Abstract
Evidence from imaging and anatomical studies suggests that the midcingulate cortex (MCC) is a dynamic hub lying at the interface of affect and cognition. In particular, this neural system appears to integrate information about conflict and punishment in order to optimize behavior in the face of action-outcome uncertainty. In a series of meta-analyses, we show how recent human electrophysiological research provides compelling evidence that frontal-midline theta signals reflecting MCC activity are moderated by anxiety and predict adaptive behavioral adjustments. These findings underscore the importance of frontal theta activity to a broad spectrum of control operations. We argue that frontal-midline theta provides a neurophysiologically plausible mechanism for optimally adjusting behavior to uncertainty, a hallmark of situations that elicit anxiety and demand cognitive control. These observations compel a new perspective on the mechanisms guiding motivated learning and behavior and provide a framework for understanding the role of the MCC in temperament and psychopathology.
Keywords: anterior cingulate cortex (ACC), anxiety, behavioral inhibition, cognitive control, emotion, error-related negativity (ERN), feedback-related negativity (FRN), N2, post-error slowing, theta
1 Introduction
The rostral cingulate cortex, the thick belt of cortex encircling the genu and body of the corpus callosum (Fig. 1A), plays a central role in neuroscientific models of emotion and cognition (Etkin et al., 2011; Lindquist et al., 2012; Pessoa, 2008; Shenhav et al., 2013). Work to understand these two basic domains has profoundly influenced contemporary perspectives on more complex psychological phenomena, including psychopathology, pain, social processes, and the nature of executive control (Behrens et al., 2009; Etkin et al., 2011; Grupe and Nitschke, 2013; Iannetti et al., 2013). There is a growing consensus that the dorsal region of the rostral cingulate, the midcingulate cortex (MCC), is sensitive to both the elicitation of negative affect and the need for cognitive control, suggesting that the MCC implements a common, domain general process (Botvinick, 2007; Etkin et al., 2011; Pereira et al., 2010; Pessoa, 2008). Indeed, a recent meta-analysis of functional imaging studies demonstrates that the elicitation of both negative affect and cognitive control are associated with activation of an overlapping region in the anterior MCC (Fig. 1B) (Shackman et al., 2011). This overlap is consistent with anatomical evidence suggesting that the MCC represents a hub where information about pain, threat, and other more abstract forms of potential punishment can be synthesized and used to modulate regions involved in expressing fear and anxiety, executing goal-directed behaviors, and biasing the focus of selective attention (Fig. 1C–D) (Shackman et al., 2011).
Figure 1. The Adaptive Control Hypothesis (TACH).
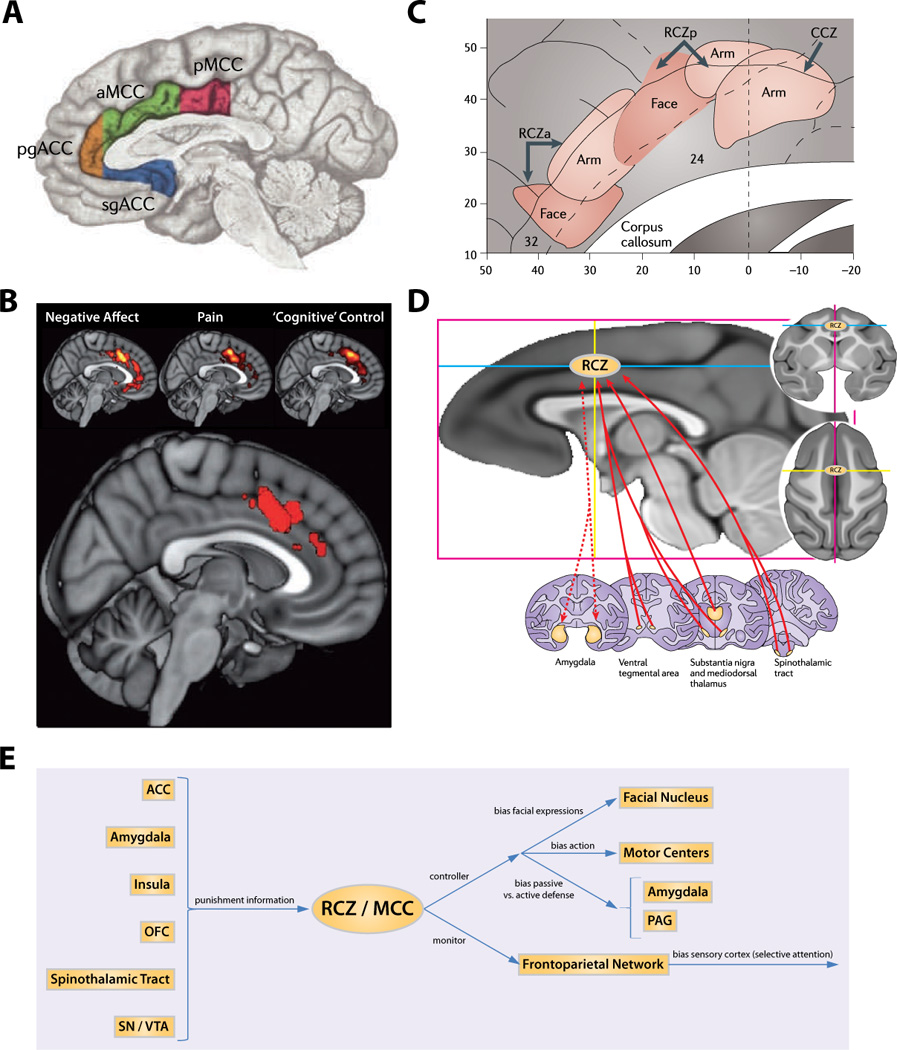
In humans and other primates, the rostral cingulate (architectonic areas 24, 25, 32 and 33)— a thick belt of cortex encircling the rostral corpus callosum — is among the most prominent features on the mesial surface of the brain. Much of the constituent gray matter lies buried within the cingulate sulci. (A) The four major subdivisions of the human rostral cingulate. Supracallosal cingulate is designated the midcingulate cortex (MCC) and is divided into anterior (aMCC; green) and posterior (pMCC; magenta) subdivisions. Cingulate territory lying anterior and ventral to the corpus callosum is designated the anterior cingulate cortex (ACC) and is approximately divided into pregenual (pgACC; orange) and subgenual (sgACC; blue) subdivisions by the coronal plane at the anterior tip of the genu. (B) Negative affect, pain and cognitive control activate a common region within aMCC. This map depicts the results of a coordinate-based meta-analysis (CBMA) of 380 activation foci (192 experiments involving >3,000 subjects). The upper panel shows thresholded activation likelihood estimate maps for each domain. The lower panel depicts the region of three-way overlap within aMCC (areas 32', a24b'/c'). (C) The MCC harbors somatotopically-organized premotor areas. Shown here are provisional locations of the cingulate premotor areas, the rostral and caudal cingulate zones (RCZ, CCZ). Somatotopy in RCZ and CCZ are based on human imaging studies. The cluster identified by the meta-analysis corresponds to the location of RCZ. The abundant projections from aMCC to motor centers would permit it to use information about punishment, feedback and other aversive reinforcers to optimize aversively-motivated instrumental actions. This stands in contrast with other cortical regions, such as the OFC and insula, that lack strong ties with motor centers. (D) Subcortical connnectivity of the rhesus homologue to human RCZ. This area receives substantial inputs from the spinothalamic system, which relays nociceptive information from the periphery to RCZ via the mediodorsal nucleus of the thalamus. Dopaminergic inputs to RCZ arise from the substantia nigra and, to a lesser extent, the ventral tegmental area. RCZ projects to the ventral striatum, including the core region of nucleus accumbens, and has robust reciprocal connections with the lateral basal nucleus of the amygdala. Dotted arrows indicate reciprocal connections. (E) The Adaptive Control Hypothesis (TACH). We have previously argued that MCC implements adaptive control by integrating information about punishment arriving from subcortical regions (Panel D), insula, orbitofrontal cortex (OFC) and elsewhere in order to bias responding in situations where the optimal course of action is uncertain or entails competition between alternative courses. Control signals generated in aMCC and directed at the amygdala or periaqueductal gray (PAG) might serve to resolve conflict between passive and active defensive behaviors. Another possibility is that aMCC directly biases aversively-motivated actions through its connections with motor centers, but indirectly biases selective attention through its connections with the frontoparietal network. It is also possible that these different mechanisms are functionally segregated at a finer level of resolution (e.g., intermingled networks) or are organized along overlapping gradients within MCC. Abbreviations: anterior cingulate cortex (ACC), caudal cingulate zone (CCZ), midcingulate cortex (MCC), orbitofrontal cortex (OFC), periaqueductal gray (PAG), rostral cingulate zone (RCZ), substantia nigra (SN), ventral tegmental area (VTA). Panels A–D adapted from (Shackman et al., 2011).
Despite this progress, the functional significance of activity in the rostral cingulate remains incompletely understood. The objective of the present review is to highlight recent advances in understanding the adaptive control system that have been made using electrophysiological measures indicative of MCC activity. A key focus will be on investigations characterized by frontal midline theta (FMΘ) signals: ~4–8Hz oscillations recorded from sensors on the scalp overlying the MCC. Using meta-analytic techniques to synthesize the human electrophysiology literature, we provide evidence that anxious individuals show larger FMΘ control signals and that larger control signals are, in turn, associated with a more cautious or inhibited response set following errors and punishment. Collectively these observations support the idea that FMΘ reflects a common mechanism, a lingua franca, for implementing adaptive control in a variety of contexts involving uncertainty about actions and their motivationally-significant potential outcomes (Cavanagh et al., 2012b). More broadly, they provide a neurobiologically-grounded framework for conceptualizing the mechanisms that confer increased risk for the development of anxiety and other psychiatric disorders.
1.1 The Adaptive Control Hypothesis (TACH)
On the basis of brain imaging and anatomical evidence, it has been hypothesized that MCC activity reflects control processes that optimize responses made in the face of uncertainties about instrumental actions and their potentially aversive outcomes (Fig. 1E) (Shackman et al., 2011), a perspective that we term The Adaptive Control Hypothesis (TACH). Put simply, TACH suggests that anxiety and negative affect tend to involve the same processes described by cognitive control theories in order to solve similar problems (see also Grupe and Nitschke, 2013). We suggest that this is a domain-general function of the MCC.
Control processes are engaged when automatic or habitual responses are insufficient to support goal-directed behavior (Botvinick et al., 2001; Norman and Shallice, 1986; Shenhav et al., 2013). This occurs when there is uncertainty about the optimal course of action (e.g., probabilistic learning), when potential actions are associated with the possibility of error or punishment, or when there is competition between alternative courses of action (e.g., flee/freeze, go/no-go). These features are hallmarks of dangerous environments, as in studies of fear, anxiety, and pain (Choi et al., 2010; Steenland et al., 2012). Not surprisingly, optimal instrumental behavior in threatening environments has long been thought to require control processes to monitor risk and generate the biasing signals required to resolve response uncertainty and avoid potentially catastrophic actions (Dehaene et al., 1998; Gray and McNaughton, 2000; Norman and Shallice, 1986; Rushworth and Behrens, 2008). Importantly, a growing body of behavioral and biological evidence indicates that errors, like punishments and other kinds of control prompts, are experienced as unpleasant and facilitate avoidance, reinforcing the possibility that MCC makes a similar contribution to ‘cognitive’ and ‘affective’ control (Dreisbach and Fischer, 2012; Kool et al., 2010; Lindström et al., 2013; Schouppe et al., 2012).
1.2 FMΘ reflects signals of the need for control
Neuronal control signals generated within the MCC propagate to the scalp, where they can be measured using well-established electroencephalographic (EEG) techniques. In particular, MCC-related control processes are reflected in a variety of event-related potential (ERP) components elicited by novel information, conflicting stimulus-response requirements, punishing feedback, and the realization of errors (Fig. 2A). For example, the presentation of cue arrays associated with conflicting response options (as in the Eriksen flanker task) elicits the N2, a negative potential that peaks approximately 300 ms after the onset of conflicting cue arrays (for a review, see: Folstein and Van Petten, 2008). Likewise, unexpected punishment elicits a similar signal, the feedback-related negativity (FRN) (for a review, see: Walsh and Anderson, 2011). ERP control signals can also be elicited by endogenous activities (i.e. internal error signals), as with the error-related negativity (ERN), a negative potential peaking approximately 80ms after the commission of an error (for a review, see: Gehring et al., 2012). While the cerebral generators of these scalp-recorded signals remains a matter of active research, and likely includes contributions from other brain regions (Bonini et al., 2014; Cohen et al., 2008; Emeric et al., 2010), a variety of evidence implicates the MCC as a key generator, including EEG source estimation (Gehring et al., 2012; van Noordt and Segalowitz, 2012; Walsh and Anderson, 2012), EEG-informed fMRI (Becker et al., 2014; Debener et al., 2005; Edwards et al., 2012; Hauser et al., 2014; Huster et al., 2011), MEG (Doñamayor et al., 2011), and invasive recordings in humans and monkeys (Cohen et al., 2008; Gemba et al., 1986; Tsujimoto et al., 2010, 2006; Wang et al., 2005; Womelsdorf et al., 2010a, 2010b).
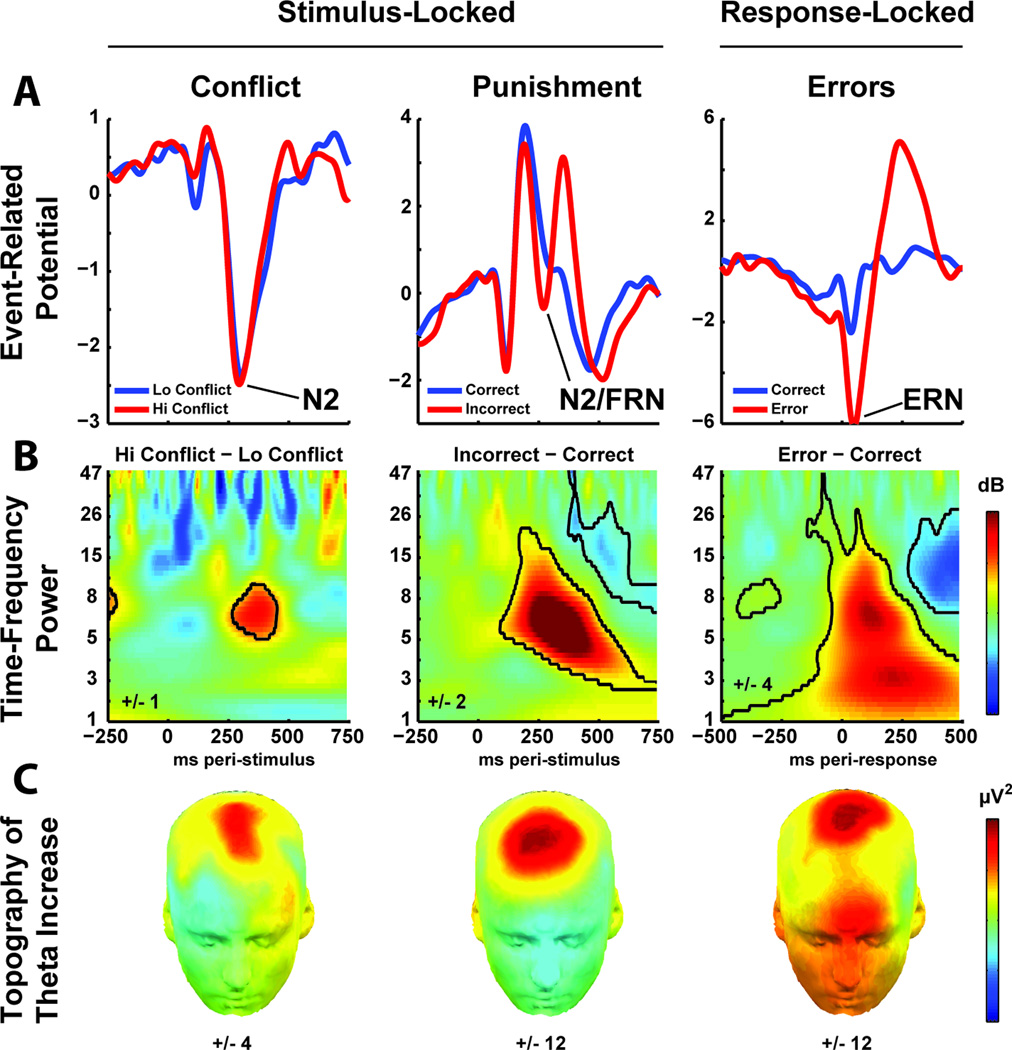
Figure 2. A variety of events indicating a need for control are associated with a similar neuroelectrical signature in the theta band (~4–8 Hz) over mid-frontal sites.
Rows depict different components of the event-related electrophysiological signal, columns show different events associated with increased demands for adaptive control. (A) Event-related potential (ERP) components in the time-domain. N2: an ERP component evoked by exogenous cues of novelty or conflict. Feedback Related Negativity (FRN): An N2-like component evoked by exogenous feedback signaling loss or punishment. Error Related Negativity (ERN): A massive ERP component evoked by commission errors. While these ERP components (i.e., peaks and troughs in the wave) are related to learning and adaptive control, they represent a small fraction of ongoing neural dynamics: signal averaging in the time-domain imposes a substantial reduction in potentially meaningful information. (B) The full spectral dynamics of event-related neuroelectrical activity depicted in time-frequency plots. Here, significant increases in power to conflict, punishment and error are outlined in black, revealing a common feature in the theta band (~4–8Hz). (C) Scalp topography of event-related theta activity. The distribution of theta power bursts is consistently maximal over the frontal midline. Data and statistical tests from (Cavanagh et al., 2012b).
Although data derived using these ERP components have played a crucial role in the development of formal models of cognitive control and reinforcement learning (Holroyd and Coles, 2002; Yeung et al., 2004), there are theoretical and methodological advantages to focusing on the spectral characteristics of these signals rather than the separate ERP components. Spectral methods decompose complex signals into different contributions of frequency, power and phase angle over time, each of which can differentially contribute to information representation. Spectral decomposition has revealed that the ERN (errors), FRN (punishment), and N2 (conflict) share a common signature in the theta band (Cavanagh et al., 2009; Luu et al., 2004, 2003; Trujillo and Allen, 2007; Yordanova et al., 2004) (Fig. 2B–C). It was recently proposed that this family of theta signals reflect canonical phase-locked activities that are used for the temporal organization of distributed neuronal ensembles (Cavanagh et al., 2012b). Neural reactions to conflict, punishment, and error manifest as variations of these obligatory theta band phase dynamics, particularly via power increases (Cohen and Donner, 2013). In the context of this common spectral perspective, we refer to this collection of control-sensitive EEG signals as FMθ. While these ERP components are partially dissociable, emphasizing their common dominant FMΘ processes offers an appropriately broad methodological and theoretical perspective.
2. Meta-analyses of FMΘ support for TACH
As the electrophysiological literature has grown, it is increasingly difficult to integrate new data with extant models of adaptive control and reinforcement learning (Shackman et al., 2011; Shenhav et al., 2013). Meta-analytic techniques provide an important tool for overcoming this challenge. Here we used random-effects meta-analytic techniques (Borenstein et al., 2009) to synthesize the voluminous electrophysiology literature and understand the relationships among FMθ control signals, dispositional anxiety, and controlled adjustments of behavior.
2.1 Dispositional anxiety
TACH and other models argue that negative emotions, such as anxiety and fear, are tightly integrated with control processes implemented in the MCC (Proudfit et al., 2013; Shackman et al., 2011). This implies that anxiety should systematically covary with differences in control-sensitive electrophysiological signals generated in the MCC. That is, one would expect a substantial degree of functional convergence (i.e. convergent validity; Campbell and Fiske, 1959). Here, we used a random-effects meta-analysis to provide a comprehensive assessment of the evidence for convergence between measures of dispositional anxiety and FMΘ control signals (for more focused meta-analyses, see: Mathews et al., 2012; Moser et al., 2013). The decision to focus on dispositional anxiety was motivated in part by work demonstrating that ERP measures of conflict monitoring and control themselves represent trait-like individual differences (Hämmerer et al., 2013; Leue et al., 2013; Olvet and Hajcak, 2009a, 2009b; Segalowitz et al., 2010; Weinberg and Hajcak, 2011).
2.2.1 Adaptive control of behavior
A central claim of TACH and other prominent models of cognitive control and reinforcement learning is that a circuit centered on the MCC tunes future instrumental behavior in the face of action-outcome uncertainty (Shackman et al., 2011; Shenhav et al., 2013). Despite strong claims, there is inconsistent evidence that MCC activity predicts behavioral adjustments made in response to conflict, errors, punishment, and other prompts for increased top-down control. Here we used random-effects meta-analytic techniques to test whether electrophysiological control signals generated in the MCC (i.e. FMΘ) predict behavioral adjustments following errors and punishments.
2.2.2 Post-error slowing
Errors, like other kinds of punishments, are associated with adaptive changes in in subsequent behavior. In particular, errors are associated with a speed/accuracy tradeoff in subsequent behavior, characterized by a slower, more cautious response style on the next trial (Dutilh et al., 2012; Rabbitt, 1966). It is natural to ask whether the amplitude of the ERN predicts the degree of subsequent post-error slowing. Many studies have investigated this question as an inter-individual phenomenon: testing whether subjects with larger error signals slow down more after making mistakes. Studies employing this between-subjects analytic strategy have revealed inconsistent evidence for a predictive relationship between MCC control signals and behavioral adjustments (see: Weinberg, Riesel, & Hajcak, 2011). Moreover, there is evidence that ERN amplitude and post-error slowing are pharmacologically dissociable (for a review, see: Jocham & Ullsperger, 2009), raising the possibility of distinct substrates.
The ambiguity, however, reflects an over-focus on between-subject examinations of individual differences and a failure to examine within-subject trial-to-trial brain-behavior relationships. Trial-by-trial correlations between neural signals and behavior offer the most stringent correlational test of brain-behavior relations (Lim et al., 2009). Here, we used a random-effects meta-analysis to systematically test whether the magnitude of error-related MCC signals, indexed by the ERN or spectral measures of FMΘ power, predicts post-error slowing. Furthermore, moderation analyses allowed us to assess whether the strength of this relationship is influenced by the use of inter-individual versus intra-individual analytic strategies.
2.2.3 Lose-switch behavior
Adaptation to punishment often involves a ‘lose-switch’ strategy, where alternate behavioral responses are chosen following punishment. Here, we used a third random-effects meta-analysis to test whether the magnitude of feedback-related MCC signals, indexed by the FRN or spectral measures of FMΘ power, predicts the active avoidance of cues associated with punishment (i.e., ‘lose-switch’), as TACH and other control models claim. Yet, not all investigations should be expected to show explicit relationships between punishment signaling and immediate switching. As detailed in section 4.2.2 below, we predict that this punishment-avoidance relationship should be only be apparent when the task does not involve the long-term integration of stimulus-response-reward relationships, since switching after every punishment would then be maladaptive. Put simply, if the best response in a given state still leads to punishment on 20% of trials, an optimal agent may plan to weather these temporary disappointments in the service of reaping long-term benefits.
2.3.1 General methods
We conducted three independent meta-analyses. The first aimed to test whether dispositional anxiety predicts trait-like individual differences in control-sensitive frontal-midline signals (i.e., ERN, FRN, and N2; no studies employing time-frequency measures of FMΘ were identified). The aim of the other meta-analyses was to test whether these same scalp-recorded neurophysiological measures consistently predict behavioral adjustments following prompts for enhanced control. Specifically, we assessed whether FMθ signals predict response slowing on trials following commission errors (post-error slowing) and whether FMθ signals predict the subsequent active avoidance of cues associated with punishment (lose-switch).
2.3.2 Study identification
Pubmed searches were used to generate a pool of potential articles for each meta-analysis (March 2012). Both used the search terms: “(ERN OR "error-related negativity" OR ERSP OR time-frequency OR "event-related spectral perturbation" OR FRN OR "feedback-related negativity" OR N2 OR "frontal theta" OR "midline theta" OR "theta band").” For the dispositional anxiety meta-analysis, this was combined with the search terms “(anxiety OR anxious OR avoidance OR avoidant OR BIS OR EDA OR electrodermal OR fear* OR FPS OR inhibited OR nervous OR neurotic OR punishment* OR SCR OR "skin conductance" OR STAI OR startle OR stress*)”. This yielded 2,204 abstracts. For the remaining meta-analyses, this was combined with the search terms: “(aversive OR conflict OR NoGo OR accuracy OR "post-error" OR "post-response slowing" OR "reaction time" OR "response time" OR Laming OR learning OR “lose-switch” OR probabilistic OR Rabbit)”. This yielded an additional 2,467 abstracts.
At least one of the authors read the abstracts and identified a subset for in-depth evaluation. The preliminary review of abstracts was meant to be inclusive; articles were excluded only if the abstract clearly indicated that the article did not meet inclusion/exclusion criteria (e.g., nonhuman sample). This was supplemented by personal communications with investigators aimed at identifying file-drawer, in-press, or in-preparation manuscripts, as well as backward citation checks on articles and reviews chosen for in-depth evaluations. Altogether, 354 reports were comprehensively evaluated by at least one of the authors.
Inclusion criteria for all meta analyses included: (a) English language; (b) use of a cognitive conflict, gambling, or probabilistic learning task, and (c) sufficient statistics from a continuous (e.g., regression) or categorical (e.g., ANOVA on extreme groups) analysis of relations between EEG amplitudes and individual differences in dispositional anxiety or behavioral measures. Studies of developmental or geriatric populations were included. A wide range of self-report and behavioral indexes of dispositional anxiety were included (e.g., behavioral inhibition, harm avoidance, neuroticism, and trait anxiety), consistent with a growing consensus that these measures reflect a broad underlying dimension of anxiety-proneness and anxious distress (Caspi et al., 2005). Exclusion criteria included: (a) the absence of previously unpublished inferential statistics (e.g., reviews); (b) pharmacological manipulations; (c) analyses of data obtained from participants with diagnosed psychopathology or who were explicitly enrolled on the basis of sub-clinical psychopathology. Studies of psychopathology and pharmacology were only included in cases where the authors reported effect sizes separately for psychiatrically-healthy or unmedicated controls and the study otherwise met our inclusion/exclusion criteria. Based on these criteria, 76 studies, incorporating nearly 2,300 psychiatrically-healthy subjects, were used in one or more of our meta-analyses.
2.3.3 Effect sizes
To maximize independence, the mean effect size was used in cases where multiple conditions or trait measures were reported. A similar rule was applied in cases where effects for continuous analyses were reported separately for participants with high and low levels of dispositional anxiety (e.g. Meyer et al., 2012). Exceptions were made for cases where the condition manipulation coincided with one of our candidate moderator variables (Olvet and Hajcak, 2009c; West and Travers, 2008) and for two cases where extensive follow-up analyses were performed on a subset of conditions (De Pascalis et al., 2010; Moadab et al., 2010). In cases where multiple conditions or measures were collected, but an effect size was only reported for the single condition or measure that reached significance (Boksem et al., 2008; Pailing and Segalowitz, 2004), that was used. In cases where multiple ERP components were reported, each component was treated as an independent sample (Amodio et al., 2008; Boksem et al., 2006; Moadab et al., 2010).
Reported effect sizes were transformed as necessary to Pearson’s correlation coefficient using the formula:
where F = t2 and dfe = degrees of freedom for the error term, equal to the sample size minus 2 in cases with no additional covariates. Tests in which higher levels of anxiety or larger behavioral adjustments predicted a more negative component (i.e., larger amplitude ERN, FRN, N2) were treated as positive effects. If an effect was reported as nonsignificant but specific information was not provided, the effect size was conservatively assigned a value of r = 0 (unbiased estimate). Confidence intervals were transformed from z to r for figures, thus they reflect the likely range of effect size in future studies, and do not directly reflect the confidence range for the statistical test in the sample reported here. Thus, in some cases, significant effects are associated with a 95% confidence interval that, when back-transformed to r, includes 0. Both r and z values are reported below.
2.3.4 Random-effects meta-analyses
Analyses were performed using the metafor package (Viechtbauer, 2010) written for R (version 2.14.1; http://www.R-project.org). Analyses were performed using Z-transformed correlation coefficients and an approximation to the unbiased estimates of the sampling variances (Hedges, 1989). As described above, effect sizes and 95% confidence intervals were shown in figures as back-transformed correlation coefficients. For omnibus tests, random-effects models were estimated using maximum likelihood (ML). Random-effects models have the advantage of permitting unconditional inferences about the mean effect size in the population of studies from which the sampled studies are drawn (Borenstein et al., 2009).
2.3.5 Moderator analyses
The potential impact of both categorical and continuous moderator variables was assessed using a series of planned contrasts implemented as ML mixed-effects linear models, one for each candidate moderator variable. Specifically, we tested whether relations with dispositional anxiety were significantly moderated by the kind of control prompt: commission error (ERN), feedback (FRN), or response conflict (N2). Likewise, for the meta-analysis of post-error adjustments, we tested whether the strength of FMΘ-behavior relations differed as a function of being computed across (inter-individual) or within subjects (intra-individual). There were no significant differences in age or sex distributions between the inter- and intra-individual subgroups for this analysis. This contrast could not be performed with adequate reliability for the lose-switch meta-analysis given the limited number of studies employing intra-individual analytic techniques (2/7; 29%).
2.3.6 Evaluation of potential publication bias
Publication bias occurs when positive (‘significant’) results are more likely to be published than negative (‘non-significant’) results, leading to an overestimate of the true population effect size. Potential publication biases were first assessed using funnel plots, which depict the effect size as a function of the standard error of the effect (i.e. an index of sample size) and serve as a diagnostic aid for detecting publication bias and other systematic heterogeneities. Absent publication bias, effect sizes are expected to be symmetrically distributed about the mean without regard to standard error; visual evidence of a lower-rightward asymmetry (e.g. smaller studies with larger standard errors tending to report larger effects) is suggestive of publication bias. The robustness of omnibus effect sizes was assessed by computing the Fail-safe N (Rosenthal, 1979) using the MAc package (version 1.1; http://CRAN.R-project.org/package=MAc) for R. This provides an estimate of the number of additional non-significant results that would have to exist for the omnibus estimate of effect size to be rendered non-significant.
3 Meta-Analysis Results
3.1 Convergence between measures of anxiety and ‘cognitive’ signals generated in MCC
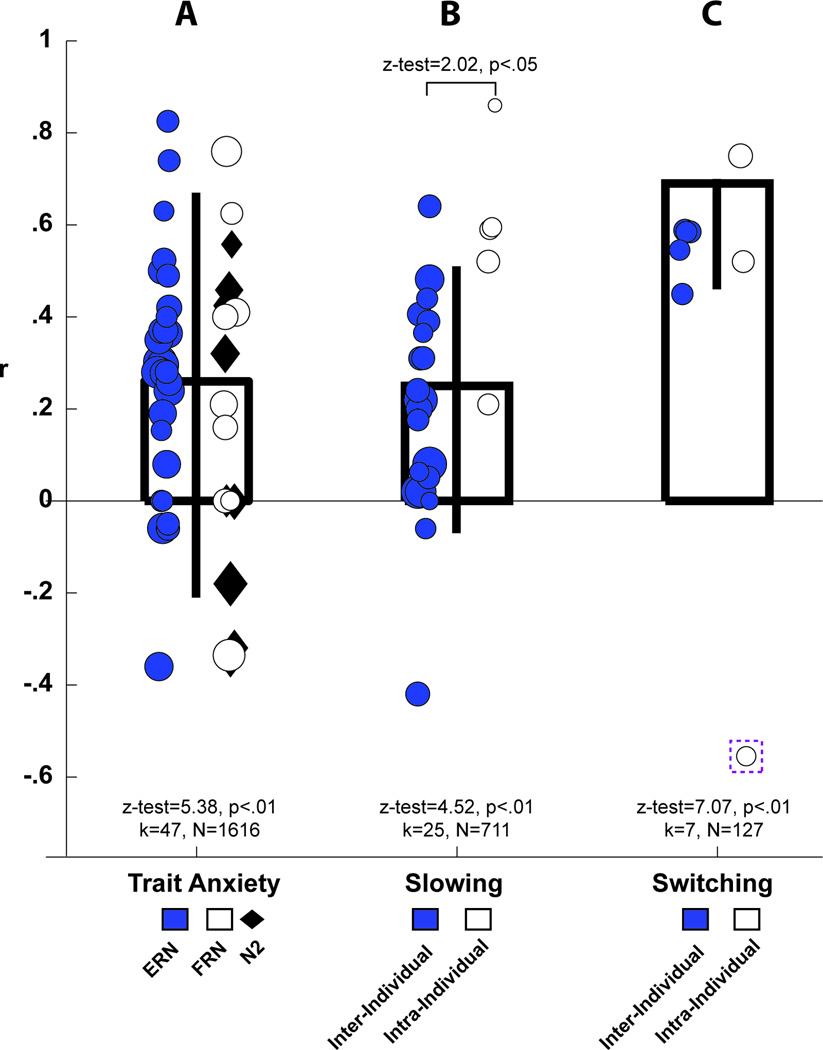
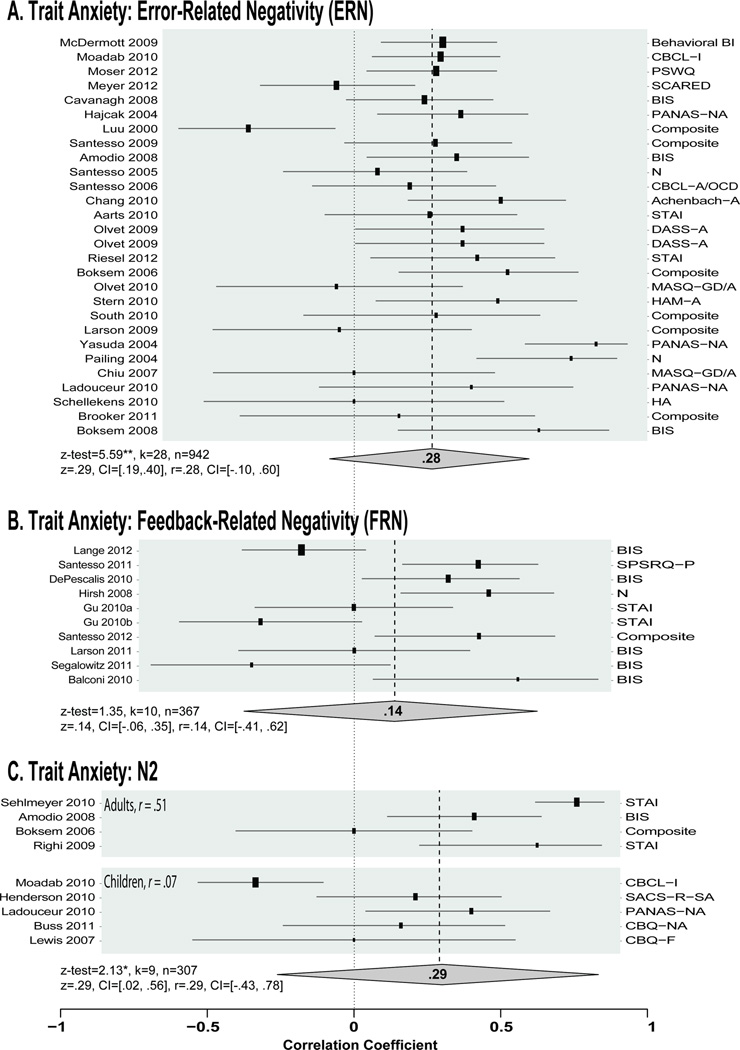
As shown in Fig. 3A, individuals with higher levels of dispositional anxiety show enhanced frontal-midline control signals when performing standard, emotionally-neutral cognitive control tasks (z-test=5.38, p<.01; mean z=.26, CI: .17, .34, mean r=.26, CI: −.21, .67). This relationship supports the hypothesis that anxiety and other kinds of negative affect are tightly integrated with cognitive control processes in the MCC (Shackman et al., 2011). The strength of this association was similar to a recent estimate derived solely from the ERN literature (Moser et al., 2013) and did not significantly differ among signals evoked by response conflict (N2), commission errors (ERN), or negative feedback (FRN), z-tests<1.42, ps>.15), consistent with the idea that these three signals are sensitive to the same underlying FMΘ processes (Cavanagh et al., 2012b).
Figure 3. FMϑ is consistently related to dispositional anxiety and predicts aversively-motivated behavioral adjustments.
(A) Individuals characterized by greater dispositional anxiety show larger FMθ signals in response to conflict, punishment, and error. There was not a significant difference between response-locked error signals (filled circles) and cue-locked signals of punishment (empty circles) or conflict (filled diamonds). (B–C) Larger control signals predict a more cautious or inhibited response set following punishment or errors. (B) Larger error-related FMθ signals predict greater post-error slowing on the subsequent trial. This was observed both inter-individually (filled circles: individuals with larger error signals showed increased behavioral adjustments) and intra-individually (empty circles: trial-to-trial differences in control signals predicted proportional variation in post-error slowing) analyses. There was a significant moderating effect of this level of anlaysis, where intra-individual studies had a significantly larger relationship between error signals andresponse slowing (z-test=2.02, p<.05). (C) Larger FMθ responses to worse-than-expected feedback predict an increased probability of switching to the alternative among studies employing both inter-individual (filled circles) and intra-individual (empty circles) analytic strategies. The empty circle outlined by a dashed box indicates a study where feedback must be integrated over time and rapid switching would be maladaptive, this study was excluded a priori from this meta anlaysis on these grounds. Statistics were determined using random-effects meta-analyses (k: number of studies; n: number of participants). Error bars depict the random-effects estimate of the brain-behavior correlations (±95% CI). Each circle is centered on the correlation of each study, with the size of the circle scaled by the sample size (10*log10(N)). Larger numbers indicate a larger absolute relationship (i.e. the sign of the voltage potential is not taken into account).
3.2 FMΘ predicts post-error slowing
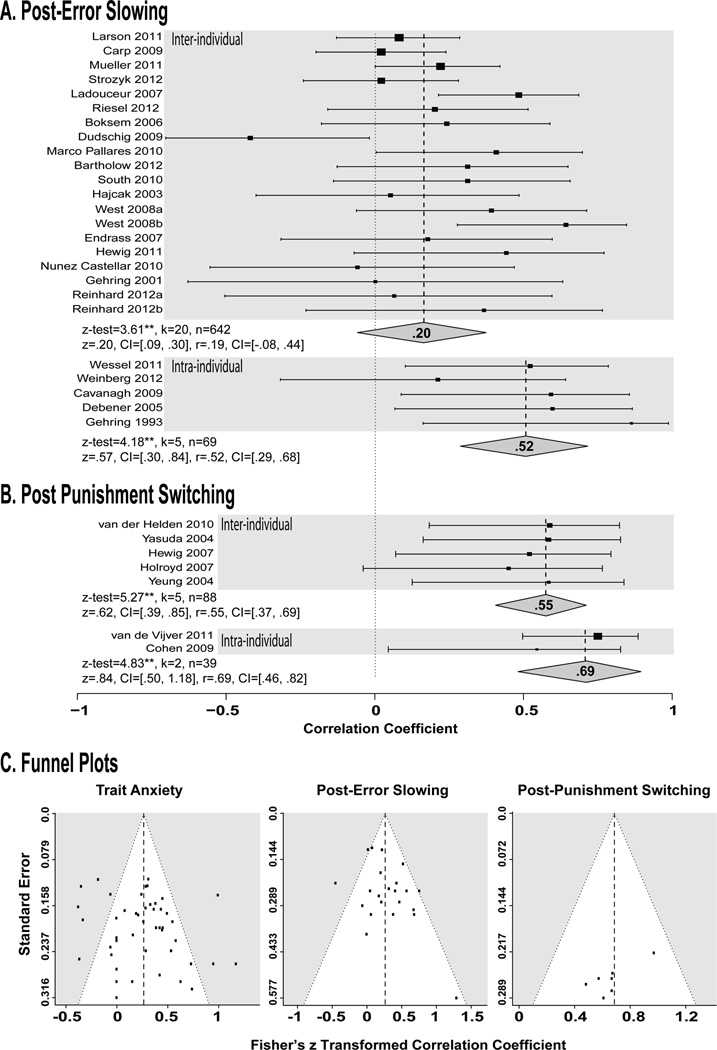
As shown in Fig. 3B, larger error signals predict greater response time slowing on the trial following an error (z-test=4.52, p<.01, mean z=.24, CI: .14, .34; mean r=.24, CI: −.07, .51), providing important evidence that MCC signals serve to regulate behavior in situations where reflexive or habitual actions are inadequate. Moreover, studies assessing the relationship between trial-by-trial fluctuations in the amplitude of MCC error signals and slowing reported significantly stronger brain-behavior relations than those relying on traditional individual differences analyses, z-test=2.02, p<.05. While this moderation analysis is limited by the few studies that have formally investigated trial-to-trial effects, the intra-individual method remains a more direct test of the functional brain-behavior relationship associated with adaptive control. These findings highlight the sensitivity of the intra-individual analytic technique and support the hypothesis that FMΘ signals support the adaptive regulation of behavior when there is a risk of negative outcomes.
3.3 FMΘ predicts lose-switch behavior
As shown in Fig. 3C, individuals characterized by larger FMθ amplitudes to punishment were more likely to actively avoid that option on the following trial (z-test=7.07, p<.01; mean z=.69, CI: .50, .88; mean r=.69, CI: −.46, .70). Taken with the post-error slowing results, this indicates that FMθ signals play an important role in using information about uncertain negative outcomes to optimize avoidance behaviors. While this lose-switch tendency is adaptive for simple decision making and instrumental learning tasks, it is maladaptive when integrating reinforcement history over a longer time period. Consistent with this principle, the only investigation that involved longer-term probabilistic integration yielded a strong negative correlation between FMθ and subsequent behavior (it was removed a priori from the meta-analytic summary; see section 2.2.3).
3.4 Individual Effect Sizes, Funnel plots and fail-safe N results
Effect sizes for each study incorporated in the meta-analyses are depicted in Figures 3 and 4. Visual inspection of the funnel plots for each meta-analysis revealed no evidence of publication biases (Figure 4C). While there is an outlying point in the switching meta-analysis funnel plot, this study had the largest N and largest effect size, and is thus not suggestive of a bias to report findings that capitalize on chance from a small N analysis. While funnel plots are prone to inaccuracy and misinterpretation, especially those with fewer than 10 samples (Lau et al., 2006; Sterne et al., 2011; Terrin et al., 2005), we included them here for all major meta-analyses for completeness. Additional tests of potential publication bias bolster these conclusions, as the results of the fail-safe N analyses show that a substantial number of additional null results (i.e., unpublished or to-be-published studies) would be required for any of the meta-analyses to yield null results (Dispositional Anxiety: 1061; Post-Error Slowing: 204; Lose-Switch: 62). Collectively, these findings demonstrate that the meta-analytic results are robust.
Figure 4. Forest plots of the relationship between FMϑ and trait anxiety (as Pearson’s r).
Forest plots display each study included in the meta-analysis in descending order of sample size (also indicated by the size of the box). Here the forest plots are separated by the type of eliciting event and specifically associated ERP component (A: error/ERN, B: punishment/FRN, C: conflict/N2). Psychometric instruments used to assess dispositional anxiety are detailed on the right of each plot.
4. Understanding the role of FMΘ in affective, cognitive, and behavioral control
The present results, summarized in Figure 3, demonstrate that anxious individuals are characterized by heightened FMθ signals in response to a range of control prompts. The similarity of these relations across signals evoked by high-conflict cues, errors, and negative feedback is consistent with the idea that that FMθ reflects a common mechanism, a lingua franca, implementing adaptive control in a variety of contexts involving uncertainty about actions and their outcomes (Cavanagh et al., 2012b). More broadly, these relations strengthen claims that ‘emotional’ and ‘cognitive’ control processes are functionally integrated in the MCC (Shackman et al., 2011). Importantly, the results of the post-error slowing and lose-switch meta-analyses indicate that larger FMθ signals in response to errors and punishment, in turn, predict a more cautious and avoidant pattern of instrumental behavior on subsequent trials. These observations provide compelling evidence that a circuit centered on the MCC contributes to the adaptive regulation of instrumental behavior in the face of action-outcome uncertainty, a central claim of TACH and other, more computationally-explicit models of cognitive control and reinforcement learning (Shackman et al., 2010; Shenhav et al., 2013).
4.1 Dispositional anxiety and FMΘ
Our meta-analytic results demonstrate that control-sensitive FMΘ signals generated in the MCC are elevated in dispositional anxious individuals. These results provide a novel framework for conceptualizing anxiety. When extreme, dispositional anxiety is a key risk factor for the development of anxiety disorders as well as co-morbid depression and substance abuse (Barlow et al., 2013; Kotov et al., 2010). These psychiatric disorders are highly prevalent, debilitating, and challenging to treat (Bystritsky, 2006; Kessler et al., 2012), underscoring the importance of a deeper understanding of the neural systems that confer liability to dispositional anxiety.
The present results suggest that chronically elevated anxiety partially reflects heightened sensitivity to uncertain punishment and risk (e.g., of error or failure). While the anxious phenotype is complex and multidimensional, there is a growing consensus that elevated reactivity to uncertain threat is a core feature of both the anxiety disorders and trait-like individual differences in anxiety and behavioral inhibition (Barker et al., 2014; Davis et al., 2010; Grupe and Nitschke, 2013; Mushtaq et al., 2011; Reeb-Sutherland et al., 2009). Indeed, elevated anxiety in response to uncertain or ambiguous threat is more discriminative of many anxiety disorders than that elicited by certain threat and prospectively predicts the initial appearance of the disorder (Craske et al., 2012; Davis et al., 2010; Lissek et al., 2005).
Converging lines of pharmacological evidence suggest that anxious individual’s exaggerated response to uncertain threat is caused by alterations in the adaptive control network (Fig. 1), consistent with our meta-analytic results. In particular, clinically-effective pharmacological treatments for anxiety and alcohol selectively reduce anxiety elicited by uncertain threat (Bradford et al., 2013; Davis et al., 2010; Gray and McNaughton, 2000; Hefner and Curtin, 2012; Hefner et al., 2013). Importantly, these anxiolytic compounds also attenuate FMθ signals and weaken behavioral adjustments following control prompts (Bartholow et al., 2012; de Bruijn et al., 2004; Easdon et al., 2005; Ridderinkhof et al., 2002; but c.f.: Yeung and Cohen, 2006; Yeung et al., 2007). Together, these findings suggest that a circuit centered on the MCC is an important substrate for the pervasive, over-generalized distress characteristic of anxious individuals.
4.2 FMΘ and the adaptive control of instrumental behavior
Our results also demonstrate that larger error-related FMΘ signals are associated with more cautious or inhibited instrumental responses on subsequent trials. These findings are consistent with the idea that FMθ reflects the summed activities of cingulate neurons, which have been shown to be modulated by conflict, punishment, error, and behavioral adjustment. This breadth of eliciting circumstances suggests that FMθ may be interpreted as a generic signal of uncertainty indicating an enhanced need for control (c.f. Miltner et al., 1997). We discuss these ideas in more detail below, arguing that this mechanistic perspective may add to a better understanding of the nature of anxiety.
4.2.1 Cingulate neurons compute the need for control
Cingulate neurons are sensitive to errors (Amiez et al., 2005), and show enhanced and sustained activity following conflict (Sheth et al., 2012) and error (Narayanan and Laubach, 2008; Narayanan et al., 2013). Pharmacological inactivation of cingulate neurons in rats attenuates post-error slowing (Narayanan and Laubach, 2008; Narayanan et al., 2013) and in humans, MCC lesions increase error rates (Devinsky et al., 1995; Milea et al., 2003) and diminish behavioral adjustments following conflict (di Pellegrino et al., 2007; Milea et al., 2003; Sheth et al., 2012). Parallel effects have been obtained using transcranial direct current stimulation applied to the scalp overlying the frontal-midline (Reinhart and Woodman, 2014). Cingulate neurons also respond to punishments and switch cues, as well the combination of these two features (i.e. lose-switch) in monkeys (Amiez et al., 2005; Ito et al., 2003; Shima and Tanji, 1998) and humans (Williams et al., 2004). When these cells are inhibited (Shima and Tanji, 1998) or lesioned (Kennerley et al., 2006; Rushworth et al., 2007; Williams et al., 2004), lose-switch behavior is hindered and perseveration is common. Moreover, glutamatergic agonists applied directly to the ACC in rats have additionally proven both necessary and sufficient for aversive learning, putatively serving as a learning signal (Johansen and Fields, 2004).
Cingulate neurons generate theta band activities (Tsujimoto et al., 2010, 2006; Wang et al., 2005; Womelsdorf et al., 2010a, 2010b), which are proposed to be detectable on the scalp as FMθ signals. Our meta-analytic results underscore the contribution of such FMΘ signals to the active avoidance of potentially aversive outcomes. Collectively, these results provide compelling evidence that the MCC, as reflected in FMθ, serves to regulate instrumental behavior in the face of uncertainty about actions and aversive outcomes, an axiom of TACH and other prominent models of cognitive control and reinforcement learning (Shackman et al., 2011; Shenhav et al., 2013).
4.2.2 The functional significance of FMϑ signals in adaptive control
Adaptive control requires that an agent form expectations and monitor deviations (prediction errors) or potential deviations (conflict) about the need for control. FMθ appears to reflect the summed outputs of these processes, providing a plausible neurophysiological mechanism for the realization of the need for control. In contrast to more established models, we argue that FMθ reflects both prediction errors and conflict due to a common reaction to uncertainty, which signals the need for increased control.
Feedback-related FMΘ signals, in particular, appear to be sensitive to both worse-than-expected (Cavanagh et al., 2010; Chase et al., 2010; Holroyd and Coles, 2002; Ichikawa et al., 2010; Philiastides et al., 2010; Yasuda et al., 2004) and better-than-expected (Baker and Holroyd, 2011; Cavanagh et al., 2012a; Oliveira et al., 2007) outcomes, suggesting that they represent an unsigned prediction error, or simple surprise (Cavanagh et al., 2012a; Hauser et al., 2014; Holroyd et al., 2008; Sallet et al., 2013; Talmi et al., 2013). Yet additional evidence indicates that feedback-related FMΘ signals are not pure measures of surprise or feedback salience, insofar as they are disproportionately enhanced for negatively-valenced outcomes (Cavanagh et al., 2012a), consistent with invasive recordings in the nonhuman primate cingulate (Hayden et al., 2011). The apparent negativity bias of this signal may reflect an innate sensitivity of the MCC to “bad” events (Blair et al., 2006; Bush et al., 2002; Shima and Tanji, 1998; Wrase et al., 2007), or be indicative of a general need for change. While a domain-general perspective suggests that there is no substantive difference between these affective and effective tendencies, it remains an important issue to more precisely determine the information content of FMθ signals. Developing a deeper understanding of the functional significance of these signals will require that investigators employ new tasks: most of the studies described in this review relied on tasks that confound valence- and behavioral-specific information (i.e., win-stay/lose-switch).
The theoretical perspective advanced here suggests that these FMΘ measures may be most useful when interpreted in the context of how uncertainty / prediction error signals relate to adaptive control. We anticipated in section 2.2.3 that FMΘ signals will only predict rapid behavioral adaptation in cases where larger prediction errors would be expected to lead to rapid adaptation, as with conflict-induced slowing or punishment-induced switching. However, this relationship would not necessarily hold during exploration or hypothesis testing, as in long-term probabilistic or reversal learning.
4.2.3 Relationships between avoidance and anxiety as informed by FMϑ
From a translational perspective, these observations provide a foundation for understanding the neurocognitive mechanisms that underlie the maladaptive behavioral profile—excessive behavioral inhibition and heightened avoidance—that characterizes anxious individuals (Grupe and Nitschke, 2013). For example, inflated expectations about punishment magnitude or likelihood would explain enhanced avoidance of cues and contexts associated with threat and punishment. Likewise, aberrant estimates of prediction error or uncertainty (i.e., learning rate) would retard expectancy adjustments when anticipated punishments do not occur, potentially explaining anxious individuals’ difficulties learning to discriminate certain from uncertain threat and associated sustained distress and avoidance. This framework is broadly consistent with evidence that successful phobia treatment is associated with a lasting reduction in MCC activation to phobic cues as well as diminished behavioral avoidance (Hauner et al., 2012).
5 Future Challenges
The present results provide robust meta-analytic evidence that dispositionally anxious individuals are characterized by larger FMΘ signals in response to uncertain negative outcomes. Larger control signals, in turn, predict subsequent behavioral adaptation. Nonetheless, it is clear that much work remains to clarify the relationships between anxiety, aversion, FMθ, and the underlying neural circuitry. Here, we outline several of the most important challenges for future research.
First, while this is compelling evidence that scalp-recorded FMΘ signals reflect generators in the MCC, the lack of a unique solution to the inverse problem dictates that other brain areas, particularly those in the adaptive control network, are also likely to contribute to these signals (Agam et al., 2011; Emeric et al., 2010; Luu et al., 2003). Clarifying the neuronal sources of the FMΘ is particularly important for bridging the gap separating invasive work in nonhuman species from correlative neurophysiological investigations in humans (Narayanan et al., 2013).
Second, while our results demonstrate that larger FMΘ signals are associated with heightened inhibition and enhanced avoidance, it will remain important to critically assess the influence of task demands on the relationship between FMθ and behavioral adjustment. Errors appear to elicit a somewhat subtle increase in response caution and stimulus attention (Danielmeier and Ullsperger, 2011), whereas punishment in a rapidly adaptive two-alterative forced choice task predicts a clear-cut lose-switch avoidance strategy. The strategic nature of behavioral adjustments thus appear to moderate the size and consistency of observable FMθ-behavior relationships. Our results suggest that trial-by-trial analyses performed at the level of individual subjects may prove especially sensitive to detecting such relationships.
Third, it will also be useful to clarify whether behavioral adaptation reflects the direct influence of MCC on motor centers or, as some have suggested (Miller and Cohen, 2001), a consequence of MCC triggering top-down control processes implemented in the fronto-parietal network. It has been previously suggested that FMθ signals underlie the communication and implementation of control via phase synchronous relationships with distal cortical areas (Cavanagh et al., 2009). This technique provides a testable measure of directional influence preceding behavioral adjustments. Fourth, a central challenge will be to determine whether increased MCC control signals are a consequence of amplified punishment-related information arriving from distal brain regions, such as the extended amygdala (Brázdil et al., 2002; Nishijo et al., 2008; Ousdal et al., 2008), or instead reflect local changes in the way that the MCC assesses demands for control and uses it to bias learning and behavior (Figure 1).
Finally, it will be important to clarify the link between control-sensitive FMΘ signals and the development of psychopathology. Although our results provide clear evidence that dispositionally anxious individuals show amplified FMΘ signals, the clinical significance of this neural marker remains uncertain (Proudfit et al., 2013). Alterations in the ERN, in particular, have been found in patients with a remarkably broad range of diagnoses, including anxiety, obsessive-compulsive, and psychotic disorders (Mathews et al., 2012; Moser et al., 2013; Weinberg et al., 2011). This apparent lack of specificity may partially reflect the influence of transdiagnostic features, such as elevated neuroticism (Foti et al., 2013, 2012), that are shared by many psychiatric disorders (Caspi et al., 2013; Kotov et al., 2010; Tackett et al., 2013). While it is not yet known whether FMΘ plays a role in the etiology of these features, evidence from pharmacological studies indicates that these signals are attenuated by the administration of anxiolytic compounds (Bradford et al., 2013; Davis et al., 2010; Gray and McNaughton, 2000; Hefner and Curtin, 2012; Hefner et al., 2013), suggesting that the MCC-centered adaptive control network (Figure 1) may be a key site of action (i.e., anxiolysis). Future work aimed at clarifying the neurobiology of adaptive control and its underlying neurobiology in humans and other animals promises to enhance nosology, improve prognosis, and accelerate the development of novel therapeutic interventions (Borsook et al., 2006; Narayanan et al., 2013; Reinhart and Woodman, 2014).
6 Concluding Remarks
Here we have surveyed new evidence that anxiety and cognitive control are anatomically, functionally, and computationally integrated in the MCC. TACH suggests that anxiety and cognitive control often share a common need to determine an optimal course of action in the face of uncertainty about instrumental actions and their potentially aversive consequences. The present meta-analytic results reinforce this claim, demonstrating that anxious individuals show heightened FMΘ signals in response to punishment and other prompts for increased cognitive control; in turn, elevated FMΘ signals are associated with more cautious and avoidant instrumental behavior on subsequent trials. These findings suggest that key elements of adaptive control are embodied in FMθ activity and contribute to both ‘affect’ and ‘cognition’. This work provides a novel, neurobiologically-grounded framework for deciphering the contribution of adaptive control circuitry to temperament and psychopathology.
Figure 5. Forest plots of the relationship between FMϑ and behavioral control (as Pearson’s r) and funnel plots.
Here the forest plots are separated by the type of eliciting event and specifically associated EEG activity (A: error/ERN and RT slowing, B: punishment/FRN and switching), as well as the distinction between inter- and intra-individual analysis. C) Funnel plots were used to qualitatively assess the presence of publication bias. These plots display the standard error (y-axis) as a function of the effect size (x-axis). The lines of the funnel represent the range where 95% of points are expected to lie in the absence of publication bias. Asymmetrical deviations around the center line also suggest possible publication bias, especially if there are more points in the lower right corner (small N, large effect) but not in the lower left corner (small N, small effect). There was no qualitative evidence for publication bias in any meta analysis.
HIGHLIGHTS.
The midcingulate cortex is involved in adaptively regulating behavior
This domain general process is common to negative affect and cognitive control
Frontal-midline theta band EEG signals reflect these adaptations to uncertainty
Three meta analyses support the domain general nature of frontal-midline theta
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Mental Health (MH046729, MH081884, MH084051) and the University of Maryland. The authors thank Michael J Frank, Mike X Cohen, and John JB Allen, and Mickey Inzlicht for helpful comments on previous versions of this manuscript and insightful discussions of this topic. Some of this material was first presented at the 2012 meeting on the Determinants of Executive Function & Dysfunction (Boulder, CO) as well as the 2012 Wisconsin Symposium on Emotion (Madison, WI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- Agam Y, Hämälaänen MS, Lee AKC, Dyckman Ka, Friedman JS, Isom M, Makris N, Manoach DS. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proc. Natl. Acad. Sci. U. S. A. 2011;108:17556–17561. doi: 10.1073/pnas.1103475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph J-P, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur. J. Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology. 2008;45:11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Baker TE, Holroyd CB. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Barker TV, Reeb-Sutherland BC, Fox Na. Individual differences in fear potentiated startle in behaviorally inhibited children. Dev. Psychobiol. 2014;56:133–141. doi: 10.1002/dev.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Bullis JR, Comer JS, Ametaj Aa. Evidence-based psychological treatments: an update and a way forward. Annu. Rev. Clin. Psychol. 2013;9:1–27. doi: 10.1146/annurev-clinpsy-050212-185629. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, Saults JS, Wood PK. Alcohol effects on performance monitoring and adjustment: affect modulation and impairment of evaluative cognitive control. J. Abnorm. Psychol. 2012;121:173–186. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MPI, Nitsch AM, Miltner WHR, Straube T. A Single-Trial Estimation of the Feedback-Related Negativity and Its Relation to BOLD Responses in a Time-Estimation Task. J. Neurosci. 2014;34:3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Rushworth MFS. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Blair K, Marsh AA, Morton J, Vythilingam M, Jones M, Mondillo K, Pine DC, Drevets WC, Blair JR. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J. Neurosci. 2006;26:11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem Mas, Tops M, Kostermans E, De Cremer D. Sensitivity to punishment and reward omission: evidence from error-related ERP components. Biol. Psychol. 2008;79:185–192. doi: 10.1016/j.biopsycho.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Boksem MAS, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Res. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Bonini F, Burle B, Liégeois-Chauvel C, Régis J, Chauvel P, Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343:888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons.; 2009. [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat. Rev. Drug Discov. 2006;5:411–424. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ. How Bad Could It Be? Alcohol Dampens Stress Responses to Threat of Uncertain Intensity. Psychol. Sci. 2013;24:2541–2549. doi: 10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brázdil M, Roman R, Falkenstein M, Daniel P, Jurak P, Rektor I. Error processing--evidence from intracerebral ERP recordings. Exp. Brain Res. 2002;146:460–466. doi: 10.1007/s00221-002-1201-y. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt Ba, Holmes J, Dale AM, Greve D, Jenike Ma, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. U. S. A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky A. Treatment-resistant anxiety disorders. Mol. Psychiatry. 2006;11:805–814. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol. Bull. 1959;56:81–105. [PubMed] [Google Scholar]
- Caspi a, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, Moffitt TE. The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clin. Psychol. Sci. 2013;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: stability and change. Annu. Rev. Psychol. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Figueroa CM, Cohen MX, Frank MJ. Frontal Theta Reflects Uncertainty and Unexpectedness during Exploration and Exploitation. Cereb. Cortex. 2012a;22:2575–2586. doi: 10.1093/cercor/bhr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJB. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJB. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012b;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Swainson R, Durham L, Benham L, Cools R. Feedback-related Negativity Codes Prediction Error but Not Behavioral Adjustment during Probabilistic Reversal Learning. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21456. [DOI] [PubMed] [Google Scholar]
- Choi J-S, Cain CK, LeDoux JE. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn. Mem. 2010;17:139–147. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Donner TH. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 2013;110:2752–2763. doi: 10.1152/jn.00479.2013. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 2008;1238:127–142. doi: 10.1016/j.brainres.2008.07.114. [DOI] [PubMed] [Google Scholar]
- Craske MG, Wolitzky–Taylor KB, Mineka S, Zinbarg R, Waters AM, Vrshek–Schallhorn S, Epstein A, Naliboff B, Ornitz E. Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: Evidence from a longitudinal investigation. J. Abnorm. Psychol. 2012 doi: 10.1037/a0025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Ullsperger M. Post-error adjustments. Front. Psychol. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruijn ERA, Hulstijn W, Verkes RJ, Ruigt GSF, Sabbe BGC. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology. 2004 doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Varriale V, D’Antuono L. Event-related components of the punishment and reward sensitivity. Clin. Neurophysiol. 2010;121:60–76. doi: 10.1016/j.clinph.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effortful cognitive tasks. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Di Pellegrino G, Ciaramelli E, Ladavas E. The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J. Cogn. Neurosci. 2007;19:275–286. doi: 10.1162/jocn.2007.19.2.275. [DOI] [PubMed] [Google Scholar]
- Doñamayor N, Marco-Pallarés J, Heldmann M, Schoenfeld MA, Munte TF. Temporal dynamics of reward processing revealed by magnetoencephalography. Hum. Brain Mapp. 2011;32:2228–2240. doi: 10.1002/hbm.21184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach G, Fischer R. Conflicts as aversive signals. Brain Cogn. 2012;78:94–98. doi: 10.1016/j.bandc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Dutilh G, Vandekerckhove J, Forstmann BU, Keuleers E, Brysbaert M, Wagenmakers E-J. Testing theories of post-error slowing. Atten. Percept. Psychophys. 2012;74:454–465. doi: 10.3758/s13414-011-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Brain Res. Cogn. Brain Res. 2005;25:873–883. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Edwards BG, Calhoun VD, Kiehl Ka. Joint ICA of ERP and fMRI during error-monitoring. Neuroimage. 2012;59:1896–1903. doi: 10.1016/j.neuroimage.2011.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Leslie MW, Pouget P, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: Supplementary eye field. J Neurophysiol. 2010;104:1523–1537. doi: 10.1152/jn.01001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Kotov R, Bromet E, Hajcak G. Beyond the broken error-related negativity: functional and diagnostic correlates of error processing in psychosis. Biol. Psychiatry. 2012;71:864–872. doi: 10.1016/j.biopsych.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Kotov R, Hajcak G. Psychometric considerations in using error-related brain activity as a biomarker in psychotic disorders. J. Abnorm. Psychol. 2013;122:520–531. doi: 10.1037/a0032618. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J. The Error-Related Negativity (ERN/Ne) In: Luck SJ, Kappenman E, editors. Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press; 2012. pp. 231–291. [Google Scholar]
- Gemba H, Sasaki K, Brooks VB. “Error” potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neurosci. Lett. 1986;70:223–227. doi: 10.1016/0304-3940(86)90467-2. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety: An enquiry into the function of the septo-hippocampal system. Oxford University Press; 2000. [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerer D, Li S-C, Völkle M, Müller V, Lindenberger U. A lifespan comparison of the reliability, test-retest stability, and signal-to-noise ratio of event-related potentials assessed during performance monitoring. Psychophysiology. 2013;50:111–123. doi: 10.1111/j.1469-8986.2012.01476.x. [DOI] [PubMed] [Google Scholar]
- Hauner KK, Mineka S, Voss JL, Paller KA. From the Cover: Exposure therapy triggers lasting reorganization of neural fear processing. Proc. Natl. Acad. Sci. 2012 doi: 10.1073/pnas.1205242109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Stämpfli P, Drechsler R, Brandeis D, Walitza S, Brem S. The feedback-related negativity (FRN) revisited: New insights into the localization, meaning and network organization. Neuroimage. 2014;84:159–168. doi: 10.1016/j.neuroimage.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J Neurosci. 2011;31:4178–4187. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV. An unbiased correction for sampling error in validity generalization studies. J. Appl. Psychol. 1989;74:469–477. [Google Scholar]
- Hefner KR, Curtin JJ. Alcohol stress response dampening: selective reduction of anxiety in the face of uncertain threat. J. Psychopharmacol. 2012 doi: 10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Moberg CA, Hachiya LY, Curtin JJ. Alcohol stress response dampening during imminent versus distal, uncertain threat. J. Abnorm. Psychol. 2013 doi: 10.1037/a0033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45:688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Eichele T, Enriquez-Geppert S, Wollbrink a, Kugel H, Konrad C, Pantev C. Multimodal imaging of functional networks and event-related potentials in performance monitoring. Neuroimage. 2011;56:1588–1597. doi: 10.1016/j.neuroimage.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Salomons TV, Moayedi M, Mouraux A, Davis KD. Beyond metaphor: contrasting mechanisms of social and physical pain. Trends Cogn. Sci. 2013;17:371–378. doi: 10.1016/j.tics.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Ichikawa N, Siegle GJ, Dombrovski A, Ohira H. Subjective and model-estimated reward prediction: association with the feedback-related negativity (FRN) and reward prediction error in a reinforcement learning task. Int J Psychophysiol. 2010;78:273–283. doi: 10.1016/j.ijpsycho.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. (80-.). [DOI] [PubMed] [Google Scholar]
- Jocham G, Ullsperger M. Neuropharmacology of performance monitoring. Neurosci Biobehav Rev. 2009;33:48–60. doi: 10.1016/j.neubiorev.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat. Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W, McGuire JT, Rosen ZB, Botvinick MM. Decision making and the avoidance of cognitive demand. J. Exp. Psychol. Gen. 2010;139:665–682. doi: 10.1037/a0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol. Bull. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:596–597. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leue A, Klein C, Lange S, Beauducel A. Inter-individual and intra-individual variability of the N2 component: On reliability and signal-to-noise ratio. Brain Cogn. 2013;83:61–71. doi: 10.1016/j.bandc.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Lim LimS-L, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist Ka, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström BR, Mattsson-Mårn IB, Golkar A, Olsson A. In Your Face: Risk of Punishment Enhances Cognitive Control and Error-Related Activity in the Corrugator Supercilii Muscle. PLoS One. 2013;8:e65692. doi: 10.1371/journal.pone.0065692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav. Res. Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Mathews Ca, Perez VB, Delucchi KL, Mathalon DH. Error-related negativity in individuals with obsessive-compulsive symptoms: toward an understanding of hoarding behaviors. Biol. Psychol. 2012;89:487–494. doi: 10.1016/j.biopsycho.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev. Cogn. Neurosci. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milea D, Lehéricy S, Rivaud-Pechoux S, Duffau H, Lobel E, Capelle L, Marsault C, Berthoz a, Pierrot-Deseilligny C. Antisaccade deficit after anterior cingulate cortex resection. Neuroreport. 2003;14:283–287. doi: 10.1097/00001756-200302100-00026. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. J. Cogn. Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Moadab I, Gilbert T, Dishion TJ, Tucker DM. Frontolimbic activity in a frustrating task: covariation between patterns of coping and individual differences in externalizing and internalizing symptoms. Dev. Psychopathol. 2010;22:391–404. doi: 10.1017/S0954579410000131. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front. Hum. Neurosci. 2013;7:466. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq F, Bland AR, Schaefer A. Uncertainty and cognitive control. Front. Psychol. 2011;2:249. doi: 10.3389/fpsyg.2011.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat. Neurosci. 2013:1–10. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J. Neurophysiol. 2008;100:520–525. doi: 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijo H, Hori E, Tazumi T, Ono T. Neural correlates to both emotion and cognitive functions in the monkey amygdala. Behav. Brain Res. 2008;188:14–23. doi: 10.1016/j.bbr.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self-Regulation. Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. J Cogn Neurosci. 2007;19:1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. Reliability of error-related brain activity. Brain Res. 2009a;1284:89–99. doi: 10.1016/j.brainres.2009.05.079. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009b;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The effect of trial-to-trial feedback on the error-related negativity and its relationship with anxiety. Cogn. Affect. Behav. Neurosci. 2009c;9:427–433. doi: 10.3758/CABN.9.4.427. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Jensen J, Server A, Hariri AR, Nakstad PH, Andreassen OA. The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging Go-NoGo task. Neuroscience. 2008;156:450–455. doi: 10.1016/j.neuroscience.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Pereira MG, de Oliveira L, Erthal FS, Joffily M, Mocaiber IF, Volchan E, Pessoa L. Emotion affects action: Midcingulate cortex as a pivotal node of interaction between negative emotion and motor signals. Cogn. Affect. Behav. Neurosci. 2010;10:94–106. doi: 10.3758/CABN.10.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat. Rev. Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Biele G, Vavatzanidis N, Kazzer P, Heekeren HR. Temporal dynamics of prediction error processing during reward-based decision making. Neuroimage. 2010;53:221–232. doi: 10.1016/j.neuroimage.2010.05.052. [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Inzlicht M, Mennin DS. Anxiety and error monitoring: the importance of motivation and emotion. Front. Hum. Neurosci. 2013;7:636. doi: 10.3389/fnhum.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt PMA. CHOICE-RESPONSE TASKS i. Exp. Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Pérez-Edgar K, Henderson HA, Lissek S, Chronis-Tuscano A, Grillon C, Pine DS, Fox NA. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RMG, Woodman GF. Causal Control of Medial-Frontal Cortex Governs Electrophysiological and Behavioral Indices of Performance Monitoring and Learning. J. Neurosci. 2014;34:4214–4227. doi: 10.1523/JNEUROSCI.5421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GPH. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science (New York, N.Y.) 2002 doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychol. Bull. 1979;86:638–641. [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sallet J, Camille N, Procyk E. Modulation of feedback-related negativity during trial-and-error exploration and encoding of behavioral shifts. Front. Neurosci. 2013;7:209. doi: 10.3389/fnins.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouppe N, De Houwer J, Richard Ridderinkhof K, Notebaert W. Conflict: Run! Reduced Stroop interference with avoidance responses. Q. J. Exp. Psychol. 2012 doi: 10.1080/17470218.2012.685080. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Murphy TI, Homan D, Chantziantoniou DK, Khan S. Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology. 2010;47:260–270. doi: 10.1111/j.1469-8986.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010;51:1319–1333. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter Ha, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth Sa, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488:218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. (80-.). [DOI] [PubMed] [Google Scholar]
- Steenland HW, Li X-Y, Zhuo M. Predicting Aversive Events and Terminating Fear in the Mouse Anterior Cingulate Cortex during Trace Fear Conditioning. J. Neurosci. 2012 doi: 10.1523/JNEUROSCI.5566-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JPT. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 2011;343:302–307. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- Tackett JL, Lahey BB, van Hulle C, Waldman I, Krueger RF, Rathouz PJ. Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. J. Abnorm. Psychol. 2013;122:1142–1153. doi: 10.1037/a0034151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Atkinson R, El-Deredy W. The feedback-related negativity signals salience prediction errors, not reward prediction errors. J. Neurosci. 2013;33:8264–8269. doi: 10.1523/JNEUROSCI.5695-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J. Clin. Epidemiol. 2005;58:894–901. doi: 10.1016/j.jclinepi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J. Neurophysiol. 2006;95:2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y, Sasaki K. Theta oscillations in primate prefrontal and anterior cingulate cortices in forewarned reaction time tasks. J. Neurophysiol. 2010;103:827–843. doi: 10.1152/jn.00358.2009. [DOI] [PubMed] [Google Scholar]
- Van Noordt SJR, Segalowitz SJ. Performance monitoring and the medial prefrontal cortex: a review of individual differences and context effects as a window on self-regulation. Front. Hum. Neurosci. 2012;6:197. doi: 10.3389/fnhum.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. [Google Scholar]
- Walsh MM, Anderson JR. Learning from delayed feedback: neural responses in temporal credit assignment. Cogn. Affect. Behav. Neurosci. 2011;11:131–143. doi: 10.3758/s13415-011-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MM, Anderson JR. Learning from experience: event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neurosci. Biobehav. Rev. 2012;36:1870–1884. doi: 10.1016/j.neubiorev.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Longer term test-retest reliability of error-related brain activity. Psychophysiology. 2011;48:1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Hajcak G. Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motiv. Emot. 2011;36:84–100. [Google Scholar]
- West R, Travers S. Tracking the temporal dynamics of updating cognitive control: an examination of error processing. Cereb. Cortex. 2008;18:1112–1124. doi: 10.1093/cercor/bhm142. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A. 2010a;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Vinck M, Leung LS, Everling S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavio. Front Hum Neurosci. 2010b;4:210. doi: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schiagenhauf F, Beck A, Cohen MX, Knutson B, Heinz A. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007;36:1253–1262. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Yasuda A, Sato A, Miyawaki K, Kumano H, Kuboki T. Error-related negativity reflects detection of negative reward prediction error. Neuroreport. 2004;15:2561–2565. doi: 10.1097/00001756-200411150-00027. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD. The Impact of Cognitive Deficits on Conflict Monitoring. Psychol. Sci. 2006;17:164–171. doi: 10.1111/j.1467-9280.2006.01680.x. [DOI] [PubMed] [Google Scholar]
- Yeung N, Ralph J, Nieuwenhuis S. Drink alcohol and dim the lights: the impact of cognitive deficits on medial frontal cortex function. Cogn. Affect. Behav. Neurosci. 2007;7:347–355. doi: 10.3758/cabn.7.4.347. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004;22:590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]