Abstract
Neurodevelopmental delays in intensive care neonates are common but difficult to predict. In children, hemisphere differences in cortical processing of speech are predictive of cognitive performance. We hypothesized that hemisphere differences in auditory event-related potentials in intensive care neonates are predictive of neurodevelopment in infancy, even in those born preterm. Event-related potentials to speech sounds were prospectively recorded in 57 infants (gestational age 24–40 weeks) prior to discharge. The Developmental Assessment of Young Children was performed at 6 and 12 months. Hemisphere differences in mean amplitudes increased with postnatal age (P < .01) but not with gestational age. Greater hemisphere differences were associated with improved communication and cognitive scores at 6 and 12 months, but decreased in significance at 12 months after adjusting for socioeconomic and clinical factors. Auditory cortical responses can be used in intensive care neonates to help identify infants at higher risk for delays in infancy.
Keywords: auditory event-related potentials, lateralization, neonatal intensive care, preterm, neurodevelopment
Infants hospitalized in intensive care nurseries are at high risk for poor neurodevelopmental outcomes.1,2 Preterm infants suffer from the interruption of normal brain development, often in the early third trimester that is an essential period of neuronal arborization and synaptogenesis.3–5 Term infants in intensive care nurseries are often admitted because of birth complications or congenital abnormalities requiring surgery. All infants, term or preterm, are further subject to the atypical sensory experiences of the intensive care environment, ranging from painful procedures and increased sound exposure to neurotoxic medications. Some studies have postulated that the intensive care nursery environment, with its abnormal auditory experiences can alter the development of normal neural processing in infants and result in poorer outcomes after discharge.6–9
In order to quantify neural functioning in infants and children, researchers, and more recently clinicians, have used event-related potential measurements, or stimulus-locked electroencephalogram (EEG). Event-related potentials characterize cortical signals in terms of time and amplitude, providing information on the strength and speed of electrical signals traveling through the brain.10 In full-term infants and children, information processing as measured by event-related potentials is highly predictive of later cognitive outcomes11 and small studies suggest the same for preterm infants.12
The specific use of auditory event-related potentials to study brain function is well established in the field of child development and an attractive methodology in infants and ill patients because it is non-invasive and does not require active participation. Auditory signal processing in infants depends both on the maturity of the primary auditory cortex and on the postnatal age of the subject.13 Maturation of the auditory system leads to structural changes in cortical organization that are evidenced in better-defined event-related potential peaks, perhaps reflecting experience-expectant development.14–16 Conversely, functional development could be reflected in more global processes of cortical specialization, such as hemisphere differences, which could be at least partially related to the development of experience-dependent neural processes supporting cognitive functions.17–19 Although nonspeech auditory stimuli typically are associated with comparable activity over both hemispheres, speech sounds tend to elicit greater amplitudes over the left hemisphere than the right,17 as early as in full-term newborns.20
Although in our prior study we demonstrated that the ability to discriminate between speech sounds correlates with gestational age at birth as well as postnatal age at testing time,21 in the present study, we hypothesized that cortical hemisphere specialization with increased amplitudes on the left would be primarily associated with postnatal age, much as it is in full-term infants.
We also hypothesized that hemisphere differences in speech sound processing in the hospital would be predictive of neurodevelopmental status throughout infancy. To test these hypotheses, we designed a prospective study to measure hemisphere differences in event-related potential responses to speech sounds in infants, prior to discharge from the intensive care nursery, and performed neurodevelopmental assessments when the infants returned to the intensive care nursery follow-up clinic at 6 and 12 months of age.
Study Design
This was a prospective, observational study of 57 infants cared for in the intensive care nursery at the Monroe Carell Jr Children’s Hospital at Vanderbilt between January 2009 and January 2012. Infants were recruited according to Vanderbilt Institutional Review Board–approved protocols. All infants received serial cranial ultrasounds to evaluate for the presence of white matter damage during their intensive care nursery stay as well as auditory brainstem testing by a pediatric audiologist prior to event-related potential response testing. Testing was performed after 32 weeks’ gestational age, when infants were considered clinically stable. Additionally, a head circumference of at least 31 cm was necessary to technically acquire event-related potential data.
Event-Related Potential Methodology
Stimuli
The stimuli included 6 computer-synthesized consonant-vowel syllables (/ba/, /da/, /ga/, /bu/, /du/, /gu/), originally employed by Stevens and Blumstein.22 The 5-formant consonant-vowels were synthesized on a Klatt (Cascade) synthesizer, so that the amplitude of individual formants was modulated as a function of the respective formant frequencies, as in natural speech. Duration of F1 transition ranged between 15 and 45 ms depending on the syllable-initial consonant as well as on the following vowel. Transition duration for all the other formants was always 40 ms and was followed by a 250-ms steady-state vowel. Rise and decay times were equivalent across stimuli.
Electrodes
A high-density array of 124 Ag/AgCl electrodes embedded in soft sponges saturated with warm saline solution (Geodesic Sensor Net without the lower eye channels; EGI) was used to record event-related potentials. This array of soft sensors does not create any localized pressure points and minimizes any potential discomfort. Electrode impedance levels were below 40 kΩ before and after testing. During acquisition, data were sampled at 250 Hz with the low-pass filter set to 30 Hz and the high-pass filter to 0.1 Hz. All electrodes were referred to vertex and then re-referenced offline to an average reference.
Procedure
Each infant was tested prior to hospital discharge in his or her single-patient intensive care nursery room while lying in the bassinet or in the caregiver’s arms with both ears unobstructed. No restraint was used beyond the typical infant swaddling routinely performed by nurses and parents. The stimuli were presented by a computer at 80 dB SPL(A) as measured at the infant’s ear through a speaker positioned approximately 1 m above the midline of the infant’s head, which ensured equal speaker-to-ear distance for both ears. All sounds were presented in random order, 25 times each, for a total of 150 trials. Inter-stimulus intervals varied randomly from 1600 to 2600 ms to prevent habituation to sound onset. Net Station software (v. 4.2; EGI) controlled recording of brain activity. Stimulus presentation was controlled by E-Prime (v. 1.2, PST Inc, Pittsburgh, PA). During the entire test session, the infant’s EEG and behavior were continuously monitored so that the stimulus presentation occurred only when the infant was in proper alignment with the speaker, and the EEG was free of motor artifacts. The entire testing session (including sensor application and removal) lasted approximately 15 minutes.
Data Analysis
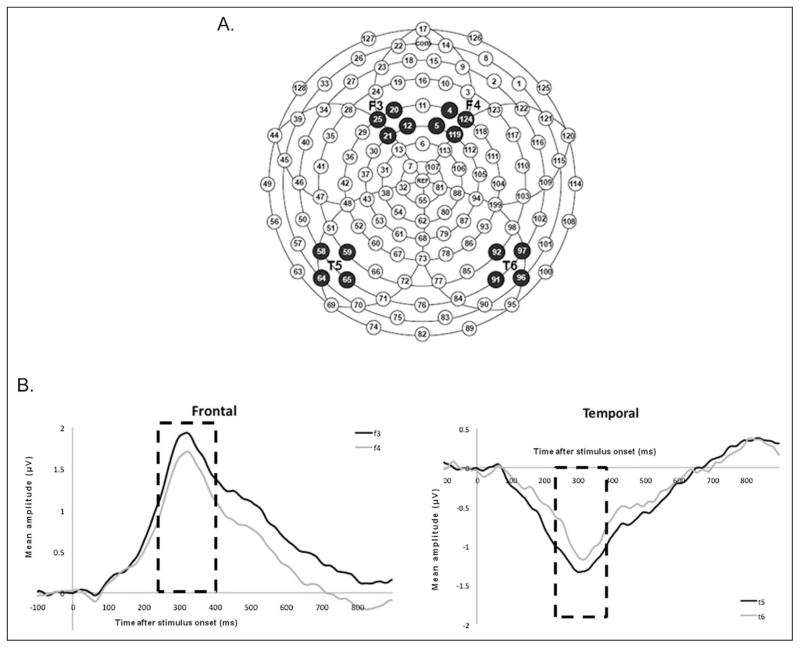
Individual event-related potentials were obtained by segmenting the ongoing EEG based on each stimulus onset to include a 100-ms pre-stimulus baseline and a 900-ms post-stimulus interval. Individual trials contaminated by movements (eg, sucking) or ocular artifacts were discarded using automated NetStation software algorithms followed by manual review. Data for electrodes with poor signal quality were reconstructed using spherical spline interpolation procedures. For a trial to be included in the remaining analyses, no more than 15 electrodes (12% of the array) could be interpolated. For a participant’s data set to be included in the overall analyses, averages for each stimulus had to be based on a minimum of 10 trials. Rejection rates were comparable across stimuli with the final averages based on 14.89 ± 3.78 trials per syllable (/ba/: 14.8 ± 3.8; /da/: 14.8 ± 3.9; /ga/: 15.0 ± 3.5; /bu/: 14.7 ± 3.5; /du/: 14.8 ± 3.85; /gu/: 15.1 ± 4.1). Overall rejection rates between infants who had follow-up and those who did not were not significantly different (P > .2) and there were no correlations between trial rejection rates and PNA. The data were then averaged across trials and sounds, referenced to an average reference, and baseline-corrected by subtracting the average microvolt value across the 100-ms prestimulus interval from each time point in the poststimulus period. Because brain responses of very young infants are often in the form of broad positive and negative deflections rather than well-defined peaks, mean amplitudes were calculated for a subset of the 124 electrodes corresponding to frontal and temporal locations in the left and right hemisphere (Figure 1A), which are the scalp locations commonly used to document brain activity associated with cortical auditory processing. Mean amplitudes within a 250- to 400-ms window after stimulus onset (represented by the interval superimposed on the averaged waveform of all normative patients in Figure 1B) were used to test the predictive association between brain responses and neurodevelopmental outcomes. This time window was selected according to previous studies in newborn infants and older children.14 Additionally, prior studies in infants showed that this window demonstrated the best variation between term and preterm infant’s response to sound.21
Figure 1.
Event-potential responses to speech sounds. (A) Electrode clusters on scalp locations. (B) Event-potential responses averaged across all study subjects. Mean amplitudes (in μV) averaged for all 57 patients in the 250- to 400-ms time window were: F3 = 1.48 (±0.19), F4 = 1.31 (±0.19), T5 = −1.1 (±0.11), T6 = −0.5 (±0.11). F3, frontal left; F4, frontal right; T5, temporal left; T6, temporal right.
Developmental Testing
During their visits to the neonatal developmental follow-up clinic at 6 and 12 months’ chronologic age, in addition to standard neurologic and physical exams, infants were tested using Developmental Assessment of Young Children, a standardized test of infant and child development. The subscales of cognitive and communication function23 were performed by trained examiners, with parent questionnaires corroborated by infant observation and challenge. Examples of communication milestones at various infancy time points include vocalization of vowels (/a/, /u/), early consonants (/g/, /k/), briefly stopping when told “no” and saying “mama” or “dada” discriminately. Examples of cognitive milestones include pulling a cloth off their face, intentionally dropping an object, imitating scribbling, or attempting to start a toy. Standardized scores were obtained for chronologic and adjusted age. Maternal socioeconomic status was scored in a continuous manner factoring in occupation, education, and family situation according to a modernized Hollingshead scoring method from 8 to 66.24
Statistical Analysis
To test the hypothesis that hemisphere differences in neural responses to speech sounds are correlated with the postnatal experience in the intensive care nursery estimated by postnatal age at testing time, linear regression was used. As previously described,21 postnatal age at testing time is not a proxy for length of stay as infants often stay several days of weeks past event-related potential testing. It corresponds to postnatal experience at testing time, encompassing all types of sensory exposure as well as any events related to reasons for hospitalization. Mean amplitudes of event-related potentials recorded over the right hemisphere were subtracted from those over the left hemisphere, and the difference served as the dependent variables. Gestational age, postnatal age, and scalp location were used as the independent variables. Additionally, we considered if infant gender, maternal education, socioeconomic status, race, or antenatal steroid use were predictive of mean amplitudes. We used generalized estimating equations with the robust sandwich estimator to account for correlation arising from repeatedly measuring amplitudes on the same subject over continuous time points. Our prespecified analysis plan included using a model with a 4-degree-of-freedom restricted cubic spline for time with all second- and third-order interactions among the predictors in order to allow for a flexible relationship with mean amplitude. Sensitivity analysis and model checking indicated that this model provided a good fit to our data, and more complex models did not alter the scientific conclusions drawn from the analysis. Model fit was summarized by the percentage of the variance in mean amplitudes explained by postnatal age and gestational age.
To test the hypothesis that lateralization of speech sound processing to the left in the neonatal period would be predictive of neurodevelopmental status in infancy, linear regression was used to evaluate the association of hemisphere differences in mean amplitudes with adjusted age Developmental Assessment of Young Children scores. In particular, we quantified lateralization as the difference in mean amplitudes between left and right scalp locations and used this difference as the predictor in separate regression models for frontal and temporal locations. The robust sandwich estimator was used to estimate all standard errors in order to relax normality and homoskedasticity assumptions that are otherwise needed for valid inference from linear regression. All analyses were conducted using the R statistics program.
Results
Characteristics of the Patient Population
Of the 57 patients studied (Table 1), 4 infants had severe white matter injury (grade III or IV intraventricular hemorrhage), 1 infant had Turner syndrome and 2 had a unilateral auditory neuropathy. Of the remaining 50 infants, 1 had a unilateral grade I hemorrhage and 2 had unilateral grade II without ventriculomegaly. There were no infants with central nervous system infections or persistent apneas. The median gestational age at birth of the study group was 28 completed weeks (interquartile range = 26–31), and the median postnatal age at testing was 2.2 (interquartile range = 1.6–2.8) months. As expected, the relationship between gestational age and postnatal age at the time of event-related potential testing followed a linear correlation (ρ = −0.61, P < .001). Infants born at earlier gestational ages were more likely to have a longer postnatal age in the intensive care nursery prior to the test session due, in large part, to the time it took for their head circumferences to reach the minimum testable size. Population characteristics also included maternal education (median score = 4, interquartile range = 4–5) corresponding to a high school education and Hollings-head socioeconomic score (median 31, interquartile range = 22–36), in between the lower second and third quintiles of the general population.
Table 1.
Population Characteristics.a
| N | ||
|---|---|---|
| Gestational age at birth in weeks | 57 | 28 (26, 31) |
| Postnatal age at ERP in months | 57 | 2.2 (1.6, 2.8) |
| Gender (male %) | 57 | 60 |
| Antenatal steroids received (%) | 57 | 61 |
| SES score | 53 | 31 (22, 36) |
| Maternal Education | 56 | 4 (4, 5) |
| DAYC scores at 6 months | ||
| Cognitive | 38 | 99 (94, 106) |
| Communication | 38 | 107 (94, 110) |
| DAYC scores at 12 months | ||
| Cognitive | 24 | 88 (85, 94) |
| Communication | 24 | 89 (82, 94) |
Abbreviations: DAYC, Developmental Assessment of Young Children; ERP, event-related potential; IQR, interquartile range; SES, socioeconomic score.
Values are median (IQR) unless otherwise indicated.
Effects of Gestational Age and Postnatal Age on Hemisphere Differences
Our first objective was to characterize how gestational and postnatal ages affect the lateralization of speech sound processing, as measured by the difference in event-related brain responses between left and right sides. To answer this question, we examined mean amplitudes averaged over all sounds and individually assessed the effects of gestational age, postnatal age, time after stimulus onset, and frontal versus temporal location as well as their interactions. We considered the entire study population, including those infants with abnormal ultra-sonographs to ensure that our sample was representative of an intensive care population.
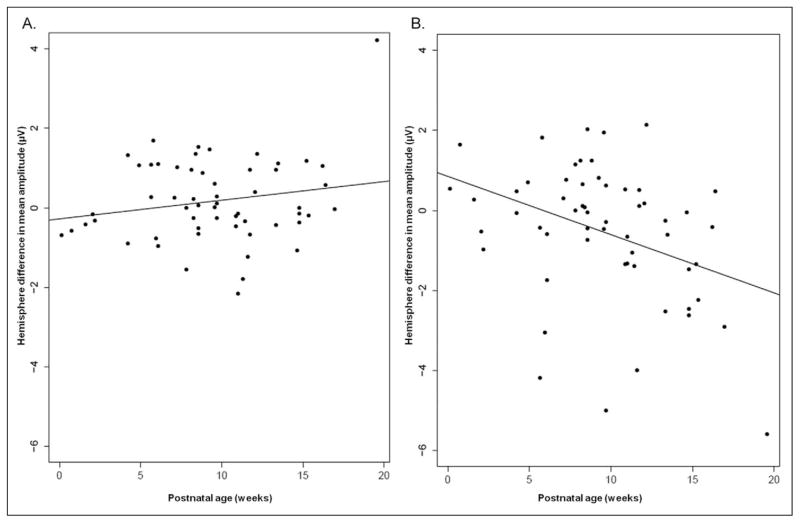
As can be seen in Table 2, all studied variables except gestational age had a significant effect on mean amplitudes (P < .001 for all). This indicated that left-right differences in mean amplitude of neural signals varied based on spatial, temporal, and experiential characteristics of the infant brain. In Figure 2, the differences in mean amplitude of the event-related potentials increase as postnatal age increases both in frontal and temporal locations (R = 0.19 and 0.37, respectively). The main effect of gestational age was not significant, and neither was the interaction of gestation and postnatal age (P = .72), suggesting that hemisphere differences were affected predominantly by the length of sound experience prior to testing. Gestational age did interact with location, with greater interhemispheric differences in temporal location than frontal as GA increased (P = .02).
Table 2.
Significance of Variable Effects on Amplitude Differences Between Left and Right Hemispheres in Response to Speech Sounds.
| Degrees of freedom | F coefficient | P valuea | |
|---|---|---|---|
| Gestational age | 8 | 1.91 | NS |
| Postnatal age | 8 | 2.45 | .01 |
| Time | 16 | 3.37 | <.001 |
| Fontal vs temporal location | 8 | 3.47 | <.001 |
| Gestational age × location | 2 | 4.11 | .02 |
| Gestational age × postnatal age | 2 | 0.33 | NS |
| Postnatal age × location | 2 | 4.49 | <.01 |
| Postnatal age × time | 4 | 1.92 | NS |
| Location × time | 4 | 3.68 | <.01 |
| Gestational age × postnatal age location | 1 | 0.48 | NS |
| Total for model | 23 | 6.48 | <.0001 |
Abbreviation: NS, not significant.
All P uncorrected
Figure 2.
Hemisphere differences in mean amplitudes responses to speech sounds as a function of postnatal age. (A) Frontal locations. (B) Temporal locations. Line represents linear regression fit.
Hemispheric Differences in Event-Related Potential Responses and Neurodevelopmental Status in Infancy
Prior event-related potential studies have shown that the lateralization of speech sound processing is a sign of increasing processing efficiency.25 Therefore, we examined the associations between the left-right hemisphere difference in mean amplitudes in the 250- to 400-ms poststimulus time window at the frontal and temporal locations and developmental outcomes at 6 and 12 months of chronologic age. In temporal locations, larger brain responses in the selected time window correspond to greater negative mean amplitude values. Our hemispheric difference measure is the subtraction of the right mean amplitude from the left mean amplitude. Therefore, in temporal regions, a more negative hemispheric difference indicates a greater response on the left side. In frontal locations, the polarity of the signal is reversed, resulting in a greater positive value when the left side has a greater response (see Figure 1). The results of the analysis examining associations between hemispheric differences and developmental outcomes are presented in Table 3.
Table 3.
Hemisphere Differences in Mean Amplitudes Are Associated With Developmental Milestone Acquisition at 6 and 12 Months.a
| Age | Scalp location | Adjusted | N | Coefficient | Confidence interval |
|---|---|---|---|---|---|
| DAYC Communication score associations | |||||
| 6 | Temporal | − | 38 | 1.9* | 0.1, 3.8 |
| 6 | Temporal | + | 38 | 1.5* | 0.1, 3.2 |
| 6 | Frontal | − | 38 | 0.5 | −4.4, 5.5 |
| 6 | Frontal | + | 38 | 1.5 | −2.5, 5.6 |
| 12 | Temporal | − | 24 | 2.4* | 0.1, 4.8 |
| 12 | Temporal | + | 24 | 1.5 | −1.7, 3.8 |
| 12 | Frontal | − | 24 | 0.2 | −4.9, 5.4 |
| 12 | Frontal | + | 24 | 1.2 | −3.8, 3.1 |
| DAYC Cognitive score associations | |||||
| 6 | Temporal | − | 38 | 0.8 | −0.8, 2.4 |
| 6 | Temporal | + | 38 | 0.2 | −1.4, 1.8 |
| 6 | Frontal | − | 38 | −2.2* | −4, −0.4 |
| 6 | Frontal | + | 38 | −1.9* | −3.6, −0.1 |
| 12 | Temporal | − | 24 | 2.0* | 0.1, 4 |
| 12 | Temporal | + | 24 | 1.4 | −1.5, 4.3 |
| 12 | Frontal | − | 24 | 1.0 | −2.5, 4.6 |
| 12 | Frontal | + | 24 | 2.0 | −1.8, 5.9 |
Abbreviation: DAYC, Developmental Assessment of Young Children.
Adjusted model includes gestational age at birth, antenatal steroid use, socioeconomic score, and gender.
Greater hemispheric differences at the temporal location were associated with the improved acquisition of communication milestones, as measured by adjusted age Developmental Assessment of Young Children scores at 6 and 12 months of age. These scores are standardized with a mean at 100 and a standard deviation of 15 points. At 6 months, for every 1-μV increase in temporal hemispheric difference (left > right), there was a 1.9-point increase in Developmental Assessment of Young Children communication score. In frontal locations, for every 1-μV decrease in the hemispheric difference value (also indicating larger response on the left side because of reversal in polarity), there was a 2.2-point increase in Developmental Assessment of Young Children cognitive score. Therefore, after accounting for polarity reversal, the associations between hemispheric differences and developmental scores demonstrated a similar trend in both temporal and frontal locations. By 12 months, only temporal hemispheric differences in brain responses remained significantly associated with both communication and cognitive outcomes.
We also examined the associations between hemispheric differences in event-related potential responses after adjusting for gestational age, gender, antenatal steroids, and maternal socioeconomic score. These clinical variables have been used in outcome studies of preterm infants as they all have the potential to affect neurodevelopment during and after intensive care discharge. Adjusting for these variables at 6 months did not significantly alter the associations between lateralization measures and outcomes; however, it did decrease the significance of the findings at 12 months.
To test the cohesiveness of our associative model between hemispheric differences in response to speech sounds and 6-month outcomes, regardless of neural injury, we separated the data of the 7 infants with potential abnormal brain development from the other 50. Hemispheric amplitude differences still fit well within the regression model predicting neurodevelopmental outcomes. These findings are illustrated in Figure 3 showing the distribution of data for the infants from the normative sample as well as atypical ones.
Figure 3.
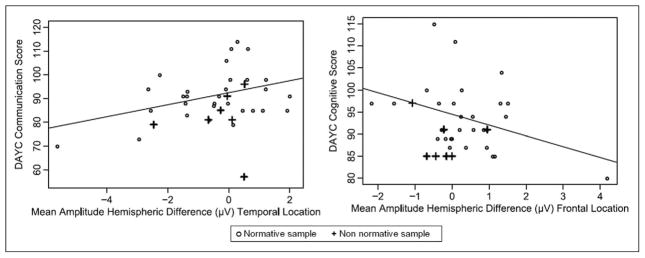
Associations of hemisphere difference in amplitudes and 6-month developmental scores. Normative sample n = 50; nonnormative sample n = 7 (4 infants with severe white matter injury [grade III or IV intraventricular hemorrhage], 1 infant with Turner syndrome, 2 with unilateral auditory neuropathy). Line represents linear regression fit. DAYC, Developmental Assessment of Young Children.
Discussion
A novel finding in the current study is that the hemispheric differences of speech sound processing can be observed even in infants born at early gestational ages and can be predictive of later developmental outcomes. Additionally, we observed an effect of gestational age on hemispheric differences in response to speech sounds in temporal locations, but this was independent of postnatal age. This finding likely reflects structural maturity differences in the auditory cortex.
In prior studies of late preterm infants, gestational age was shown to have an effect on the specific measures of auditory discrimination.26–28 Gestational age at birth has been shown to influence the ability of very-low-birth-weight infants to respond to various stimuli, both at term equivalent and at 6 months of age.29,30 However, there were no effects of postnatal age, possibly because of a very brief postnatal period used in prior studies of speech sound recognition; postnatal age in these studies did have a measurable effect on auditory memory.28,31
In our study, postnatal age had a major independent influence on hemispheric differences in speech sound processing, indicative of a more global neurodevelopmental process. Therefore, our findings demonstrate that even infants born at the earliest gestational age can therefore benefit from postnatal auditory experience. They also extend a continuum of conclusions on hemispheric lateralization of speech processing during infancy and early childhood.20 In particular, Molfese and others showed that in term infants, lateralization of event-related potential responses to the left hemisphere of event-related potential responses evolves throughout infancy and thus is a sign of maturation.25,32
Hemisphere differences in speech sound processing have been shown in numerous studies of term infants to be related to later cognitive and communication outcomes.11,33,34 This holds true for preterm infants as well. Greater hemispheric differences of event-related potential responses in temporal regions correlated well with communication milestone acquisition at 6 and 12 months and cognitive functioning at 12 months. The loss of significance at 12 months after adjusting for socioeconomic and clinical factors can be due to small sample size or the possible increasing contributions of home environment to neurodevelopmental outcomes at older ages. It can also indicate the need for larger studies incorporating event-related potential variables with important neurodevelopmental influences in predictive models.
Numerous studies have shown that the ability to process auditory signals is directly associated with the development of receptive and expressive language35,36 and could be used to predict atypical development in the context of dyslexia and other communication disorders.34,37,38 It is tempting to speculate that brain responses at the temporal scalp reflect functionality of the underlying auditory cortex relevant to the development of communicative processes, especially because functional magnetic resonance imaging in the fetus has shown this correspondence.39 Similarly, a relationship between hemisphere differences in frontal locations and higher cognitive processes rather than communication development could be suggestive of the importance of the cortico-cortical connections for better outcomes.40,41 However, the direct correspondence between event-related potential responses at specific scalp locations and activity of underlying brain structures cannot be ascertained, even in preterm infants whose extremely thin skulls can interfere less with signal transmission.10
An association between event-related potential response measures of sound processing efficiency in infancy and later cognitive outcomes has been previously established in older children.11 The event-related potential response results of the present study confirm a similar association in ill and preterm infants.
Although this study addresses the associations between brain maturity, postnatal age in the intensive care nursery, and neural processing, it is limited by the use of postnatal age as a proxy for postnatal sound experience. It does not account for variations in noxious exposures that could affect auditory processing, yet do not constitute direct sound exposure. For example, the combination of ototoxic agents and genetic susceptibility can also affect the impact of length of stay in the intensive care unit.42 Moreover, the study was not sufficiently powered to assess numerous other clinical variables associated with variability in developmental outcomes, such as days of ventilation or infectious episodes or breast milk versus formula feeding. Many of these clinical variables could affect the developing brain and contribute to the distribution of event-related potential response measurements in otherwise normal subjects.
The value of the current study lies in demonstrating an association between event-related potential response patterns and meaningful neurodevelopmental outcomes in early infancy. Future studies should address the possibility of using event-related potential measures of sound discrimination in screening for developmental disorders of speech and language in neonatal populations at highest risk for neural injury. Additionally, event-related potential response methodology can prove useful in assessing the immediate and longer term effects of neuroprotective or therapeutic interventions on neural processing efficiency prior to and following discharge from the intensive care nursery.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a Hazinski-Turner Award from Vanderbilt University to NLM, NICHD grant P30HD15052 to Vanderbilt Kennedy Center, and by a grant of the Gerber Foundation to JLA. Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University, supported by grant 1 UL1 RR024975 from NCRR/NIH.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
All patients and parents were recruited according to Vanderbilt IRB # 040526, 040881, 070377, 081415 approved protocols.
Author Contributions
NLM formulated the hypothesis, obtained funding, designed the study analysis, tested the patients in the follow-up clinic, calculated and entered the neurobehavioral data, and wrote all drafts of the manuscript. JLA designed the complementary study, obtained grant funding, and reviewed all versions of the manuscript. APK obtained and processed all electrophysiology data, designed the electrophysiology acquisition program, and provided reference to established literature in the field as well as co-wrote and edited all drafts. JCS designed, performed, and wrote the draft of the statistical analysis and reviewed all versions of the manuscript.
References
- 1.Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, Marlow N. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- 5.Peterson BS. Brain imaging studies of the anatomical and functional consequences of preterm birth for human brain development. Ann N Y Acad Sci. 2003;1008:219–237. doi: 10.1196/annals.1301.023. [DOI] [PubMed] [Google Scholar]
- 6.Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Neonatology. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 7.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. J Am Med Assoc. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn P, Zores C, Astruc D, Dufour A, Casper C. Développement sensoriel des nouveau-nés grands prématurés et environnement physique hospitalier. Arch de Pédiatrie. 2011;18(suppl 2):S92–S102. doi: 10.1016/S0929-693X(11)71097-1. [DOI] [PubMed] [Google Scholar]
- 9.Brown G. NICU noise and the preterm infant. Neonat Network. 2009;28:165–173. doi: 10.1891/0730-0832.28.3.165. [DOI] [PubMed] [Google Scholar]
- 10.Key AP, Dove GO, Maguire MJ. Linking brainwaves to the brain: an ERP primer. Dev Neuropsychol. 2005;27:183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- 11.Molfese DL, Molfese VJ, Beswick J, Jacobi-Vessels J, Molfese PJ, Key AP, Starkey G. Dynamic links between emerging cognitive skills and brain processes. Dev Neuropsychol. 2008;33:682–706. doi: 10.1080/87565640802418647. [DOI] [PubMed] [Google Scholar]
- 12.Mikkola K, Kushnerenko E, Partanen E, et al. Auditory event-related potentials and cognitive function of preterm children at five years of age. Clin Neurophysiol. 2007;118:1494–1502. doi: 10.1016/j.clinph.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Key A, Lambert E, Aschner J, Maitre N. Influence of gestational age and postnatal age on speech sound processing in NICU infants. Psychophysiology. 2012;49:1–41. doi: 10.1111/j.1469-8986.2011.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushnerenko E, Ceponiene R, Balan P, Fellman V, Naatanen R. Maturation of the auditory change detection response in infants: a longitudinal ERP study. Neuroreport. 2002;13:1843–1848. doi: 10.1097/00001756-200210280-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kushnerenko E, Ceponiene R, Balan P, Fellman V, Huotilaine M, Näätäne R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport. 2002;13:47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- 16.Ceponiene R, Rinne T, Näätänen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.Peelle JE. The hemispheric lateralization of speech processing depends on what “speech” is: a hierarchical perspective. Front Human Neurosci. 2012;6:309. doi: 10.3389/fnhum.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- 19.Belin P, Zilbovicius M, Crozier S, et al. Lateralization of speech and auditory temporal processing. J Cogn Neurosci. 1998;10:536–40. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- 20.Molfese DL, Freeman RBJ, Palermo DS. The ontogeny of brain lateralization for speech and nonspeech stimuli. Brain Lang. 1975;2:356–368. doi: 10.1016/s0093-934x(75)80076-9. [DOI] [PubMed] [Google Scholar]
- 21.Key APF, Lambert EW, Aschner JL, Maitre NL. Influence of gestational age and postnatal age on speech sound processing in NICU infants. Psychophysiology. 2012;49:720–731. doi: 10.1111/j.1469-8986.2011.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens KN, Blumstein SE. Invariant cues for place of articulation in stop consonants. J Acoust Soc Am. 1978;64:1358–1368. doi: 10.1121/1.382102. [DOI] [PubMed] [Google Scholar]
- 23.Voress JMT. Developmental Assessment of Young Children. Austin, TX: PRO-ED; 1998. [Google Scholar]
- 24.Hollingshead AB. Four factor index of social status. Hollings-head; 1975. [Google Scholar]
- 25.Mento G, Suppiej A, Altoe G, Bisiacchi PS. Functional hemispheric asymmetries in humans: electrophysiological evidence from preterm infants. Eur J Neurosci. 2010;31:565–574. doi: 10.1111/j.1460-9568.2010.07076.x. [DOI] [PubMed] [Google Scholar]
- 26.deRegnier RA, Wewerka S, Georgieff MK, Mattia F, Nelson CA. Influences of postconceptional age and postnatal experience on the development of auditory recognition memory in the newborn infant. Dev Psychobiol. 2002;41:216–225. doi: 10.1002/dev.10070. [DOI] [PubMed] [Google Scholar]
- 27.deRegnier RA. Neurophysiologic evaluation of brain function in extremely premature newborn infants. Semin Perinatol. 2008;32:2–10. doi: 10.1053/j.semperi.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 28.deRegnier RA, Nelson CA, Thomas KM, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137:777–784. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- 29.Bisiacchi PS, Mento G, Suppiej A. Cortical auditory processing in preterm newborns: an ERP study. Biol Psychol. 2009;82:176–185. doi: 10.1016/j.biopsycho.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Fellman V, Kushnerenko E, Mikkola K, Ceponiene R, Leipälä J, Näätänen R. Atypical auditory event-related potentials in preterm infants during the first year of life: a possible sign of cognitive dysfunction? Pediatr Res. 2004;56:291–297. doi: 10.1203/01.PDR.0000132750.97066.B9. [DOI] [PubMed] [Google Scholar]
- 31.Therien JM, Worwa CT, Mattia FR, deRegnier R-AO. Altered pathways for auditory discrimination and recognition memory in preterm infants. Dev Med Child Neurol. 2004;46:816–824. doi: 10.1017/s0012162204001434. [DOI] [PubMed] [Google Scholar]
- 32.Molfese DL. Left hemisphere sensitivity to consonant sounds not displayed by the right hemisphere: electrophysiological correlates. Brain Lang. 1984;22:109–127. doi: 10.1016/0093-934x(84)90082-8. [DOI] [PubMed] [Google Scholar]
- 33.Molfese VJ, Molfese DL, Modgline AA. Newborn and preschool predictors of second-grade reading scores: an evaluation of categorical and continuous scores. J Learn Disabil. 2001;34:545–554. doi: 10.1177/002221940103400607. [DOI] [PubMed] [Google Scholar]
- 34.Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72:238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- 35.Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: a case for the preeminence of temporal processing. Ann N Y Acad Sci. 1993;682:27–47. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- 36.Bailey PJ, Snowling MJ. Auditory processing and the development of language and literacy. Br Med Bull. 2002;63:135–146. doi: 10.1093/bmb/63.1.135. [DOI] [PubMed] [Google Scholar]
- 37.Guttorm TK, Leppanen PHT, Richardson U, Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. J Learn Disabil. 2001;34:534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- 38.Jansson-Verkasalo E, Korpilahti P, Jantti V, et al. Neurophysiologic correlates of deficient phonological representations and object naming in prematurely born children. Clin Neurophysio. 2004;115:179–187. doi: 10.1016/s1388-2457(03)00319-5. [DOI] [PubMed] [Google Scholar]
- 39.Jardri R, Pins D, Houfflin-Debarge V, et al. Fetal cortical activation to sound at 33 weeks of gestation: a functional MRI study. NeuroImage. 2008;42:10–18. doi: 10.1016/j.neuroimage.2008.04.247. [DOI] [PubMed] [Google Scholar]
- 40.Tyler LK, Marslen-Wilson WD, Randall B, et al. Left inferior frontal cortex and syntax: function, structure and behaviour in patients with left hemisphere damage. Brain. 2011;134(pt 2):415–431. doi: 10.1093/brain/awq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodd JM. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex. 2004;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerman E, Lahav A. Ototoxicity in preterm infants: effects of genetics, aminoglycosides, and loud environmental noise. J Perinatol. 2013;33:3–8. doi: 10.1038/jp.2012.105. [DOI] [PubMed] [Google Scholar]