Abstract
The protective effect of ischemic postconditioning (IPostC) against stroke has been well-established, and the underlying mechanisms are known to involve inhibited-inflammation and free radical production. Nevertheless, how IPostC affects protein expression of iNOS, nitrotyrosine, and COX-2 has not been characterized. In addition, the role of the galectin-9/Tim-3 cell signaling pathway—a novel inflammatory pathway—in IPostC has not been studied. We examined whether iNOS, nitrotyrosine, and COX-2, as well as galectin-9/Tim-3 are involved in the protective effects of IpostC in a rat focal ischemia model. Western blot and confocal immunofluoresent staining results indicate that IPostC significantly inhibited Tim-3 expression, and that galectin-9 expression was also inhibited. In addition, IPostC attenuated production of iNOS and nitrotyrosine, but not COX-2, suggesting that IPostC has distinct effects on these inflammatory factors. Furthermore, the inflammation inhibitor minocycline blocked Tim-3 and iNOS expression induced by stroke. Taken together, we show that the galectin-9/Tim-3 cell signaling pathway is involved in inflammation induced by stroke, and IPostC may reduce infarction by attenuating this novel pathway as well as the inflammatory factors iNOS and nitrotyrosine, but not COX-2.
Keywords: stroke, ischemic postconditioning, Tim-3, iNOS
Introduction
The concept of ischemic postconditioning (IPostC) against cerebral ischemia is well-established both by measurements of infarct size and by behavioral testing (Zhao, 2009; Zhao, 2011; Zhao et al., 2012), but its underlying protective mechanisms remain elusive. Previous studies suggest that IPostC reduces ischemic injury by inhibiting the hyperemic response and ameliorating later reduction in cerebral blood flow (CBF) and metabolism after reperfusion (Gao et al., 2008a; Ren et al., 2008), thus blocking ROS overproduction and lipid peroxidation (Abas et al., 2010), and inhibiting apoptosis (Xing et al., 2008b). IPostC also improves neuronal survival cell signaling pathways, including the Akt, mTOR and Erk pathways(Gao et al., 2008a; Pignataro et al., 2008; Xie et al., 2013). Furthermore, IPostC inhibits inflammation after stroke, as it reduces myeloperoxidase (MPO) activity, an indicator of leukocyte accumulation, attenuates IL-1β and TNF-α mRNA expression as well as ICAM-1 protein expression (Xing et al., 2008a), and blocks TLR-4 receptor expression (Feng et al., 2011).
Despite extensive studies, however, the effects of IPostC on various basic inflammatory factors and free radical generators have not been characterized. For example, cycloxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) are two well-known free radical generators (Yin et al., 2007; Zawadzka et al., 2012), and nitrotyrosine is a marker for iNOS activity (Nanetti et al., 2007). COX-2 is inducible in inflammatory tissue, and its production is involved in inflammation after stroke. iNOS and nitrotyrosine are critical for microglia/macrophage mediated-inflammation after stroke (Yin et al., 2007; Zawadzka et al., 2012). To our surprise, although IPostC is well-known to inhibit free radical generation and inflammation, how IPostC affects COX-2, iNOS and nitrotyrosine has not been reported.
In addition, Tim-3, a member of the T cell immunoglobulin and mucin domain (Tim) family, has double roles in regulating the inflammatory response (Anderson et al., 2007). When expressed on CD4+T help 1 (Th1) cells, Tim-3 is activated by its ligand galectin-9, which causes calcium influx and cell aggregation, resulting in Th1 cell death. Tim-3 therefore inhibits the inflammatory response by eliminating Th1 cells (Su et al., 2008). However, when Tim-3 is expressed on macrophage, microglia and dendritic cells, it promotes the inflammatory response (Anderson et al., 2007). In addition, Tim-3 is also expressed on nerve cells in the brain (Gielen et al., 2005). Most recently, we demonstrated that Tim-3 is upregulated in ischemic neurons, and its expression is inhibited by remote preconditioning (Wei et al., 2012). However, how IPostC affects these inflammatory pathways has not been investigated.
In this study we examined the protective effects of IPostC on the galectin-9/Tim-3 pathway, and on the production of COX-2, iNOS and nitrotyrosine. We found that IPostC blocked the galectin-9/Tim-3 pathway and iNOS and nitrotyrosine production, but it did not alter COX-2 activities, suggesting that postconditioning has distinct effects on these cell signaling pathways after stroke.
Materials and Methods
Animal experiments were conducted according to the protocols approved by the Stanford Intuitional Animal Care and Use Committee and the NIH Guidelines for Care and Use of Laboratory Animals. Animals were housed under a 12:12 hour light:dark cycle with food and water available ad libitum.
Focal cerebral ischemia model and Ischemic postconditioning
The stroke model and IPostC were performed as described (Zhao et al., 2006). Male Sprague-Dawley rats (250g 350g) were used. Anesthesia was induced by 5% isoflurane and maintained with 2% to 3% isoflurane during surgery and early reperfusion. Core body temperature was monitored with a rectal probe and maintained at 37 C during whole experiments. The bilateral common carotid arteries (CCAs) were separated and a suture was circled around each artery. The distal middle cerebral artery (MCA) was exposed. For ischemia induction, the bilateral CCA were occluded by aneurysms clips first, and then the left distal MCA was cauterized above rhinal fissure. The aneurysms clips were released 30 min later. IPostC was performed as described (Zhao et al., 2006)(Fig. 1). In brief, after releasing the bilateral CCAs for 30 seconds, the CCAs were occluded by tightening the sutures for 10 seconds. A total of 3 cycles of 30 second reperfusions with 10 second occlusions was performed.
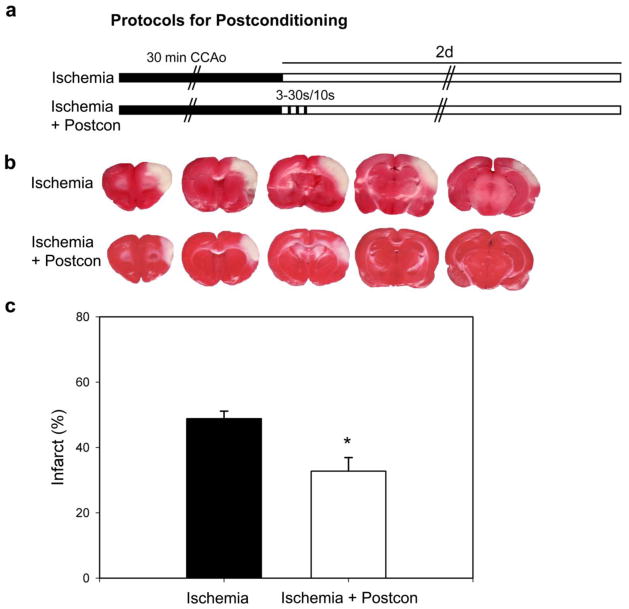
Fig. 1. Ischemic postcondtioning reduced infarct size after focal ischemia.
A. Diagram showing the ischemic postconditioning protocol, which was induced 30 s after CCA release, with 3 cycles of 30 s reperfusion and 10 s occlusion. B. Representative TTC staining from rats receiving focal ischemia with and without postconditioning. C. Postconditioning reduced infarct size. Infarct size in the ischemic cortex was measured at each level and normalized to the non-ischemic contralateral cortex. An average value from the 5 levels was calculated and presented. CCAo, common carotid artery occlusion. Control ischemia, n = 6; postconditioning, n = 7. * P < 0.05, vs control.
Drug injection
To test whether protein expression of Tim-3 is involved in inflammation, we tested whether the inflammation inhibitor minocycline inhibited Tim-3 expression. Minocycline hydrochloride (M9511-250MG, SIGMA) was dissolved in saline. The freshly made solution was intraperitoneally ( i.p ) administrated (50mg/kg) at 60 min before and 8 hours after the MCA occlusion. The dose of minocycline and the injection protocol were adopted from previous studies (Fox et al., 2005; Koistinaho et al., 2005; Tang et al., 2010). Rat brains were harvested 24 hours after MCA occlusion for western blot (n = 6).
Immunofluorescence staining and confocal microscopy
Rat brains were harvested 24 hours after stroke and IPostC for immunostaining as described previously(Zhao et al., 2005). Rats were transcardially perfused with ice-cold phosphate-buffered saline (pH 7.4) and 4% paraformaldehyde solution. Brains were post-fixed in 4% paraformaldehyde solution and cytoprotected in 20% sucrose at 4°C overnight. We stained cryostat sections (40μm) with antibodies against Tim-3 (1:100; Santa Cruz Biotechnology; sc-30326), galectin-9 (1:50; Santa Cruz Biotechnology; sc-19292), Cox-2 (1:400; Cayman; 160106), nitrotyrosine (1:50; Chemcon, 92590 ). Fluorescent-stained sections were analyzed by confocal microscopy.
Western blot
To examine the effect of IPostC on protein expressions, rats were randomly assigned into different groups with or without IPostC, euthanized at 1, 5, and 24 hours after stroke (each group n = 6 ), and rat brains were harvested for western blot as described previously (Gao et al., 2008b). Animals receiving sham surgery without stroke were used as controls. Samples were lysed with RIPA buffer (10-mmol/L TRIS, 140-mmol/L Nacl, 1% Triton, 1% Na-deoxycholate, 0.1% SDS, 0.5-mmol/L phenylmethylsulfonyl fluoride and supplemented with cocktail inhibitors; Roche). Extracts were homogenized and insoluble debris removed by centrifugation at 6000g for 10 minutes at 4°C. Protein concentration in the resulting supernatants was calculated using a Pierce protein assay kit according to the manufactures instructions (Pierce, IL, USA). Equal amounts of protein samples (13ul) were loaded, separated using 4–15% SDS-polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA, USA) electrophoresis and then transferred to a Trans-Blot nitrocellulose membrane (BioRad, CA, U.S.A.). Membranes were scanned using Typhoon trio (GE Healthcare). We use primary antibodies against Tim-3 (1:500; Santa Cruz Biotechnology; sc-30326), Cox-2 (1:1000; Cayman; 160106 ), iNOS ( 1:10000; BD Biosciences 610431 ), β-actin (1:10000; SIGMA; A3854-200μL ). The densities of the protein bands were analyzed with ImageQuant software (Molecular Dynamics). The quantification was conducted by the same person who performed the Western blots.
Statistical analysis
Data are presented as means ± SEM. Statistical comparisons were made using a one-way or two-way ANOVA and Student’s t test. P < 0.05 indicates statistical significance.
Results
We first confirmed the protective effect of IPostC against stroke (Fig. 1). After 30 minutes ischemia, IPostC was induced by 3 cycles of a 30 second reperfusion followed with 10 seconds of bilateral CCA occlusion. The results show that infarct sizes measured by TTC staining 2 days post-stroke were significantly reduced by IPostC (Fig. 1).
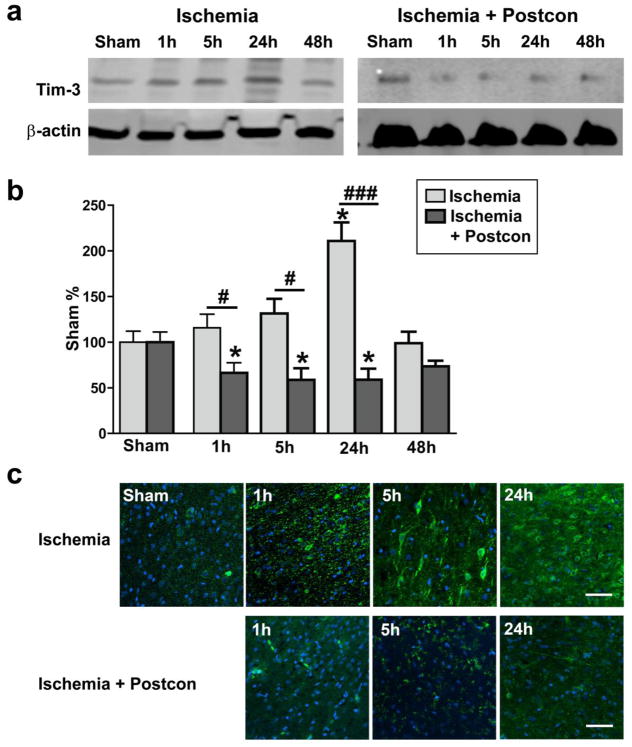
We then investigated whether IPostC inhibited protein expression in the novel galectin-9/Tim-3 cell signaling pathway, which is involved in inflammation. We previously confirmed that Tim-3 is up-regulated in ischemic neurons after stroke (Wei et al., 2012). In this study both western blot and immunofluorescent staining results indicated that protein levels of Tim-3 were significantly increased at 24 hours after stroke, which were inhibited by IPostC (Fig. 2). Furthermore, confocal immunofluorescent staining suggested that galectin-9 was weakly expressed in brain tissue (Fig. 3). Its expression, however, was promoted in blood vessels as early as 1 hour post-stroke, and increased in neurons from 5 hours to 24 hours after stroke, which was inhibited by IPostC.
Fig. 2. Increased Tim-3 expression after stroke was inhibited by postconditioning.
Representative protein bands of Tim-3 and beta-actin are presented for rats receiving control ischemia alone (A) and ischemia plus postconditioning (D). Bar graph indicating that Tim-3 increased as early as 1h after stroke, peaked at 24h after stroke (B) and was inhibited by postconditioning (E). *, ***, vs sham, P < 0.05, 0.001, respectively, vs sham. #, ###, P < 0.05, 0.001, respectively, between the two indicated groups. N = 6/group. These results were further confirmed by immunofluorescent confocal microscopy in control ischemic (C) and postconditioning rats (F). Scale bar, 50 μm.
Fig. 3. Postconditioning inhibited galectin-9 expression induced by stroke.
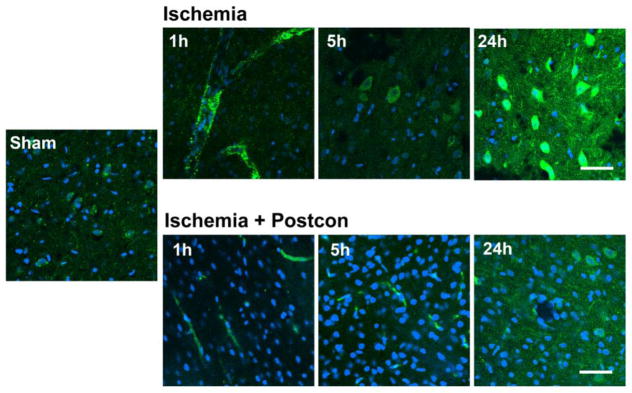
Double staining of galection-9 (green) and DAPI (Bblue) was conducted and examined by confocal microscopy. The results indicate that galectin-9 was expressed on blood vessels 1h after stroke, and on neurons from 5 h to 24 h. Such expression was inhibited by postconditioning. Scale bar, 50 μm.
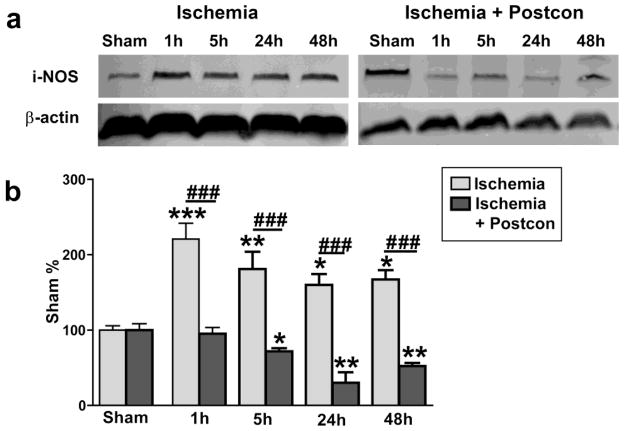
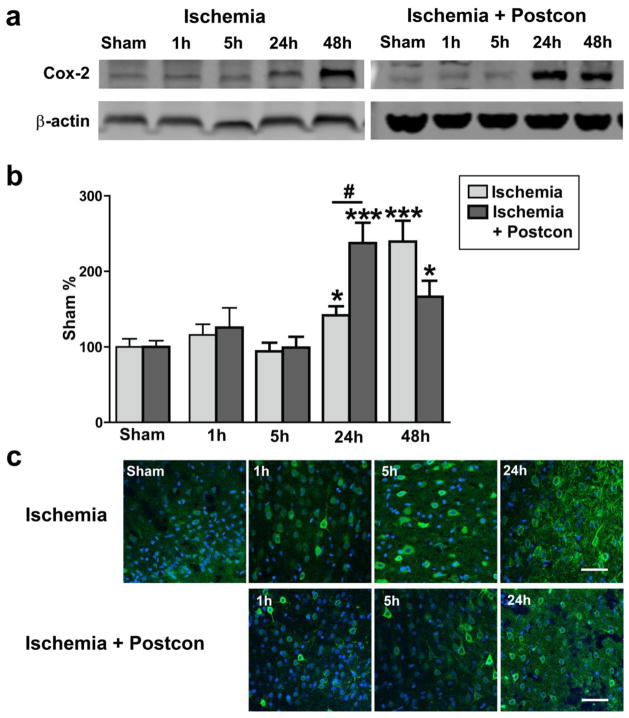
We further examined the effects of IPostC on some other critical molecules related to free radical production and inflammation, including iNOS, nitrotyrosine (a product derived from iNOS activity) and COX-2. Western blot analysis showed that iNOS protein levels were increased from 1 to 24 hours, which were blocked by IPostC (Fig. 4). Consistent with this finding, immunostaining showed that increased-nitrotyrosine levels after stroke were inhibited by IPostC (Fig. 5). Nevertheless, although COX-2 protein levels were significantly increased from 24 to 48 hours post-stroke, IPostC did not alter its protein levels (Fig. 6).
Fig. 4. Postconditioning blocked iNOS expression after stroke.
Representative western blot protein bands of iNOS are shown for control ischemia (A) and postconditioning (C). Results suggest that iNOS was significantly increased from 1 h to 48 h after stroke in rats receiving control ischemia (B). Postconditioning inhibited iNOS expression (D).*, **, *** vs sham, P < 0.05, 0.01. 0.001, respectively; ###, P < 0.001, respectively, between the two indicated groups. n = 6/group. Scale bar, 50 μM.
Fig. 5. Postconditioning blocked nitrotyrosine expression.
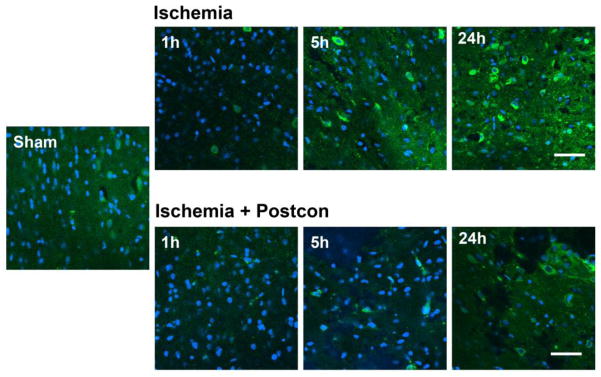
Double staining of nitrotyrosine (green) and DAPI (DAPI) was conducted and pictures were taken by confocal microscopy. Immunostaining suggests that nitrotyrosine expression was increased from 1h to 24h after stroke in control ischemic rats, which was attenuated by postconditioning. Scale bar, 50 μM.
Fig. 6. Postconditioning had no protective effects on COX-2 expression induced by stroke.
Representative western blot protein bands of COX-2 are presented for control ischemia (A) and postconditioning (D. Results suggest that protein levels of COX-2 were significantly increased at 24h and 48h (B), which were not attenuated by postconditioning (E). Confocal microscopy results indicate that COX-2 was expressed in the ischemic peri-infarct region after stroke (C), and postconditioning did not change its expression pattern (F). *, *** vs sham, P < 0.05, 0.001; #, P < 0.05, respectively, between the two indicated groups. n = 6/group. Scale bar, 50 μM.
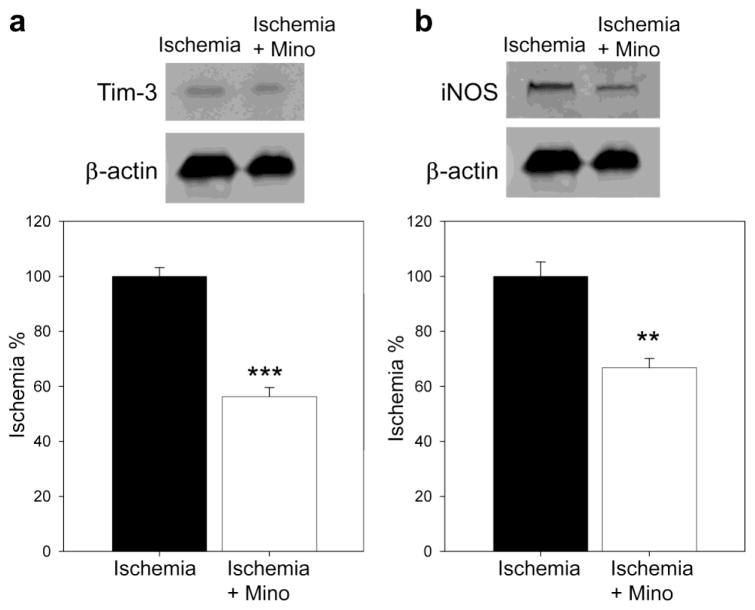
Last, to confirm Tim-3’s involvement in inflammation, we examined the effect of minocycline, a well-known inflammation inhibitor, on protein levels of Tim-3, together with iNOS (Fig. 7). Western blot results showed that minocycline did inhibit both Tim-3 and iNOS protein expression 24 hours after stroke.
Fig. 7. Effect of the inflammation inhibitor minocycline on protein expression of Tim-3 and iNOS 24h after stroke.
Representative protein bands of Tim-3 (A) and iNOS (B) are shown. Statistical analyses suggest that minocycline injection attenuated increases in Tim-3 and iNOS, but promoted COX-2 expression. Mino, minocycline. **, *** vs stroke, P < 0.05, 0.001, respectively. N=5–6/group.
Discussion
The protective effects of IPostC against stroke are well-established, but the underlying protective mechanisms are not completely understood. We and others have demonstrated that IPostC promotes the Akt/mTOR neuronal survival cell-signaling pathways and the Bcl-2/Bcl-XL anti-apoptotic pathways, while it inhibits pro-apoptotic pathways, including the cytochrome c/caspase pathway (Zhao, 2009). In addition, the effects of IPostC on inflammation after stroke have been explored (Xing et al., 2008a). Previous studies have shown that IPostC inhibits leukocyte accumulation, IL-1β and TNF-α mRNA expression, ICAM-1 protein, IL-6, and TLR-4 as well as matrix metalloproteinase 9 (MMP9) activity (Feng et al., 2011; Liu et al., 2012; Xing et al., 2008b). Most recently, we showed that IPostC had no acute protection in T cell deficient nude rats, though it reduced brain injury measured 1 month post-stroke, suggesting that T-cell-mediated brain inflammation is involved in the protective effects of IPostC (Xie et al., 2013). We have also showed that IPostC significantly blocked increases in inflammatory cell populations in the brain, including microglia/macrophages, CD4 and CD8 T cells, as well as B lymphocytes (Joo et al., 2013).
Despite these extensive studies, whether or not IPostC reduces protein levels of iNOS, nitrotyrosine and COX-2 has not been examined. Here we show for the first time that IPostC blocked increases in iNOS and nitrotyrosine expression but not COX-2, suggesting that IPostC has distinct effects on these inflammatory factors. It is well-known that iNOS contributes to neuronal injury and inflammation after stroke (Han et al., 2002; Karabiyikoglu et al., 2003; Limbourg et al., 2002), and that nitrotyrosine is a marker of NO-dependent products derived from iNOS; its expression indicates cell damage and inflammation (Hirabayashi et al., 2000; Martinez-Murillo et al., 2007; Nanetti et al., 2007). We showed that iNOS protein levels increased as early as 1 hour after stroke, maintained high levels for up to 24 hours, and that nitrotyrosine expression was also elevated. We also showed these increases were significantly attenuated by IPostC. Although COX-2 is also involved in ROS activity and the inflammatory response, our results suggest it is not altered by IPostC, and therefore may not be important for the protective effects of IPostC.
We also show for the first time that IPostC attenuated galectin-9 and Tim-3 protein expression induced by stroke. Galectin-9/Tim-3 is a relatively novel pathway that regulates immune and inflammatory responses (Anderson and Anderson, 2006; Nakae et al., 2007; Sanchez-Fueyo et al., 2003). Galectin-9/Tim-3 proteins were originally identified in T cells, macrophages and dendritic cells. Later mRNA levels of Tim-3 were found to be increased in the mouse brain 3 days after stroke (Zhao et al., 2011). We recently reported that galectin-9/Tim-3 proteins were upregulated in ischemic neurons and remote preconditioning inhibited their expression. Consistent with our previous findings, we further showed that IPostC inhibited galectin-9/Tim-3 activity.
To confirm that the Tim-3 pathway is indeed involved in stroke-induced inflammation, we tested the hypothesis that the inflammatory inhibitor minocycline (Joo et al., 2013) inhibits Tim-3 expression. Indeed our results showed that protein levels of Tim-3 and iNOS, both induced by stroke, were inhibited by minocycline.
In conclusion, we demonstrated that IPostC attenuated increases in protein levels of galectin-9 and Tim-3 as well as iNOS and nitrotyrosine, but not COX-2. We conclude that inhibition of the galectin-9/Tim-3 pathway may contribute to the protective effects of IPostC.
Acknowledgments
We wish to thank Ms. Elizabeth Hoyte for figure preparation and Ms. Cindy H. Samos for manuscript editing. This study was supported by AHA grant in Aid and 1R01NS 064136-01A1 (HZ)
References
- Abas F, et al. Neuroprotective effects of postconditioning on lipid peroxidation and apoptosis after focal cerebral ischemia/reperfusion injury in rats. Turk Neurosurg. 2010;20:1–8. [PubMed] [Google Scholar]
- Anderson AC, Anderson DE. TIM-3 in autoimmunity. Curr Opin Immunol. 2006;18:665–9. doi: 10.1016/j.coi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Anderson AC, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–3. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- Feng R, et al. Toll-like receptor 4 is involved in ischemic tolerance of postconditioning in hippocampus of tree shrews to thrombotic cerebral ischemia. Brain Res. 2011;1384:118–27. doi: 10.1016/j.brainres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Fox C, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138–49. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, et al. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008a;86:2505–11. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- Gao X, et al. The Akt signaling pathway contributes to postconditioning’s protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008b;105:943–55. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen AW, et al. Expression of T cell immunoglobulin- and mucin-domain-containing molecules-1 and -3 (TIM-1 and -3) in the rat nervous and immune systems. J Neuroimmunol. 2005;164:93–104. doi: 10.1016/j.jneuroim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Han HS, et al. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. 2002;22:3921–8. doi: 10.1523/JNEUROSCI.22-10-03921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi H, et al. Nitrotyrosine generation via inducible nitric oxide synthase in vascular wall in focal ischemia-reperfusion. Brain Res. 2000;852:319–25. doi: 10.1016/s0006-8993(99)02117-4. [DOI] [PubMed] [Google Scholar]
- Joo SP, et al. Ischemic postconditioning protects against focal cerebral ischemia by inhibiting brain inflammation while attenuating peripheral lymphopenia in mice. Neuroscience. 2013;243:149–57. doi: 10.1016/j.neuroscience.2013.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabiyikoglu M, et al. Attenuation of nitric oxide synthase isoform expression by mild hypothermia after focal cerebral ischemia: variations depending on timing of cooling. J Neurosurg. 2003;98:1271–6. doi: 10.3171/jns.2003.98.6.1271. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, et al. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–7. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- Limbourg FP, et al. Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J Clin Invest. 2002;110:1729–38. doi: 10.1172/JCI15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XR, et al. Ischemic postconditioning diminishes matrix metalloproteinase 9 expression and attenuates loss of the extracellular matrix proteins in rats following middle cerebral artery occlusion and reperfusion. CNS Neurosci Ther. 2012;18:855–63. doi: 10.1111/j.1755-5949.2012.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Murillo R, et al. The nitric oxide donor LA 419 decreases brain damage in a focal ischemia model. Neurosci Lett. 2007;415:149–53. doi: 10.1016/j.neulet.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Nakae S, et al. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–8. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanetti L, et al. Reactive oxygen species plasmatic levels in ischemic stroke. Mol Cell Biochem. 2007;303:19–25. doi: 10.1007/s11010-007-9451-4. [DOI] [PubMed] [Google Scholar]
- Pignataro G, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:232–41. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- Ren C, et al. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS One. 2008;3:e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fueyo A, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- Su EW, et al. TIM-1 and TIM-3 proteins in immune regulation. Cytokine. 2008;44:9–13. doi: 10.1016/j.cyto.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, et al. Minocycline reduces neuronal death and attenuates microglial response after pediatric asphyxial cardiac arrest. J Cereb Blood Flow Metab. 2010;30:119–29. doi: 10.1038/jcbfm.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, et al. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One. 2012;7:e30892. doi: 10.1371/journal.pone.0030892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, et al. Ischemic post-conditioning facilitates brain recovery after stroke by promoting Akt/mTOR activity in nude rats. J Neurochem. 2013 doi: 10.1111/jnc.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, et al. Ischemic post-conditioning protects brain and reduces inflammation in a rat model of focal cerebral ischemia/reperfusion. J Neurochem. 2008a doi: 10.1111/j.1471-4159.2008.05276.x. [DOI] [PubMed] [Google Scholar]
- Xing B, et al. Ischemic Postconditioning Inhibits Apoptosis After Focal Cerebral Ischemia/Reperfusion Injury in the Rat. Stroke. 2008b doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- Yin W, et al. Preconditioning suppresses inflammation in neonatal hypoxic ischemia via Akt activation. Stroke. 2007;38:1017–24. doi: 10.1161/01.STR.0000258102.18836.ca. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, et al. Early steps of microglial activation are directly affected by neuroprotectant FK506 in both in vitro inflammation and in rat model of stroke. J Mol Med (Berl) 2012;90:1459–71. doi: 10.1007/s00109-012-0925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, et al. Increased T cell Immunoglobulin and Mucin Domain 3 Positively Correlate with Systemic IL-17 and TNF-alpha Level in the Acute Phase of Ischemic Stroke. J Clin Immunol. 2011 doi: 10.1007/s10875-011-9534-6. [DOI] [PubMed] [Google Scholar]
- Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–85. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. The Protective Effects of Ischemic Postconditioning against Stroke: From Rapid to Delayed and Remote Postconditioning. Open Drug Discov J. 2011;5:138–147. doi: 10.2174/1877381801002010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, et al. From rapid to delayed and remote postconditioning: the evolving concept of ischemic postconditioning in brain ischemia. Curr Drug Targets. 2012;13:173–87. doi: 10.2174/138945012799201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, et al. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–21. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao H, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]