Abstract
Danon disease is a rare X-linked disorder comprising hypertrophic cardiomyopathy, skeletal myopathy, intellectual disability, and retinopathy; mutations of the lysosome-associated membrane protein gene LAMP2 are responsible. Most affected persons exhibit “private” point mutations; small locus rearrangements have recently been reported in four cases. Here, we describe the clinical, pathologic, and molecular features of a male proband and his affected mother with Danon disease and a small LAMP2 microduplication. The proband presented at age 12 years with exercise intolerance, hypertrophic cardiomyopathy, and increased creatine kinase. Endomyocardial biopsy findings were nonspecific, showing myocyte hypertrophy and reactive mitochondrial changes. Quadriceps muscle biopsy demonstrated the characteristic autophagic vacuoles with sarcolemma-like features. LAMP2 tissue immunostaining was absent; however, LAMP2 sequencing was normal. Deletion/duplication testing by multiplex ligation-dependent probe amplification (MLPA) assay revealed a 1.5kb microduplication containing LAMP2 exons 4 and 5. RT-PCR studies were consistent with the inclusion of these two duplicated exons in the final spliced transcript, resulting in a frameshift. The proband’s mother, who had died following cardiac transplantation due to suspected myocarditis at age 35, was reviewed and was shown to be affected upon immunostaining of banked myocardial tissue. This case constitutes the second report of a pathogenic microduplication in Danon disease, and illustrates a number of potential diagnostic pitfalls. Firstly, given the imperfect sensitivity of LAMP2 sequencing, tissue immunostaining and/or MLPA should be considered as a diagnostic adjunct in the workup for this disorder. Secondly, the pathological findings in myocardium may be falsely indicative of relatively common conditions such as myocarditis.
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2013_277) contains supplementary material, which is available to authorized users.
Introduction
Danon disease (OMIM #300257) is a rare X-linked disorder originally described as a Pompe disease phenocopy with normal acid maltase activity (Danon et al. 1981). The cardinal features of this condition are hypertrophic cardiomyopathy, preexcitation and tachyarrhythmias, and skeletal myopathy, with some affected persons manifesting intellectual disability and/or pigmentary retinopathy. Symptoms in affected males are highly penetrant, progressive, and severe, with mean onset in the preadolescent years; survival beyond 25 years is unlikely without cardiac transplantation (Boucek et al. 2011). Although heterozygous females have an attenuated phenotype, potentially fatal cardiac sequelae (preexcitation, arrhythmias, and cardiomyopathy) do occur in a large proportion, and median female survival is approximately 45 years (Boucek et al. 2011; Miani et al. 2012). The disease process is characterized pathologically by progressive interstitial fibrosis, muscle fiber hypertrophy, periodic-acid-Schiff-positive sarcoplasmic vacuolation, and myofibrillar disarray (Murakami et al. 1995; Sugie et al 2005). Affected tissues accumulate abnormal autophagosomes containing bulk cytoplasmic debris and glycogen, bounded by a membrane expressing peculiar sarcolemma-like features (e.g., dystrophin and sarcoglycan staining) (Danon et al. 1981; Sugie et al. 2005; Tanaka et al. 2000; González-Polo et al 2005).
Danon disease results from loss-of-function mutations of the lysosome-associated membrane protein gene LAMP2 (Nishino et al. 2000). LAMP2’s three protein products (LAMP2-A, LAMP2-B, and LAMP2-C) are integral membrane proteins, sharing a common N-terminal (luminal) domain, but with distinct transmembrane domains and cytoplasmic tails. These proteins permit the targeted import of cytoplasmic proteins (LAMP2-A, LAMP2-B) and RNA (LAMP2-C) into lysosomes for degradation (Cuervo and Dice 1996; Bandyopadhyay et al. 2008; Kaushik et al. 2011; Demirel et al. 2012; Fujiwara et al. 2013). The majority of reported Danon disease alleles are private, with nonsense and frameshift mutations predominating (Boucek et al 2011). Recently, nonrecurrent microdeletions and microduplications at the LAMP2 locus have been demonstrated in four unrelated cases (Yang et al 2010; Majer et al 2013). Herein, we report the clinical, pathological, and molecular findings in a mother and son with Danon disease due to a small intragenic LAMP2 microduplication, a novel category of mutation in this disorder.
Methods
Human Subjects—All studies were undertaken with the prior free and informed consent of the patient or legally responsible guardian, according to principles of the October 2008 revision of the Declaration of Helsinki. Written, informed consent was obtained prior to publication. In the case of the proband, clinical data were obtained in the course of providing standard clinical genetic care, rather than under a research protocol; clinical data for patient’s mother were gathered by retrospective chart review after receiving the appropriate written permissions.
Molecular Analyses—Genomic DNA was isolated from blood leukocytes by salt precipitation on the Gentra Autopure LS workstation (Qiagen, Valencia, California). LAMP2 sequencing and MLPA analysis were performed by a third-party clinical sequencing facility (Emory Genetics Laboratory, Decatur, GA) according to standard protocols. For RNA studies, total RNA was extracted from cultured skin fibroblasts using the PerfectPure RNA Cultured Cell Kit (5 Prime, Hamburg, Germany); first-strand cDNA synthesis was performed with the SuperScript III system (Invitrogen, Carlsbad, California) and oligo-(dT) priming. PCR analysis was performed according to standard protocols; products were then sequenced by standard fluorescent dideoxy terminator sequencing and separated on a 3730xl DNA analyzer (Applied Biosystems, Foster City, California). LAMP2 primers used for genomic PCR (Fig. 3b) are ATGGAATTCTGATGGCCAA (forward) and GCTGCAGCTGAACATCACT (reverse). Primers used for LAMP2 RT-PCR (Fig. 3c–d) are CTGGCTTTTC CTGGATTGCG (forward) and TAGAGCAGTGTGAGAACGGC (reverse).
Fig. 3.
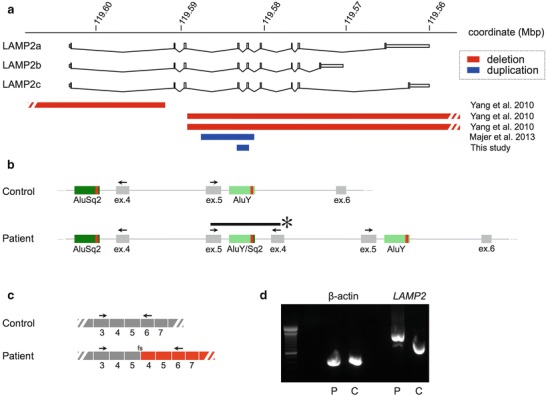
Disruption of LAMP2 by a small microduplication. (a) Overview of all reported LAMP2 microdeletions (red) and microduplications (blue). Our patient’s duplication encompasses LAMP2 exons 4 and 5. B: Genomic PCR, which amplifies a novel junction fragment (*) in the patient (primer sites indicated with arrows); sequencing of this fragment situates the breakpoints within a 31bp region of high identity (red bar) between two flanking Alu repeats (data not shown). (c) The resulting spliced transcript contains an extra copy of exons 4 and 5 (344 coding bases), with an ensuing frameshift (fs). Arrows indicate primers used for RT-PCR shown in panel D. (d) RT-PCR (fibroblast RNA) yields a ~344bp larger product in the patient, consistent with the splicing scheme depicted in panel C
Results
Clinical Presentation–The proband, a male, was referred to our center at age 12 for evaluation of leg weakness and reduced exercise tolerance. Pregnancy, delivery, and early developmental milestones were normal. He presented initially to his primary care physician at age 6 for fatigability, poorly coordinated running gait, and post-exertional thigh pain; a persistently elevated creatine kinase (900–1,630 U/L) was documented. Weakness, more pronounced proximally in the lower extremities, followed an indolent course, such that he remained able to walk unassisted. The only other presenting complaint was poor academic performance, for which he received special schooling from age 9 onward. Family history was significant for his mother having undergone catheter ablation and defibrillator implantation at age 32 for persistent ventricular tachycardia; she subsequently developed severe heart failure, and died of perioperative complications following cardiac transplant at age 35. Pertinent physical examination findings in the proband included a partial Gower’s sign, proximal lower extremity weakness (4+), intact reflexes, and an S3-S4 “gallop.” Selected investigations were as follows: EMG was normal. ECG (Fig. 1) showed impressive left ventricular hypertrophy with a shortened PR interval, compatible with preexcitation and nonspecific intraventricular conduction delay. Plain chest film showed cardiomegaly with enlarged central suprahilar pulmonary vessels. Echocardiography showed an impressive degree of concentric left ventricular hypertrophy (Fig. 2; 10.1007/8904_2013#MOESM1_ESM.doc), which, despite significant progression over 4 years, remained nonobstructive. Holter monitor demonstrated only rare supraventricular ectopy, and very rare ventricular ectopy, the predominant rhythm being sinus. Electrophysiology study revealed no accessory pathway, compatible with a diagnosis of “pseudopreexcitation”; however, he developed inducible ventricular tachycardia deteriorating to ventricular fibrillation. On formal psychometric testing (WISC-IV), his full-scale IQ was scored as “borderline” (third centile). Cranial MRI appearance was nonspecific, showing a few tiny bilateral foci of increased T2 white matter signal intensity.
Fig. 1.
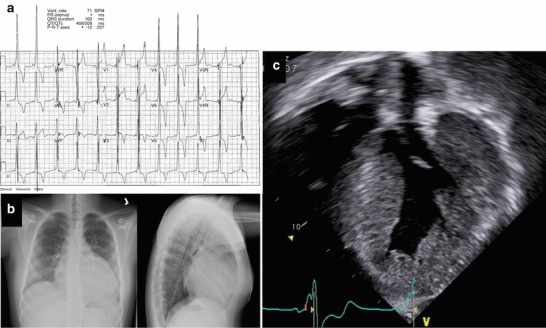
Hypertrophic cardiomyopathy. (a) Standard 12-lead ECG showing shortened PR interval (~80 ms), left axis deviation with LV hypertrophy, nonspecific intraventricular conduction delay, and diffuse T-wave inversions. The patient was asymptomatic during the study. (b) Plain films showing massive cardiomegaly, prominent perihilar vessels, enlarged cardiothoracic ratio (~0.68), and absence of retrosternal clear space. (c) Echocardiogram shows massive LV hypertrophy (see also 10.1007/8904_2013#MOESM1_ESM.doc)
Fig. 2.
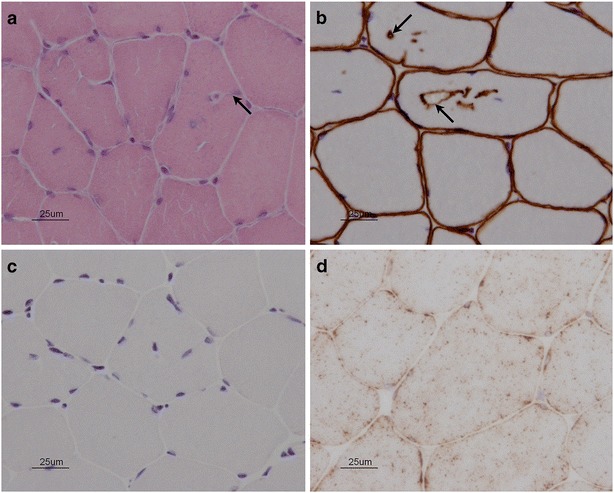
Vacuolar myopathy with absent LAMP2 in skeletal muscle. (a) Hematoxylin and eosin-stained section shows cytoplasmic vacuolation. Arrow indicates a vacuole in continuity with plasma membrane. (b) Adhalin (alpha-sarcoglycan) stain. This marker, ordinarily present in the plasma membrane, also highlights vacuolar membranes. (c) Absent LAMP2 immunostaining in the proband. (d) Normal LAMP2 immunostaining in control muscle tissue
A diagnostic endomyocardial biopsy was performed at 13 years of age. This showed a degree of myocyte hypertrophy, but was otherwise unremarkable, with no glycogen accumulation or abnormal inclusions, and normal-appearing contractile filaments. Electron microscopy showed a nonspecific reactive increase in the number of mitochondria; mitochondria were also seen to demonstrate more than the usual number of cristae. The pathological examination of the native heart of the proband’s mother had shown myocyte hypertrophy and extensive chronic inflammation, with muscle fiber necrosis, fibrofatty infiltration, and biventricular thinning (more pronounced in the right ventricle). The epicardium was described as having significant, virtually diffuse inflammation. These had been described in the original pathology summary as indicative of an active myocarditis, with changes consistent with possible arrhythmogenic right ventricular dysplasia (ARVD).
As a definitive diagnosis remained elusive, the proband further underwent a quadriceps muscle biopsy (Fig. 2). Routine stains showed no evidence of myofiber necrosis, regeneration, atrophy, or hypertrophy. Vacuolated fibers containing basophilic, granular, periodic acid-Schiff-positive material were identified. Electron microscopy showed patches of myofibrillar disarray, but no abnormal glycogen accumulation. Collections of subsarcolemmal mitochondria were seen, but again without any specific mitochondrial abnormality. Respiratory chain histochemical stains were normal; ATPase stain showed mild clustering of type I fibers. Plasma membranes exhibited normal staining for dystrophin, sarcoglycans alpha through delta, dysferlin, merosin, and caveolin. Importantly, these same stains also highlighted the rim of many of the abnormal vacuoles. LAMP-2 immunostaining was absent in skeletal muscle, in the proband’s banked myocardial biopsy, and in banked myocardium from the proband’s mother. Somewhat unexpectedly, LAMP2 sequencing in the proband was normal; multiplex ligation-dependent probe amplification (MLPA) analysis identified a small intragenic microduplication of approximately 1.5kb, containing exons 4 and 5 (minimal hg19 coordinates chrX:119581626–119583100) (Fig. 3a). This interval is bounded by Alu repeats showing 83% overall sequence identity, suggesting nonhomologous recombination as a likely mechanism. Confirmatory genomic PCR and RT-PCR analyses (Fig. 3b-d) confirmed the presence of an additional copy of LAMP2 exons 4 and 5 (344 coding bases) into the final spliced transcript, with an ensuing frameshift. X-inactivation studies could not be undertaken in the proband’s mother due to limitations on the small amount of (paraffin-embedded) tissue remaining.
Discussion
Although consensus guidelines have yet to be established, follow-up for our patient consists of regular clinical assessment of Cardiology and Metabolics, Neurology (regarding his weakness), appropriate rehabilitative services (physical and occupational therapy), and the ongoing involvement of a genetic counselor. An implantable cardioverter-defibrillator has been offered but (so far) declined. He is currently being assessed for possible cardiac transplantation, a difficult decision given this family’s prior experience.
This report constitutes the second, and smallest, reported LAMP2 microduplication of which we are aware. Unlike microdeletions, which should be readily detectable in males due to their failure to amplify, microduplications are likely to be missed unless sought specifically using MLPA or a comparable technique. As this case illustrates, LAMP2 immunofluorescence is a useful diagnostic adjunct in males, with the caveats that “leaky” or nontruncating (e.g., missense) mutations may exhibit normal immunoreactivity (van der Kooi et al. 2008). In cases in which myocardial tissue is unavailable, or a less invasive testing option is preferred, alternative assays for LAMP2 protein expression have been described in peripheral blood leukocytes (by immunoblot) and in cultured skin fibroblasts (by immunofluorescence) (Fanin et al. 2006; Alroy et al. 2010). Because the presenting features of Danon disease (weakness, hypertrophic cardiomyopathy, arrhythmias, and intellectual disability) are individually rather common, a minimally invasive approach might be to combine leukocyte immunoblot with LAMP2 sequencing in patients with compatible clinical features.
Also noteworthy in this case is that routine endomyocardial examination was initially nondiagnostic in both affected members of this pedigree. The reason for the discordant cardiac and skeletal muscle findings in our proband is unclear, but is unlikely to be due to differing tissue expression, as the duplication identified should completely abolish expression of all three LAMP2 isoforms. Although we are unaware of prior reports of “false-negative” endomyocardial biopsy in patients with characteristic skeletal muscle findings, the reciprocal situation (vacuolar myopathy in cardiac but not skeletal muscle biopsy) has been described (Taylor et al. 2007). Findings in the proband’s mother illustrate that the later stages of illness are sufficiently destructive that characteristic findings may not be readily apparent. In light of the surprisingly high sample prevalence of 6/75 (8%) and 3/50 (6%) of LAMP2 mutations in two recent molecular screens of unselected hypertrophic cardiomyopathy patients (Arad et al. 2005; Cheng et al 2012), a high index of suspicion for this condition is warranted, and routine screening using a combination of methods appears appropriate.
Acknowledgments
The authors would like to gratefully acknowledge the participation of the patient and family, without whom this work could not have been completed.
Electronic Supplementary Material
Progressive hypertrophic cardiomyopathy
Synopsis
The diagnosis of Danon disease was established in a mother and son with a small intragenic LAMP2 microduplication; myocardial pathologies and LAMP2 sequencing having been initially misleading.
Compliance with Ethics Guidelines
Conflict of Interest: Matthew Lines, Stacy Hewson, William Halliday, Peter Sabatini, Tracy Stockley, Anne Dipchand, Sarah Bowdin, and Komudi Siriwardena declare that they have no conflict of interest.
Informed Consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of 1975, as revised in 2008. Informed consent was obtained from each patient (or legal delegate) for inclusion in the study. Additional informed consent was obtained from each patient identified in this report.
Author Contributions
Matthew Lines: Analysis/interpretation of data, drafting and revision of manuscript
Stacy Hewson: Analysis/interpretation of data, critical revision of manuscript
William Halliday: Analysis/interpretation of data (pathology), critical revision of manuscript
Peter Sabatini: Analysis/interpretation of data (molecular), critical revision of manuscript
Tracy Stockley: Analysis/interpretation of data (molecular), critical revision of manuscript
Anne Dipchand: Analysis/interpretation of data (echocardiography), critical revision of manuscript
Sarah Bowdin: Analysis/interpretation of data (clinical), critical revision of manuscript
Komudi Siriwardena: Conception and design, critical revision of manuscript, guarantor of manuscript
Footnotes
Competing interests: None declared
Contributor Information
Komudi Siriwardena, Email: komudi.siriwardena@sickkids.ca.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Alroy J, Pfannl R, Slavov D, Taylor MR. Electron microscopic findings in skin biopsies from patients with Danon disease. Ultrastruct Pathol. 2010;34:333–336. doi: 10.3109/01913123.2010.499024. [DOI] [PubMed] [Google Scholar]
- Arad M, Maron BJ, Gorham JM, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352:362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol. 2008;28:5747–5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucek D, Jirikowic J, Taylor M. Natural history of Danon disease. Genet Med. 2011;13:563–568. doi: 10.1097/GIM.0b013e31820ad795. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Cui Q, Tian Z, et al. Danon disease as a cause of concentric left ventricular hypertrophy in patients who underwent endomyocardial biopsy. Eur Heart J. 2012;33:649–656. doi: 10.1093/eurheartj/ehr420. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Danon MJ, Oh SJ, DiMauro S. Lysosomal glycogen storage disease with normal acid maltase. Neurology. 1981;31:51–57. doi: 10.1212/WNL.31.1.51. [DOI] [PubMed] [Google Scholar]
- Demirel Ö, Jan I, Wolters D, et al. The lysosomal polypeptide transporter TAPL is stabilized by interaction with LAMP-1 and LAMP-2. J Cell Sci. 2012;125:4230–4240. doi: 10.1242/jcs.087346. [DOI] [PubMed] [Google Scholar]
- Fanin M, Nascimbeni AC, Fulizio L, Spinazzi M, Melacini P, Angelini C. Generalized lysosome-associated membrane protein-2 defect explains multisystem clinical involvement and allows leukocyte diagnostic screening in Danon disease. Am J Pathol. 2006;168:1309–1320. doi: 10.2353/ajpath.2006.050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Furuta A, Kikuchi H, et al. Discovery of a novel type of autophagy targeting RNA. Autophagy. 2013;9:403–409. doi: 10.4161/auto.23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Polo RA, Boya P, Pauleau AL, et al. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Bandyopadhyay U, Sridhar S, et al. Chaperone-mediated autophagy at a glance. J Cell Sci. 2011;124:495–499. doi: 10.1242/jcs.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer F, Pelak O, Kalina T et al (2013) Mosaic tissue distribution of the tandem duplication of LAMP2 exons 4 and 5 demonstrates the limits of Danon disease cellular and molecular diagnostics. J Inh Metab Dis [Epub ahead of print] (PMID: 23716275/doi 10.1007/s10545-013-9617-z) [DOI] [PubMed]
- Miani D, Taylor M, Mestroni L, et al. Sudden death associated with danon disease in women. Am J Cardiol. 2012;109:406–411. doi: 10.1016/j.amjcard.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Murakami N, Goto Y, Itoh M, et al. Sarcolemmal indentation in cardiomyopathy with mental retardation and vacuolar myopathy. Neuromuscul Disord. 1995;5:149–155. doi: 10.1016/0960-8966(94)00046-C. [DOI] [PubMed] [Google Scholar]
- Nishino I, Fu J, Tanji K, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- Sugie K, Noguchi S, Kozuka Y, et al. Autophagic vacuoles with sarcolemmal features delineate Danon disease and related myopathies. J Neuropathol Exp Neurol. 2005;64:513–522. doi: 10.1093/jnen/64.6.513. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Guhde G, Suter A, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- Taylor MR, Ku L, Slavov D, et al. Danon disease presenting with dilated cardiomyopathy and a complex phenotype. J Hum Genet. 2007;52:830–835. doi: 10.1007/s10038-007-0184-8. [DOI] [PubMed] [Google Scholar]
- van der Kooi AJ, van Langen IM, Aronica E, et al. Extension of the clinical spectrum of Danon disease. Neurology. 2008;70:1358–1359. doi: 10.1212/01.wnl.0000309219.61785.b3. [DOI] [PubMed] [Google Scholar]
- Yang Z, Funke BH, Cripe LH, et al. LAMP2 microdeletions in patients with Danon disease. Circ Cardiovasc Genet. 2010;3:129–137. doi: 10.1161/CIRCGENETICS.109.901785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Progressive hypertrophic cardiomyopathy


