Abstract
Background
HIV-1 shedding from the female genital tract is associated with increased sexual and perinatal transmission, and has been broadly evaluated in cross-sectional studies. However, few longitudinal studies have evaluated how the immune microenvironment effects shedding.
Methods
Thirty-nine HIV-1–infected women had blood, cervicovaginal lavage (CVL), and biopsies of the uterine cervix taken quarterly for up to five years. Cytokines/chemokines were quantified by Luminex assay in CVL and cellular phenotypes were characterized using immunohistochemistry in cervical biopsies. Comparisons of cytokine/chemokine concentrations and the percent of tissue staining positive for T cells were compared using generalized estimating equations between non-shedding and shedding visits across all women, and within a subgroup of women who intermittently shed HIV-1.
Results
Genital HIV-1 shedding was more common when plasma HIV-1 was detected. Cytokines associated with cell growth (IL-7), Th1 cells/inflammation (IL-12p70), and fractalkine were significantly increased at shedding visits compared to non-shedding visits within intermittent shedders and across all subjects. Within intermittent shedders and across all subjects FOXP3+ T cells were significantly decreased at shedding visits. However, there were significant increases in CD8+ cells and proportions of CD8+FOXP3+ T cells associated with HIV-1 shedding.
Conclusions
Within intermittent HIV-1 shedders, decreases in FOXP3+ T cells at the shedding visit suggests that local HIV-1 replication leads to CD4 T cell depletion, with increases in the proportion of CD8+FOXP3+ cells. HIV-1–infected cell loss may promote a cytokine milieu that maintains cellular homeostasis and increases immune suppressor cells in response to HIV-1 replication in the cervical tissues.
Keywords: HIV-1, women, genital shedding, cytokines/chemokines, CD8+FOXP3+ cells
INTRODUCTION
Globally, transmission of human immunodeficiency-1 (HIV-1) occurs predominantly through heterosexual transmission. Of concern is transmission that involves women, particularly those not receiving antiretroviral therapy (ART), because they are at the nexus of sexual and perinatal HIV-1 transmissions. Trauma,1 inflammation and immune activation,2-6 sexually transmitted infections,7 phase of the menstrual cycle,8,9 and changes in presence of Lactobacillus species,10-12 have been associated with HIV-1 shedding from the female genital tract (FGT). These studies are often cross-sectional and do not take into account subject-specific variation in inflammatory markers or the vaginal microenvironment that can drive HIV-1 shedding. Longitudinal studies of women who intermittently shed HIV-1 provide an opportunity to evaluate changes in the genital tract microenvironment that could provide insight into an important public health issue.
CD4+ T helper cells are the primary target for HIV-1 infection in vivo and the most likely source of HIV-1 shedding. Th1, Th2, Th17, and TReg helper cells are detected at the uterine cervix from women with cervical cancer.13 However, only Th17 cells from the uterine cervix have been examined early after HIV-1 infection and were apparently depleted compared with HIV-1 negative women.14,15 As inflammation has been associated with HIV-1 shedding, we hypothesized T helper cells and cytokines associated with inflammation (Th1 and Th17) would be increased in the uterine cervix at shedding compared with non-shedding visits in HIV-1 infected women. The immune milieu was assessed for T helper subsets (Th1, Th17, and TReg) using immunohistochemical staining of uterine cervical biopsies and for inflammatory cytokine/chemokine profiles in cervicovaginal lavage (CVL) using multiplexed Luminex assay in the same women with and without HIV-1 shedding. Additionally, a subset of women with intermittent HIV-1 genital shedding was evaluated longitudinally.
METHODS
Study Population and Design
Fifty-seven HIV-1-infected women from Seattle, WA and Rochester, NY had blood, cervical secretions, and cervical biopsy specimens taken every 3-4 months for up to five years from 2003-2008. Women with CVL and formalin fixed paraffin embedded (FFPE) cervical biopsy samples and with clinical assessments of genital health (sexually transmitted infections (STI), bacterial vaginosis (BV), cervicitis, and yeast) were included in the current study. Genital HIV-1 shedding was defined as HIV-1 RNA detected at >30 copies/mL. Subjects were categorized as described5 into non-shedders (HIV-1 never detected in CVL), intermittent shedders (at least 1 visit with and without shedding) or as persistent shedders (HIV-1 detected in CVL at all visits) based on shedding data from all visits in the parent study (median 6 visits, interquartile range (IQR): 5-10 visits). In the current study a smaller number of visits were assayed for each outcome due to limited sample availability (Supplemental Figure 1). All women provided informed consent through the University of Washington or University of Rochester Institutional Review Boards for participation in this study.
Specimen processing and assessments for STI and genital health were evaluated at each study visit as described elsewhere.3,16 Briefly, these included detection of: BV by gram stain using the Nugent criteria; Neisseria gonorrhoeaa and Chlamydia trachomatis by a combined nucleic acid test (Aptima Combo2, Gen Probe, San Diego) using urine or genital secretions; Lactobacillus and Candida albicans assessed by culture; and Trichomonis vaginalis by culture using the In-Pouch TV (BioMed Diagnostics, White City OR). Human papilloma virus (HPV) positivity was assessed via questionnaire and via pelvic examination. Serology at enrollment was used for detection of herpes simplex virus one (HSV-1), HSV-2, and cytomegalovirus (CMV).
Luminex assay for quantification of cytokines and chemokines
Ten mL of 1 × phosphate buffered saline (PBS) was used to wash the uterine cervix as described16 and divided into 1.5 mL aliquots and frozen at −80° C until thawed for cytokine assessments. Cytokines were selected to evaluate cell trafficking or growth (e.g. fractalkine, RANTES, SDF-1, TGF-β1, and IL-8; or IL-2, IL-7 and IL-15), and different T helper populations associated with inflammation (Th1: IFN-γ, IL-2, IL-12p40, IL-12p70; TReg: TGF-β1 and IL-10; Th17: TGF-β1, IL-6, IL-17, IL-21, IL-23) or general inflammation (IL-6, IL-10, TNF-α, sCD40L). Multiplexed Luminex assays were performed for fractalkine, IFN-γ, TNF-α, sCD40L, IL-2, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-15, IL-17 and in a separate multiplex plate for SDF-1, IL-21 and IL-23 (Millipore, Bellirica, MA). Single-plex assays were performed for TGF-β1 and RANTES (Luminex). Fifty uL of CVL fluid was assayed using secretions collected with 7 mL of 1 × PBS.16 Standard curves were used to establish the lower limit of quantification (LLQ); when factors were below the manufacturer's LLQ cytokine concentrations were set at half of LLQ. All longitudinal samples from a subject were assayed in duplicate on the same plate to avoid inter-plate variation. Intra-plate coefficient of variation (CV) was <2% (median 1.68% interquartile range (IQR): (0.34, 3.47) across cytokines, while inter-plate variation was higher (median, 6.48, IQR: 3.57, 12.62). Six cytokines had > 80% of assays below the LLQ (IFNγ, IL-2, IL-17, IL-21, IL-23, and SDF-1) and were not included in additional analyses.
Formalin Fixation of Cervical Biopsies and Immunohistochemistry (IHC)
Cervical biopsies were collected as described17 using Baby Tischler forceps and placed into 4% paraformaldehyde for four hours followed by 70% ethanol prior to embedding in paraffin. Cervical biopsies were cut into 5-micron sections using a Leica RM 2235 Microtome (Leica, Buffalo Grove, IL) and placed onto Superfrost Plus slides (Superfrost Plus, Fisher Scientific, Waltham, MA). Fixed tissues were deparaffinized in xylene and rehydrated in 100%, 95% and 70% ethanol followed by double distilled water.18
Antigen retrieval was performed for 60 min in a vegetable steamer in DIVA Decloaker (BioCare, Concord, CA) followed by cooling to room temperature (RT), then rinsed with 1 × Tris-buffered saline (TBS)/0.1% Tween. Endogenous peroxidases were quenched using 3% H202 peroxidase applied for 10 minutes (min) followed by a wash (submerging three times in 1x TBS/0.1%Tween). Sniper (BioCare) was applied to tissue sections for 10 min to block non-specific binding. The following rabbit anti-human antibodies (Ab) were applied individually: polyclonal FOXP3 (Abcam, Cambridge, MA), polyclonal T-bet (Santa Cruz Biotechnology, Dallas TX, clone H210), polyclonal RORγ (Abcam), or Ki67 (Dako, Carpinteria, CA, clone MIB-1) and incubated for 30 min at RT then washed and incubated with mouse anti-human Ab against CD3 (Biocare, Concord, CA, clone PS1) or CD8 (Santa Cruz Biotechnology, clone UCH-T4) for 30 min at RT. Sections were washed then incubated with MACH II (BioCare) containing secondary anti-mouse and anti-rabbit Ab conjugated to horse radish peroxidase or alkaline phosphatase, respectively. CD3 and CD8 were developed using Vulcan Red (BioCare) followed by development of FOXP3, T-bet, or Ki67 with DAB (BioCare). Attempts were made to optimize ROR-γ Ab staining, but background was too high for evaluation. Slides were counterstained with hematoxylin, rinsed with water and 1x TBS, and dehydrated with 95% then 100% ethanol then cover-slipped using VectaMount (Vector Labs, Burlingame, CA).18 Sections were scanned using a Nanozoomer 2.0 HT (Hamamatsu, San Jose, CA) and analyzed using Visiopharm software (Broomfield, CO). All cervical sections were counterstained with hematoxylin and evaluated microscopically for presence of histological abnormalities.
Statistical Analysis
A Fisher's exact test was used to compare the proportion of visits where a plasma viral load was detected in the absence or presence of genital HIV-1 shedding. A Kruskal-Wallis test was used to compare age at enrollment between shedding categories, and a Fisher's exact test was used to compare race by shedding status and the proportion of women with and without human papilloma virus (HPV). Generalized estimating equations (GEE) with an independent correlation matrix and robust standard errors was used to evaluate CD4 count and number of WBC in cervix or vagina across all study visits between never, intermittent, and persistent shedders.
Generalized estimating equations were used to evaluate associations between HIV-1 shedding, BV, yeast, and cervicitis and used for log10 transformed cytokine/chemokine concentrations and the percent of tissue staining positive for different markers. Analyses were adjusted for log10 HIV-1 plasma viral load, BV positivity, cervicitis, and yeast, and accounted for multiple visits within a woman. Measures were initially compared between non-shedding and shedding visits within intermittent shedders, followed by comparing non-shedding and shedding visits across all women. Subsequent comparisons were performed within non-shedding visits between never–shedders and intermittent shedders, within shedding visits between the intermittent and persistent shedders, and between never and persistent shedders. All analyses were conducted using Stata SE v12 (StataCorp, College Station, Texas). All tests were two-sided and p-values <0.05 were considered significant.
RESULTS
Study Visits and Subject Characteristics
Fifty-seven women (38 from Seattle and 19 from Rochester) were enrolled in the original study; 39 (30 from Seattle and 9 from Rochester) subjects had CVL, FFPE samples and physical assessments of genital health and were included in the present sub-study. Cytokines assessments included 39 subjects with 50 non-shedding and 31 shedding visits, whereas IHC assessments included 27 subjects with 29 non-shedding and 14 shedding visits. Twenty-one women were classified as non-shedders (based on median of 6 (IQR 3-9) study visits), 14 as intermittent shedders (based on median of 8 (IQR 5-10) study visits), and four as persistent shedders (based on median of 5 (IQR 3-11) study visits). Within intermittent shedders there were 14 subjects for cytokine assessments with 20 non-shedding and 21 shedding visits, while for IHC assessments there were 10 subjects with 9 non-shedding and 10 shedding visits (Supplemental Figure 1). The majority of the 39 subjects were either African American (59%) or White women (26%) with fewer from other racial/ethnic groups [Pacific Islander (5%), Hispanic (5%), American Indian, (3%) and other (3%)].
Genital Shedding, Plasma Viral Load, and Sexually Transmitted Infections
Genital shedding was more frequently detected when plasma viral loads were detectable; of the non-shed visits 25/50 (50%) had detectable plasma viral load whereas all shed visits (31/31, 100%) had detectable plasma viral load (p <0.001). The median genital HIV-1 concentration at shedding visits was less than 1,000 copies/mL (median 771 copies/mL, IQR: 126 – 5,270 copies/mL). There were no significant associations between age at enrollment and HIV-1 shedding (p=0.25), or in longitudinal analysis adjusting for plasma viral load between CD4 count (p=0.08), the number of WBC in the cervix (p=0.32) or vagina (p=0.70) and HIV-1 shedding category. African American women made up 59% of this cohort, and represented a significantly larger proportion of intermittent (93%) than never (38%) or persistent shedders (50%; p=0.002). None of the women in this cohort had detectable N. gonorrhoeaa or C. trachomatis. Three women had T. vaginalis detected at a total of four study visits; two of these women had persistent genital shedding. Twenty-two of 39 women were BV positive at 33/81 (41%) study visits, and 10/39 women had yeast at 20/81 (25%) study visits. Thirteen of the 39 women reported having HPV, but there were no significant differences between shedding categories (p=1.00). Similar to our previous reports,3,12 BV and yeast were significantly associated with HIV-1 shedding, however after adjusting for plasma viral load only BV remained significantly associated with shedding (mean log10 difference (β) 1.42, IQR: 0.81, 2.02, p<0.0001). Because STI were of low prevalence, they were not included in additional analyses. However, BV, cervicitis, and yeast were adjusted for in the current analysis.
Cytokines/Chemokines Associated with HIV-1 Genital Shedding
Within the intermittent shedders after adjustment for plasma viral load, BV, cervicitis, and yeast, fractalkine, IL-7, and IL-12p70 remained significantly increased at shedding visits compared with non-shedding visits (Table 1). Within intermittent shedders mean log10 IL-10 trended higher at shedding visits (β 0.16, IQR: −0.03, 0.34, p=0.10). Across all subjects when comparing shedding to non-shedding visits fractalkine (β 0.39, IQR: 0.17, 0.61; p=0.001), IL-7 (β 0.29, IQR: 0.10, 0.47; p=0.003), IL-10 (β 0.23, IQR: 0.08, 0.38; p=0.003), IL12p70 (β 0.18, IQR: 0.08, 0.29; p=0.001) and sCD40L (β 0.17, IQR: −0.001, 0.34; p=0.05) were significantly increased at shedding visits. Six cytokines had concentrations below LLQ for >80% of their evaluations and were therefore excluded from the final analysis.
Table 1.
Cytokines and chemokines associated with cell growth and cellular homeostasis are associated with genital HIV-1 shedding.
| Cytokine or Chemokine | Never shedders* (n= 21 subjects) Median
concentration (IQRπ) |
Intermittent shedders* (n=14 subjects)
Median concentration (IQRπ) |
Persistent shedders* (n=4 subjects)
Median concentration (IQRπ) |
|
|---|---|---|---|---|
| Non-shed visits (n=30)^ | Non-shed visits (n=20) | Shed visits (n=21)^ | Shed visits (n=10)^ | |
| FRACTALKINE | 47.3 (16.9, 92.0) | 18.3† (8.0, 61.6) | 58.1†,£ (31.9, 153) | 47.1£ (22.7, 64.3) |
| RANTES | 4.8 (1.6, 9.2) | 5.4 (4.5, 7.7) | 5.9 (5.2, 22.5) | 6.1 (1.6, 13.8) |
| sCD40L | 1.6¥ (1.6, 6.7) | 1.6 (1.6, 1.6) | 1.6 (1.6, 1.6) | 1.6¥ (1.6, 4.5) |
| TGF-β1 | 26.0£ (4.9, 84.7) | 92.0£ (17.0, 125) | 36.3 (4.9, 200) | 13.9 (4.9, 50.9) |
| TNF-α | 1.6 (1.6, 1.6) | 1.6 (1.6, 3.3) | 1.6 (1.6, 4.6) | 1.6 (1.6, 3.8) |
| IL-6 | 3.5 (1.6, 9.4) | 3.9 (3.3, 5.3) | 4.0 (1.6, 6.5) | 3.9 (1.6, 8.4) |
| IL-7 | 8.2 (1.6, 14.7) | 6.7† (1.6, 12.2) | 13.0† (8.1, 21.2) | 6.8 (4.4, 14.9) |
| IL-8 | 659£ (315, 2,429) | 917£ (378, 2,600) | 1,039£ (550, 2,663) | 533£ (268, 639) |
| IL-10 | 3.8¥ (1.6, 4.5) | 1.6 (1.6, 2.6) | 1.6£ (1.6, 4.4) | 4.5¥,£ (4.3, 7.0) |
| IL-12p40 | 9.8 (6.2, 17.4) | 6.3 (3.9, 13.1) | 7.6 (6.3, 14.3) | 6.1 (3.5, 9.8) |
| IL-12p70 | 3.3 (1.6, 4.1) | 1.6† (1.6, 1.6) | 3.2† (1.6, 3.9) | 3.5 (1.6, 4.6) |
| IL-15 | 1.6¥ (1.6, 3.7) | 1.6 (1.6, 3.4) | 1.6 (1.6, 3.6) | 2.5¥ (1.6, 6.9) |
Comparisons were made using generalized estimating equations (GEE) with an independent correlation matrix and robust standard errors, after adjusting for log10 HIV-1 plasma viral load, the presence or absence of bacterial vaginosis, cervicitis and yeast of the following groups: 1) Non-shedding and shedding visits within intermittent shedders 2) Non-shedding visits between never shedders and intermittent shedders 3) Shedding visits between intermittent and persistent shedders 4) and never shedders to persistent shedders.
IQR, Interquartile range
Bold indicates statistical significance ≤ 0.001 within intermittent shedders. Cytokines where the concentration was below the lower limit of quantification (LLQ) had concentration set at half of LLQ. Six cytokines had > 80% of the assays below the LLQ (IFNγ, IL-2, IL-17, IL-21, IL-23, and SDF-1) and were not included in the final analyses.
Bold indicates statistical significance < 0.05 at non-shedding visits between never and intermittent shedders or between shedding visits between intermittent and persistent shedders.
Bold indicates statistical significance < 0.05 comparing never shedders to persistent shedders.
The number of visits was variable across all study groups for each cytokine due to specimen depletion: for never shedders RANTES and TGF-β1 had 29 study visits; for intermittent shedders at the shedding visit TGF-β had 20 visits and IL-8 had 19 visits; for persistent shedders RANTES had 7 visits and TGF-β1 had 8 visits.
We further explored whether there were differences in cytokines concentrations between shedding categories. Fractalkine was slightly lower within intermittent shedders at non-shedding visits compared to never shedders, but did not reach significance. However, fractalkine was significantly higher at shedding visits from intermittent shedders than within persistent shedders. There were no significant differences in fractalkine between never and persistent shedders (Table 1). IL-7 concentrations were not significantly different between never and persistent shedders, and intermittent shedders at non-shedding visits. IL-10 was significantly higher in the persistent shedders compared with intermittent shedders at shedding visits. IL-12p70 was slightly lower at non-shedding visits from intermittent shedders compared to never shedders, but this did not reach significance. IL-12p70 concentrations were similar between never and persistent shedders (Table 1).
T cell Subtypes Associated with Genital Shedding
Within intermittent shedders the median percent of tissues staining positive for T cell markers were generally lower at shedding visits compared to non-shedding visits (Table 2). After adjusting for plasma viral load, BV, cervicitis, and yeast, within the intermittent shedders there was a significant decrease in the percent of tissue staining FOXP3+ (β −0.24, IQR: −1.39, 0.05; p<0.0001), and tissues staining positive for CD3+FOXP3+ (β −0.67, IQR: −1.39, 0.05; p=0.07) and T-bet+ cells (β 0.67, IQR: −1.42, 0.08; p=0.08) trended lower at shedding compared to non-shedding visits (Table 2). When comparing non-shedding to shedding visits across all subjects the percent of tissue staining positive for FOXP3+ remained significantly decreased with HIV-1 shedding (β −0.21, IQR: −0.38, −0.05; p=0.01).
Table 2.
HIV-1 shedding is associated with decreases in FOXP3+ and T-bet+ cells in women with intermittent shedding and gradual increases in CD8+FOXP3+ T cells.
| T cell populations | Never shedders* (n=14 subjects) Median percent (%) of
tissues staining positive (IQR) |
Intermittent shedders* (n=10 subjects)
Median percent (%) of tissues staining positive (IQR) |
Persistent shedders* (n=3 subjects)
Median percent (%) of tissues staining positive (IQR) |
|
|---|---|---|---|---|
| Non-shed visits (n=18)^ | Non-shed visits (n=9)^ | Shed visits (n=10)^ | Shed visits (n= 3)^ | |
| CD3+ | 0.16 (0.06, 0.32) | 0.65 (0.19, 1.47) | 0.24 (0.14, 1.11) | 1.01 (0.02, 2.03) |
| CD8+ | 0.98¥ (0.66, 2.34) | 2.06 (1.27, 5.32) | 1.70£ (0.70, 2.62) | 8.66¥,£ (7.78, 9.53) |
| FoxP3+ | 0.03¥ (0.02, 0.08) | 0.05† (0.03, 0.07) | 0.04†,£ (0.02, 0.07) | 0.03¥,£ (0.01, 0.07) |
| T-bet+ | 0.10 (0.03, 0.52) | 0.07 (0.04, 0.20) | 0.03£ (0.01, 0.05) | 0.26£ (0.10, 2.83) |
| CD3+ FoxP3+ | 0.02 (0.01, 0.06) | 0.03 (0.01, 0.31) | 0.01 (0.00, 0.19) | 0.14 (0.00, 0.19) |
| CD3+ T-bet+ | 0.48¥ (0.23, 0.82) | 2.33 (0.63, 2.61) | 0.80 (0.52, 1.86) | 0.70¥ (0.50, 0.71) |
| CD8+ FoxP3+ | 0.20¥ (0.08, 0.37) | 0.22* (0.16, 0.75) | 0.18†,£ (0.10, 0.33) | 1.07¥,£ (0.19, 1.94) |
| Ki67+ | 1.28¥ (0.76, 1.69) | 1.50 (0.87, 3.23) | 0.97¥,£ (0.68, 1.46) | 0.96£ (0.63, 1.30) |
Comparisons were made using generalized estimating equations (GEE) with an independent correlation matrix and robust standard errors, after adjusting for log10 HIV-1 plasma viral load, the presence or absence of bacterial vaginosis, cervicitis and yeast of the following groups: 1) Non-shedding and shedding visits within intermittent shedders 2) Non-shedding visits between never shedders and intermittent shedders 3) Shedding visits between intermittent and persistent shedders 4) and never shedders to persistent shedders.
Bold indicates statistical significance < 0.05 within intermittent shedders.
Bold indicates statistical significance < 0.05 at non-shedding visits between never and intermittent shedders or between shedding visits between intermittent and persistent shedders.
Bold indicates statistical significance < 0.05 comparing never shedders to persistent shedders.
The number of visits per T cell populations for staining was variable across study groups due to specimen depletion: for never shedders FOXP3+ had 20 study visits, CD3+ and CD3+FOXP3+ had 19 visits, and CD3+T-bet+ had 17 visits; for intermittent shedders at non-shedding visits Ki67+ had 7 visits, while at shedding visits Ki67+ had 9 visits, and CD8+, T-bet+, CD3+T-bet+, and CD8+FOXP3+ had 8 visits; for persistent shedders CD3+ had 4 visits, and CD8+ CD8+FOXP3+, and Ki67+ had two visits.
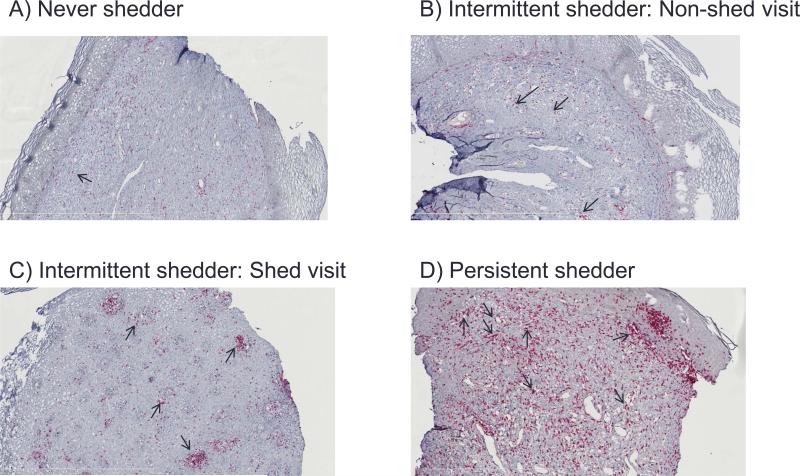
When T cell populations were evaluated by category there was a gradual increase in the percent of tissues staining positive for CD8+ and CD8+FOXP3+ T cells from non-shedders to intermittent and persistent shedders, with persistent shedders having significantly higher CD8+ and CD8+FOXP3+ T cells (CD8+ β 0.41, IQR: 0.11, 0.72; p=0.008; CD8+FOXP3+ β 0. 1.02, IQR: 0.50, 1.54; p<0.0001) (Figure 1 and Table 2). Conversely, there was a gradual decrease in the tissue staining Ki67+ from non-shedders to intermittent (β −0.06, IQR: −0.12, −0.37; p=0.03) and persistent shedders with persistent shedders having significantly less proliferation compared with never shedders (β −0.59, IQR: −0.81, −0.37; p <0.0001) (Table 2). Comparing never shedders to intermittent shedders, non-shedding visits showed no significant differences in T cell populations. A significant decrease in FOXP3+ T cells was noted in persistent shedders compared with never shedders (β −0.61, IQR: −1.13, −0.10; p =0.02). CD3+ T-bet cells were significantly increased in persistent shedders compared with non-shedders (Table 2).
Figure 1.
Intermittent and persistent HIV-1 shedding are associated with increases in CD8+FOXP3+ T cells. Immunohistochemistry (IHC) of uterine cervix biopsies from a never shedder, intermittent shedder, and persistent shedder showing increased staining of CD8+ and CD8+FOXP3+ T cells associated with HIV-1 shedding. Polyclonal rabbit anti-human FOXP3 followed by incubation with mouse anti-human CD8. Mach II containing ployclonal secondary anti-mouse and anti-rabbit antibody conjugated to horse radish peroxidase and alkaline phosphatase, respectivly were incubated as described in materials and methods. FOXP3 was developed using DAB (brown) and CD8 was developed using Vulcan Red (pink). Tissue sections were scanned using a Nanozoomer and analyzed using Visiopharm software at 10x magnification. Arrows indicate cells that are CD8+FOXP3+ dual stained.
Th17 cells were not evaluated in these subjects because of high background staining. No clinically significant histological abnormalities were observed in cervical biopsies upon microscopic examination in women from this study.
DISCUSSION
The primary findings from this study are increases in cytokine/chemokine profiles associated with cell growth and recruitment of Th1 or TReg cells, most apparent in intermittent shedders, when subjects had HIV-1 shedding from the genital tract. Women with intermittent shedding further showed decreases in FOXP3+, with trends towards decreasing CD3+FOXP3 and T-bet+ T cells at shedding visits in contrast to our original hypothesis. There were significant increases in cytokines/chemokines accompanied by decreases in cell populations observed in the overall analysis. However, longitudinally when we evaluate intermittent shedders, who contribute both non-shedding and shedding visits, the associations become stronger and in the IHC analysis these associations became more apparent. Specifically, we observed significant increases in chemokines that promote recruitment of Th1 and FOXP3+ T cells to tissue sites (IL-7, fractalkine),19,20 and cytokines that maintain memory T cells (IL-7) and Th1/inflammation (IL-12p70). Importantly, the mucosal microenvironment associated with active HIV-1 shedding in this cohort is consistent with local viral replication1,2,5,21 in the genital tract or associated lymph nodes rather than the passive transfer of virus from plasma to genital tissues.22 The decreased number of FOXP3+ and T-bet+ T cells in the cervix during genital shedding may be due to increased cell death associated with viral replication,23 changes in T cell phenotype due to down-regulation of FOXP3+ associated with increases in IL-7,24,25 or may reflect trafficking of these cells to local lymph nodes where HIV-1 is thought to primarily replicate.26-28 This work is to our knowledge, the first to evaluate the relationship of Th1 and TReg subsets at the uterine cervix and genital HIV-1 shedding. As CD4+ T helper cells represent the major target in vivo for HIV-1 infection it will be important to better characterize these subtypes in the FGT within larger cohorts of HIV-1 infected women.
Previous reports support our findings that HIV-1 shedding correlates with chemokines/cytokines that attract Th1 and TReg cells to the genital tract; i.e., fractalkine,19,31-35 but this is not consistent across studies.6 Increased concentrations of IL-7, a critical factor for maintenance of memory T cells in tissues36 has been reported, but was no longer significant after controlling for false discovery rates.34 Our current analysis did not adjust for false discovery rates, although the factors identified in this study were consistently significant in cross-sectional and longitudinal analysis after adjusting for plasma viral load and genital BV, cervicitis, and yeast, suggesting the signals are robust. IL-10 and IL-12p70 were also associated with HIV-1 shedding in this and other reports,4,35,37,38 but in this cohort only IL-12p70 remained significant after adjusting for BV, cervicitis, and yeast. We observed unique cytokine patterns within intermittent shedders, particularly elevated pro-inflammatory cytokine (TGF-β and IL-8) concentrations suggest the intermittent shedders are different from never or persistent shedders. These data suggest that our intermittent shedders were a “homogenous” population that may ordinarily have more inflammation or other underlying conditions possibly due to the prevalence of African American women in this group.39-41 In this study 1/3 of the women reported a history of HPV, however the proportion of women with HPV was similar across categories. Other differences in study outcomes may include how cytokines at or below the LLQ are evaluated,4,31,42,43 sensitivities between assay platforms at the LLQ (multiplexed Luminex assays vs. ELISA),1,4,6,35,38 sample processing1,32,35 and volumes used to assay for cytokines.4,42
Across shedding categories we observed a gradual increase in the percent of tissue staining positive for CD8+FOXP3+ T cells in parallel with decrease in cell proliferation (Ki67+ cells) a relationship that has not been previously described. These data suggest sustained viral replication may promote expansion of CD8+FOXP3+ TReg cells due to chronic antigen expression at sites of replication as noted in SIV infected macaques29 that may inhibit cellular proliferation30 and suppress the immune response to HIV-1. Our data are in agreement with cross-sectional studies showing increased or stable CD3+ and CD8+ T cells,44,45 but differ from another study showing increases in Ki67+ cells associated with genital HIV-1 shedding2 rather than the decreases observed here. The increases in CD8+FOXP3+ T cells with concurrent decreases in cell proliferation suggest accumulation of TReg cells at the uterine cervix may contribute to shedding in some HIV-1 infected women. Discrepancies across studies may be attributed to different study populations (Kenya vs. United States) with different ART histories (naïve vs. experienced),44 differences in the samples collected,2 and assays used to assess T cell populations.2,45 This work expands on previous studies showing Th17 cells are depleted at the uterine cervix early after HIV-1 infection14,48 by demonstrating Th1 and TReg cells are either depleted or migrate to sites of viral replication in women with chronic infection when HIV-1 is actively being shed. While we were unable to evaluate whether Th17 cells were depleted during HIV-1 shedding it is likely that all CD4+ T cell subsets that are permissible for HIV-1 infection are depleted.
There are several strengths to the current study including the dynamic complexity of studying cytokines and cells in the FGT and examining within an individual as she transitions from a non-shedding to a shedding time point. The data emphasize the strength of longitudinal studies, as increases in cytokine/chemokine concentrations and changes in T cell populations were mostly or only apparent within women that contributed a non-shedding and shedding visit for the study. The fact that this detailed and longitudinal evaluation largely confirmed what has been observed by us3,12 and others31-35 in cross-sectional studies is encouraging and implies that our collective understanding of what is happening in the FGT is expanding.
The limitations to the current study include the relatively small number of women available to perform analysis of transitions from non-shedding and shedding visits. Th17 and Th2 cells were not evaluated in this study because of high background with RORγ antibody and depletion of cervical biopsy samples, so these results remain exploratory.
In summary, cytokine/chemokine profiles associated with recruitment and maintenance of Th1 and TReg cells coupled with decreases in FOXP3+ and T-bet+ cells at mucosal sites were associated with HIV-1 shedding in the FGT. Increases in CD8+FOXP3+ T cells at the uterine cervix suggest women with chronic shedding may develop a more immunosuppressive environment that inhibits immune responses to HIV-1 shedding. Further research is required to confirm and extend these results to better understand the microenvironment that promotes HIV-1 shedding within larger longitudinal cohorts. Defining the microenvironment in the FGT associated with genital HIV-1 shedding is critically important to advance our understanding of sexual and perinatal mechanisms that promote HIV-1 transmission and might eventually provide insight into the development of targeted vaccine or microbicide interventions.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the women who participated in the Women's HIV Interdisciplinary Network (WHIN) study and the work of the clinical personnel (Lauren Asaba, Jane Reid, and Norma Nunez).
Source of Funding: This study was supported by a grant from the National Institute of Health (NIH) to R.W. Coombs (PO1 HD40540-04) and J. Hitti (NIH R21 AI080439-02-S-1). This publication was supported in part by the University of Washington Center for AIDS Research (P30 AI027757), an NIH funded program.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Lawn SD, Subbarao S, Wright TC, Jr., et al. Correlation between human immunodeficiency virus type 1 RNA levels in the female genital tract and immune activation associated with ulceration of the cervix. J Infect Dis. 2000 Jun;181(6):1950–1956. doi: 10.1086/315514. [DOI] [PubMed] [Google Scholar]
- 2.Jaspan HB, Liebenberg L, Hanekom W, et al. Immune activation in the female genital tract during HIV infection predicts mucosal CD4 depletion and HIV shedding. J Infect Dis. 2011 Nov 15;204(10):1550–1556. doi: 10.1093/infdis/jir591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell C, Hitti J, Paul K, et al. Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retrov. 2011 Jan;27(1):35–39. doi: 10.1089/aid.2010.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blish CA, McClelland RS, Richardson BA, et al. Genital Inflammation Predicts HIV-1 Shedding Independent of Plasma Viral Load and Systemic Inflammation. J Acq Iimmun Def Synd (1999) 2012 Dec 1;61(4):436–440. doi: 10.1097/QAI.0b013e31826c2edd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homans J, Christensen S, Stiller T, et al. Permissive and protective factors associated with presence, level, and longitudinal pattern of cervicovaginal HIV shedding. J Acq Iimmun Def Synd (1999) 2012 May 1;60(1):99–110. doi: 10.1097/QAI.0b013e31824aeaaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold BC, Keller MJ, Shi Q, et al. Plasma and mucosal HIV viral loads are associated with genital tract inflammation in HIV-infected women. J Acq Iimmun Def Synd (1999) 2013 Aug 1;63(4):485–493. doi: 10.1097/QAI.0b013e3182961cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanton C, Weiss HA, Le Goff J, et al. Correlates of HIV-1 genital shedding in Tanzanian women. PLoS One. 2011;6(3):e17480. doi: 10.1371/journal.pone.0017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichelderfer PS, Coombs RW, Wright DJ, et al. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. AIDS (London, England) 2000 Sep 29;14(14):2101–2107. doi: 10.1097/00002030-200009290-00005. [DOI] [PubMed] [Google Scholar]
- 9.Curlin ME, Leelawiwat W, Dunne EF, et al. Cyclic Changes in HIV Shedding From the Female Genital Tract During the Menstrual Cycle. J Infect Dis. 2013 May;207(10):1616–1620. doi: 10.1093/infdis/jit063. [DOI] [PubMed] [Google Scholar]
- 10.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9(6):e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mane A, Kulkarni S, Ghate M, Risbud A, Thakar M. HIV-1 RNA shedding in the female genital tract is associated with reduced quantity of Lactobacilli in clinically asymptomatic HIV-positive women. Diagn Microbiol Infect Dis. 2013 Jan;75(1):112–114. doi: 10.1016/j.diagmicrobio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell C, Balkus JE, Fredricks D, et al. Interaction between lactobacilli, bacterial vaginosis-associated bacteria, and HIV Type 1 RNA and DNA Genital shedding in U.S. and Kenyan women. AIDS Res Hum Retrov. 2013 Jan;29(1):13–19. doi: 10.1089/aid.2012.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adurthi S, Mukherjee G, Krishnamurthy H, et al. Functional tumor infiltrating TH1 and TH2 effectors in large early-stage cervical cancer are suppressed by regulatory T cells. Int J Gynecol Cancer. 2012 Sep;22(7):1130–1137. doi: 10.1097/IGC.0b013e318262aa53. [DOI] [PubMed] [Google Scholar]
- 14.El Hed A, Khaitan A, Kozhaya L, et al. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J Infect Dis. 2010 Mar 15;201(6):843–854. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinnon LR, Nyanga B, Chege D, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011 Dec 1;187(11):6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell CM, Balkus J, Agnew KJ, et al. Bacterial vaginosis, not HIV, is primarily responsible for increased vaginal concentrations of proinflammatory cytokines. AIDS Res Hum Retrov. 2008 May;24(5):667–671. doi: 10.1089/aid.2007.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bull ME, Learn GH, McElhone S, et al. Monotypic human immunodeficiency virus type 1 genotypes across the uterine cervix and in blood suggest proliferation of cells with provirus. J Virol. 2009 Jun;83(12):6020–6028. doi: 10.1128/JVI.02664-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Path. 2009 Feb;5(2):e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraticelli P, Sironi M, Bianchi G, et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001 May;107(9):1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabin RL, Alston MA, Sircus JC, et al. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J Immunol. 2003 Sep 15;171(6):2812–2824. doi: 10.4049/jimmunol.171.6.2812. [DOI] [PubMed] [Google Scholar]
- 21.Wright TC, Jr., Subbarao S, Ellerbrock TV, et al. Human immunodeficiency virus 1 expression in the female genital tract in association with cervical inflammation and ulceration. Am J Obstet Gynecol. 2001 Feb;184(3):279–285. doi: 10.1067/mob.2001.108999. [DOI] [PubMed] [Google Scholar]
- 22.Iversen AK, Larsen AR, Jensen T, et al. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J Infect Dis. 1998;177(5):1214–1220. doi: 10.1086/515266. [DOI] [PubMed] [Google Scholar]
- 23.Shearer WT. HIV infection and AIDS. Prim Care. 1998 Dec;25(4):759–774. doi: 10.1016/s0095-4543(05)70086-5. [DOI] [PubMed] [Google Scholar]
- 24.Levy Y, Sereti I, Tambussi G, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012 Jul;55(2):291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013 Jan 3;121(1):29–37. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 26.Chen JJ, Huang JC, Shirtliff M, et al. CD4 lymphocytes in the blood of HIV(+) individuals migrate rapidly to lymph nodes and bone marrow: support for homing theory of CD4 cell depletion. J Leukoc Biol. 2002 Aug;72(2):271–278. [PubMed] [Google Scholar]
- 27.Andersson J, Boasso A, Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005 Mar 15;174(6):3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 28.Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci US A. 2007 Feb 27;104(9):3390–3395. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George J, Cofano EB, Lybarger E, et al. Early short-term antiretroviral therapy is associated with a reduced prevalence of CD8(+)FoxP3(+) T cells in simian immunodeficiency virus-infected controller rhesus macaques. AIDS Res Hum Retrov. 2011 Jul;27(7):763–775. doi: 10.1089/aid.2010.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki M, Jagger AL, Konya C, et al. CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells. J Immunol. 2012 Sep 1;189(5):2118–2130. doi: 10.4049/jimmunol.1200122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Harthi L, Kovacs A, Coombs RW, et al. A menstrual cycle pattern for cytokine levels exists in HIV-positive women: implication for HIV vaginal and plasma shedding. AIDS (London, England) 2001 Aug 17;15(12):1535–1543. doi: 10.1097/00002030-200108170-00011. [DOI] [PubMed] [Google Scholar]
- 32.Iversen AK, Attermann J, Gerstoft J, Fugger L, Mullins JI, Skinhoj P. Longitudinal and cross-sectional studies of HIV-1 RNA and DNA loads in blood and the female genital tract. Eur J Obstet Gynecol Reprod Biol. 2004 Dec 1;117(2):227–235. doi: 10.1016/j.ejogrb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Rebbapragada A, Howe K, Wachihi C, et al. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. J Acq Iimmun Def Synd (1999) 2008 Dec 15;49(5):520–522. doi: 10.1097/QAI.0b013e318189a7ca. [DOI] [PubMed] [Google Scholar]
- 34.Blish CA, Dogan OC, Jaoko W, et al. Cellular immune responses and susceptibility to HIV-1 superinfection: a case-control study. AIDS (London, England) 2012 Mar 13;26(5):643–646. doi: 10.1097/QAD.0b013e3283509a0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukura LR, Ghosh M, Fahey JV, Cu-Uvin S, Wira CR. Genital tract viral load in HIV Type 1-positive women correlates with specific cytokine levels in cervical-vaginal secretions but is not a determinant of infectious virus or anti-HIV activity. AIDS Res Hum Retrov. 2012 Nov;28(11):1533–1539. doi: 10.1089/aid.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007 Jun;19(3):320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Zara F, Nappi RE, Brerra R, Migliavacca R, Maserati R, Spinillo A. Markers of local immunity in cervico-vaginal secretions of HIV infected women: implications for HIV shedding. Sex Transm Infect. 2004 Apr;80(2):108–112. doi: 10.1136/sti.2003.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummins JE, Christensen L, Lennox JL, et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retrov. 2006 Aug;22(8):788–795. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 39.Suthanthiran M, Li B, Song JO, et al. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci US A. 2000 Mar 28;97(7):3479–3484. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fantasia J, Lin CB, Wiwi C, et al. Differential levels of elastin fibers and TGF-beta signaling in the skin of Caucasians and African Americans. J Dermatol Sci. 2013 Jun;70(3):159–165. doi: 10.1016/j.jdermsci.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Lee SB, Kanasaki K, Kalluri R. Circulating TGF-beta1 as a reliable biomarker for chronic kidney disease progression in the African-American population. Kidney Int. 2009 Jul;76(1):10–12. doi: 10.1038/ki.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowley-Nowick PA, Ellenberg JH, Vermund SH, Douglas SD, Holland CA, Moscicki AB. Cytokine profile in genital tract secretions from female adolescents: impact of human immunodeficiency virus, human papillomavirus, and other sexually transmitted pathogens. J Infect Dis. 2000 Mar;181(3):939–945. doi: 10.1086/315311. [DOI] [PubMed] [Google Scholar]
- 43.Spear GT, Zariffard MR, Chen HY, et al. Positive association between HIV RNA and IL-6 in the genital tract of Rwandan women. AIDS Res Hum Retrov. 2008 Jul;24(7):973–976. doi: 10.1089/aid.2008.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirbod T, Kimani J, Tjernlund A, et al. Stable CD4 expression and local immune activation in the ectocervical mucosa of HIV-infected women. J Immunol. 2013 Oct 1;191(7):3948–3954. doi: 10.4049/jimmunol.1301220. [DOI] [PubMed] [Google Scholar]
- 45.Gibbs A, Hirbod T, Li Q, et al. Presence of CD8+ T cells in the ectocervical mucosa correlates with genital viral shedding in HIV-infected women despite a low prevalence of HIV RNA-expressing cells in the tissue. J Immunol. 2014 Apr 15;192(8):3947–3957. doi: 10.4049/jimmunol.1302826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim A, French MA, Price P. CD4+ and CD8+ T cells expressing FoxP3 in HIV-infected patients are phenotypically distinct and influenced by disease severity and antiretroviral therapy. J Acq Iimmun Def Synd (1999) 2009 Jul 1;51(3):248–257. doi: 10.1097/QAI.0b013e3181a74fad. [DOI] [PubMed] [Google Scholar]
- 47.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006 May 11;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 48.Chaput N, Louafi S, Bardier A, et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009 Apr;58(4):520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.