Abstract
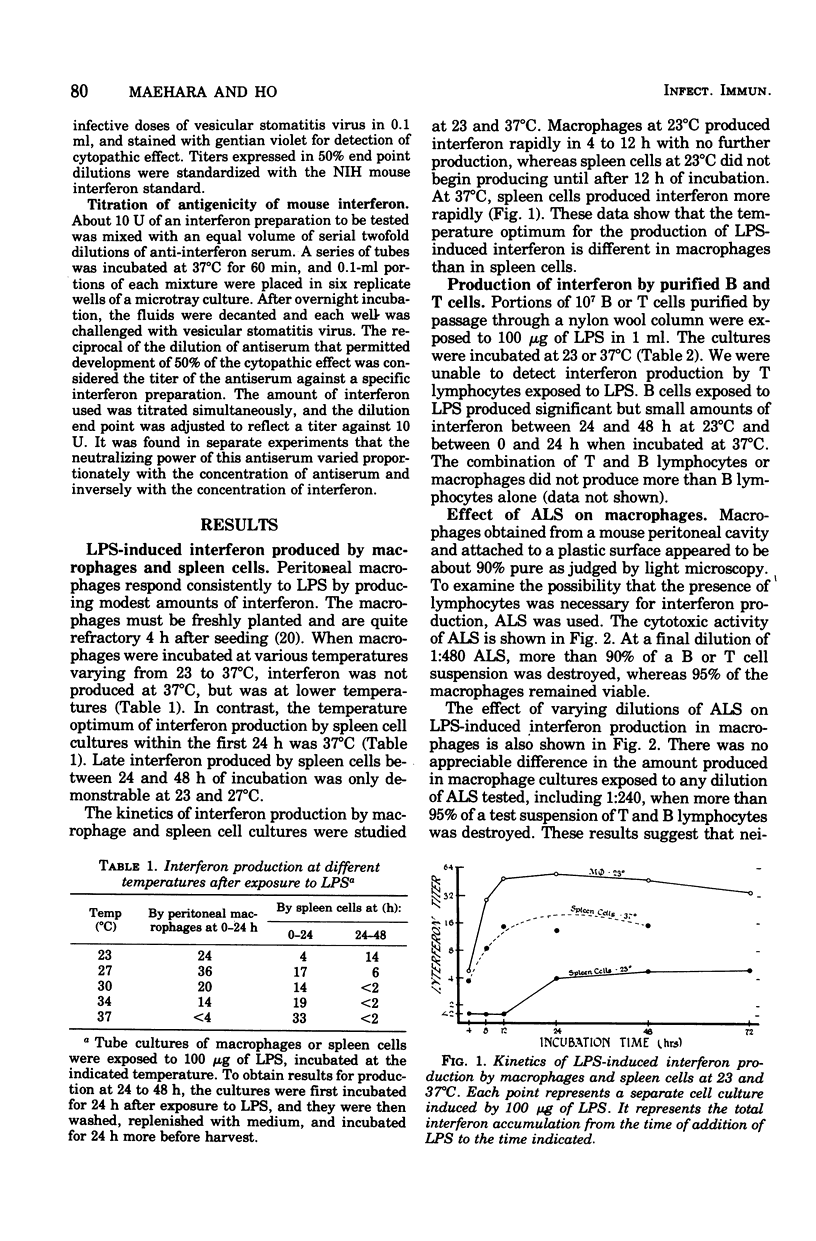
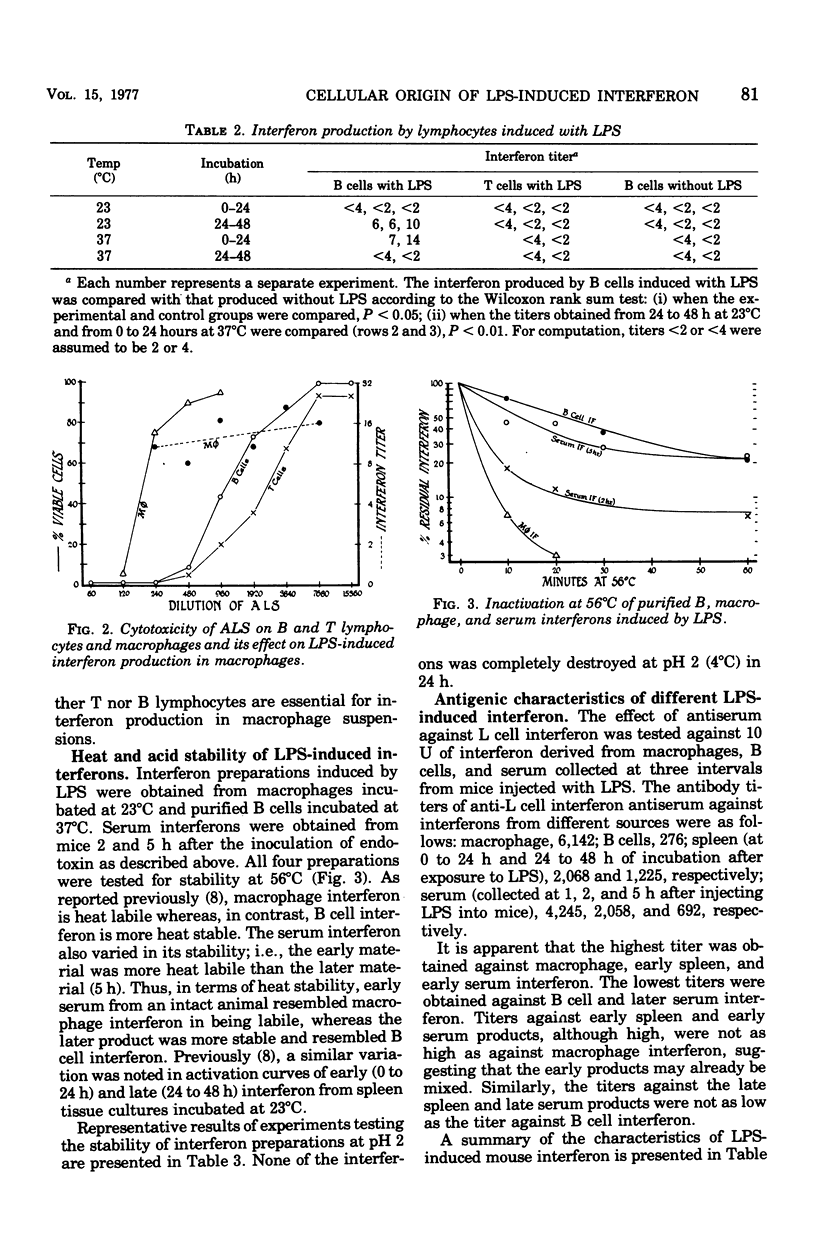
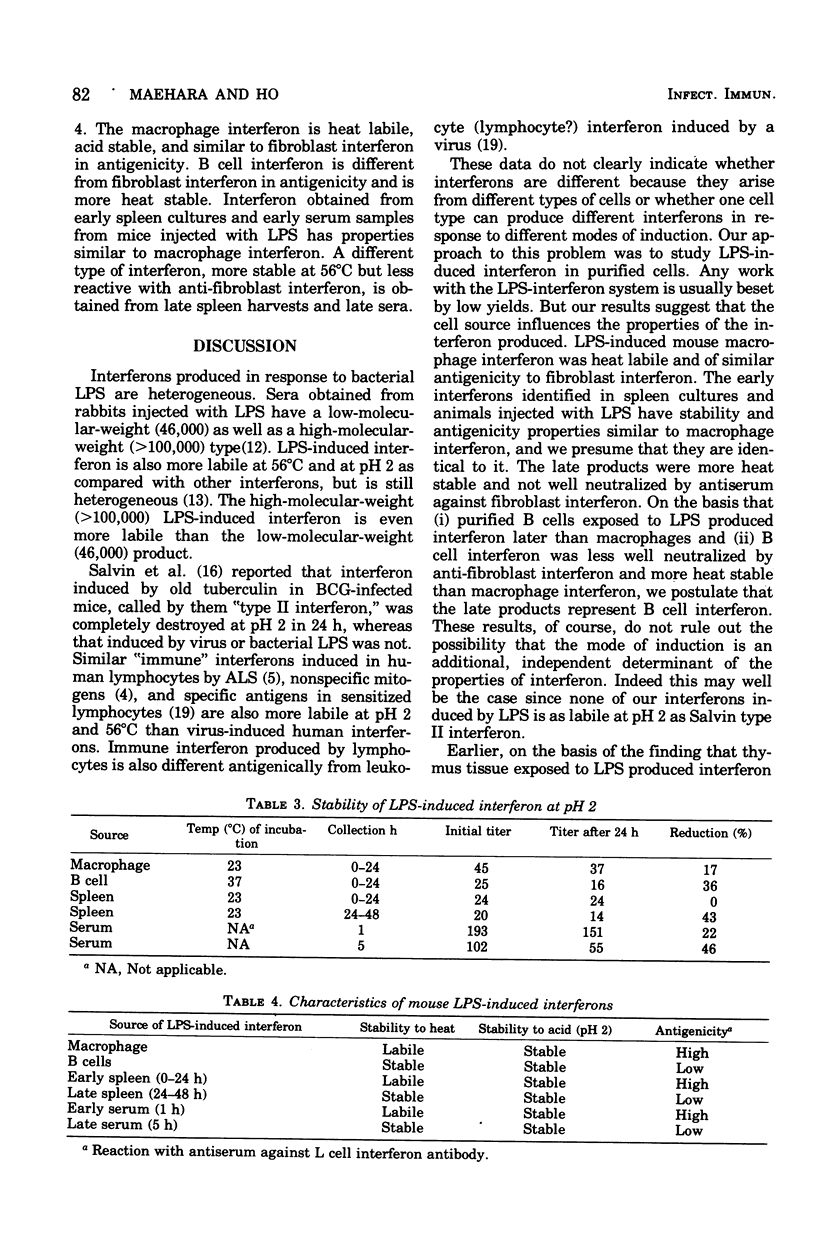
Bacterial lipopolysaccharide (LPS) induces interferons with different properties in mouse macrophages and B lymphocytes. Macrophage interferon is labile at 56 degrees C and is neutralized by anti-mouse fibroblast interferon at a dilution of 1:6,142. B cell interferon is more heat stable and is neutralized by the same antiserum only at a dilution of 1:276. Serum obtained early (1 h) after an intravenous injection of 100 mug of LPS resembled macrophage interferon, whereas serum obtained at later times resembled more and more B cell interferon. The diverse cellular origin of LPS-induced interferon may explain the broad hyporesponsiveness produced by LPS in animals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., Page R. C. Effects of endotoxin on macrophages and other lymphoreticular cells. J Infect Dis. 1973 Jul;128(Suppl):212–219. doi: 10.1093/infdis/128.supplement_1.s212. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borecký L., Lackovic V., Waschke K. Enhancing effect of anti-endotoxic serum on interferon induction in mouse peritoneal cells by endotoxin. Acta Virol. 1968 Nov;12(6):551–553. [PubMed] [Google Scholar]
- Davis R. C., Cooperband S. R., Mannick J. A. Preparation and in vitro assay of effective and ineffective antilymphocyte sera. Surgery. 1969 Jul;66(1):58–64. [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. PPD-stimulated interferon: in vitro macrophage-lymphocyte interaction in the production of a mediator of cellular immunity. Cell Immunol. 1971 Dec;2(6):602–613. doi: 10.1016/0008-8749(71)90008-6. [DOI] [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Catinot L., de Vomecourt A., Sanceau J. Synthesis of interferon in human lymphocytes stimulated "in vitro" by antilymphocytic serum. Rev Eur Etud Clin Biol. 1972 Jan;17(1):20–26. [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Ho M., Armstrong J. A. Interferon. Annu Rev Microbiol. 1975;29:131–161. doi: 10.1146/annurev.mi.29.100175.001023. [DOI] [PubMed] [Google Scholar]
- Ho M., Breinig M. C., Maehara N. Cellular basis of interferon formation and hyporeactivity after exposure to bacterial lipopolysaccharide. J Infect Dis. 1976 Jun;133 (Suppl):A30–A36. doi: 10.1093/infdis/133.supplement_2.a30. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Ke Y. H., Ho M. Characterization of virus- and endotoxin-induced interferons obtained from the serum and urine of rabbits. J Virol. 1967 Oct;1(5):883–890. doi: 10.1128/jvi.1.5.883-890.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y. H., Ho M. Studies on the physico-chemical inactivation of rabbit inerferons. Proc Soc Exp Biol Med. 1968 Nov;129(2):433–438. doi: 10.3181/00379727-129-33337. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Yasui O., Masuzumi M. Studies on early-appearing interferon in vitro. Production of endotoxin-induced interferon by mouse spleen cells cultured in vitro. Proc Soc Exp Biol Med. 1969 Jun;131(2):487–494. doi: 10.3181/00379727-131-33908. [DOI] [PubMed] [Google Scholar]
- Kojima Y. Sites of interferon production in rabbits induced by bacterial endotoxin. Kitasato Arch Exp Med. 1970 Jun;43(1):35–44. [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Wagner R. R. Rabbit macrophage interferons. I. Conditions for biosynthesis by virus-infected and uninfected cells. J Exp Med. 1967 Apr 1;125(4):559–577. doi: 10.1084/jem.125.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Valle M. J., Jordan G. W., Haahr S., Merigan T. C. Characteristics of immune interferon produced by human lymphocyte cultures compared to other human interferons. J Immunol. 1975 Jul;115(1):230–233. [PubMed] [Google Scholar]
- Waschke K., Borecký L., Lackovic V. Release of interferon by mouse peritoneal cells in vitro. The requirement of contact with endotoxin and the temperature dependence of release. Acta Virol. 1969 Sep;13(5):393–400. [PubMed] [Google Scholar]
- Wheelock E. F. Interferon-Like Virus-Inhibitor Induced in Human Leukocytes by Phytohemagglutinin. Science. 1965 Jul 16;149(3681):310–311. doi: 10.1126/science.149.3681.310. [DOI] [PubMed] [Google Scholar]


