ALT: 1,500 U/L), the CK18-to-FK18 ratio was 1:2.33 These biomarker data illustrate the fundamental differences between an apoptotic process with secondary necrosis (galactosamine/endotoxin) and a primary necrotic process (hepatic ischemia-reperfusion injury). However, if one assumes that the minor increase of CK18 at 3h reperfusion represents apoptotic cell death, the percentage of apoptosis compared to necrosis would be estimated to be below 1% of total cell death. This would explain why it is difficult to detect caspase-3 activity in the liver or plasma and apoptotic bodies in tissue sections. Nevertheless, the temporary and minor (<3-fold) increase in apoptosis over baseline is unlikely to represent a relevant injury mechanism and may account for a failed attempt to induce apoptosis that is overwhelmed by hepatic stress. Consistent with this conclusion, the caspase inhibitor, at a dose that is highly effective in apoptosis models,35 did not attenuate injury or the number of TUNEL-positive cells. Thus, the DNA strand breaks indicated by the TUNEL staining were not caused by the caspase-activated DNase but more likely by other DNases activated during necrosis. This could involve endonuclease G and apoptosis inducing factor derived from damaged mitochondria as has been reported for acetaminophen hepatotoxicity.55,56
Although only a temporary, minor increase in CK18 levels was detected during warm ischemia-reperfusion in mice, recent reports indicated some elevation of CK18 between 24–72h of reperfusion during human liver transplantation.49,50 During the early reperfusion period, there was only a substantial increase in FK18 suggesting predominantly necrotic cell death during the first 24h of reperfusion in humans.49 However, a pancaspase inhibitor given to the transplant recipient did not improve preservation injury47 suggesting that apoptotic cell death may be of limited relevance during reperfusion. Because the human studies included only a limited number of patients, these results need further confirmation.
HMGB1 can be passively released by necrotic cells42 or the acetylated form can be actively secreted by activated macrophages.43 Our data indicate an immediate release of HMGB1 parallel to ALT, miR-122 and FK18 during the early and mid-phase of reperfusion (1–12h). At 24h, HMGB1 levels dropped substantially. It remains unclear if this drop of plasma HMGB1 levels suggests the end of the active injury phase and the discrepancy between miR-122 and FK18 levels may be caused by the shorter half-life of HMGB1 or if it reflects an exhaustion of HMGB1 in hepatocytes. A similar drop and disconnect to miR-122 and FK18 has not been observed during 3 days of bile duct ligation.33 Further studies are needed to address this issue. However, the actively secreted acetylated form was only detectable at 12 and 24h of reperfusion suggesting a later recruitment and activation of tissue macrophages. Thus, the early release of HMGB1 from necrotic cells may contribute to formation of cytokines, which are involved in neutrophil recruitment.14,57 Recently, it has been described that mutually exclusive, redox-dependent, post-translational modifications on HMGB1 regulate its cytokine-inducing (disulfide HMGB1) and chemotactic/cell recruitment (full reduced HMGB1) functions.58–60 An investigation into the redox-dependent modifications of HMGB1 in these models may offer further insights into these mechanisms that might yield novel biomarkers or pathways for therapeutic intervention.
In summary, our data using several plasma biomarkers of apoptosis and necrosis together with histological evaluation demonstrated consistently extensive necrotic cell damage and inflammation during hepatic ischemia-reperfusion in a murine model. No evidence from morphological assessment, TUNEL staining, various plasma biomarkers or pharmacological intervention with a pancaspase inhibitor suggest a relevant contribution of apoptotic cell death to the overall injury. In fact, quantitative comparison of FK18 (necrosis) and CK18 (apoptosis) release indicate only a temporary and very minor degree of apoptosis (3-fold above baseline cell turnover at 3h of reperfusion). These data suggest that the focus of future research should be on the elucidation of necrotic signaling mechanisms to identify relevant targets, which may be used to attenuate hepatic ischemia-reperfusion injury.
Acknowledgments
Financial Support:
This work was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916, and by grants from the National Center for Research Resources 5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM12345 and, from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 from the National Institute of Environmental Health Sciences. DJA, REJ and BKP would like to acknowledge the financial support from the Medical Research Council (G0700654). DJA would also like to acknowledge additional financial support from a Wellcome Trust Research Fellowship.
Abbreviations
- AcHMGB1
hyper-acetylated high mobility group box 1 protein
- ALT
alanine aminotransferase
- CK18
caspase-cleaved fragment of cytokeratin-18
- FK18
full length cytokeratin-18
- Gal/ET
galactosamine/endotoxin
- H&E
hematoxylin and eosin
- HMGB1
high mobility group box 1 protein
- miR-122
microRNA-122
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling assay
REFERENCES
- 1.Adkison D, Höllwarth ME, Benoit JN, Parks DA, McCord JM, Granger DN. Role of free radicals in ischemia-reperfusion injury to the liver. Acta Physiol Scand Suppl. 1986;548:101–107. [PubMed] [Google Scholar]
- 2.Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988;81:1240–1246. doi: 10.1172/JCI113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metzger J, Dore SP, Lauterburg BH. Oxidant stress during reperfusion of ischemic liver: no evidence for a role of xanthine oxidase. Hepatology. 1988;8:580–584. doi: 10.1002/hep.1840080324. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 6.Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289:G760–G767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- 7.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264:G801–G809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 9.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, et al. The role of cytokine networks in the local liver injury following hepatic ischemia/reperfusion in the rat. Hepatology. 1996;23:506–514. doi: 10.1002/hep.510230315. [DOI] [PubMed] [Google Scholar]
- 10.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 11.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 13.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90:665–677. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115–136. doi: 10.1016/0009-2797(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 16.Rüdiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149–159. [PubMed] [Google Scholar]
- 17.Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652–1660. doi: 10.1002/hep.510270626. [DOI] [PubMed] [Google Scholar]
- 18.Kohli V, Selzner M, Madden JF, Bentley RC, Clavien PA. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation. 1999;67:1099–1105. doi: 10.1097/00007890-199904270-00003. [DOI] [PubMed] [Google Scholar]
- 19.Cursio R, Gugenheim J, Ricci JE, Crenesse D, Rostagno P, Maulon L, et al. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. FASEB J. 1999;13:253–261. doi: 10.1096/fasebj.13.2.253. [DOI] [PubMed] [Google Scholar]
- 20.Natori S, Higuchi H, Contreras P, Gores GJ. The caspase inhibitor IDN-6556 prevents caspase activation and apoptosis in sinusoidal endothelial cells during liver preservation injury. Liver Transpl. 2003;9:278–284. doi: 10.1053/jlts.2003.50019. [DOI] [PubMed] [Google Scholar]
- 21.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 22.Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 23.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- 24.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 25.Koh EJ, Yoon SJ, Lee SM. Losartan protects liver against ischaemia/reperfusion injury through PPAR-γ activation and receptor for advanced glycation end-products down-regulation. Br J Pharmacol. 2013;169:1404–1416. doi: 10.1111/bph.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai C, Shi X, Korff S, Zhang J, Loughran PA, Ruan X, et al. CD14 contributes to warm hepatic ischemia-reperfusion injury in mice. Shock. 2013;40:115–121. doi: 10.1097/SHK.0b013e318299d1a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue S, et al. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J Hepatol. 2013;59:1200–1207. doi: 10.1016/j.jhep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- 30.Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 32.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273:524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa T, Ito Y, Wijeweera J, Liu J, Malle E, Farhood A, et al. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1385–G1395. doi: 10.1152/ajpgi.00246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaeschke H, Farhood A, Cai SX, Tseng BY, Bajt ML. Protection against TNF-induced liver parenchymal cell apoptosis during endotoxemia by a novel caspase inhibitor in mice. Toxicol Appl Pharmacol. 2000;169:77–83. doi: 10.1006/taap.2000.9035. [DOI] [PubMed] [Google Scholar]
- 36.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 37.Jaeschke H, Williams CD, Farhood A. No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology. 2011;53:718–719. doi: 10.1002/hep.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schattenberg JM, Galle PR, Schuchmann M. Apoptosis in liver disease. Liver Int. 2006;26:904–911. doi: 10.1111/j.1478-3231.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- 39.Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24:85–89. doi: 10.1111/j.1478-3231.2004.0906.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bivén K, Erdal H, Hägg M, Ueno T, Zhou R, Lynch M, et al. A novel assay for discovery and characterization of pro-apoptotic drugs and for monitoring apoptosis in patient sera. Apoptosis. 2003;8:263–268. doi: 10.1023/a:1023672805949. [DOI] [PubMed] [Google Scholar]
- 42.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 43.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- 45.Künstle G, Leist M, Uhlig S, Revesz L, Feifel R, MacKenzie A, et al. ICE-protease inhibitors block murine liver injury and apoptosis caused by CD95 or by TNF-alpha. Immunol Lett. 1997;55:5–10. doi: 10.1016/s0165-2478(96)02642-9. [DOI] [PubMed] [Google Scholar]
- 46.Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- 47.Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, et al. Clinical Trial of the Pan-Caspase Inhibitor, IDN-6556, in Human Liver Preservation Injury. Am J Transplant. 2007;7:218–225. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 48.Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 1999;442:117–121. doi: 10.1016/s0014-5793(98)01640-8. [DOI] [PubMed] [Google Scholar]
- 49.Ulukaya S, Ulukaya E, Alper I, Yilmaztepe-Oral A, Kilic M. Soluble cytokeratin 18 biomarkers may provide information on the type of cell death during early ischemia and reperfusion periods of liver transplantation. Clin Transplant. 2010;24:848–854. doi: 10.1111/j.1399-0012.2009.01177.x. [DOI] [PubMed] [Google Scholar]
- 50.Brenner T, Rosenhagen C, Brandt H, Schmitt FC, Jung GE, Schemmer P, et al. Cell death biomarkers as early predictors for hepatic dysfunction in patients after orthotopic liver transplantation. Transplantation. 2012;94:185–191. doi: 10.1097/TP.0b013e318254397c. [DOI] [PubMed] [Google Scholar]
- 51.Ogasawara J, Watanabe-Fukunage R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 52.Antoine DJ, Harrill AH, Watkins PB, Park BK. Safety biomarkers for drug-induced liver injury – current status and future perspectives. Toxicol Res. 2014;3:75–85. [Google Scholar]
- 53.McGill MR, Jaeschke H. Mechanistic biomarkers in acetaminophen-induced hepatotoxicity and acute liver failure: from preclinical models to patients. Expert Opin Drug Metab Toxicol. 2014;10:1005–1017. doi: 10.1517/17425255.2014.920823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 56.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 57.Lentsch AB. Regulatory mechanisms of injury and repair after hepatic ischemia/reperfusion. Scientifica (Cairo) 2012;2012:513192. doi: 10.6064/2012/513192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang H, Lundbäck P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antoine DJ, Harris HE, Andersson U, Tracey KJ, Bianchi ME. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med. 2014;20:135–137. doi: 10.2119/molmed.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]