Abstract
Calcium homeostasis during lactation is critical for maternal and neonatal health. We previously showed that nonneuronal/peripheral serotonin [5-hydroxytryptamine (5-HT)] causes the lactating mammary gland to synthesize and secrete PTHrP in an acute fashion. Here, using a mouse model, we found that genetic inactivation of tryptophan hydroxylase 1 (Tph1), which catalyzes the rate-limiting step in peripheral 5-HT synthesis, reduced circulating and mammary PTHrP expression, osteoclast activity, and maternal circulating calcium concentrations during the transition from pregnancy to lactation. Tph1 inactivation also reduced sonic hedgehog signaling in the mammary gland during lactation. Each of these deficiencies was rescued by daily injections of 5-hydroxy-L-tryptophan (an immediate precursor of 5-HT) to Tph1-deficient dams. We used immortalized mouse embryonic fibroblasts to demonstrate that 5-HT induces PTHrP through a sonic hedgehog-dependent signal transduction mechanism. We also found that 5-HT altered DNA methylation of the Shh gene locus, leading to transcriptional initiation at an alternate start site and formation of a variant transcript in mouse embryonic fibroblasts in vitro and in mammary tissue in vivo. These results support a new paradigm of 5-HT-mediated Shh regulation involving DNA methylation remodeling and promoter switching. In addition to having immediate implications for lactation biology, identification and characterization of a novel functional regulatory relationship between nonneuronal 5-HT, hedgehog signaling, and PTHrP offers new avenues for the study of these important factors in development and disease.
Maternal calcium levels need to rise during lactation to sustain milk synthesis and meet maternal needs (1). Bone is the primary source of calcium for milk synthesis (1), and lactating women lose 6%–10% of their bone mass over a 6-month lactation period (2). All mammals experience impaired maternal calcium homeostasis during the early periparturient period, whereas in some species such as the bovine and the canine, this can result in a clinical form of hypocalcemia (3–5). Understanding the molecular mechanisms of bone calcium mobilization during the late pregnancy/early lactation period has therapeutic implications for treatment of lactation-induced hypocalcemia, particularly in the bovine.
Serotonin [5-hydroxytryptamine (5-HT)], derived from nonneuronal sources, is critical for calcium homeostasis during lactation. 5-HT biosynthesis is mediated by tryptophan hydroxylase 1 (TPH1), which converts L-tryptophan into 5-hydroxy-L-tryptophan (5-HTP) (6). After this rate-limiting step, aromatic l-amino acid decarboxylase converts 5-HTP to 5-HT, which exerts its actions by signaling through more than 15 receptors (7). Mammary-derived 5-HT synthesis directs a variety of physiological pathways associated with lactation (6, 8–11). We have previously shown in Tph1-deficient mice that PTHrP is not present in the mammary gland on day 10 of lactation, and a single dose of 5-HTP increases the PTHrP in the mammary gland and circulation, and feeding rats with supplemental 5-HTP during the pregnancy to lactation transition increases PTHrP and calcium in circulation and milk (12, 13). PTHrP is essential for milk formation and maintenance of circulating maternal calcium (2, 14, 15). However, it is not known how 5-HT induces PTHrP in the mammary gland, and the downstream pathways that mobilize calcium from the bone during lactation, particularly during the late pregnancy/early lactation period.
Previous studies in bone and cartilage development and breast cancer metastases to bone identified the Hedgehog (Hh) signaling pathway as an upstream activator of PTHrP synthesis (16–18). Upon binding of the Hh ligand to its 12-pass transmembrane protein receptor patched-1 (PTCH1), smoothened (SMO) is activated and translocates to the primary cilium. This triggers a complex downstream signaling cascade that stimulates Hh target gene transcription through glioblastoma (GLI) transcription factors 1, 2, and 3 (19). Indian Hedgehog as well as sonic hedgehog (SHH), the most widely expressed mammalian Hh ligand, activates PTHrP synthesis in bone and cartilage (18, 20). However, it has not been determined whether SHH also activates PTHrP synthesis in lactating mammary gland.
The goal of this study is to determine the mechanism by which nonneuronal 5-HT activates mammary PTHrP synthesis and bone calcium mobilization during the late pregnancy/early lactation time period. Overall, our findings reveal the necessity of peripheral 5-HT in controlling maternal calcium levels during lactation by inducing mammary gland PTHrP synthesis and bone calcium mobilization through epigenetic regulation of Shh DNA methylation.
Materials and Methods
Animal care, experimental design, and treatments
All experimental procedures were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Pregnant C57BL6/J mice were randomly assigned to individual cages from day 13 of pregnancy until day 10 of lactation. Experimental groups included the following: 1) wild-type (WT) mice (WT, n = 7), 2) Tph1 knockout mice [Tph1−/− (21), n = 7], and 3) rescues [Tph1−/− (5-HTP) mice, n = 7]. In group 3, mice were administered 5-HTP by ip injection [100 mg/kg·d from d 13 of pregnancy to d 10 of lactation, (22)], whereas group 1 and 2 mice were injected with saline alone. Litter size was standardized to six pups per dam, regardless of their sex.
Data and sample collection
Serum and plasma samples were harvested from maxillary venous blood (on d 1, d 5, and d 10 of lactation). Femurs and mammary glands (number 4 gland) were harvested on day 10 of lactation. Tissue was stored at −80°C until used. One femur was fixed in 4% paraformaldehyde overnight at 4°C dehydrated in ethanol, decalcified in Decalcifier-I (Leica Biosystems; number 3800440), embedded in paraffin, and cut into sections (5 μm).
RNA and protein extraction
Total RNA was isolated from bone and mammary gland with the use of TRI reagent (Molecular Research; number TR118) and was reverse transcribed (1 μg) to cDNA (Bio-Rad Laboratories; number 1708841) according to the manufacturer's instructions. Mammary gland protein was extracted in radioimmunoprecipitation assay buffer containing 10 μL/mL of Halt protease and phosphatase inhibitors cocktail (Thermo Scientific; number 78443). Protein concentrations were determined with the bicinchoninic acid assay (Thermo Scientific; number 23225, number 23227).
Determination of 5-HT and PTHrP in the circulation and the mammary gland
Serum 5-HT concentrations were determined by an ELISA (Enzo Life Sciences; number ADI-900–175), according to the manufacturer's instructions. The intraassay coefficient of variation (CV) was 2.6%. Plasma PTHrP concentrations were determined by an immunoradiometric assay (IRMA; Active PTHrP IRMA; Beckman Coulter; number DSL8100) according to the manufacturer's instructions. The intraassay CV was 7.5%. Mammary gland concentration of 5-HT was determined by an EIA (enzyme immunoassay kit; Enzo Life Sciences; number ADI-900–175), using 50 μg of protein per sample analyzed. The intraassay CV was 6.6%. Mammary gland concentration of PTHrP was measured by an IRMA (Active PTHrP IRMA; Beckman Coulter; number DSL8100), and the interassay CV was 7.5%. Serum was diluted 1:50 to detect 5-HT concentrations within the parameters of the assay.
Serum calcium determination
Total serum calcium concentrations were measured with a calcium assay kit (Cayman Chemical Co; number 700550) according to the manufacturer's instructions. The intraassay CV was 3.4%.
Real-time RT-PCR
Real-time RT-PCR was conducted with the CFX96 Touch real-time PCR detection system (Bio-Rad Laboratories). Reaction mixtures and cycling conditions were as previously described (12).
Bone histology
Sectioned femurs were stained using tartrate-resistant alkaline phosphatase (acid phosphatase leukocyte kit; Sigma; number 387) to identify osteoclasts (OCs). Three random images per slide were obtained. Two independent researchers blinded to the treatments quantified the OC number and the total area per slide (average of three images per slide) using ImageJ software (National Institutes of Health, Bethesda, Maryland). All images were captured (×20 objective lens magnification) using a Zeiss Axio Vert A1 fluorescent microscope and processed using Q-capture pro 7 software (Q Imaging).
Methylated DNA immunoprecipitation (MeDIP)
Genomic DNA was isolated using the QIAGEN DNeasy blood and tissue kit (number 69504) according to the manufacturer's instructions. MeDIP was performed on 4 μg of genomic DNA as described previously (23). The enriched methylated DNA was quantified by real-time PCR as described previously (23), using gene-specific primers for Shh-F1/R1 [transcriptional start site (TSS)-1]: 5′-TCTCAGGGTTAACATCAGAAGACA-3′ and 5′-AATGGTAGCAAGGCTGGAGA-3′ and Shh-F2/R2 (TSS2): 5′-GAGCGAGGAAGGGAGAGC-3′ and 5′-CGGAACCTGAGGACTTGTGA-3′ (Entrez gene identification 20423). Results were analyzed using the 2-ΔΔCt method and expressed as enrichment over IgG (24). The imprinted Igf2 gene (5′-CCTCCTCGCCTCAGACTCC-3′ and 5′-GGACAGCACGGAGAGAAACAG-3′) was used as a positive control.
In vitro cell culture experiments
Carrier-free recombinant human SHH ligand (R&D Systems) was resuspended at 100 μg/mL in sterile PBS containing 0.1% BSA. The anti-SHH monoclonal neutralizing 5E1 antibody was purchased from the Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City, Iowa). Vismodegib (vis; LC Labs) was dissolved in dimethylsulfoxide at a stock concentration of 15 μM. 5-HT (Acros Organics) was dissolved in DMEM with 1% fetal calf serum at a stock concentration of 2 mM. Intact immortalized mouse embryonic fibroblasts (iMEFs) and Gli2−/−3−/− iMEFs (25) were plated in 24-well plates (Falcon) at 2.0 × 105 cells/well in 400 μL DMEM media with 10% fetal calf serum. Cells were allowed to attach overnight and media were replaced with DMEM containing 1% fetal calf serum with or without the indicated combinations of 0.4 μg/mL Shh peptide (or BSA alone), 8 μg/mL 5E1 antibody, 50 nM vis (or dimethylsulfoxide alone), and 200 μM 5-HT. After 48 hours, RNA was harvested from cells as previously described (19). To study the in vitro DNA methylation, iMEFs were plated and treated as described above. After 48 hours genomic DNA was harvested and MeDIP was performed. Total RNA extraction, cDNA synthesis, and MeDIP-real-time PCR for the cell culture experiment were performed as described for the in vivo study.
Statistical analyses
Prism version 6.0b (GraphPad Software) was used to assess differences between groups (Student's t test) or among groups (one way and two way ANOVA) followed by Tukey's post hoc test method to detect differences between treatment groups. Normality and outlier (using Grubbs test, extreme studentized deviate method) tests were performed. Welch's correction was applied if the sample populations had unequal variances. Differences between means were considered significant at a value of P < .05. Data are reported as mean values ± SEM.
Results
Tph1 gene is required to maintain circulating 5-HT levels and for mammary 5-HT production
We previously showed that a single injection of 5-HTP in Tph1-deficient dams increased circulating and mammary PTHrP (13). Herein we determined the sustained effects of TPH1/5-HT on PTHrP in dams during late pregnancy/early lactation. To this end, we first tested whether TPH1 is required to maintain circulating 5-HT levels during the late pregnancy/early lactation period. Tph1−/− dams were deficient in circulating 5-HT throughout the lactation period (d 1, d 5, and d 10) compared with WT animals (P < .038, Figure 1A). To bypass the requirement of Tph1 in peripheral 5-HT synthesis, exogenous 5-HTP was administered daily to dams from day 7 prior to parturition through day 10 of lactation. Exogenous 5-HTP rescued the circulating 5-HT deficiency in Tph1-deficient dams (P < .012, Figure 1A). We then tested whether TPH1 maintains mammary 5-HT synthesis during lactation. Tph1−/− dams were deficient in mammary 5-HT, and this deficiency was rescued by giving exogenous 5-HTP to Tph1-deficient dams (P < 0.018, Figure 1B). These results establish that TPH1 maintains circulating and mammary 5-HT levels during lactation and that 5-HTP can substitute for this TPH1 requirement.
Figure 1.
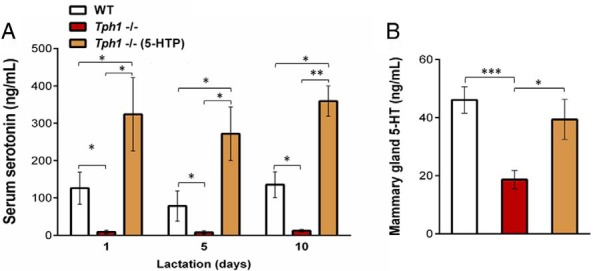
Genetic ablation of Tph1 gene in mice decreased circulating and mammary gland 5-HT levels. WT mouse dams were given saline and Tph1−/− dams were given either saline or daily injections of exogenous 5-HTP [100 mg/kg; Tph1−/−(5-HTP)], from pregnancy day 13 to lactation day 10. A, Serum 5-HT concentration determined on day 1, day 5, and day 10 of lactation. B, Mammary 5-HT protein concentration determined on day 10 of lactation. All values are means ± SEM (n = 7 per group). *, P < .05; **, P < 0.01; ***, P < .001.
Peripheral 5-HT regulates calcium homeostasis by activating OCs through the induction of mammary PTHrP expression during lactation
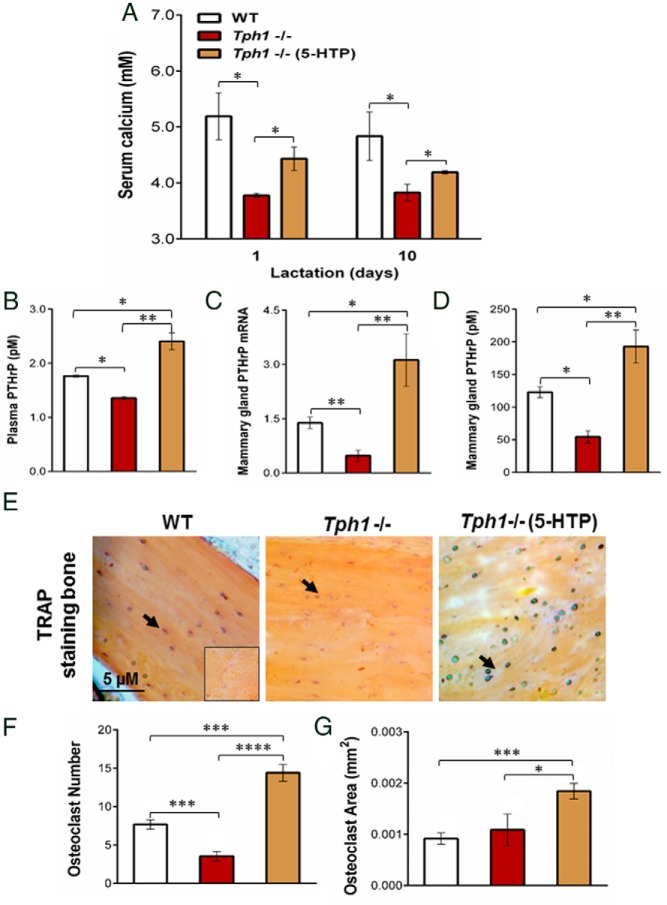
Previously we demonstrated that feeding supplemental 5-HTP during the late pregnancy/early lactation period in rats resulted in increased serum calcium concentrations as well as increased OC numbers and size (12). Here we wanted to determine the contribution of peripheral 5-HT to calcium homeostasis during the late pregnancy/early lactation period using mice genetically deficient in peripheral 5-HT production. Tph1−/− mice had decreased total serum calcium concentrations compared with WT mice on day 1 and day 10 of lactation. This deficiency was attenuated by administration of exogenous 5-HTP to Tph1-deficient mice (P < .05, Figure 2A). Because PTHrP mediates bone mineral resorption during lactation (2), we evaluated whether peripheral 5-HT is required to maintain PTHrP concentrations during the late pregnancy/early lactation period. Tph1−/− mice had decreased plasma and mammary PTHrP concentrations when compared with WT on day 10 of lactation (P < .024, Figure 2, B and D). Plasma and mammary gland PTHrP concentrations were increased by chronic administration of exogenous 5-HTP to Tph1-deficient dams (P < .041, Figure 2, B and D). Similarly, Tph1−/− mice had decreased PTHrP mRNA expression in the mammary gland on day 10 of lactation when compared with WT, and this was also restored by giving exogenous 5-HTP to Tph1-deficient dams (P < .0024, Figure 2C).
Figure 2.
Lack of peripheral 5-HT decreases blood circulating calcium and bone OC activity during lactation through PTHrP activation. WT mouse dams were given saline and Tph1−/− dams were given either saline or daily injections of exogenous 5-HTP [100 mg/kg; Tph1−/−(5-HTP)], from pregnancy day 13 to lactation day 10. A, Total serum calcium content determined on day 1 and day 10 of lactation. B–D, Abundance of plasma circulating PTHrP, mammary gland PTHrP mRNA, and protein determined on lactation day 10. E, Tartrate-resistant acid phosphatase (TRAP) staining of bone femurs conducted on day 10 of lactation to identify OCs (black arrows). F–G, OC number and area per group. All values are reported as means ± SEM (n = 7 per group). *, P < .05; **, P < 0.01; ***, P < .001.
We next examined whether Tph1−/− dams exhibited differences in bone resorption during lactation. Tartrate-resistant acid phosphatase staining was used to visualize and count the OC number and surface area in demineralized bone sections as indices of OC activity (26) (Figure 2E). The femur OC number was reduced in Tph1−/− dams compared with the WT and was restored by chronic administration of exogenous 5-HTP to those mice (P < .001, Figure 2F). The OC area was not different in Tph1−/− mice femurs compared with WT, but chronic administration of exogenous 5-HTP increased OC area in Tph1-deficient dams (P < .0001, Figure 2G).
Peripheral 5-HT activates Hh signaling in the lactating mammary gland
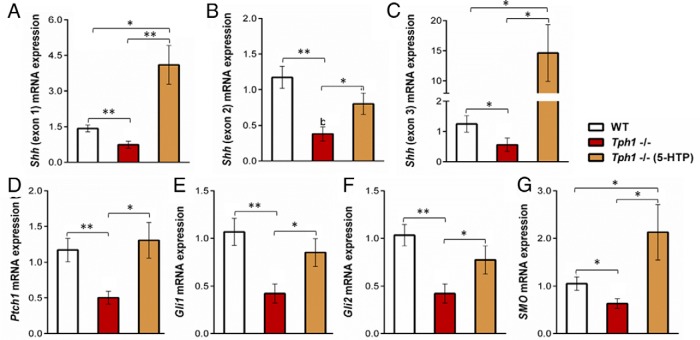
Expression of Hh signaling pathway members in the lactating mammary gland has never been examined, and whether 5-HT can activate this pathway in mammary tissue has never been addressed. The Shh ligand mRNA abundance was significantly lower in Tph1−/− mammary glands compared with WT mammary glands for the three Shh exons evaluated (P < .05, Figure 3, A–C), and this defect was restored by chronic administration of exogenous 5-HTP to Tph1-deficient mice. To test whether these changes in Shh mRNA content were associated with changes in Hh pathway activation, we examined the expression of Hh target genes, Ptch1, Gli1, Gli2, and Smo. The mRNA abundance of these target genes was reduced in Tph1−/− dams compared to WT dams (P < .039), and exogenous 5-HTP restored their expression in Tph1-deficient mice (Figure 3, D–H).
Figure 3.
Peripheral 5-HT deficiency down-regulates several key Hh pathway members in the lactating mammary gland. WT mouse dams were given saline and Tph1−/− dams were given either saline or daily injections of exogenous 5-HTP [100 mg/kg; Tph1−/−(5-HTP)] from pregnancy day 13 to lactation day 10. A–C, Shh ligand (exons 1, 2, and 3). D–G, Ptch1, Gli1, Gli2, and Smo mammary gland mRNA expression on day 10 of lactation. All values are reported as means ± SEM (n = 7 per group). *, P < .05; **, P < 0.01.
5-HT induces PTHrP by inducing Shh and activating the Hh pathway
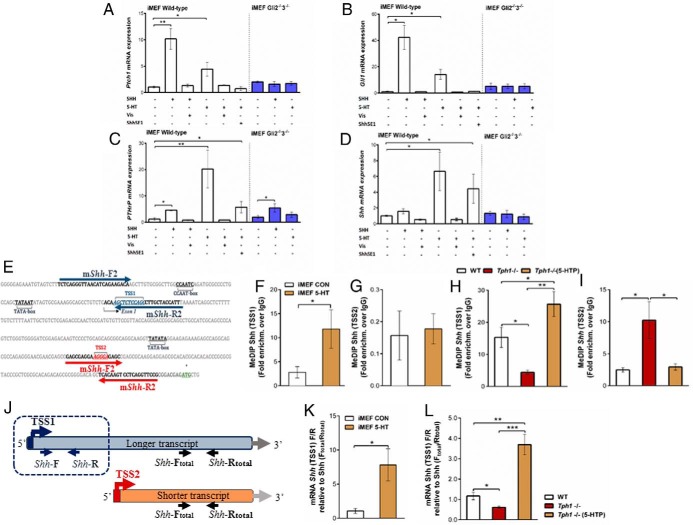
To dissect the mechanism by which 5-HT regulates PTHrP, in vitro assays were conducted using SHH-responsive iMEF cell lines (25). Extensive chemical and genetic tools associated with this established in vitro system enable us to resolve detailed interactions between 5-HT, Hh signaling, and PTHrP. Treatment of iMEF WT cells with 5-HT or SHH peptide increased abundance of Hh pathway target genes Gli1 and Ptch1, as well as PTHrP (P < .01, Figure 4, A–C). Induction of these genes by the SHH peptide and 5-HT was completely blocked by vis, a potent synthetic SMO antagonist (Figure 4, A and C). 5-HT treatment also increased Shh mRNA abundance (P = .041, Figure 4D), consistent with the notion that SHH peptide mediates Hh pathway activation and induces PTHrP mRNA expression. Indeed, addition of the SHH-neutralizing antibody Shh5E1 significantly blocked 5HT-mediated induction of Gli1, Ptch1, and PTHrP (Figure 4, A–C). Finally, in cells lacking both Gli2 and Gli3 (Gli2−/−3−/−), 5-HT treatment failed to induce Gli1 and Ptch1 expression, whereas PTHrP induction was greatly muted relative to that seen in 5-HT-treated WT iMEF cells (P = .014, Figure 4, A–C).
Figure 4.
Peripheral 5-HT induces PTHrP expression by activating canonical Hh signaling in vitro and in vivo. WT or Gli2/3-deficient (Gli2−/−3−/−) iMEFs were grown for 48 hours in control media or in media supplemented with SHH peptide (0.4 μg/mL), 5-HT (20 μM), the SMO antagonist vis (50 nM), or the SHH-neutralizing 5E1 antibody (Shh5E1; 8 μg/mL). A–D, Ptch1, Gli1, PTHrP, and Shh mRNA abundance quantified by real-time quantitative PCR. E, Diagrammatic representation of the mouse Shh gene 5′ flanking region. TATA boxes are located 33 and 52 bp upstream of the putative TSSs, TSS2 and TSS1, respectively (adapted from Ref. 28). F–I, The DNA methylation landscape at each TSS was determined by MeDIP-quantitative PCR, in vitro (F–G) using WT iMEFs (grown for 48 hours in control media or media supplemented with 200 μM 5-HT), and in vivo (H–I), using WT and Tph1−/− mice given either saline or 5-HTP (100 mg/kg) daily injections. J–L, To determine whether 5-HT influenced the fraction of Shh transcripts deriving from TSS2, the mRNA abundance of a unique 216-bp region (J) was quantified by real-time quantitative PCR in vitro (K) and in vivo (L). Results were normalized to total Shh mRNA, determined by measuring the mRNA abundance of a 125-bp region shared by both TSSs (Shh-total). Results are means ± SEM (n = 4 per group). *, P < .05; **, P < 0.01; ***, P < .001. Con, control.
5-HT establishes the pattern of Shh promoter methylation in vitro and in vivo
The human Shh gene contains two TSSs, TSS1 and TSS2 (27). The relative DNA methylation status at the two TSS sites impacts which one is used to initiate transcription. The mouse Shh locus contains two relatively well-conserved sequence regions that appear to correspond to the human canonical TSS1, and the upstream TSS2 (Figure 4E). We used MeDIP-quantitative PCR to examine whether 5-HT treatment influences the DNA methylation landscape of the mouse Shh gene locus in iMEF cells. 5-HT treatment significantly increased TSS1 DNA methylation compared with vehicle control treatment (P = .048, Figure 4F), whereas TSS2 DNA methylation was unchanged (Figure 4G). We next evaluated whether peripheral 5-HT impacts Shh TSS DNA methylation in the mammary gland during lactation in vivo. Tph1-deficient mammary glands exhibited less Shh TSS1 DNA methylation and more TSS2 DNA methylation relative to WT mammary glands (P < .028, Figure 4, H and I). Administration of exogenous 5-HTP to Tph1−/− dams increased DNA methylation at TSS1 and decreased DNA methylation at TSS2 compared with untreated Tph1−/− dams (P < .006, Figure 4, H and I). The resulting DNA methylation pattern in 5-HTP-treated Tph1−/− dams was similar to the WT dams. No differences in the DNA methylation of the imprinted gene Igf2 (positive control) were detected between treatment groups (data not shown).
5-HT enhances Shh expression and increases DNA methylation at the downstream Shh transcriptional initiation site (TSS1)
Our results are consistent with the hypothesis that 5-HT-induced methylation of TSS1 decreases transcription from TSS1 and increases transcription from the hypomethylated TSS2. To test this hypothesis, we measured mRNA abundance of the 216-bp unique region of the Shh transcript originating from TSS2. This sequence is absent from TSS1-intitiated Shh transcripts. TSS2-initiated Shh mRNA abundance was normalized to total Shh mRNA abundance to determine the fraction of Shh mRNA initiated by TSS2 (Figure 4J, Shh-F/R and Shh-Ftotal/Rtotal, respectively, primer sequences in Table 1). Treatment of iMEF cells with 5-HT increased the fraction of TSS2-initiated Shh mRNA by 5-fold (P = .031, Figure 4K). Importantly, we found that this mechanism of 5-HT action was paralleled in lactating mouse mammary gland in vivo. The fraction of TSS2-initiated Shh mRNA was lower in Tph1−/− mice compared with the wild types (P = .023), and exogenous 5-HTP increased TSS2-initiated Shh mRNA by 4-fold in Tph1−/− mammary glands (P = .009, Figure 4L).
Table 1.
Primer sequences for the studied genes quantified by real-time PCR
| Genes | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| RPS15 | GTTGAAGGTCTTGCCGTTGT | TTGAGAAAGGCCAAAAAGGA |
| Tph1, exon 2 | TTCACCATGATTGAAGACAAC | TCCGACTTCATTCTCCAAGG |
| PTHrP | TTCCTGCTCAGCTACTCCGT | GATGGACTTGCCCTTGTCAT |
| mShh-F1/R1 | TCTCAGGGTTAACATCAGAAGACA | AATGGTAGCAAGGCTGGAGA |
| mShh-F2/R2 | GAGCGAGGAAGGGAGAGC | CGGAACCTGAGGACTTGTGA |
| Shh-F/R | CTCCGATGTGTTCCGTTACC | GCCTGGCTCTTTCTCTTCCT |
| Shh-Ftotal/Rtotal | CTGGCCAGATGTTTTCTGGT | GGCTAAAGGGGTCAGCTTTT |
| Shh-exon1 | AAAAGCTGACCCCTTTAGC | CTGAGTCATCAGC |
| Shh, exon2 | TTAAATGCCTTGGCCATCTC | CAGTGGATGTGAGCTTTGGA |
| Shh, exon3 | CCTGCTATGCTCCTGCTTTC | GTGGCGGTTACAAAGCAAAT |
| Gli1 | GGCAGGGAAGAGAGCAGACT | ACTGCCTGCTGGGGAGTG |
| Gli2 | AGAGCTCCGGGCTTTGTC | TCCATGCCACTGTCATTGTT |
| Gli3 | GAGCCAGCTTTCCTGTCTTG | ATCAAAATGGAGGCACAAGG |
| Ptch1 | GGATTAAAGGCAGCTAATCTC | GCCTCTTCTCCTATCTTCTGA |
| SMO | GACTCCGTGAGTGGCATCTG | GTGGCAGCTGAAGGTGATGA |
Discussion
Calcium homeostasis during lactation is critical to all mammalian species for milk formation and maternal health (1). Of particular interest is periparturient hypocalcemia, which is a significant financial burden to the US dairy industry, with the estimated cost for treatment between $150 million and $300 million annually (4). It is therefore critically important to identify mechanisms of calcium homeostasis during lactation (1, 28). We showed previously that nonneuronal 5-HT induces the mammary synthesis of PTHrP, a master regulator of bone calcium mobilization (2), and it has been previously determined that osteoclastogenesis is reduced in male Tph1 null animals (22). However, the mechanisms of how 5-HT regulates PTHrP induction during the late pregnancy/early lactation period had not been resolved. Here, using the Tph1-deficient mouse model, we made the surprising discovery that 5-HT induces canonical Hh signaling by remodeling SHH promoter methylation patterns, leading to a Hh-dependent activation of mammary PTHrP synthesis (Figure 5). This is the first evidence of Hh signaling contributing to systemic calcium homeostasis during lactation. This pathway has been extensively studied in fetal mammary gland development. Several Hh signaling molecules are present in ectodermal placodes that give rise to mammary gland buds (29) and mammary placode formation is impaired in mice with mutations in Gli3, which encodes a transcriptional repressor of the Hh pathway (30). Although Hh pathway members are expressed in adult mammary epithelial and stromal cells (31), canonical Hh signaling does not appear to be required for postpubertal ductal branching morphogenesis or for gestational alveologenesis (31, 32). Instead, our results reveal a novel role for Hh signaling in initiation of mammary gland endocrine signaling during lactation.
Figure 5.
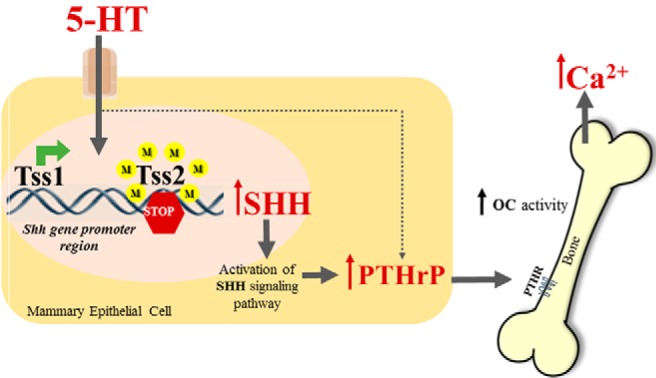
Model illustrating how nonneuronal 5-HT acts through the Hh signaling pathway to regulate PTHrP. Alteration of the methylation status of the Shh gene promoter by 5-HT increases Shh mRNA expression, which then initiates signal transduction through Smo and the Glis, culminating in the regulation of Hh target genes, including PTHrP. Secreted PTHrP exerts its paracrine effects in bone, leading to OC activation impacting blood calcium (Ca2+) levels. M, methyl group.
The Hh pathway is essential for the development of numerous tissues and organs and more recently has been implicated in mechanisms of tissue repair, regeneration, and cancer. Although the transduction cascade between SHH/PTCH1 and the GLI transcription factors is well characterized, how Shh transcription is regulated remains largely unknown (33). Kitazawa et al (27) demonstrated that the 5′ flanking region of the human SHH gene has two TSSs, from which two distinct transcripts are initiated. However, little attention has been given to how initiation from these transcription start sites is orchestrated and how it impacts SHH expression. A recent study showed that DNA methylation of SHH TSS can function as a transcriptional regulatory mechanism in human mammary cells grown in vitro (34) and that the pharmacological inhibition of DNA methylation induces SHH transcription from the upstream TSS (34). Our study extends these findings and supports a novel regulatory mechanism by which an endogenous factor, 5-HT, remodels DNA methylation of the Shh promoter, shifting transcriptional initiation to the upstream TSS (TSS2) from the TSS1 and increasing Shh mRNA abundance (Figure 5). The switching of TSS in the Shh promoter region has been previously described, but we are the first to demonstrate this occurrence in response to administration of 5-HT.
The results described here demonstrate that 5-HT, Hh, and PTHrP comprise an essential regulatory pathway in the lactating mammary gland that maintains calcium homeostasis during lactation (Figure 5). Importantly, the parallel operation of this pathway in a more generalizable and tractable iMEF assay suggests potential broader relevance in development and disease. For example, Hh and 5-HT signaling pathway members are expressed in the developing brain and face (33), and disruption of either pathway causes craniofacial malformations (35–38). Yet whether 5-HT activates Hh signaling during craniofacial development has yet to be assessed. Likewise, Hh and PTHrP activity have been linked in breast cancer (16, 39), but whether 5-HT activates their expression has not been examined. The potential implications of the disruption of this pathway for human health are highlighted by the widespread use of selective 5-HT reuptake inhibitors (selective serotonin reuptake inhibitors). Selective serotonin reuptake inhibitors are the most widely prescribed class of antidepressant drugs in the United States and have been associated with adverse bone health such as increased bone loss and risk of fracture (40, 41). Moreover, our findings are potentially applicable for the treatment of periparturient hypocalcemia in dairy cattle. In conclusion, the studies presented here provide a foundation for mechanistic examination of this pathway and its disruption across a wide range of biological systems.
Acknowledgments
The 5E1 antibody developed by Jessell and Brenner-Morton was obtained from the Developmental Studies Hybridoma Bank, created by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health and maintained at Department of Biology, The University of Iowa, Iowa City, Iowa. The Tph1−/− animals were a gift from Dr Nelson D. Horseman (Department of Molecular and Cellular Physiology, University of Cincinnati, Cincinnati, Ohio). We thank Drs Xin Sun and Linda Schuler (University of Wisconsin-Madison, Madison, Wisconsin) for their thoughtful discussions and comments.
This work was supported by The National Institute of Health, Grant DK099328 to C.M.V. and Grant R00DE022101 to R.J.L, and USDA/Hatch Grant 142-PRJ74RE to L.L.H.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CV
- coefficient of variation
- GLI
- glioblastoma
- Hh
- Hedgehog
- 5-HT
- 5-hydroxytryptamine
- 5-HTP
- 5-hydroxy-L-tryptophan
- iMEF
- immortalized mouse embryonic fibroblast
- IRMA
- immunoradiometric assay
- MeDIP
- methylated DNA immunoprecipitation
- OC
- osteoclast
- PTCH1
- protein receptor patched-1
- SHH
- sonic hedgehog
- SMO
- smoothened
- TPH1
- tryptophan hydroxylase 1
- TSS
- transcriptional start site
- vis
- vismodegib
- WT
- wild type.
References
- 1. Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18(6):832–872 [DOI] [PubMed] [Google Scholar]
- 2. Wysolmerski JJ. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. In: Zaidi M, ed. Skeletal Biology and Medicine. Vol 11922010:161–169. Ann NY Acad Sci. 2010;1192:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goff JP. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet J. 2008;176(1):50–57 [DOI] [PubMed] [Google Scholar]
- 4. Reinhardt TA, Lippolis JD, McCluskey BJ, Goff JP, Horst RL. Prevalence of subclinical hypocalcemia in dairy herds. Vet J. 2011;188(1):122–124 [DOI] [PubMed] [Google Scholar]
- 5. Davidson AP. Reproductive causes of hypocalcemia. Top Companion Anim Med. 2012;27(4):165–166 [DOI] [PubMed] [Google Scholar]
- 6. Matsuda M, Imaoka T, Vomachka AJ, et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell. 2004;6(2):193–203 [DOI] [PubMed] [Google Scholar]
- 7. Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195(1):198–213 [DOI] [PubMed] [Google Scholar]
- 8. Collier RJ, Hemandez LL, Horseman ND. Serotonin as a homeostatic regulator of lactation. Domest Anim Endocrinol. 2012;43(2):161–170 [DOI] [PubMed] [Google Scholar]
- 9. Hernandez LL, Collier JL, Vomachka AJ, Collier RJ, Horseman ND. Suppression of lactation and acceleration of involution in the bovine mammary gland by a selective serotonin reuptake inhibitor. J Endocrinol. 2011;209(1):45–54 [DOI] [PubMed] [Google Scholar]
- 10. Hernandez LL, Stiening CM, Wheelock JB, Baumgard LH, Parkhurst AM, Collier RJ. Evaluation of serotonin as a feedback inhibitor of lactation in the bovine. J Dairy Sci. 2008;91(5):1834–1844 [DOI] [PubMed] [Google Scholar]
- 11. Pai VP, Horseman ND. Biphasic regulation of mammary epithelial resistance by serotonin through activation of multiple pathways. J Biol Chem. 2008;283(45):30901–30910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laporta J, Peters TL, Weaver SR, Merriman KE, Hernandez LL. Feeding 5-hydroxy-L-tryptophan during the transition from pregnancy to lactation increases calcium mobilization from bone in rats. Domest Anim Endocrinol. 2013;44(4):176–184 [DOI] [PubMed] [Google Scholar]
- 13. Hernandez LL, Gregerson KA, Horseman ND. Mammary gland serotonin regulates parathyroid hormone-related protein and other bone-related signals. Am J Physiol Endocrinol Metab. 2012;302(8):E1009–E1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40(4):795–826 [DOI] [PubMed] [Google Scholar]
- 15. Mamillapalli R, VanHouten J, Dann P, et al. Mammary-specific ablation of the calcium-sensing receptor during lactation alters maternal calcium metabolism, milk calcium transport, and neonatal calcium accrual. Endocrinology. 2013;154(9):3031–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterling JA, Oyajobi BO, Grubbs B, et al. The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells. Cancer Res. 2006;66(15):7548–7553 [DOI] [PubMed] [Google Scholar]
- 17. Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9(7):873–886 [DOI] [PubMed] [Google Scholar]
- 18. Jemtland R, Divieti P, Lee K, Segre GV. Hedgehog promotes primary osteoblast differentiation and increases PTHrP mRNA expression and iPTHrP secretion. Bone. 2003;32(6):611–620 [DOI] [PubMed] [Google Scholar]
- 19. Lipinski RJ, Gipp JJ, Zhang JX, Doles JD, Bushman W. Unique and complimentary activities of the Gli transcription factors in Hedgehog signaling. Exp Cell Res. 2006;312(11):1925–1938 [DOI] [PubMed] [Google Scholar]
- 20. Tavella S, Biticchi R, Schito A, et al. Targeted expression of SHH affects chondrocyte differentiation, growth plate organization, and Sox9 expression. J Bone Miner Res. 2004;19(10):1678–1688 [DOI] [PubMed] [Google Scholar]
- 21. Holtje M, Winter S, Walther D, et al. The vesicular monoamine content regulates VMAT2 activity through Gα(q) mouse platelets—evidence for autoregulation of vesicular transmitter uptake. J Biol Chem. 2003;278(18):15850–15858 [DOI] [PubMed] [Google Scholar]
- 22. Chabbi-Achengli Y, Coudert AE, Callebert J, et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc Natl Acad Sci USA. 2012;109(7):2567–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keil KP, Abler LL, Mehta V, et al. DNA methylation of E-cadherin is a priming mechanism for prostate development. Dev Biol. 2014;3(14):00048–00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-δδC) method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 25. Lipinski RJ, Bijlsma MF, Gipp JJ, Podhaizer DJ, Bushman W. Establishment and characterization of immortalized Gli-null mouse embryonic fibroblast cell lines. BMC Cell Biol. 2008;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamp EC, Drexler HG. Biology of tartrate-resistant acid phosphatase. Leukemia Lymphoma. 2000;39(5–6):477–484 [DOI] [PubMed] [Google Scholar]
- 27. Kitazawa S, Kitazawa R, Tamada H, Maeda S. Promoter structure of human sonic hedgehog gene. Biochim Biophys Acta Gene Struct Express. 1998;1443(3):358–363 [DOI] [PubMed] [Google Scholar]
- 28. Wysolmerski JJ. Parathyroid hormone-related protein: an update. J Clin Endocrinol Metab. 2012;97(9):2947–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michno K, Boras-Granic K, Mill P, Hui CC, Hamel PA. Shh expression is required for embryonic hair follicle but not mammary gland development. Dev Biol. 2003;264(1):153–165 [DOI] [PubMed] [Google Scholar]
- 30. Veltmaat JM, Relaix F, Kratochwil K, et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development. 2006;133(12):2325–2335 [DOI] [PubMed] [Google Scholar]
- 31. Lewis MT, Ross S, Strickland PA, et al. The Gli2 transcription factor is required for normal mouse mammary gland development. Dev Biol. 2001;238(1):133–144 [DOI] [PubMed] [Google Scholar]
- 32. Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–1113 [DOI] [PubMed] [Google Scholar]
- 33. Geng X, Speirs C, Lagutin O, Inbal A, et al. Haploinsufficiency of Six3 fails to activate sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell. 2008;15(2):236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. ten Haaf A, Franken L, Heymann C, et al. Paradox of sonic hedgehog (SHH) transcriptional regulation: alternative transcription initiation overrides the effect of downstream promoter DNA methylation. Epigenetics. 2011;6(4):466–478 [DOI] [PubMed] [Google Scholar]
- 35. Tsukiji N, Amano T, Shiroishi T. A novel regulatory element for Shh expression in the lung and gut of mouse embryos. Mech Dev. 2014;131:127–136 [DOI] [PubMed] [Google Scholar]
- 36. Shuey DL, Sadler TW, Lauder JM. Serotonin as a regulator of craniofacial morphogenesis—site specific malformations following exposure to serotonin uptake inhibitors. Teratology. 1992;46(4):367–378 [DOI] [PubMed] [Google Scholar]
- 37. Reisoli E, De Lucchini S, Nardi I, Ori M. Serotonin 2B receptor signaling is required for craniofacial morphogenesis and jaw joint formation in Xenopus. Development. 2010;137(17):2927–2937 [DOI] [PubMed] [Google Scholar]
- 38. Lipinski RJ, Song C, Sulik KK, et al. Cleft lip and palate results from hedgehog signaling antagonism in the mouse: phenotypic characterization and clinical implications. Birth Defects Res Part A Clin Mol Teratol. 2010;88(4):232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Das S, Tucker JA, Khullar S, Samant RS, Shevde LA. Hedgehog signaling in tumor cells facilitates osteoblast-enhanced osteolytic metastases. Plos One. 2012;7(3):e34374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eom C-S, Lee H-K, Ye S, Park SM, Cho K-H. Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res. 2012;27(5):1186–1195 [DOI] [PubMed] [Google Scholar]
- 41. Shea MLO, Garfield LD, Teitelbaum S, et al. Serotonin-norepinephrine reuptake inhibitor therapy in late-life depression is associated with increased marker of bone resorption. Osteoporos Int. 2013;24(5):1741–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol (Clifton, NJ). 2000;132:365–386 [DOI] [PubMed] [Google Scholar]