Abstract
The lack of stability is a challenge for most heterogeneous catalysts. During operations, the agglomeration of particles may block the active sites of the catalyst, which is believed to contribute to its instability. Recently, titanium oxide (TiO2) was introduced as an alternative support material for heterogeneous catalyst due to the effect of its high surface area stabilizing the catalysts in its mesoporous structure. TiO2 supported metal catalysts have attracted interest due to TiO2 nanoparticles high activity for various reduction and oxidation reactions at low pressures and temperatures. Furthermore, TiO2 was found to be a good metal oxide catalyst support due to the strong metal support interaction, chemical stability, and acid-base property. The aforementioned properties make heterogeneous TiO2 supported catalysts show a high potential in photocatalyst-related applications, electrodes for wet solar cells, synthesis of fine chemicals, and others. This review focuses on TiO2 as a support material for heterogeneous catalysts and its potential applications.
1. Introduction
1.1. Essential Principle of Catalyst
The catalysis industry is a billion-dollar industry that accounts for the manufacture of 60% of all the chemicals that are utilized for most chemical processes [1–6]. Some of the products derived from catalytic processes include polymers [2], plastics [3], pharmaceuticals [4], and detergents [5]. After decades of research, systematic information on the catalytic properties of many catalysts has been established and accumulated. From the literature, it is concluded that one of the major limitations of the catalytic reaction is separation and distribution [7–9]. Many of real catalysts are made up of small (in the nanometer range) sizes. This consequently brought about the uncertainly and nonuniformity of the materials involved, the preparation methods, and surface conditions [8]. Heterogeneous catalyst was introduced to overcome the separation and distribution problems.
1.2. Importance of Heterogeneous Catalyst
Heterogeneous catalysts have become a crucial part of many industrial activities, such as organic synthesis, oil refining, and pollution control [10–15]. Modern heterogeneous catalysts consist of several elements in precise proportions [12]. Currently, heterogeneous catalysts are optimized for the greatest reaction rate, which in turn results in optimal selectivity [11–13]. It is possible to improve the heterogeneous catalyst activity over modifying the support by approaches such as nanotechnology and nanoscience or controlling the pore structure [14–16]. For heterogeneous catalysis, the problem of catalyst separation and recovery from the reaction matrix are addressed by using various catalyst supports to immobilize the particle [15]. This in turn provides a large enough surface area for the heterogeneous catalyst for it not to dissolve into the solution matrix [16]. Therefore, the heterogeneous catalyst with broad supports such as Al2O3, TiO2, ZrO2, ZnO, and others is applied based on its broad availability and cost-effective modes of synthesis.
1.3. Importance of Heterogeneous Catalyst Support
Recently, the importance of an appropriate catalyst's support material has been of huge interest. The idea is that the main catalyst should be dispersed on a suitable support to make the catalytic nanoparticles stable and obtain optimal performance and decrease the amount of costly metal being utilized, which accordingly decrease the total catalyst expenses [11, 15]. Furthermore, with porous characteristics, support materials offer a high dispersion of nanoparticle catalyst and simplify electron transfer, both of which contribute to better catalytic activities [17–20].
However, the heterogeneous catalyst support may sometime exert a structural effect, brought about by textural and active phase-linked effect [18]. Thus, the selection on support heterogeneous catalyst must retain its specific properties, such as porosity, surface area, dispersion, selectivity, and activity [19–21]. The morphology and pores size of the selected support materials play an important role in enhancing the heterogeneous catalyst's stability and performance [20].
According to the literature, the support of the heterogeneous catalyst can be alumina [22], zeolites [23], carbon nanofibers [19], active carbon [17], and metal oxides [13], such as TiO2, La2O3, CeO2, MnO2, and ZrO2. TiO2 is a recognized heterogeneous catalyst support that is broadly utilized in fuel processing due to its tunable porous surface and distribution, high thermal stability, and mechanical strength [24, 25]. Being used in this manner contributes to the ability of TiO2 to develop Lewis acidity as well as redox properties [25].
2. TiO2: In General
TiO2 has proven to be one of the promising n-type semiconductors due to its wide band gap (3.2 eV) under ultraviolet light [24]. Additionally, possessing high physical and chemical stability as well as the high refractive index makes this material widely researched [25]. Due to its electronic and optical properties, it can be utilized in several fields, such as solar cells, photocatalyst, sensors, and self-cleaning [26]. In electrochemistry, TiO2 based materials play a key role due to their high conductivity and stability in alkaline and acid media. TiO2 exists in three crystalline forms; anatase and rutile are the most common types, and the crystalline size of the rutile is always larger than the anatase phase. Brookite is the third structural form, an orthorhombic structure, which is rarely utilized, and is of no interest for most applications [27–30]. Rutile phase is the most thermally stable among the three phases. Brookite and anatase crystalline, above 600°C, experience a phase transition and convert into the rutile phase [28, 29]. The anatase phase contains zigzag chains of octahedral molecules linked to each other, while the rutile consists of linear chains of opposite edge-shared octahedral structure [29–32]. Generally, the anatase-to-rutile phase transformation occurs between 600–700°C, but, for certain applications, it is required that TiO2 anatase be stable at 900°C [31]. Generally, the anatase TiO2 nanoparticles are stabilized by the addition of cations [32].
The synthesis techniques of TiO2 usually require high temperatures to crystallize the amorphous material into one of the phases of TiO2, such as brookite, anatase, and rutile, consequently leading to larger particles and typically nonporous materials [33–35]. Recently, low temperature synthesis methods resulted in crystalline TiO2 with a higher degree of control over the formed polymorph and its intra- or interparticle porosities [32]. There are reports on the formation of crystalline nanoscale TiO2 particle via solution based approach without thermal treatment with special focus on the resulting polymorphs, surface area, particle dimensions, and crystal morphology [34]. There are exceptional emphases on the sol-gel method via glycosylated precursor and also the miniemulsion method [30–32].
TiO2, due to its nontoxicity, long-term photo stability, and high effectiveness, has been widely utilized in mineralizing toxic and nonbiodegradable environmental contaminants. TiO2 possesses good mechanical resistance and stabilities in acidic and oxidative environments. These properties make TiO2 a prime candidate for heterogeneous catalyst support.
2.1. TiO2: As a Heterogeneous Catalysis
It has been demonstrated that TiO2 improve the performance of catalysts [35–39], allowing the modulation of catalytic activities for many reactions, including dehydrogenation [38, 39], hydrodesulphurization [37], water gas shift [36], and thermal catalytic decomposition [35].
There are also some obvious drawbacks in using TiO2 as a heterogeneous catalysis. The limitations included small specific surface areas, low quantum efficiency, and low adsorption abilities [36, 37]. Furthermore, both costs and difficulties in the separation of catalyst from the reaction media and inadequacy for continuous processing limited the applications of TiO2 as a heterogeneous catalyst in large-scale industries [38]. Despite these drawbacks, a number of studies have focused on catalytic reaction with TiO2 as a support material. The mesoporous TiO2 of pure anatase phase with sharp pore distribution and large surface area was synthesized to increase the degree of distribution and homogeneity of immobilized catalyst [39, 40]. The influences of TiO2 support on heterogeneous catalysts affect electronic effects and bifunctional mechanism [41]. TiO2, as a catalyst support, enforces an electronic effect where the hypo-d-electronic Ti3+ promotes electrocatalytic features of hyper-d-electronic noble catalyst surface atoms [42]. This, in turn, decreases the adsorption energy of CO intermediates, while enhancing the mobility of CO groups. At the same time, the adsorption of OH species on TiO2 tends to facilitate the conversion of the catalytically toxic CO intermediates in CO2, thus improving the durability of the heterogeneous catalyst [43, 44]. Both factors indirectly assist the dispersion and anchor of the heterogeneous catalyst particle [44]. Further improvement in the catalytic stability and activity of the heterogeneous catalysts involves modifying the TiO2 support material with semiconductor metal oxides.
3. TiO2: As Support in Heterogeneous Catalysis
Among different material candidates such as nitrides, perovskites, and carbides, TiO2 based catalyst support materials are known to have excellent properties [44], due to TiO2 nanoparticles high chemical and thermal stability. TiO2 based catalyst supports have outstanding resistance towards corrosion in different electrolytic media. TiO2 can be regarded as a support for heterogeneous catalysts which guarantees stability in electrochemical environment and commercial availability [45]. Meanwhile, strong interactions between the catalytic particles and mesoporous TiO2 have been recorded, which, in the end, resulted in both improved catalytic stability and activity. TiO2 as a catalyst support material also indicated a certain degree of proton conductivity, which may potentially enhance the regime of the triple phase boundary for catalytic reactions [44–46]. In general, the advantages and disadvantages of the other heterogeneous catalyst system are listed in Table 1.
Table 1.
List of advantages and disadvantages of heterogeneous catalysis system.
| Type of catalysis support | Advantages | Disadvantages |
|---|---|---|
| Organic polymer | (i) Easy and versatile functionalization, especially for the polymer containing aryl group (ii) Hydrocarbon polymers are chemically inert-support does not interfere with catalytic groups (iii) It can be prepared with a width range of physical properties (porosity, surface area, and solution characteristics) |
(i) It has poor heat transfer ability (ii) It has poor mechanical properties which prevent from the pulverization during stirring process in reactor (iii) Commercial polymers are not always very defined and often contain unknown impurities (iv) Physical properties vary widely depending on molecular weight and chemical nature |
|
| ||
| Metal | (i) It can induce some catalytic activity as homogeneous catalyst but more selectivity (ii) It is easy to separate from the product (iii) It gives rise to less corrosion (iv) It can be used for long periods without sign of deterioration in properties |
(i) Optimization of the reaction condition is more complex because there are more variables (ii) leaching problem brought by the Van de Waals link between the catalyst |
|
| ||
| Carbon | (i) It has high surface area due to porous structure (ii) It has relatively small amount of chemically bonded heteroatoms (mainly O2 and H2) |
It could not be used for hydrogenation reaction >700 K or in the presence of O2 > 500 K because it may become gasified to yield methane and CO2, respectively |
|
| ||
| Dendrimer | (i) It coordinates strongly with metal catalyst (ii) It leads to recyclable catalyst system and does not suffer from mass transfer limitation (iii) It has well-defined macromolecular structure to precisely control catalyst support (iv) Uniform distribution |
(i) It suffers from diminished activity due to the reduction in accessibility accessibility (ii) It depends on swelling properties influenced by catalytic performance |
3.1. TiO2: As Support in Metal Heterogeneous Catalysis
The study of metal nanoparticle on TiO2 support is important in heterogeneous catalysis due to the size and nature of the interaction of a metal nanoparticle with TiO2 support [45]. This interaction strongly influences the determination of catalytic activity and selectivity of the metal heterogeneous catalyst [46]. Reduction and oxidation at elevated temperature are compulsory steps in the preparation of metal supported TiO2 heterogeneous catalysts [47, 48]. However, both treatments caused morphological changes to the dispersed metal nanoparticles from the sintering of TiO2. Therefore, it is important that the optimal conditions for catalyst supported TiO2 preparation be optimized, both in terms of pretreatment and activation [48, 49]. Besides, depending on the particular metal heterogeneous catalyst, different morphological changes will result from metal-TiO2 support interaction [50–52], such as sintering [50], alloy formation [52] encapsulation, and interdiffusion [51].
Among the TiO2 modifications, anatase is frequently utilized as a catalyst support for metal heterogeneous catalyst due to its high specific surface area and strong interaction with metal nanoparticles [37, 40]. There are only a few studies reporting a rutile catalyst support which resulted in higher catalytic activity compared to anatase, such as the oxidation of toluene, xylene, and benzene over rutile-supported Cu catalyst. In comparison, rutile is preferred as a model support for particles of metals in surface science studies [53–55], due to its high crystal phase's thermodynamic stability. Furthermore, it is indicated that rutile and anatase differ noticeably in their ability of fixing particles of metals onto their respective surface [49, 55]; whereas the strong metal support interaction is normally shown on anatase, this effect is not as significant on rutile. Inopportunely, the thermodynamic stability of TiO2 is comparatively low, and calcination would usually lead to the collapse of the porous structures [54]. Additionally, it is reported that calcination above 465°C has always resulted in the phase transition from anatase to rutile [36]. The phase transition could be connected to the growth of crystal size, which results in a severe reduction in specific surface area [35]. Consequently, this should also influence the overall catalytic performance of metal heterogeneous catalysts.
3.1.1. Au/TiO2 Heterogeneous Catalyst
Gold (Au) is an excellent catalyst for the oxidation of alcohol by molecular O2 in the liquid phase with high activity, selectivity, and promising resistance to deactivation [56–58]. The catalytic performance of Au heterogeneous catalyst is mainly determined via the particle size and properties of the support [57]. Amongst all catalyst supports, TiO2 was determined to be a good support for the Au heterogeneous catalyst system due to the strong interaction in metal support, chemical stability, and acid-base properties [44, 46].
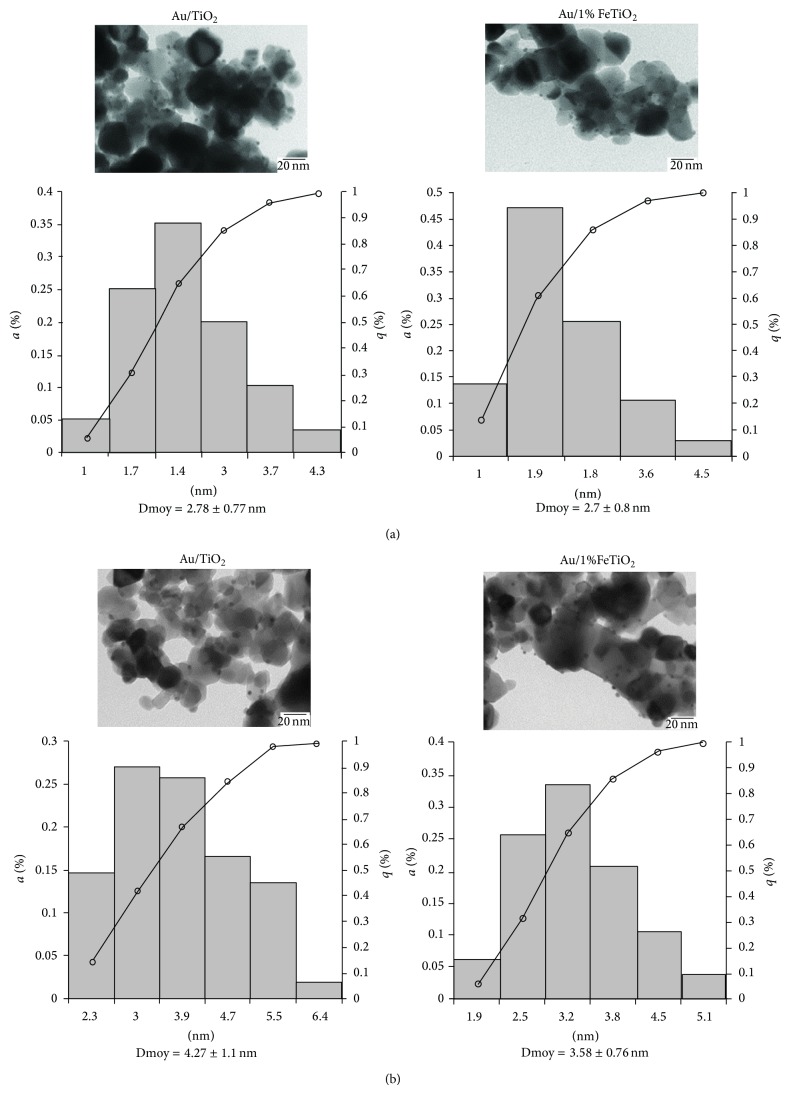
Au-supported TiO2 nanoparticle has been prepared by changing the several different synthesis parameters, including precipitation pH, drying pretreatment, Au-cluster size/morphology, catalyst conditions, the nature of the support, acid-base treatment of TiO2 support, loading of TiO2 nanoparticle, incorporation of impurities, CO adsorption, catalytic reaction conditions, and chemical/electronic state in catalysts [53, 58]. The valence band of Au/TiO2 indicates the presence of the Au 5d band, with a significant contribution to the O 2p nonbonding states, which probably is derived from TiO2 structure [46]. Therefore, the morphology images showed an interaction among the particles of Au with TiO2 support affecting the Ti–O bonds at the surface, which leads to a lower Ti 2p binding energy in an intact Au/TiO2 compared to native TiO2 (Figure 1) [59–62]. Generally, Au nanoparticles, as a catalyst, have a negative charge [57]. This is due to the electron transfer from oxygen vacancies of the TiO2 acting as a catalyst support [58].
Figure 1.
TEM analysis of gold supported on TiO2 and 1% FeTiO2 before (a) and after (b) thermal treatment [59].
The transformation was clearly observed in the case of acetate to ketenylidene reaction [60] (Figure 2). Thus, the charge density and level binding energy of Au is 0.15–0.45 eV lower than that in pure Au [56]. It is concluded that the presence of TiO2 as a support in Au is necessary for the crystallization of the support. This, in turn, reduces the number of Ti–OH functions to be proportional to the deposition of Au [60].
Figure 2.
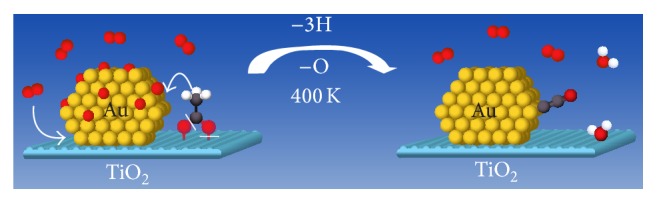
This shows the conversion of acetate to ketenylidene at the perimeter site of the Au/TiO2 catalyst. The acetate, which adsorbs on TiO2, undergoes dehydrogenation (oxidation) and the deoxygenation to form ketenylidene on the gold [60].
The aforementioned theory brought about the next advantage of Au/TiO2 hybrid catalyst. The incorporation of Au is believed to prevent radiation-induced changes in the Au/TiO2 heterogeneous catalyst composition, particularly for as—synthesized and dried samples [63, 64]. For example, X-ray irradiation limits a detectable further reduction in the Au/TiO2 due to the presence of Au3+ state and binding energy of the Au itself [57]. The binding energy and half-width of the Au 4f7/2 spectra of Au particles deposited on the TiO2 in a range of concentrations were sensitive to irradiation. Indeed, the local heating mechanism occurred at higher X-ray concentrations [65]. Exposure of Au/TiO2 heterogeneous catalyst to X-ray irradiation induces the breaking of Ti–O bonds, Ti4+ state reduction, and the O2 desorption from the surface layer [64–66]. This is demonstrated by an increase in the fraction of Ti3+ species, a nonmonotonic diminution of the O2 −/Ti4+ atomic ratio, improvement of the valence band, and the resultant variation of core binding energy [65]. Even though Ti 2p and O 1s binding energies in TiO2 remain almost unaffected under extended X-ray irradiation, in Au/TiO2, a gradual increase in the Ti 2p binding energy is observed [67]. Thus, the effect of X-ray irradiation of Au/TiO2 is relatively smaller than Au due to the direct evidence for charge transfer processes triggered by the production of O2 vacancies in TiO2 as a catalyst support [63, 64].
Many scientists have documented that Au/TiO2, as a heterogeneous catalyst, showed higher reaction activities for the oxidation of primary alcohols to carboxylic acid compared to Au/zeolite [68]. The comparative study of CO oxidation reaction and deactivation behavior between the mesoporous Au/TiO2 and Au demonstrated that Au/TiO2 has greater catalytic selectivity and activity due to its higher surface area. Another study found that the deposition of Au into TiO2 led to the highly active heterogeneous catalyst for the oxidation of methanol [69–71]. The catalytic performance was determined to increase with Au loading, provided that the deposited particle size remained unchanged [70–72]. In this case, the methoxy species bound to the oxide surface are reasonable reaction intermediates, with the formation of significant bound on TiO2 [71]. The catalytic performance was correlated with the number of Au atoms. Thus, the methanol oxidation occurs at the interface with O2 being activated at Au nanoparticle and the oxide acting as a reservoir of methoxy species (Figure 3) [73–75].
Figure 3.
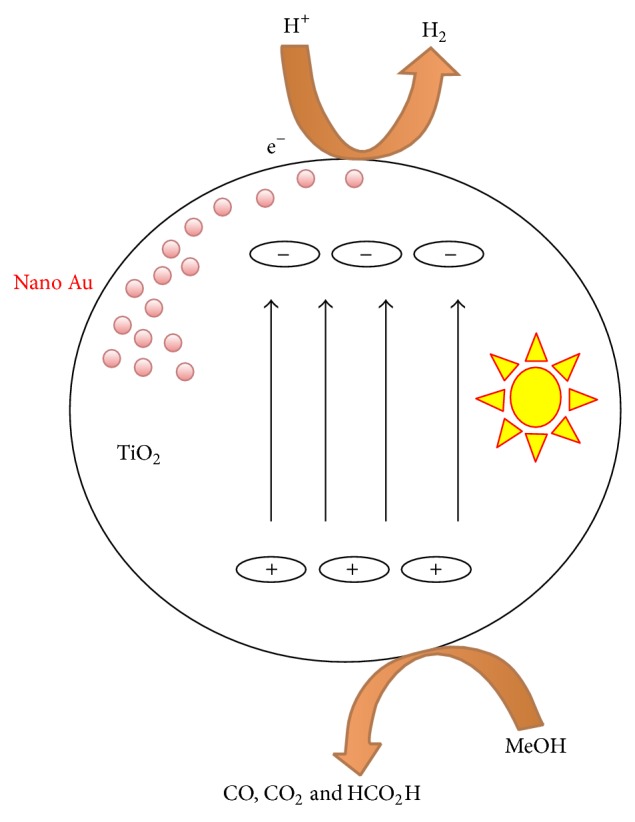
Au/TiO2 catalysts, easily prepared in situ from different Au precursors and TiO2, generate H2 from H2O/alcohol mixtures [73].
Furthermore, there are several reports on the catalytic application of Au/TiO2 on the removal of CO and NOx. Most of these reports investigated the catalytic and photocatalytic of Au/TiO2 in the gas and liquid phase reactions, as well as their reaction mechanisms [74]. In other studies a higher stability was observed for Au/TiO2 heterogeneous catalyst compared to Au/Co3O4, which indicates a comparatively fast deactivation [70, 72]. This is supported by the aforementioned report; a significant enhancement in the stability of Au particles against calcination can be realized by utilizing TiO2 as a catalyst support [71]. With these promising properties, Au/TiO2 catalyst was also found to catalyze the epoxidation of propylene, hydrogenation reaction, water-gas shift reaction, and the oxidation of alkanes [68–76].
Meanwhile, the porosity and phase transformation are other factors that affect the catalytic performance of Au/TiO2 as a heterogeneous catalyst [77, 78]. It is estimated that various TiO2 crystalline phases, as a catalyst support, could affect the interaction of support-metal, electronic density, oxidation state, Au-size, and Au dispersion of deposit material in heterogeneous catalyst system. In a comparative study of the activity of porous and nonporous Au/TiO2, heterogeneous catalyst prepared from different crystalline phase of TiO2 included the anatase, rutile, and rutile+anatase [69, 79]. For example, certain studies found that the activity of nonporous Au/TiO2 heterogeneous catalyst decreased from brookite via anatase to rutile for catalyst activated by calcination at 300°C, while, after reduction at 150°C, the activities became rather comparable [80, 81]. Similar observation was reported by other studies for mesoporous TiO2 acting as support for Au heterogeneous catalyst [82]. The tendency in the increment for Au particle sintering during calcination at 300°C is brookite, anatase, and rutile, followed by mesoporous. In contrast, such effect is insignificant at the reduction of 150°C [82]. Some studies reported that the crystalline phase of TiO2 influences also the deactivation of Au/TiO2 heterogeneous catalyst with comparable surface area of 46–54 m2 g−1, increased in the order of Au/TiO2 anatase and Au/TiO2 nonporous rutile/anatase and followed by Au/TiO2 rutile [83]. Contrarily, some studies concluded that the crystalline phase has no significant influence on the catalytic performance of the unconditioned Au/TiO2 heterogeneous catalyst [84]. This was different, as the Au/TiO2 heterogeneous catalyst was calcined prior to the reaction, where the Au/TiO2 rutile+anatase heterogeneous catalyst indicated a significantly higher stability than Au/TiO2 rutile and Au/TiO2 anatase [85]. After calcination at 250°C, the activities are comparable to those of the unconditioned heterogeneous catalyst and are similar for all catalysts. However, calcination at 350°C leads to lower activities for the mesoporous rutile or anatase, while the rutile+anatase supported Au retains its respective activities [83]. The catalytic performance of Au-supported TiO2 anatase being superior to other heterogeneous catalyst was examined for the decrease of NOx using propane due to the smaller size of the Au particles on TiO2 anatase [86, 87].
Although Au/TiO2 is active as a heterogeneous catalyst, it often complied with quick deactivate process which limits its commercial applications [70–74]. In general, in order for the Au particle to indicate higher performance at near subambient temperatures, its cluster size has to be less than 5 nm [65]. Therefore, some attempt has to be made to synthesize Au hydroxide [AuOx(OH)4−2x]n deposited on the TiO2 support, followed by drying in the air. The idea is to prevent the in situ formation and the agglomeration of metallic Au nanoparticles, as seen with coprecipitation [64]. Another approach is by synthesizing nearly monodispersed thiol-protected Au nanoparticles and is deposited into TiO2 as a support. In this case, the thiol ligands were utilized to control the size of the cluster [82, 88]. The advantages offered by both methods included a better control size of Au nanoparticle due to ex situ synthesis and the formation of Au cluster in the solution prior to the deposition on the TiO2 as a catalyst support [88].
In conclusion, the most common factors affecting the Au/TiO2 heterogeneous catalyst activity are the size of the Au nanoparticles, preparation method, pretreatment method, Au loading, the oxidation state of Au, and the binding strength to TiO2 as a catalyst support.
3.1.2. Co/TiO2 Heterogeneous Catalyst
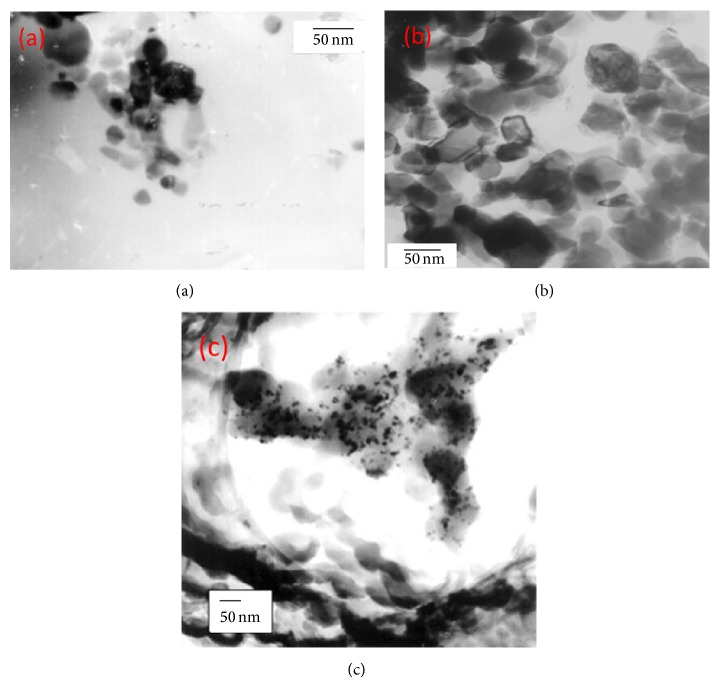
The activity of cobalt supported TiO2 (Co/TiO2) heterogeneous catalyst is greatly related to the TiO2 crystal phase and the loading of Co3+ ions on the catalyst support [89–91]. The case of Co/TiO2, TiO2 in the rutile phase is more appropriate as a catalyst support material than those with a 100% anatase phase [25, 90]. Therefore, Co/TiO2 heterogeneous catalyst synthesized with more than 15% rutile phase is shown to have 4 times higher CO conversion rate than those that only consist of the anatase phase [92, 93]. Furthermore, the Co/TiO2, when modified with alkaline earth metals, resulted in a greater CO conversion rate, whereas modification with Mn and V resulted in high C5+ and low methane selectivity, respectively [94–96]. Furthermore, with only 0.8% Ca modification, Co/TiO2 obtained the highest CO conversion rate and site-time yield of C5+ products. The conversion rate is 1.3–1.5 times higher than cosupported SiO2 catalyst (Figure 4) [97]. Therefore, the CO conversion reaction rate over Co/TiO2 catalyst was proportionately amplified by increasing the surface area of Co [93].
Figure 4.
(a) TEM micrograph of Co/TiO2 (673)-I catalyst after reduction-oxidation-reduction cycle at 773-623-623 K. (b) TEM micrograph of Co/TiO2 (973)-I catalyst after reduction-oxidation-reduction cycle at 773-623-773. (c) TEM micrograph of Co/TiO2 (673)-SG catalyst after reduction-oxidation-reduction cycle at 773-623-773 K [97].
Actually, the production of highly dispersed Co on TiO2 as a catalyst support requires a strong interaction [91, 92]. Nevertheless, too strong of an interaction generates the TiO2 compound as a suboxide at an interface, which is highly resistant to reduction [89]. In this case, while it has known that the dominant surface sites of TiO2 support consist of two main sites, which are Ti3+ and Ti4+, the surface site's effect on the formation of Co to TiO2 interface indicates that the reaction should be structured to be insensitive and based on the number of the exposed Co metal sites [93–99]. It is also proposed that this growth in the reaction rate might be due to the strong Co to TiO2 interaction altering the catalytic properties [100].
3.1.3. Ni/TiO2 Heterogeneous Catalyst
Nickel (Ni) supported TiO2 (Ni/TiO2), as a heterogeneous catalyst, is another kind of important Ni-based catalyst. This is due to the strong interaction between Ni as a metal and TiO2 as a catalyst support [101–103]. For example, the catalytic properties of supported Ni/TiO2 heterogeneous catalysts prepared by the incipient wetness impregnation method were evaluated for the vapor phase hydrogenation of maleic anhydride [104]. It was discovered that the hydrogenation process of maleic anhydride is strongly affected by the calcination temperature of Ni/TiO2. The Ni/TiO2 heterogeneous samples recorded an optimum catalytic performance with 96% maleic anhydride conversion as the calcination temperature reached 1023 K [104, 105]. This is attributed to the change of surface properties of TiO2 support to the increase of calcination temperature. In addition, the strong metal support interaction between Ni and rutile surface over Ni/TiO2 catalyst was the key reason for the better activity of the catalyst in acetophenone hydrogenation [105]. However, the deactivation of Ni/TiO2 heterogeneous catalyst occurred as the carbonaceous species of maleic anhydride was deposited onto the Ni surface [106, 107]. To regenerate the catalytic performance of Ni/TiO2, thermal treatment in the oxidant atmosphere approach is applied.
3.1.4. Pd/TiO2 Heterogeneous Catalyst
Anatase TiO2 effectively engenders OH species. Thus, palladium-supported TiO2 (Pd/TiO2) anatase possesses excellent catalytic activity vis-à-vis methanol electrooxidation. There are some reports claiming that Pd-supported TiO2 anatase heterogeneous catalyst demonstrated a greater activity than TiO2 rutile in selective hydrogenation reactions due to the superior metal supporting the behavior of TiO2 anatase [108–112]. Consequently, negligible CO intermediates are produced in direct formic acid fuel cells and formic acid electrooxidation, which consequently possess striking catalytic activity. In contradiction, the acetoxylation of toluene study indicates that Pd supported by rutile TiO2 has greater selectivity of almost 100% without any deactivation of the catalyst compared to the ones supported by anatase [110–113], due to the high thermal stability of rutile. Meanwhile, the treatment of Pd/TiO2 anatase heterogeneous catalyst by an H2 reduction at 200°C resulted in greater reactivity in hydrogenation of alkadienes than that of the Pd/TiO2 in rutile phase [112]. The strong metal support interaction was only observed in the anatase phase of supported Pd heterogeneous catalyst [109, 110]. There is also an attempt to synthesize the genesis of the Pd cluster on TiO2-grafted SiO2. The results showed that TiO2 anchors Pd particles during air calcination and maintains its small ensemble during H2 reduction [114, 115]. These observations serve to demonstrate how TiO2, as a catalyst support, influences the structure and catalytic performance, specifically for sulfur-resistant catalysts [114]. It was also concluded that TiO2-grafted SiO2 offers better support due to the presence of high surface area and thermal stability for Pd heterogeneous catalyst compared to pure TiO2 [116–118].
3.1.5. Pt/TiO2 Heterogeneous Catalyst
The dispersion and loading of platinum (Pt) nanoparticles onto TiO2 support is controlled by the structure and porosity of TiO2. Consequently, a suitable combination of passable electronic conductivity and nanostructured morphology with controlled porosity could result in very promising Pt/TiO2 heterogeneous catalysts [119–124]. For example, a novel electrocatalyst based on mesoporous TiO2 supported Pt nanoparticles indicates a high stability under accelerated stress test conditions and activity compared to commercial Pt-supported carbon catalyst [121, 122]. Some studies have investigated the influence of the reductive treatment on structural properties of Pt/TiO2, with their catalytic activity for formaldehyde oxidation [123]. It is claimed that the enhanced catalytic performance is reflected by a uniform dispersion of Pt nanoparticles and the interaction between Pt and TiO2 [124]. Thus, the application of Pt as a heterogeneous catalyst becomes more encouraging, especially in electrochemical and photoelectrochemical context [125, 126]. For example, H2 production could be significantly improved by photocatalytic water splitting over Pt/TiO2 nanosheets compared to native Pt [126].
Several methods have applied in the preparation of Pt nanoparticle deposited on TiO2 substrates included underpotential deposition [127–129], hydrothermal treatment [130, 131], photoassisted reduction [132], and vacuum deposition [133, 134]. Electrodeposition would be a straightforward approach for the synthesis of Pt-supported TiO2, which is highly required for the production of complex electrode architectures in fuel cells [132–134]. This in turn resulted in the H2 evolution rate of 3 wt% Pt/TiO2, which was relatively greater than that of 3 wt% Pt/Al2O3. Other studies found that TiO2, as a support material, improves Pt O2 reduction activity by assisting mechanisms such as reactant surface diffusion and O2 spillover [135–137]. Furthermore, Pt/TiO2 showed similar performance to Pt/C in H2-fuel cell operated at 60°C and 0.8 V (Figure 5) [136].
Figure 5.
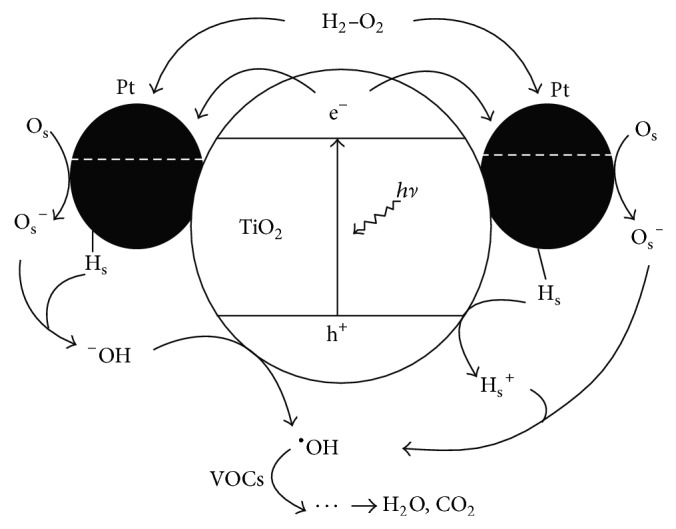
Proposed mechanism for the photochemical generation of dOH radicals on a Pt/TiO2 catalyst in the coexistence of H2 and O2 [136].
Certain studies on the effect of particle size indicated that amorphous TiO2 could powerfully suppress the O2 reduction reaction activity of supported Pt at smaller sizes [138–140]. For example, a series of Pt/TiO2 heterogeneous catalyst with various Pt particle sizes was prepared and tested for low temperature CO oxidation [139]. The result indicated that the Pt/TiO2 heterogeneous catalyst that resulted in superior activity at the weight percent of Pt in Pt/TiO2 heterogeneous catalyst was 5.0%, with the complete conversion temperature being 120°C [141–143].
3.2. TiO2: As Support in Metal Oxide Heterogeneous Catalysis
Metal oxides signify one of the important and broadly used categories of heterogeneous catalysts. Metal oxides are utilized for both their redox and their acid-base properties and constitute the main family of catalyst in the heterogeneous category. Furthermore, certain metal salts and organometallic compounds using a heterogeneous catalyst precursor have the tendency to be decomposed via exposure to light irradiation. Some reports show that, as the oxide-containing catalyst under light irradiation analysis needs great care, considerable damage is possible even at low irradiation dose. For catalytic applications, metal oxides have also been employed for various applications such as gas sensor, the photocatalyst thin film, and fuel cells. TiO2 is often used to modify the supported metal oxide heterogeneous catalyst due to properties such as reducible surface and possible electron transfer via the spontaneous alignment of the Fermi levels.
3.2.1. CuO/TiO2 Heterogeneous Catalyst
It has been reported that copper oxide (CuO) heterogeneous catalyst is highly active for CO oxidation and lower in cost compared to other noble metal heterogeneous catalysts [144–147]. CuO has additional advantages, such as a high thermal stability and the fact that it is economical [145–149]. The highest activities of CuO heterogeneous catalyst are attributed to the synergy between the Cu species and the support, especially in the case of TiO2 as support material. For example, the increment of activity for CO oxidation over the CuO/TiO2 heterogeneous catalyst is attributed to the sites for CO chemisorption that is responsible for O2 activation [150, 151]. Furthermore, since CO oxidation over the supported metal heterogeneous catalyst takes place at the metal support interface, TiO2, as reducible oxide, could provide the intrinsic activity to the entire reaction [149]. Therefore, only a small amount of CuO is loaded onto TiO2 and influence of TiO2 crystalline phase, which are indicative of the fact that the support system has higher efficiency and selectivity than the CO oxidation being examined [152].
On the other hand, CuO/TiO2 heterogeneous catalyst is also found suitable for NO reductions [150]. Rutile TiO2 is the most stable phase compared to anatase and brookite, and Cu-based heterogeneous catalysts show promising activity towards NO reduction [151]. Therefore, it is necessary and important to further approach the change of heterogeneous catalyst surface with plasma-assisted processing. NO reduction by CO reaction was comparatively studied over microwave plasma pretreated CuO/TiO2 heterogeneous catalysts employing transmission electron microscopy, H2 temperature programmed reduction, and in situ Fourier transform infrared spectroscopy [152–154]. The CuO/TiO2 heterogeneous catalytic performances indicated that a remarkable improvement in activity and selectivity was achieved after microwave plasma pretreatment, which depends on the microwave plasma pretreatment time [153]. The results also suggested that high active oxygen species are formed on the surface of plasma pretreated CuO/TiO2 heterogeneous catalysts, which led to the easy oxidation of NO to NO2 at low temperatures even without the introduction of any additional O2 gas. Therefore, these high active oxygen species should play an important role in the exaltation of the catalytic performances of the CuO/TiO2 heterogeneous catalysts [155]. It is found that CuO supported on the anatase phase of TiO2-support calcined at 400°C demonstrated better catalytic activity than those supported on TiO2 calcined at 500 or 700°C. Among all of the investigated heterogeneous catalysts with CuO loading from 2% to 12%, the CuO/TiO2 heterogeneous catalyst with 8 wt% CuO loading exhibited the highest catalytic activity [156]. The optimum calcination temperature of the CuO/TiO2 heterogeneous catalysts was recorded at 300°C.
CuO/TiO2 is also known as a promising heterogeneous catalyst in the photocatalytic applications. Whilst it is widely recognized that CuO facilitates charge separation and acts as a H2O reduction site, conjecture still exists as to the exact nature of the dispersed CuO, specifically on TiO2 and the optimum CuO loading for efficient H2 production [157, 158]. The surface chemistry of the CuO/TiO2 heterogeneous catalyst is the subject of a number of investigations to explain the excellent activity of the photocatalysts. It is generally recognized that CuO exists in several different forms on TiO2, with the specification depending on the CuO content and catalyst pretreatment conditions [159]. Experimental evidence suggested that, at low CuO loadings, Cu2+ is highly dispersed on TiO2 as a surface complex or CuO monolayer. This, in turn, can easily reduce the CO or H2 at low temperatures [150]. For example, CuO/TiO2 photocatalyst is very active in the reduction of water under sacrificial conditions (95 vol.% H2O, 5 vol.% CH3OH) and UV excitation, with the optimal CuO loading of 5 to 10 wt% of the heterogeneous catalyst system [153]. The high catalytic activity caused photo-citation of electrons in the conduction bands of both CuO and TiO2, followed by the migration of the conduction band electrons in TiO2 into the conduction band of CuO heterogeneous catalyst [155, 156]. The accumulation of excess electrons in the conduction band of CuO caused a negative shift in the Fermi level of CuO to give the required overvoltage necessary for H2O reduction [154]. However, once the CuO loading exceeds the dispersion capacity, nanocrystalline of the CuO begins to form. CuO is itself inactive for H2 production from water or alcohol-water mixtures under UV or visible irradiation, since the conduction band of CuO is more positive than the H2O/H2 redox potential [160–162]. Accordingly, the onset of the CuO nanoparticle formation should coincide with a decrease in the photocatalytic activity of CuO/TiO2 photocatalysts for H2 production, as the presence of inert CuO nanoparticles will reduce the number of surface sites on TiO2 available for photoreactions [161, 162]. Experimental evidence to support this hypothesis is currently lacking, which motivates the present investigation.
CuO/TiO2 heterogeneous catalyst could be synthesized by a deposition-precipitation method. For example, CuO has been deposited on TiO2 nanotubes with Cu/Ti atom ratio of 10 and then evaluated by H2 generation activity in methanol H2O mixtures under UV excitation. It is reported that the optimal CuO loading is around 1.3 wt%, giving the H2 production rate of 2061 μmol g−1 h−1[161]. Meanwhile, the CuO/TiO2 heterogeneous catalyst prepared by a complex precipitation method indicates the highest H2 production observed in 10 wt% of the CuO loading [162]. Another study evaluated the performance of 2.5 wt% CuO-TiO2 photocatalyst for H2 production from methanol and found that 27–29% of H2 evolution at rate of 1350 μmol g−1 h−1 is produced for ethanol-H2O mixture of 900–1000 g−1 h−1 [158, 159]. It was recorded that 1 wt% CuO/TiO2 photocatalyst is active for H2O splitting under visible light in the presence of triethanolamine.
3.2.2. V2O5/TiO2 Heterogeneous Catalyst
Initially, vanadium oxide (V2O5) supported TiO2 heterogeneous catalyst was applied for the o-xylene oxidation to phthalic anhydride reaction. Then, the potential of V2O5/TiO2 was recorded in the pollution abatement of NO with NH3 process [163, 164]. Thus, V2O5/TiO2 is one of the most efficient catalysts in the oxidative dehydrogenation of propane, with only a few byproducts [165]. A stable deposit of TiO2 in V2O5/TiO2 heterogeneous catalyst system was established with dip coating of V2O5 in an aqueous suspension of titanium isopropoxide [166, 167].
Furthermore, V2O5/TiO2 heterogeneous catalyst found another promising application in the dehydrogenation of alkanes and selective oxidation of alcohols/alkanes [168]. In this respect, the activity and product distribution of the heterogeneous catalyst for methanol oxidation strongly depend on its surface acidity and redox ability, and this can be regulated through proper modifications [169]. The heterogeneous catalyst of V2O5 supported on TiO2 exhibited high conversion of methanol and selectivity of dimethylmethane for the oxidation of methanol under mild conditions [170]. The catalytic oxidation of V2O5/TiO2 heterogeneous catalyst was investigated for 1,2-dichlorobenzene [171], and it was surmised that the catalytic oxidation of 1,2-dichlorobenzene on chemical vapor condensation which prepared V2O5/TiO2 heterogeneous catalyst demonstrated excellent performance at lower temperatures [171]. The surface structure of TiO2 allows the development of the Lewis acidity, as well as redox properties. Consequently, redox properties TiO2 modified by the presence of V2O5 lead to an electronic interaction between this support and V2O5 species [169]. V2O5 loading is a key point for the activity, and it has been suggested that a single redox surface site participates in the kinetically significant steps, with the formation of crystalline V2O5 being detrimental to oxidation activity [168, 172].
The effect on surface acidity and redox ability of V2O5/TiO2 is also found to be interesting for the selective catalytic reduction by NH3. The proposed mechanism suggested that NH3 is activated and reacts from a strongly adsorbed state with gaseous or weakly adsorbed NO [173–175]. It is believed that reliable structure-activity relationship is based on the understanding of the V2O5/TiO2 heterogeneous catalyst molecular structure under operating conditions [174]. Thus, a dual-site mechanism involving a surface V2O5 redox site and an adjacent nonreducible site appears to be more favorable. Furthermore, the reported influence of specific oxide supports, along with the observed stability of terminal V=18O bonds during NH3 reaction, suggests that the V–O-support bond is involved in the rate-determining step [167–169]. Similarly, the NH3 action of transition for V2O5/TiO2 heterogeneous catalyst correlates well with the extent of interactions between the active phase and the support [172]. The Raman studies of V2O5/TiO2 heterogeneous catalyst revealed that the presence of multiple structures of surface V2O5 species on TiO2, including monomeric and polymeric V2O5 species at submonolayer coverage [175, 176]. Therefore, Raman studies of V2O5/TiO2 heterogeneous catalysts under SCR or reducing (NH3) conditions are scarce.
The influence on preparation procedure towards surface of V2O5 state plays an important role in selective catalytic reduction. Some studies have investigated the surface of V2O5 prepared by incipient wetness impregnation, with TiO2 being a support, and found that both isolated and polymeric surface V2O5 species existed with medium VO surface coverage [177–179]. This is supported by other studies mentioning that polymeric V2O5 species demonstrated greater catalytic activity than monomeric V2O5 [178]. This is due to the greater mobility of lattice O2 atoms resultant experience faster reduction and reoxidation by gaseous O2 [179, 180]. Furthermore, the redox properties of V2O5 are modified by the incorporation of TiO2, leading to an electronic interaction between TiO2 and V2O5. There are some reports discussing the fact that the addition of molybdenum oxides (MoO2) species to V2O5/TiO2 heterogeneous catalyst causes dramatic changes in the 1,2-dichlorobenzene oxidation, most importantly, by improving the catalytic activity [181, 182].
3.2.3. MnO/TiO2 Heterogeneous Catalyst
Manganese oxides (MnO), containing several types of labile oxygen, which are necessary to complete the catalytic cycle, have relatively high activity, but their optimal temperature (above 150°C) is still high [183–185]. For example, the activity and selectivity of pure MnO on NO conversion reached 90% selectivity [184]. To enhance the catalytic activity, MnO are usually supported on TiO2 as a carrier. In this regard, the dispersion of the MnO towards TiO2 support had an important influence on the reaction, since crystalline MnO contributed little to activity [186, 187]. This, in turn, possesses profound surface acid-base properties and provides high surface area, strong mechanical strength, and high thermal stability [188].
Existing research on supported MnO to TiO2 heterogeneous catalyst for NO oxidation demonstrated high catalytic activity [189–192]. For example, a series of MnO/TiO2 heterogeneous catalyst prepared by the deposition-precipitation method and the sample with the Mn/Ti ratio of 0.3 showed a superior activity for NO catalytic oxidation to NO2 [188]. Therefore, the maximum NO conversion over the MnO/TiO2 heterogeneous catalyst could reach 89% in 250°C [189]. Indeed, MnO/TiO2 heterogeneous catalyst showed an interesting development as a highly active heterogeneous catalyst with low pollution for the low temperature NO conversion process [190]. Meanwhile, some studies reported on MnO/TiO2 heterogeneous catalyst being prepared by sol-gel, impregnation, and coprecipitation methods for low-temperature selective catalytic reduction of NO with NH3 [191, 192]. Strong interaction, high concentration of hydroxyl groups, large surface area, and high concentration of amorphous Mn on the surface might be the main reasons for the excellent performance of the catalysts [191].
It was documented that the crystal phase of the TiO2 influences catalysts' activity [193–197]. Therefore, some studies on the effect of the crystalline phase of TiO2 towards the catalytic performance of MnO/TiO2 heterogeneous catalyst were carried out in [194], and it was discovered that compared to anatase and rutile anatase+rutile resulted in better dispersion of MnO on the support surface, suppressed the agglomeration of catalyst particles, and produced more Mn2O3, which is more active for the oxidation of NO [195, 196]. In addition, anatase+rutile enhanced the reduction of MnO, especially for Mn2O3, and the formation of easily desorbed O2− generated from the Mn3+–O bond [197].
3.2.4. RuO2/TiO2 Heterogeneous Catalyst
Ruthenium oxide supported TiO2 (RuO2/TiO2) heterogeneous catalyst found an attraction in the oxidation process [198, 199]. Generally, RuO2/TiO2 heterogeneous catalysts are prepared by the impregnation of Ru salt, followed by calcination, with limited control on the properties of the RuO2 species formation [199]. Furthermore, a recent development of green method to prepare calibrated RuO2 nanoparticles has been developed and analyzed. It is expected that, for this kind of preparation method, the RuO2/TiO2 heterogeneous catalyst exhibits outstanding oxidation activity [200].
3.3. TiO2: As Support in Bimetallic Heterogeneous Catalysis
In this area, the availability, affordability, and lack of toxicity of TiO2 as a robust solid with outstanding photochemical stability make it an attractive support for the bimetallic heterogeneous catalyst. TiO2 is used for its well-known ability to interact with bimetallic through the formation of Ti3+ ions. Generally, any electronic conductivity of TiO2 is due to the presence of Ti3+ ions. There are two ways to create Ti3+ ions in the TiO2 structure. The formation of Ti3+ ions either through the O2 vacancy creation or through shear planes by introducing appropriate donor dopants. It is well known that strong bimetallic support interaction occurred as the bimetallic catalyst was reduced by H2. The H2 reduction over TiO2 supported bimetallic catalyst generates O2 vacancies in the form of coordinate unsaturated cations in the vicinity of active bimetallic, which in the end results in changes in the catalytic activity and stability.
3.3.1. PdNi/TiO2 Heterogeneous Catalyst
Spherical TiO2 nanoparticles were used to synthesize the PdNi-supported TiO2 electrocatalyst for methanol oxidation [201]. It was found that the electrocatalytic activity of PdNi/TiO2 catalyst is much more promising, better than the antipoisoning capability, and comparatively favorable as compared to commercial 31 PtRu-supported carbon [201]. The methanol oxidation mechanism of the PdNi/TiO2 heterogeneous catalyst mainly results from the high catalytic activity of the hybrid system without UV light illumination. Therefore, PdNi/TiO2 catalyst might become a promising candidate for a direct-methanol fuel cell.
3.3.2. AuCu/TiO2 Heterogeneous Catalyst
The AuCu/TiO2 heterogeneous catalyst was studied for several other reactions, including water, gas shift, and total oxidation of methane, ethane, propane, and epoxidation of propane. The incorporation of Au into Cu complements each other in terms of electronic properties, O2 mobility, and surface stability [202, 203]. The interaction of Au and Cu in AuCu/TiO2 bimetallic heterogeneous catalyst was analyzed for methanol oxidation, and it was found that the catalytic activity and selectivity of the bimetallic heterogeneous catalyst system are greater than the one with the monolithic catalyst [202].
3.3.3. CoMn/TiO2 Heterogeneous Catalyst
A series of CoMn/TiO2 heterogeneous catalysts, with a composition range of 2–12 wt% containing 25% Co and 75% Mn, have been prepared by the coimpregnation method [204–206]. The produced CoMn/TiO2 heterogeneous catalyst was tested in Fischer-Tropsch synthesis for the production of C2–C4 olefins [205], and it was discovered that the heterogeneous catalyst containing 8 wt% (CoMn)/TiO2 is the optimal formulation for the production of C2–C4 olefins. It should also be pointed out that the operating conditions, such as the H2/CO molar feed ratio, temperature, Gas Hourly Space Velocity, and total reaction pressure affect the heterogeneous catalytic performance of an optimal catalyst [204].
4. Applications of TiO2 Supported Heterogeneous Catalysis
4.1. Environmental Security: Photocatalysis
Recently, TiO2-supported semiconductor is extensively used to mineralize toxic and nonbiodegradable environmental pollutants due to its high effectiveness, long-term photostability, and nontoxicity [66, 76]. This is also attributed to the limitation of most semiconductors, such as low quantum efficiency, small specific surface area, and low adsorption ability. This in turn limits the efficiency of the photocatalyst. On the other hand, both costly and difficult separation of reaction media and the inadequacy for continuous processing are some of the restrictive factors [110]. As a result, a number of studies have focused on the immobilization of semiconductor materials onto porous TiO2 nanoparticles [156]. This is believed to not only promote photocatalytic reactions by offering more active sites but also allow the recycling and reuse of semiconductor as a heterogeneous catalyst. Some data also reported that porous TiO2 nanoparticle has shown some advantages in the preparation of highly supported catalysts due to its special physicochemical properties, including high adsorption capabilities [66].
Indeed, it is well known that TiO2, with its crystallographic forms, small particle size, and highly porous structure, greatly influence the photocatalytic performance of composite materials [76]. Among metal oxides suitable for photocatalytic processes, TiO2 is the most widely used, due to both its high photocatalytic activity and its chemical/photocorrosion stability in the reaction conditions. TiO2 has increased the photoactivities, due to the photoinduced electron-hole pairs on its surface that can be harvested to increase electron transfer and chemical reactivity. The semiconductor nature of TiO2 has made it possible for the utilization of UV-Visible radiation to harvest the conduction band electrons that are subsequently used to reduce metallic ions onto TiO2's surface [66, 156]. Therefore, the immobilization of semiconductor on a TiO2 nanoparticle can exhibit a higher photodecomposition of organic and inorganic pollutant compared to nonsupported semiconductors [110].
4.2. Chemical Reaction/Conversion
As a versatile metal/metal oxide supported TiO2 heterogeneous catalyst, it is broadly studied in a variety of mild oxidation reactions, such as ethane to acetic acid, ethanol to acetaldehyde, and oxidative dehydrogenation of propane to propylene [8, 9, 16]. For example, various TiO2 supported catalysts, including Au/TiO2, Pd/TiO2, Co/TiO2, and Pt/TiO2, have recently been developed for frequent industrial applications, including the hydrosulfurization of hydrocarbon oils, the epoxidation of propane, and the selective catalytic reduction of NO [36, 43, 46–48]. However, since these aforementioned reactions are powerfully exothermic, it is important to avoid the hot spots that are responsible for structural damages and early deactivation of heterogeneous catalyst [53, 56–59]. Generally, the presence of hot spot leading from products productions included CO2, especially in oxidation reaction [60]. As an alternative, recent studies focused on the 3D structure of metal/metal oxide supported TiO2 with an open structure [63]. The open structure of metal/metal oxide supported TiO2 catalyst favor efficient heat and mass transfers between the gaseous reactants, the catalytic active phase, and the wall of the chemical reactors.
Furthermore, metal oxide supported TiO2 heterogeneous catalysts are commonly used in several industrial important reactions, including selective reduction of NO by NH3 [109, 121]. It should be pointed out that metal oxide supported TiO2 heterogeneous catalyst demonstrated excellent performance in slurry reactions [123, 128]. Indeed, TiO2, utilized as support for metal oxide, attracts considerable interest due to its favorable properties of low pressure drop, thermal shock resistance, chemical durability, low manufacturing cost, and high structural strengths [134–136, 160].
4.2.1. Small Molecules Transformation
Recently, heterogeneous catalyst supported TiO2 for small molecules transformation has received considerable attention because of its simplicity and the advantages, over most other methods of preparing highly pure mixed oxides and a variety of other materials [13]. This included sulfides and phosphates, which are obtained under very similar experimental conditions. The preparation of metal particles with TiO2 supported catalyst is commonly applied because of its mild reducing performance which has a chelating effect, which avoids agglomeration of particles during preparation [57]. The synthesis of mono- or polymetal particles of Co, Ni, Cu, and noble metals in submicrometer and -nanometer size range has been reported and the materials obtained by catalyzed supported of TiO2 show homogenous phase composition, narrow particle distribution, and high specific surface area [203]. For example, polyol-mediated preparation of nanoscale oxides can carry out by dissolving a suitable metal precursor (acetate, alcoholate, and halogenide) in diethylene glycol or other polyalcohol with assisted of heterogeneous catalyst supported TiO2. During this step, the surface of growing particles will be immediately complexed by TiO2 as a catalyst support material, which limit grain growth [49].
Heterogeneous catalyst supported TiO2 also applied for the nitrate to nitrite reduction with bimetallic catalyst. It is expected that H2 molecules produced through the reaction and adsorbed on noble metal subsequently reduces nitrite to harmless N2 gas [207]. A wide range of metal pairs included Au-Pd, Sn-Pd, Ni-Rh, Rh-Cu, and Pd-Cu supported on TiO2 were extensively studied to maximize the efficiency of nitrate reduction to N2 gas. In advances, some studies focus on the effect of pH and zwitterionic buffer on catalytic nitrate reduction by Cu-Pd supported TiO2 and found that the nitrate reduction decreased from 100% to 72% as suspension of pH increased from 6 to 10 of which range was kept by zwitterionic buffers [207] (Figure 6).
Figure 6.
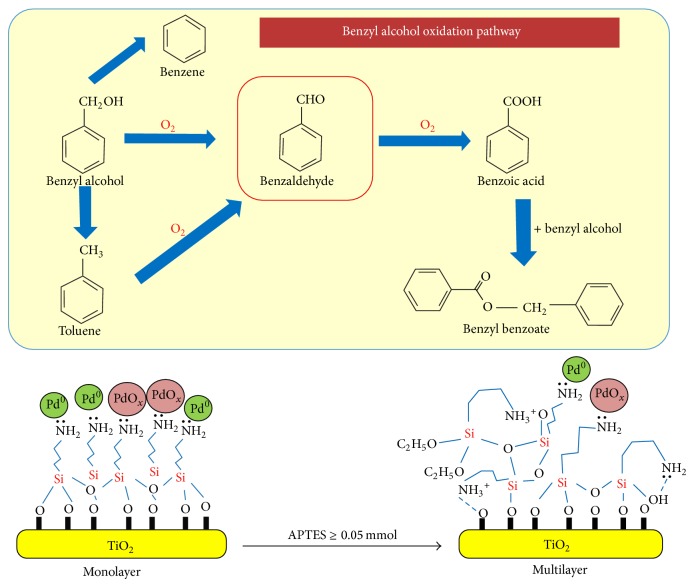
Surface functionalized TiO2 supported Pd catalysts for solvent-free selective oxidation of benzyl alcohol [207].
4.2.2. Organic Synthesis
It also revealed that Pd catalyst supported TiO2 could be functionalized with various amounts of 3-aminopropyltriethoxysilane via a post synthesis grafting method combined with electroless deposition of Pd [108]. The supported catalyst system gave promising catalytic properties in the solvent-free selective oxidative of benzyl alcohol. It correlated well with the highest amount of Pd distribution (with particle size of 3.4 nm) with the 1% of Pd-supported TiO2. Increasing of surface basicity via the hydrolysis of NH2 suggested enhancing the dehydrogenation of benzyl alcohol and, as a consequence, the selectivity towards benzaldehyde [109–111]. In addition, the presence of TiO2 support gave high catalytic activity in benzyl alcohol oxidation, emphasizing that the reduction of PdOx species by the adsorbed benzyl alcohol is an essential step to form highly active metallic PdO sites [112].
4.2.3. Organic Reactions
Another important application of heterogeneous catalyst supported TiO2 derived from the Fisher-Tropsch synthesis, which can convert various carbon sources (coal, natural gas, and biomass) into long chain hydrocarbon via syngas [103]. It is a promising way to produce environmentally benign fuels with no sulfur and nitrogen compounds [116]. In this case, certain transition metals supported with TiO2 have frequently applied as catalyst. Among them, Co/TiO2 is considered as the preferred catalyst due to its high selectivity for long chain linear paraffin, high resistance toward deactivation by water, and low activity for the competitive water gas shift reaction [25]. Several studies indicated that activity of Co catalyst depended on the number of exposed metal sites [90, 93, 95, 96]. Therefore, the Co/TiO2 catalyst system may increase the dispersion of active Co metal species. Meanwhile, TiO2 is suitable for the practical application due to the low cost, safety, and the chemical stability. Furthermore, it is reported that the strength of Co support interaction of Co/TiO2 was in the middle of those on Co/SiO2 and Co/Al2O3 [99, 100]. It found that the activity of the Co/TiO2 catalyst for Fischer-Tropsch synthesis largely depended on the crystal phase of TiO2 support, the reduction degree of Co, and the surface area of Co metal. Rutile TiO2 gives more optimum reduction degree of Co with almost 60% as compared to the anatase [204, 205].
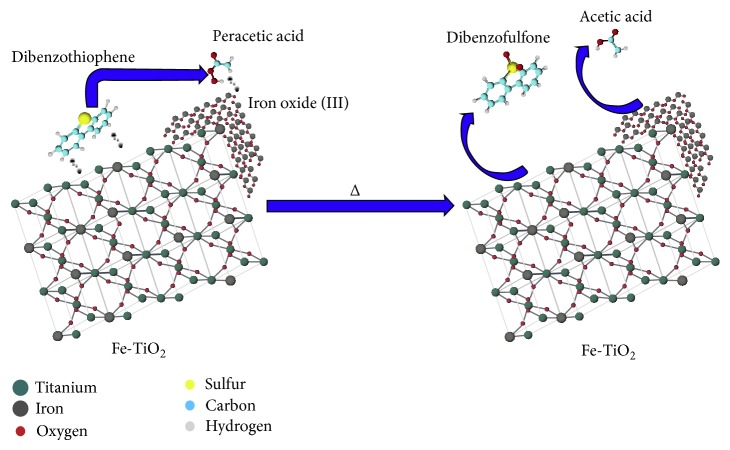
Another interesting application of TiO2 supported catalyst is the use of Fe/TiO2 in petroleum refining industries. Several routes can apply the Fe/TiO2 catalyst system including oxidation, adsorption, hydrodesulfurization, oxidative desulfurization, and biodesulfurization for organosulfur compounds removal from crude oil and refined petroleum products [2, 59]. This is due to the surface of Fe/TiO2 catalyst possess electron-hole pairs and free OH radicals [92]. Highly reactive OH radicals can also be formed by the reaction of the hole with OH–, which are able to generate oxidizing radicals in the presence of peroxides and may perform the oxidative desulfurization better due to recombination reactions occurring via free radical mechanisms [204]. Rather than that, the hydrophilic-hydrophobic character of Fe/TiO2 facilitate dibenzothiophene oxidation as a phase transfer catalyst. The coordination of acetic acid and peroxyacetic acid with Fe3+ and Ti4+ present at the Fe/TiO2 surface which in turn perform a superoxides its surface [208] (Figure 7).
Figure 7.
Oxidative removal of dibenzothiophene in a biphasic system using sol-gel Fe/TiO2 catalysts and H2O2 promoted with acetic acid [208].
4.3. Electrochemical Applications
TiO2 is an interesting material to evaluate as a heterogeneous catalyst support for electrochemical applications, not only because of its high stability cathode potentials in acidic and hydrous environment [19, 34], but also because of the recent reports introducing Ti-containing material into the electrodes [118, 142]. Substoichiometric TinO2n−2 has been studied and performs well as a catalyst support in cathodes [90, 126]. It has been reported that once metals are deposited on TiO2, it indicates an increased electrochemically active area [139, 142]. The incorporation of TiO2 into the cathode indicated the improved methanol tolerance and its proton conductor properties [111].
5. Conclusion and Suggestions
TiO2 is a reducible metal oxide and strongly reacts with noble metals compared to other metal oxides. For this reason, TiO2 has attracted much attention for application as heterogeneous catalyst support in many reactions. It is inferred that catalyst support on TiO2 with different structures might exhibit different physicochemical properties and catalytic activities. Generally, pure TiO2 possesses abysmal electronic conductivity. It is proposed that substoichiometric TiO2 is prepared in order to improve its conductivity. Although the electronic conductivity improved by utilizing TiO2 as a heterogeneous catalyst support, the stability was compromised after extensive polarization at high oxygen electrode potentials. Therefore, another method that could improve electronic conductivity is to dope TiO2 with n-type dopants, including niobium (Nb) or tantalum (Ta). It has been reported in literature that TiO2, with a/an rutile/anatase structure doped Nb, had a significantly greater electronic conductivity compared to the native TiO2. The presence of Ti3+ species was observed, which was induced by the partial replacement of Ti4+ by Nb5+. Therefore, in all cases, Nb TiO2 is both electrochemically and thermally stable, which can further promote explorations for heterogeneous catalyst support.
Acknowledgments
This work is financially supported by University Malaya Research Grant UMRG RP022-2012E and Fundamental Research Grant Scheme (FRGS: FP049-2013B) by Universiti Malaya and Ministry of High Education (MOE), Malaysia.
Conflict of Interests
The authors declare that all the written materials related to the text, tables, and figures for paper of “Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis” do not have any conflict of interests concerning the validity of research and financial gain.
References
- 1.Adam F., Appaturi J. N., Iqbal A. The utilization of rice husk silica as a catalyst: review and recent progress. Catalysis Today. 2012;190(1):2–14. doi: 10.1016/j.cattod.2012.04.056. [DOI] [Google Scholar]
- 2.Pellecchia C., Mazzeo M., Pappalardo D. Isotactic-specific polymerization of propene with an iron-based catalyst: polymer end groups and regiochemistry of propagation. Macromolecular Rapid Communications. 1998;19(12):651–655. [Google Scholar]
- 3.Lee K.-H., Noh N.-S., Shin D.-H., Seo Y. Comparison of plastic types for catalytic degradation of waste plastics into liquid product with spent FCC catalyst. Polymer Degradation and Stability. 2002;78(3):539–544. doi: 10.1016/S0141-3910(02)00227-6. [DOI] [Google Scholar]
- 4.Pollard D. J., Woodley J. M. Biocatalysis for pharmaceutical intermediates: the future is now. Trends in Biotechnology. 2007;25(2):66–73. doi: 10.1016/j.tibtech.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty A., Dunn J. L., Rateri D. L., Heinecke J. W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. Journal of Clinical Investigation. 1994;94(1):437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanan M. W., Nocera D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+ Science. 2008;321(5892):1072–1075. doi: 10.1126/science.1162018. [DOI] [PubMed] [Google Scholar]
- 7.Hu L., Yang X., Dang S. An easily recyclable Co/SBA-15 catalyst: Heterogeneous activation of peroxymonosulfate for the degradation of phenol in water. Applied Catalysis B: Environmental. 2011;102(1-2):19–26. doi: 10.1016/j.apcatb.2010.11.019. [DOI] [Google Scholar]
- 8.Kazuya Y., Noritaka M. Supported ruthenium catalyst for the heterogeneous oxidation of alcohols with molecular oxygen. Angewandte Chemie International Edition. 2002;41(23):4538–4542. doi: 10.1002/1521-3773(20021202)41:23<4538::AID-ANIE4538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Mori K., Hara T., Mizugaki T., Ebitani K., Kaneda K. Hydroxyapatite-supported palladium nanoclusters: a highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. Journal of the American Chemical Society. 2004;126(34):10657–10666. doi: 10.1021/ja0488683. [DOI] [PubMed] [Google Scholar]
- 10.Wan K. T., Davis M. E. Design and synthesis of a heterogeneous asymmetric catalyst. Nature. 1994;370(6489):449–450. doi: 10.1038/370449a0. [DOI] [Google Scholar]
- 11.Shibasaki-Kitakawa N., Honda H., Kuribayashi H., Toda T., Fukumura T., Yonemoto T. Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst. Bioresource Technology. 2007;98(2):416–421. doi: 10.1016/j.biortech.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Liu R., Jin R., An J., Zhao Q., Cheng T., Liu G. Hollow-shell-structured nanospheres: a recoverable heterogeneous catalyst for rhodium-catalyzed tandem reduction/lactonization of ethyl 2-acylarylcarboxylates to chiral phthalides. Chemistry—An Asian Journal. 2014;9(5):1388–1394. doi: 10.1002/asia.201301543. [DOI] [PubMed] [Google Scholar]
- 13.Leng Y., Liu J., Jiang P., Wang J. Organometallic-polyoxometalate hybrid based on V-Schiff base and phosphovanadomolybdate as a highly effective heterogenous catalyst for hydroxylation of benzene. Chemical Engineering Journal. 2014;239:1–7. doi: 10.1016/j.cej.2013.10.092. [DOI] [Google Scholar]
- 14.Cong P., Doolen R. D., Fan Q., Giaquinta D. M., Guan S., McFarland E. W., Poojary D. M., Self K., Turner H. W., Weinberg W. H. High-throughput synthesis and screening of combinatorial heterogeneous catalyst libraries. Angewandte Chemie. 1999;38(4):484–488. doi: 10.1002/(SICI)1521-3773(19990215)38:4<483::AID-ANIE483>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Uysal B., Oksal B. S. New heterogeneous B(OEt)3-MCM-41 catalyst for preparation of α,β-unsaturated alcohols. Research on Chemical Intermediates. 2013 doi: 10.1007/s11164-013-1498-0. [DOI] [Google Scholar]
- 16.Yamaguchi K., Yoshida C., Uchida S., Mizuno N. Peroxotungstate immobilized on ionic liquid-modified silica as a heterogeneous epoxidation catalyst with hydrogen peroxide. Journal of the American Chemical Society. 2005;127(2):530–531. doi: 10.1021/ja043688e. [DOI] [PubMed] [Google Scholar]
- 17.Planeix J. M., Coustel N., Coq B., Brotons V., Kumbhar P. S., Dutartre R., Geneste P., Bernier P., Ajayan P. M. Application of carbon nanotubes as supports in heterogeneous catalysis. Journal American Chemical Society. 1994;116(17):7935–7936. doi: 10.1021/ja00096a076. [DOI] [Google Scholar]
- 18.Kent P. D., Mondloch J. E., Finke R. G. A four-step mechanism for the formation of supported-nanoparticle heterogenous catalysts in contact with solution: the conversion of Ir(1,5-COD)Cl/γ-Al2O3 to Ir(0)~170/ γ-Al2O3 . Journal of the American Chemical Society. 2014;136(5):1930–1941. doi: 10.1021/ja410194r. [DOI] [PubMed] [Google Scholar]
- 19.Dobrzeniecka A., Kulesza P. J. Electrocatalytic activity toward oxygen reduction of RuSxN y catalysts supported on different nanostructured carbon carriers. ECS Journal of Solid State Science and Technology. 2013;2(12):M61–M66. doi: 10.1149/2.006312jss. [DOI] [Google Scholar]
- 20.Astruc D., Lu F., Aranzaes J. R. Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angewandte Chemie—International Edition. 2005;44(48):7852–7872. doi: 10.1002/anie.200500766. [DOI] [PubMed] [Google Scholar]
- 21.Crudden C. M., Sateesh M., Lewis R. Mercaptopropyl-modified mesoporous silica: a remarkable support for the preparation of a reusable, heterogeneous palladium catalyst for coupling reactions. Journal of the American Chemical Society. 2005;127(28):10045–10050. doi: 10.1021/ja0430954. [DOI] [PubMed] [Google Scholar]
- 22.Solsona B. E., Edwards J. K., Landon P., Carley A. F., Herzing A., Kiely C. J., Hutchings G. J. Direct synthesis of hydrogen peroxide from H2 and O2 using Al2O3 supported Au-Pd catalysts. Chemistry of Materials. 2006;18(11):2689–2695. doi: 10.1021/cm052633o. [DOI] [Google Scholar]
- 23.Corma A., Iglesias M., del Pino C., Sánchez F. New rhodium complexes anchored on modified USY zeolites. A remarkable effect of the support on the enantioselectivity of catalytic hydrogenation of prochiral alkenes. Journal of the Chemical Society—Series Chemical Communications. 1991;(18):1253–1255. [Google Scholar]
- 24.Kraeutler B., Bard A. J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on TiO2 powder and other substrates. Journal of the American Chemical Society. 1978;100(13):4317–4318. doi: 10.1021/ja00481a059. [DOI] [Google Scholar]
- 25.Eschemann T. O., Bitter J. H., de Jong K. P. Effects of loading and synthesis method of titania-supported cobalt catalysts for Fischer-Tropsch synthesis. Catalysis Today. 2014;228:89–95. doi: 10.1016/j.cattod.2013.10.041. [DOI] [Google Scholar]
- 26.Mor G. K., Shankar K., Paulose M., Varghese O. K., Grimes C. A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Letters. 2006;6(2):215–218. doi: 10.1021/nl052099j. [DOI] [PubMed] [Google Scholar]
- 27.D'Agata A., Fasulo S., Dallas L. J., Fisher A. S., Maisano M., Readman J. W., Jha A. N. Enhanced toxicity of “bulk” titanium dioxide compared to “fresh” and “aged” nano-TiO2 in marine mussels (Mytilus galloprovincialis) Nanotoxicology. 2014;8(5):549–558. doi: 10.3109/17435390.2013.807446. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y.-G., Hu Y.-S., Sigle W., Maier J. Superior electrode performance of nanostructured mesoporous TiO2 (Anatase) through efficient hierarchical mixed conducting networks. Advanced Materials. 2007;19(16):2087–2091. doi: 10.1002/adma.200602828. [DOI] [Google Scholar]
- 29.Xu J., Li K., Shi W., Li R., Peng T. Rice-like brookite titania as an efficient scattering layer for nanosized anatase titania film-based dye-sensitized solar cells. Journal of Power Sources. 2014;260:233–242. doi: 10.1016/j.jpowsour.2014.02.092. [DOI] [Google Scholar]
- 30.Chen M.-M., Sun X., Qiao Z.-J., Ma Q.-Q., Wang C.-Y. Anatase-TiO2 nanocoating of Li4Ti5O12 nanorod anode for lithium-ion batteries. Journal of Alloys and Compounds. 2014;601:38–42. doi: 10.1016/j.jallcom.2014.02.130. [DOI] [Google Scholar]
- 31.Fujimoto M., Koyama H., Konagai M., Hosoi Y., Ishihara K., Ohnishi S., Awaya N. TiO2 anatase nanolayer on TiN thin film exhibiting high-speed bipolar resistive switching. Applied Physics Letters. 2006;89(22) doi: 10.1063/1.2397006.223509 [DOI] [Google Scholar]
- 32.Grosso D., Soler-Illia G. J. D. A. A., Crepaldi E. L., Cagnol F., Sinturel C., Bourgeois A., Brunet-Bruneau A., Amenitsch H., Albouy P. A., Sanchez C. Highly Porous TiO2 Anatase Optical Thin Films with Cubic Mesostructure Stabilized at 700°C. Chemistry of Materials. 2003;15(24):4562–4570. doi: 10.1021/cm031060h. [DOI] [Google Scholar]
- 33.Ramimoghadam D., Bagheri S., Abd Hamid S. B. Biotemplated synthesis of anatase titanium dioxide nanoparticles via lignocellulosic waste material. BioMed Research International. 2014;2014 doi: 10.1155/2014/205636.205636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J., Polleux J., Lim J., Dunn B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. Journal of Physical Chemistry C. 2007;111(40):14925–14931. doi: 10.1021/jp074464w. [DOI] [Google Scholar]
- 35.Kominami H., Kalo J.-I., Takada Y., Doushi Y., Ohtani B., Nishimoto S.-I., Inoue M., Inui T., Kera Y. Novel synthesis of microcrystalline titanium(IV) oxide having high thermal stability and ultra-high photocatalytic activity: thermal decomposition of titanium(IV) alkoxide in organic solvents. Catalysis Letters. 1997;46(1-2):235–240. doi: 10.1023/A:1019022719479. [DOI] [Google Scholar]
- 36.Bagheri S., Shameli K., Abd Hamid S. B. Synthesis and characterization of anatase titanium dioxide nanoparticles using egg white solution via Sol-Gel method. Journal of Chemistry. 2013;2013 doi: 10.1155/2013/848205.848205 [DOI] [Google Scholar]
- 37.Palcheva R., Dimitrov L., Tyuliev G., Spojakina A., Jiratova K. TiO2 nanotubes supported NiW hydrodesulphurization catalysts: characterization and activity. Applied Surface Science. 2013;265:309–316. doi: 10.1016/j.apsusc.2012.11.001. [DOI] [Google Scholar]
- 38.Liang G., He L., Cheng H., Li W., Li X., Zhang C., Yu Y., Zhao F. The hydrogenation/dehydrogenation activity of supported Ni catalysts and their effect on hexitols selectivity in hydrolytic hydrogenation of cellulose. Journal of Catalysis. 2014;309:468–476. doi: 10.1016/j.jcat.2013.10.022. [DOI] [Google Scholar]
- 39.Luo Q., Beller M., Jiao H. Formic acid dehydrogenation on surfaces—a review of computational aspect. Journal of Theoretical and Computational Chemistry. 2013;12(7) doi: 10.1142/S0219633613300012.1330001 [DOI] [Google Scholar]
- 40.Nolan M. Modifying ceria (111) with a TiO2 nanocluster for enhanced reactivity. Journal of Chemical Physics. 2013;139(18) doi: 10.1063/1.4829758.184710 [DOI] [PubMed] [Google Scholar]
- 41.Si L., Huang Z., Lv K., Tang D., Yang C. Facile preparation of Ti3+ self-doped TiO2 nanosheets with dominant {0 0 1} facets using zinc powder as reductant. Journal of Alloys and Compounds. 2014;601:88–93. doi: 10.1016/j.jallcom.2014.02.141. [DOI] [Google Scholar]
- 42.Julkapli N. M., Bagheri S., Abd Hamid S. B. Recent advances in heterogeneous photocatalytic decolorization of synthetic dyes. The Scientific World Journal. 2014;2014 doi: 10.1155/2014/692307.692307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sui X.-L., Wang Z.-B., Yang M., Huo L., Gu D.-M., Yin G.-P. Investigation on C-TiO2 nanotubes composite as Pt catalyst support for methanol electrooxidation. Journal of Power Sources. 2014;255:43–51. doi: 10.1016/j.jpowsour.2014.01.001. [DOI] [Google Scholar]
- 44.Bamwenda G. R., Tsubota S., Nakamura T., Haruta M. The influence of the preparation methods on the catalytic activity of platinum and gold supported on TiO2 for CO oxidation. Catalysis Letters. 1997;44(1-2):83–87. doi: 10.1023/A:1018925008633. [DOI] [Google Scholar]
- 45.Tauster S. J., Fung S. C., Baker R. T. K., Horsley J. A. Strong interactions in supported-metal catalysts. Science. 1981;211, article 4487 doi: 10.1126/science.211.4487.1121. [DOI] [PubMed] [Google Scholar]
- 46.Kim T. S., Stiehl J. D., Reeves C. T., Meyer R. J., Mullins C. B. Cryogenic CO oxidation on TiO2-supported gold nanoclusters precovered with atomic oxygen. Journal of the American Chemical Society. 2003;125(8):2018–2019. doi: 10.1021/ja028719p. [DOI] [PubMed] [Google Scholar]
- 47.Lietti L., Forzatti P., Bregani F. Steady-state and transient reactivity study of TiO2-supported V2O5-WO3 De-NOx catalysts: relevance of the vanadium-tungsten interaction on the catalytic activity. Industrial and Engineering Chemistry Research. 1996;35(11):3884–3892. doi: 10.1021/ie960158l. [DOI] [Google Scholar]
- 48.Lin S. D., Bollinger M., Vannice M. A. Low temperature CO oxidation over Au/TiO2 and Au/SiO2 catalysts. Catalysis Letters. 1993;17(3-4):245–262. doi: 10.1007/BF00766147. [DOI] [Google Scholar]
- 49.Gallardo Amores J. M., Sanchez Escribano V., Busca G. Anatase crystal growth and phase transformation to rutile in high-area TiO2, MoO3-TiO2 and other TiO2-supported oxide catalytic systems. Journal of Materials Chemistry. 1995;5(8):1245–1249. doi: 10.1039/jm9950501245. [DOI] [Google Scholar]
- 50.Yan W., Mahurin S. M., Pan Z., Overbury S. H., Dai S. Ultrastable Au nanocatalyst supported on surface-modified TiO2 nanocrystals. Journal of the American Chemical Society. 2005;127(30):10480–10481. doi: 10.1021/ja053191k. [DOI] [PubMed] [Google Scholar]
- 51.Ren X., Zhang H., Cui Z. Acetylene decomposition to helical carbon nanofibers over supported copper catalysts. Materials Research Bulletin. 2007;42(12):2202–2210. doi: 10.1016/j.materresbull.2007.01.007. [DOI] [Google Scholar]
- 52.Park K.-W., Seol K.-S. Nb-TiO2 supported Pt cathode catalyst for polymer electrolyte membrane fuel cells. Electrochemistry Communications. 2007;9(9):2256–2260. doi: 10.1016/j.elecom.2007.06.027. [DOI] [Google Scholar]
- 53.Yan W., Chen B., Mahurin S. M., Schwartz V., Mullins D. R., Lupini A. R., Pennycook S. J., Dai S., Overbury S. H. Preparation and comparison of supported gold nanocatalysts on anatase, brookite, rutile, and P25 polymorphs of TiO2 for catalytic oxidation of CO. Journal of Physical Chemistry B. 2005;109(21):10676–10685. doi: 10.1021/jp044091o. [DOI] [PubMed] [Google Scholar]
- 54.Went G. T., Leu L.-J., Bell A. T. Quantitative structural analysis of dispersed vanadia species in TiO2(Anatase)-supported V2O5 . Journal of Catalysis. 1992;134(2):479–491. doi: 10.1016/0021-9517(92)90336-G. [DOI] [Google Scholar]
- 55.Francisco M. S. P., Mastelaro V. R. Inhibition of the anatase-rutile phase transformation with addition of CeO2 to CuO-TiO2 system: raman spectroscopy, X-ray diffraction, and textural studies. Chemistry of Materials. 2002;14(6):2514–2518. doi: 10.1021/cm011520b. [DOI] [Google Scholar]
- 56.Carrettin S., McMorn P., Johnston P., Griffin K., Hutchings G. J. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide. Chemical Communications. 2002;(7):696–697. doi: 10.1039/b201112n. [DOI] [PubMed] [Google Scholar]
- 57.Porta F., Prati L., Rossi M., Coluccia S., Martra G. Metal sols as a useful tool for heterogeneous gold catalyst preparation: Reinvestigation of a liquid phase oxidation. Catalysis Today. 2000;61(1):165–172. doi: 10.1016/S0920-5861(00)00370-9. [DOI] [Google Scholar]
- 58.Fang W., Chen J., Zhang Q., Deng W., Wang Y. Hydrotalcite-supported gold catalyst for the oxidant-free dehydrogenation of benzyl alcohol: studies on support and gold size effects. Chemistry—A European Journal. 2011;17(4):1247–1256. doi: 10.1002/chem.201002469. [DOI] [PubMed] [Google Scholar]
- 59.Dang T. M. D., Nguyen T. M. H., Nguyen H. P. The preparation of nano-gold catalyst supported on iron doped titanium oxide. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2010;1:1–10. doi: 10.1088/2043-6254/1/2/025011.025011 [DOI] [Google Scholar]
- 60.Arends I. W. C. E., Sheldon R. A. Activities and stabilities of heterogeneous catalysts in selective liquid phase oxidations: recent developments. Applied Catalysis A: General. 2001;212(1-2):175–187. doi: 10.1016/S0926-860X(00)00855-3. [DOI] [Google Scholar]
- 61.Oumahi C., Lombard J., Casale S., Calers C., Delannoy L., Louis C., Carrier X. Heterogeneous catalyst preparation in ionic liquids: titania supported gold nanoparticles. Catalysis Today. 2014;235:58–71. doi: 10.1016/j.cattod.2014.03.029. [DOI] [Google Scholar]
- 62.Ayati A., Ahmadpour A., Bamoharram F. F., Tanhaei B., Mänttäri M., Sillanpää M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere. 2014;107:163–174. doi: 10.1016/j.chemosphere.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 63.Zhou J., Yang X., Wang Y., Chen W. An efficient oxidation of cyclohexane over Au@TiO2/MCM-41 catalyst prepared by photocatalytic reduction method using molecular oxygen as oxidant. Catalysis Communications. 2014;46:228–233. doi: 10.1016/j.catcom.2013.12.026. [DOI] [Google Scholar]
- 64.Haruta M., Tsubota S., Kobayashi T., Kageyama H., Genet M. J., Delmon B. Low-Temperature Oxidation of CO over Gold Supported on TiO2, α-Fe2O3, and Co3O4 . Journal of Catalysis. 1993;144(1):175–192. doi: 10.1006/jcat.1993.1322. [DOI] [Google Scholar]
- 65.Schubert M. M., Hackenberg S., Van Veen A. C., Muhler M., Plzak V., Behm J. CO oxidation over supported gold catalysts—“Inert” and “active” support materials and their role for the oxygen supply during reaction. Journal of Catalysis. 2001;197(1):113–122. doi: 10.1006/jcat.2000.3069. [DOI] [Google Scholar]
- 66.Bowker M., Morton C., Kennedy J., Bahruji H., Greves J., Jones W., Davies P. R., Brookes C., Wells P. P., Dimitratos N. Hydrogen production by photoreforming of biofuels using Au, Pd and Au-Pd/TiO2 photocatalysts. Journal of Catalysis. 2014;310(1):10–15. doi: 10.1016/j.jcat.2013.04.005. [DOI] [Google Scholar]
- 67.Guzman J., Gates B. C. Catalysis by supported gold: correlation between catalytic activity for co oxidation and oxidation states of gold. Journal of the American Chemical Society. 2004;126(9):2672–2673. doi: 10.1021/ja039426e. [DOI] [PubMed] [Google Scholar]
- 68.Li G., Enache D. I., Edwards J., Carley A. F., Knight D. W., Hutchings G. J. Solvent-free oxidation of benzyl alcohol with oxygen using zeolite-supported Au and Au-Pd catalysts. Catalysis Letters. 2006;110(1-2):7–13. doi: 10.1007/s10562-006-0083-1. [DOI] [Google Scholar]
- 69.Hinojosa-Reyes M., Rodríguez-González V., Zanella R. Gold nanoparticles supported on TiO2-Ni as catalysts for hydrogen purification via water-gas shift reaction. RSC Advances. 2014;4(9):4308–4316. doi: 10.1039/c3ra45764h. [DOI] [Google Scholar]
- 70.Green I. X., Tang W., Neurock M., Yates J. T. Insights into catalytic oxidation at the Au/TiO2 dual perimeter sites. Accounts of Chemical Research. 2014;47(3):805–815. doi: 10.1021/ar400196f. [DOI] [PubMed] [Google Scholar]
- 71.Li L., Gao Y., Li H., Zhao Y., Pei Y., Chen Z., Zeng X. C. CO oxidation on TiO2 (110) supported subnanometer gold clusters: Size and shape effects. Journal of the American Chemical Society. 2013;135(51):19336–19346. doi: 10.1021/ja410292s. [DOI] [PubMed] [Google Scholar]
- 72.Nafria R., Ramírez De La Piscina P., Homs N., Morante J. R., Cabot A., Diaz U., Corma A. Embedding catalytic nanoparticles inside mesoporous structures with controlled porosity: Au@TiO2 . Journal of Materials Chemistry A. 2013;1(45):14170–14176. doi: 10.1039/c3ta13346j. [DOI] [Google Scholar]
- 73.Corma A., Garcia H. Supported gold nanoparticles as catalysts for organic reactions. Chemical Society Reviews. 2008;37(9):2096–2126. doi: 10.1039/b707314n. [DOI] [PubMed] [Google Scholar]
- 74.Chan S. C., Barteau M. A. Preparation of highly uniform Ag/TiO2 and Au/TiO2 supported nanoparticle catalysts by photodeposition. Langmuir. 2005;21(12):5588–5595. doi: 10.1021/la046887k. [DOI] [PubMed] [Google Scholar]
- 75.Liu L., Gu X., Cao Y., Yao X., Zhang L., Tang C., Gao F., Dong L. Crystal-plane effects on the catalytic properties of Au/TiO2 . ACS Catalysis. 2013;3(12):2768–2775. doi: 10.1021/cs400492w. [DOI] [Google Scholar]
- 76.Padikkaparambil S., Narayanan B., Yaakob Z., Viswanathan S., Tasirin S. M. Au/TiO2 reusable photocatalysts for dye degradation. International Journal of Photoenergy. 2013;2013 doi: 10.1155/2013/752605.752605 [DOI] [Google Scholar]
- 77.Xing M.-Y., Yang B.-X., Yu H., Tian B.-Z., Bagwasi S., Zhang J.-L., Gong X.-Q. Enhanced photocatalysis by au nanoparticle loading on TiO2 single-crystal (001) and (110) facets. Journal of Physical Chemistry Letters. 2013;4(22):3910–3917. doi: 10.1021/jz4021102. [DOI] [Google Scholar]
- 78.Arrii S., Morfin F., Renouprez A. J., Rousset J. L. Oxidation of CO on gold supported catalysts prepared by laser vaporization: direct evidence of support contribution. Journal of the American Chemical Society. 2004;126(4):1199–1205. doi: 10.1021/ja036352y. [DOI] [PubMed] [Google Scholar]
- 79.Claus P., Brückner A., Mohr C., Hofmeister H. Supported gold nanoparticles from quantum dot to mesoscopic size scale: effect of electronic and structural properties on catalytic hydrogenation of conjugated functional groups. Journal of the American Chemical Society. 2000;122(46):11430–11439. doi: 10.1021/ja0012974. [DOI] [Google Scholar]
- 80.Masatake H. Catalysis of gold nanoparticles deposited on metal oxides. CATTECH. 2002;6(3):102–115. doi: 10.1023/A:1020181423055. [DOI] [Google Scholar]
- 81.Haruta M. Gold as a novel catalyst in the 21st century: preparation, working mechanism and applications. Gold Bulletin. 2004;37(1-2):27–36. doi: 10.1007/BF03215514. [DOI] [Google Scholar]
- 82.Subramanian V., Wolf E. E., Kamat P. V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. Journal of the American Chemical Society. 2004;126(15):4943–4950. doi: 10.1021/ja0315199. [DOI] [PubMed] [Google Scholar]
- 83.Zanella R., Giorgio S., Henry C. R., Louis C. Alternative methods for the preparation of gold nanoparticles supported on TiO2 . Journal of Physical Chemistry B. 2002;106(31):7634–7642. doi: 10.1021/jp0144810. [DOI] [Google Scholar]
- 84.Visikovskiy A., Mitsuhara K., Kido Y. Role of gold nanoclusters supported on TiO2(110) model catalyst in CO oxidation reaction. Journal of Vacuum Science and Technology A Vacuum, Surfaces and Films. 2013;31(6) doi: 10.1116/1.4825117.061404 [DOI] [Google Scholar]
- 85.Boronat M., Concepción P., Corma A., González S., Illas F., Serna P. A molecular mechanism for the chemoselective hydrogenation of substituted nitroaromatics with nanoparticles of gold on TiO2 catalysts: a cooperative effect between gold and the support. Journal of the American Chemical Society. 2007;129(51):16230–16237. doi: 10.1021/ja076721g. [DOI] [PubMed] [Google Scholar]
- 86.Li J. H., Feng M. J., Jia M. L. The performance of au nanoparticle supported on mesoporous TiO2 for low-temperature CO Oxidation. Advanced Materials Research. 2013;726-731:720–724. doi: 10.4028/www.scientific.net/AMR.726-731.720. [DOI] [Google Scholar]
- 87.Vittadini A., Selloni A. Small gold clusters on stoichiometric and defected TiO2 anatase (101) and their interaction with CO: a density functional study. The Journal of Chemical Physics. 2002;117(1):353–360. doi: 10.1063/1.1481376. [DOI] [Google Scholar]
- 88.Zhong Z., Lin J., Teh S.-P., Teo J., Dautzenberg F. M. A rapid and efficient method to deposit gold particles on catalyst supports and its application for CO oxidation at low temperatures. Advanced Functional Materials. 2007;17(8):1402–1408. doi: 10.1002/adfm.200601121. [DOI] [Google Scholar]
- 89.Yu S., Ma Y., Zhi Y., Jing H., Su H. Q. Synthesis of cobalt-based catalyst supported on TiO2 nanotubes and its fischer-tropsch reaction. Integrated Ferroelectrics. 2013;147(1):59–66. doi: 10.1080/10584587.2013.790737. [DOI] [Google Scholar]
- 90.Lakadamyali F., Reynal A., Kato M., Durrant J. R., Reisner E. Electron transfer in dye-sensitised semiconductors modified with molecular cobalt catalysts: photoreduction of aqueous protons. Chemistry—A European Journal. 2012;18(48):15464–15475. doi: 10.1002/chem.201202149. [DOI] [PubMed] [Google Scholar]
- 91.Lu Y. C., Chen M. S., Chen Y. W. Hydrogen generation by sodium borohydride hydrolysis on nanosized CoB catalysts supported on TiO2, Al2O3 and CeO2 . International Journal of Hydrogen Energy. 2012;37(5):4254–4258. doi: 10.1016/j.ijhydene.2011.11.105. [DOI] [Google Scholar]
- 92.Larsson P.-O., Andersson A., Wallenberg L. R., Svensson B. Combustion of CO and toluene; characterisation of copper oxide supported on titania and activity comparisons with supported cobalt, iron, and manganese oxide. Journal of Catalysis. 1996;163(2):279–293. doi: 10.1006/jcat.1996.0329. [DOI] [Google Scholar]
- 93.Takanabe K., Nagaoka K., Nariai K., Aika K.-I. Titania-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. Journal of Catalysis. 2005;232(2):268–275. doi: 10.1016/j.jcat.2005.03.011. [DOI] [Google Scholar]
- 94.Morales F., De Groot F. M. F., Glatzel P., Kleimenov E., Bluhm H., Hävecker M., Knop-Gericke A., Weckhuysen B. M. In situ X-ray absorption of Co/Mn/TiO2 catalysts for fischer-tropsch synthesis. Journal of Physical Chemistry B. 2004;108(41):16201–16207. doi: 10.1021/jp0403846. [DOI] [Google Scholar]
- 95.Morales F., de Smit E., de Groot F. M. F., Visser T., Weckhuysen B. M. Effects of manganese oxide promoter on the CO and H2 adsorption properties of titania-supported cobalt Fischer-Tropsch catalysts. Journal of Catalysis. 2007;246(1):91–99. doi: 10.1016/j.jcat.2006.11.014. [DOI] [Google Scholar]
- 96.Feltes T. E., Espinosa-Alonso L., Smit E. D., D'Souza L., Meyer R. J., Weckhuysen B. M., Regalbuto J. R. Selective adsorption of manganese onto cobalt for optimized Mn/Co/TiO2 Fischer-Tropsch catalysts. Journal of Catalysis. 2010;270(1):95–102. doi: 10.1016/j.jcat.2009.12.012. [DOI] [Google Scholar]
- 97.Aguirre M. C., Santori G., Ferretti O., Fierro J. L. G., Reyes P. Morphological and structural features of Co/TiO2 catalysts prepared by different methods and their performance in the liquid phase hydrogenation of unsaturated aldehyde. Journal of the Chilean Chemical Society. 2006;51(1):1–10. [Google Scholar]
- 98.Rakap M., Kalu E. E., Özkar S. Hydrogen generation from the hydrolysis of ammonia borane using cobalt-nickel-phosphorus (Co-Ni-P) catalyst supported on Pd-activated TiO2 by electroless deposition. International Journal of Hydrogen Energy. 2011;36(1):254–261. doi: 10.1016/j.ijhydene.2010.09.027. [DOI] [Google Scholar]
- 99.Zhang Y., Liew K., Li J., Zhan X. Fischer-tropsch synthesis on lanthanum promoted Co/TiO2 catalysts. Catalysis Letters. 2010;139(1-2):1–6. doi: 10.1007/s10562-010-0407-z. [DOI] [Google Scholar]
- 100.Chen X., Zhang J., Huang Y., Tong Z., Huang M. Catalytic reduction of nitric oxide with carbon monoxide on copper-cobalt oxides supported on nano-titanium dioxide. Journal of Environmental Sciences. 2009;21(9):1296–1301. doi: 10.1016/S1001-0742(08)62418-3. [DOI] [PubMed] [Google Scholar]
- 101.Shinde V. M., Madras G. CO methanation toward the production of synthetic natural gas over highly active Ni/TiO2 catalyst. AIChE Journal. 2014;60(3):1027–1035. doi: 10.1002/aic.14304. [DOI] [Google Scholar]
- 102.Ullah K., Ye S., Sarkar S., Zhu L., Meng Z.-D., Oh W.-C. Photocatalytic degradation of methylene blue by NiS2-graphene supported TiO2 catalyst composites. Asian Journal of Chemistry. 2014;26(1):145–150. doi: 10.14233/ajchem.2014.15351. [DOI] [Google Scholar]
- 103.Song H., Dai M., Wan X., Xu X., Zhang C., Wang H. Synthesis of a Ni2P catalyst supported on anatase-TiO2 whiskers with high hydrodesulfurization activity, based on triphenylphosphine. Catalysis Communications. 2014;43:151–154. doi: 10.1016/j.catcom.2013.10.002. [DOI] [Google Scholar]
- 104.Huo W., Zhang C., Yuan H., Jia M., Ning C., Tang Y., Zhang Y., Luo J., Wang Z., Zhang W. Vapor-phase selective hydrogenation of maleic anhydride to succinic anhydride over Ni/TiO2 catalysts. Journal of Industrial and Engineering Chemistry. 2014 doi: 10.1016/j.jiec.2014.01.012. [DOI] [Google Scholar]
- 105.Kong W., Zhang X.-H., Zhang Q., Wang T.-J., Ma L.-L., Chen G.-Y. Hydrodeoxygenation of guaiacol over nickel-based catalyst supported on mixed oxides. Chemical Journal of Chinese Universities. 2013;34(12):2806–2813. doi: 10.7503/cjcu20130651. [DOI] [Google Scholar]
- 106.Tiwari P., Basu S. Ni infiltrated YSZ anode stabilization by inducing strong metal support interaction between nickel and titania in solid oxide fuel cell under accelerated testing. International Journal of Hydrogen Energy. 2013;38(22):9494–9499. doi: 10.1016/j.ijhydene.2012.09.094. [DOI] [Google Scholar]
- 107.Yan J., Wang H. Preparation and performance of Ni2P/TiO2-Al2O3 for hydrodenitrogenation. Advanced Materials Research. 2013;634-638(1):575–580. doi: 10.4028/www.scientific.net/AMR.634-638.575. [DOI] [Google Scholar]
- 108.Kaden W. E., Kunkel W. A., Roberts F. S., Kane M., Anderson S. L. Thermal and adsorbate effects on the activity and morphology of size-selected Pdn/TiO2 model catalysts. Surface Science. 2014;621:40–50. doi: 10.1016/j.susc.2013.11.002. [DOI] [Google Scholar]
- 109.Shutilov A. A., Zenkovets G. A., Pakharukov I. Y., Prosvirin I. P. Influence of CeO2 addition on the physicochemical and catalytic properties of Pd/TiO2 catalysts in CO oxidation. Kinetics and Catalysis. 2014;55(1):111–116. doi: 10.1134/S0023158414010121. [DOI] [Google Scholar]
- 110.Kočí K., Matějová L., Reli M., Čapek L., Matějka V., Lacný Z., Kustrowski P., Obalová L. Sol-gel derived Pd supported TiO2-ZrO2 and TiO2 photocatalysts; their examination in photocatalytic reduction of carbon dioxide. Catalysis Today. 2014;230:20–26. doi: 10.1016/j.cattod.2013.10.002. [DOI] [Google Scholar]
- 111.Cheng K., Yang F., Zhang D., Yin J., Cao D., Wang G. Pd nanofilm supported on C@TiO2 nanocone core/shell nanoarrays: a facile preparation of high performance electrocatalyst for H2O2 electroreduction in acid medium. Electrochimica Acta. 2013;105:115–120. doi: 10.1016/j.electacta.2013.05.007. [DOI] [Google Scholar]
- 112.Shahreen L., Chase G. G., Turinske A. J., Nelson S. A., Stojilovic N. NO decomposition by CO over Pd catalyst supported on TiO2 nanofibers. Chemical Engineering Journal. 2013;225(1):340–349. doi: 10.1016/j.cej.2013.03.102. [DOI] [Google Scholar]
- 113.Putdee S., Mekasuwandumrong O., Soottitantawat A., Panpranot J. Characteristics and catalytic behavior of Pd catalysts supported on nanostructure titanate in liquid-phase hydrogenation. Journal of Nanoscience and Nanotechnology. 2013;13(4):3062–3067. doi: 10.1166/jnn.2013.7403. [DOI] [PubMed] [Google Scholar]
- 114.Chen R., Jiang Y., Xing W., Jin W. Fabrication and catalytic properties of palladium nanoparticles deposited on a silanized asymmetric ceramic support. Industrial and Engineering Chemistry Research. 2011;50(8):4405–4411. doi: 10.1021/ie1022578. [DOI] [Google Scholar]
- 115.Zhu J., Jia Y., Li M., Lu M., Zhu J. Carbon nanofibers grown on anatase washcoated cordierite monolith and its supported palladium catalyst for cinnamaldehyde hydrogenation. Industrial and Engineering Chemistry Research. 2013;52(3):1224–1233. doi: 10.1021/ie302374a. [DOI] [Google Scholar]
- 116.Sheu H.-S., Lee J.-F., Shyu S.-G., Chou W.-W., Chang J.-R. Sulfur resistance enhancement by grafted TiO2 in SiO2-supported Pd catalysts: role of grafted TiO2 and genesis of Pd clusters. Journal of Catalysis. 2009;266(1):15–25. doi: 10.1016/j.jcat.2009.05.008. [DOI] [Google Scholar]
- 117.Gatla S., Madaan N., Radnik J., Kalevaru V. N., Pohl M.-M., Lücke B., Martin A., Bentrup U., Brückner A. Rutile—A superior support for highly selective and stable Pd-based catalysts in the gas-phase acetoxylation of toluene. Journal of Catalysis. 2013;297:256–263. doi: 10.1016/j.jcat.2012.10.016. [DOI] [Google Scholar]
- 118.Maheswari S., Sridhar P., Pitchumani S. Pd-TiO2/C as a methanol tolerant catalyst for oxygen reduction reaction in alkaline medium. Electrochemistry Communications. 2013;26(1):97–100. doi: 10.1016/j.elecom.2012.10.021. [DOI] [Google Scholar]
- 119.Kennedy G., Baker L. R., Somorjai G. A. Selective amplification of 34;O bond hydrogenation on Pt/TiO2: catalytic reaction and sum-frequency generation vibrational spectroscopy studies of crotonaldehyde hydrogenation. Angewandte Chemie. 2014;53(13):3405–3408. doi: 10.1002/anie.201400081. [DOI] [PubMed] [Google Scholar]
- 120.Brijaldo M. H., Passos F. B., Rojas H. A., Reyes P. Hydrogenation of m-dinitrobenzene over Pt supported catalysts on TiO2-Al2O3 binary oxides. Catalysis Letters. 2014;144(5):860–863. doi: 10.1007/s10562-014-1218-4. [DOI] [Google Scholar]
- 121.Chua Y. P. G., Gunasooriya G. T. K. K., Saeys M., Seebauer E. G. Controlling the CO oxidation rate over Pt/TiO2 catalysts by defect engineering of the TiO2 support. Journal of Catalysis. 2014;311:306–313. doi: 10.1016/j.jcat.2013.12.007. [DOI] [Google Scholar]
- 122.López G. P., López R. R., Viveros T. Dehydrocyclization of n-heptane over Pt catalysts supported on Al- and Si-promoted TiO2 . Catalysis Today. 2014;220–222:61–65. doi: 10.1016/j.cattod.2013.10.019. [DOI] [Google Scholar]
- 123.Ruiz-Camacho B., Santoyo H. H. R., Medina-Flores J. M., Álvarez-Martínez O. Platinum deposited on TiO2-C and SnO2-C composites for methanol oxidation and oxygen reduction. Electrochimica Acta. 2014;120:344–349. doi: 10.1016/j.electacta.2013.12.055. [DOI] [Google Scholar]
- 124.Rautio A.-R., Mäki-Arvela P., Aho A., Eränen K., Kordas K. Chemoselective hydrogenation of citral by Pt and Pt-Sn catalysts supported on TiO2 nanoparticles and nanowires. Catalysis Today. 2014 doi: 10.1016/j.cattod.2013.12.052. [DOI] [Google Scholar]
- 125.Chang T.-Y., Tanaka Y., Ishikawa R., Toyoura K., Matsunaga K., Ikuhara Y., Shibata N. Direct imaging of pt single atoms adsorbed on TiO2 (110) surfaces. Nano Letters. 2014;14(1):134–138. doi: 10.1021/nl403520c. [DOI] [PubMed] [Google Scholar]
- 126.Du Q., Wu J., Yang H. PtNb-TiO2 catalyst membranes fabricated by electrospinning and atomic layer deposition. ACS Catalysis. 2014;4(1):144–151. doi: 10.1021/cs400944p. [DOI] [Google Scholar]
- 127.Gan S., Liang Y., Baer D. R., Sievers M. R., Herman G. S., Peden C. H. F. Effect of platinum nanocluster size and titania surface structure upon CO surface chemistry on platinum-supported TiO2 (110) Journal of Physical Chemistry B. 2001;105(12):2412–2416. doi: 10.1021/jp003125z. [DOI] [Google Scholar]
- 128.Sun B., Vorontsov A. V., Smirniotis P. G. Role of platinum deposited on TiO2 in phenol photocatalytic oxidation. Langmuir. 2003;19(8):3151–3156. doi: 10.1021/la0264670. [DOI] [Google Scholar]
- 129.Herrmann J.-M., Disdier J., Pichat P. Photoassisted platinum deposition on TiO2 powder using various platinum complexes. The Journal of Physical Chemistry. 1986;90(22):6028–6034. doi: 10.1021/j100280a114. [DOI] [Google Scholar]
- 130.Yu J., Qi L., Jaroniec M. Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets. Journal of Physical Chemistry C. 2010;114(30):13118–13125. doi: 10.1021/jp104488b. [DOI] [Google Scholar]
- 131.Colindres S. C., García J. R. V., Antonio J. A. T., Chavez C. A. Preparation of platinum-iridium nanoparticles on titania nanotubes by MOCVD and their catalytic evaluation. Journal of Alloys and Compounds. 2009;483(1-2):406–409. doi: 10.1016/j.jallcom.2008.08.097. [DOI] [Google Scholar]
- 132.Schulz H., Mädler L., Strobel R., Jossen R., Pratsinis S. E., Johannessen T. Independent control of metal cluster and ceramic particle characteristics during one-step synthesis of Pt/TiO2 . Journal of Materials Research. 2005;20(9):2568–2577. doi: 10.1557/JMR.2005.0319. [DOI] [Google Scholar]
- 133.Shironita S., Goto M., Kamegawa T., Mori K., Yamashita H. Preparation of highly active platinum nanoparticles on ZSM-5 zeolite including cerium and titanium dioxides as photo-assisted deposition sites. Catalysis Today. 2010;153(3-4):189–192. doi: 10.1016/j.cattod.2010.04.026. [DOI] [Google Scholar]
- 134.Linsebigler A., Rusu C., Yates J. T., Jr. Absence of platinum enhancement of a photoreaction on TiO2-CO photooxidation on Pt/TiO2(110) Journal of the American Chemical Society. 1996;118(22):5284–5289. doi: 10.1021/ja953601c. [DOI] [Google Scholar]
- 135.Petallidou K. C., Polychronopoulou K., Boghosian S., Garcia-Rodriguez S., Efstathiou A. M. Water-gas shift reaction on Pt/Ce1- xTixO2-δ: the effect of Ce/Ti ratio. Journal of Physical Chemistry C. 2013;117(48):25467–25477. doi: 10.1021/jp406059h. [DOI] [Google Scholar]
- 136.Chen Y., Li D., Wang X., Wu L., Fu X. Promoting effects of H2 on photooxidation of volatile organic pollutants over Pt/TiO2 . New Journal of Chemistry. 2005;29(12):1514–1519. doi: 10.1039/b510953a. [DOI] [Google Scholar]
- 137.Ammal S. C., Heyden A. Origin of the unique activity of Pt/TiO2 catalysts for the water-gas shift reaction. Journal of Catalysis. 2013;306:78–90. doi: 10.1016/j.jcat.2013.06.014. [DOI] [Google Scholar]
- 138.Hua H., Hu C., Zha Z., Liu H., Xie X., Xi Y. Pt nanoparticles supported on submicrometer-sized TiO2 spheres for effective methanol and ethanol oxidation. Electrochimica Acta. 2013;105:130–136. doi: 10.1016/j.electacta.2013.05.002. [DOI] [Google Scholar]
- 139.Zhang C., Yu H., Li Y., Fu L., Gao Y., Song W., Shao Z., Yi B. Simple synthesis of Pt/TiO2 nanotube arrays with high activity and stability. Journal of Electroanalytical Chemistry. 2013;701:14–19. doi: 10.1016/j.jelechem.2013.04.012. [DOI] [Google Scholar]
- 140.Li X., Zheng W., Pan H., Yu Y., Chen L., Wu P. Pt nanoparticles supported on highly dispersed TiO2 coated on SBA-15 as an efficient and recyclable catalyst for liquid-phase hydrogenation. Journal of Catalysis. 2013;300:9–19. doi: 10.1016/j.jcat.2012.12.007. [DOI] [Google Scholar]
- 141.An H., Hu P., Hu X., Zhao W., Zhu B., Wang S., Zhang S., Huang W. Characterization of Pt catalysts supported by three forms of TiO2 and their catalytic activities for hydrogenation. Reaction Kinetics, Mechanisms and Catalysis. 2013;108(1):117–126. doi: 10.1007/s11144-012-0480-y. [DOI] [Google Scholar]
- 142.Shaddad M. N., Al-Mayouf A. M., Ghanem M. A., AlHoshan M. S., Singh J. P., Al-Suhybani A. A. Chemical deposition and electrocatalytic activity of platinum nanoparticles supported on TiO2 nanotubes. International Journal of Electrochemical Science. 2013;8(2):2468–2478. [Google Scholar]
- 143.Shen Y. L., Chen S. Y., Song J. M., Chen I. G. Ultra-long pt nanolawns supported on TiO2-coated carbon fibers as 3D hybrid catalyst for methanol oxidation. Nanoscale Research Letters. 2012;7:1–8. doi: 10.1186/1556-276X-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hao X.-Y., Zhang Y.-Q., Wang J.-W., Zhou W., Zhang C., Liu S. A novel approach to prepare MCM-41 supported CuO catalyst with high metal loading and dispersion. Microporous and Mesoporous Materials. 2006;88(1–3):38–47. doi: 10.1016/j.micromeso.2005.08.019. [DOI] [Google Scholar]
- 145.Xue Y., Lu G., Guo Y., Guo Y., Wang Y., Zhang Z. Effect of pretreatment method of activated carbon on the catalytic reduction of NO by carbon over CuO. Applied Catalysis B: Environmental. 2008;79(3):262–269. doi: 10.1016/j.apcatb.2007.10.027. [DOI] [Google Scholar]
- 146.Severino F., Brito J. L., Laine J., Fierro J. L. G., López Agudo A. Nature of copper active sites in the carbon monoxide oxidation on CuAl2O4 and CuCr2O4 spinel type catalysts. Journal of Catalysis. 1998;177(1):82–95. doi: 10.1006/jcat.1998.2094. [DOI] [Google Scholar]
- 147.Pasha N., Lingaiah N., Reddy P. S. S., Prasad P. S. Direct decomposition of N2O over cesium-doped CuO catalysts. Catalysis Letters. 2009;127(1-2):101–106. doi: 10.1007/s10562-008-9641-z. [DOI] [Google Scholar]
- 148.Chowdhuri A., Singh S. K., Sreenivas K., Gupta V. Contribution of adsorbed oxygen and interfacial space charge for enhanced response of SnO2 sensors having CuO catalyst for H2S gas. Sensors and Actuators B: Chemical. 2010;145(1):155–166. doi: 10.1016/j.snb.2009.11.050. [DOI] [Google Scholar]
- 149.Martínez-Arias A., Fernández-García M., Gálvez O., Coronado J. M., Anderson J. A., Conesa J. C., Soria J., Munuera G. Comparative study on redox properties and catalytic behavior for CO oxidation of CuO/CeO2 and CuO/ZrCeO4 catalysts. Journal of Catalysis. 2000;195(1):207–216. doi: 10.1006/jcat.2000.2981. [DOI] [Google Scholar]
- 150.Yao X., Zhang L., Li L., Liu L., Cao Y., Dong X., Gao F., Deng Y., Tang C., Chen Z., Dong L., Chen Y. Investigation of the structure, acidity, and catalytic performance of CuO/Ti0.95Ce0.05O2 catalyst for the selective catalytic reduction of NO by NH3 at low temperature. Applied Catalysis B: Environmental. 2014;150-151:315–329. doi: 10.1016/j.apcatb.2013.12.007. [DOI] [Google Scholar]
- 151.Maciel C. G., Silva T. D. F., Assaf E. M., Assaf J. M. Hydrogen production and purification from the water-gas shift reaction on CuO/CeO2-TiO2 catalysts. Applied Energy. 2013;112:52–59. doi: 10.1016/j.apenergy.2013.06.003. [DOI] [Google Scholar]
- 152.Rui Z., Huang Y., Zheng Y., Ji H., Yu X. Effect of titania polymorph on the properties of CuO/TiO2 catalysts for trace methane combustion. Journal of Molecular Catalysis A: Chemical. 2013;372:128–136. doi: 10.1016/j.molcata.2013.02.026. [DOI] [Google Scholar]
- 153.Zhu H., Dong L., Chen Y. Effect of titania structure on the properties of its supported copper oxide catalysts. Journal of Colloid and Interface Science. 2011;357(2):497–503. doi: 10.1016/j.jcis.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 154.Dean D., Davis B., Jessop P. G. The effect of temperature, catalyst and sterics on the rate of N-heterocycle dehydrogenation for hydrogen storage. New Journal of Chemistry. 2011;35(2):417–422. doi: 10.1039/c0nj00511h. [DOI] [Google Scholar]
- 155.Gu Y., Yang Y., Qiu Y., Sun K., Xu X. Combustion of dichloromethane using copper-manganese oxides supported on zirconium modified titanium-aluminum catalysts. Catalysis Communications. 2010;12(4):277–281. doi: 10.1016/j.catcom.2010.10.006. [DOI] [Google Scholar]
- 156.Gombac V., Sordelli L., Montini T., Delgado J. J., Adamski A., Adami G., Cargnello M., Bernai S., Fornasiero P. CuOx-TiO2 Photocatalysts for H2 production from ethanol and glycerol solutions. Journal of Physical Chemistry A. 2010;114(11):3916–3925. doi: 10.1021/jp907242q. [DOI] [PubMed] [Google Scholar]
- 157.Ren J., Liu S., Li Z., Lu X., Xie K. Oxidative carbonylation of methanol to dimethyl carbonate over CuCl/SiO2-TiO2 catalysts prepared by microwave heating: The effect of support composition. Applied Catalysis A General. 2009;366(1):93–101. doi: 10.1016/j.apcata.2009.06.042. [DOI] [Google Scholar]
- 158.Pradhan S., Reddy A. S., Devi R. N., Chilukuri S. Copper-based catalysts for water gas shift reaction: influence of support on their catalytic activity. Catalysis Today. 2009;141(1-2):72–76. doi: 10.1016/j.cattod.2008.06.026. [DOI] [Google Scholar]
- 159.Nian J.-N., Chen S.-A., Tsai C.-C., Teng H. Structural feature and catalytic performance of Cu species distributed over TiO2 nanotubes. The Journal of Physical Chemistry B. 2006;110(51):25817–25824. doi: 10.1021/jp064209w. [DOI] [PubMed] [Google Scholar]
- 160.Huang J., Wang S., Zhao Y., Wang X., Wu S., Zhang S., Huang W. Synthesis and characterization of CuO/TiO2 catalysts for low-temperature CO oxidation. Catalysis Communications. 2006;7(12):1029–1034. doi: 10.1016/j.catcom.2006.05.001. [DOI] [Google Scholar]
- 161.Altynnikov A. A., Tsikoza L. T., Anufrienko V. F. Ordering of Cu(II) ions in supported copper-titanium oxide catalysts. Journal of Structural Chemistry. 2006;47(6):1161–1169. doi: 10.1007/s10947-006-0439-9. [DOI] [Google Scholar]
- 162.Chary K. V. R., Sagar G. V., Naresh D., Seela K. K., Sridhar B. Characterization and reactivity of copper oxide catalysts supported on TiO2-ZrO2 . Journal of Physical Chemistry B. 2005;109(19):9437–9444. doi: 10.1021/jp0500135. [DOI] [PubMed] [Google Scholar]
- 163.Centi G. Nature of active layer in vanadium oxide supported on titanium oxide and control of its reactivity in the selective oxidation and ammoxidation of alkylaromatics. Applied Catalysis A: General. 1996;147(2):267–298. doi: 10.1016/S0926-860X(96)00179-2. [DOI] [Google Scholar]
- 164.Miyamoto A., Yamazaki Y., Inomata M., Murakami Y. Determination of the number of vanadium=oxygen species on the surface of vanadium oxide catalysts. 1. Unsupported vanadium pentoxide and vanadium pentoxide /titanium dioxide treated with an ammoniacal solution. Journal of Physical Chemistry. 1981;85(16):2366–2372. doi: 10.1021/j150616a015. [DOI] [Google Scholar]
- 165.Slink W. E., DeGroot P. B. Vanadium-titanium oxide catalysts for oxidation of butene to acetic acid. Journal of Catalysis. 1981;68(2):423–432. doi: 10.1016/0021-9517(81)90113-5. [DOI] [Google Scholar]
- 166.Kootenaei A. H. S., Towfighi J., Khodadadi A., Mortazavi Y. Stability and catalytic performance of vanadia supported on nanostructured titania catalyst in oxidative dehydrogenation of propane. Applied Surface Science. 2014;298:26–35. doi: 10.1016/j.apsusc.2013.12.172. [DOI] [Google Scholar]
- 167.Pan Y., Zhao W., Zhong Q., Cai W., Li H. Promotional effect of Si-doped V2O5/TiO2 for selective catalytic reduction of NO x by NH3. Journal of Environmental Sciences. 2013;25(8):1703–1711. doi: 10.1016/S1001-0742(12)60181-8. [DOI] [PubMed] [Google Scholar]
- 168.Feyel S., Schröder D., Schwarz H. Gas-phase oxidation of isomeric butenes and small alkanes by vanadium-oxide and-hydroxide cluster cations. Journal of Physical Chemistry A. 2006;110(8):2647–2654. doi: 10.1021/jp054799i. [DOI] [PubMed] [Google Scholar]
- 169.Setnička M., Čičmanec P., Bulánek R., Zukal A., Pastva J. Hexagonal mesoporous titanosilicates as support for vanadium oxide—promising catalysts for the oxidative dehydrogenation of n-butane. Catalysis Today. 2013;204:132–139. doi: 10.1016/j.cattod.2012.07.028. [DOI] [Google Scholar]
- 170.Rao K. N., Venkataswamy P., Bharali P., Ha H. P., Reddy B. M. Monolayer V2O5/TiO2-ZrO2 catalysts for selective oxidation of o-xylene: preparation and characterization. Research on Chemical Intermediates. 2012;38(3):733–744. doi: 10.1007/s11164-011-0412-x. [DOI] [Google Scholar]
- 171.Herrera J. E., Isimjan T. T., Abdullahi I., Ray A., Rohani S. A novel nanoengineered VO x catalyst supported on highly ordered TiO2 nanotube arrays for partial oxidation reactions. Applied Catalysis A General. 2012;417-418:13–18. doi: 10.1016/j.apcata.2011.12.016. [DOI] [Google Scholar]
- 172.Chin S., Jurng J., Lee J.-H., Moon S.-J. Catalytic conversion of 1,2-dichlorobenzene using V2O5/TiO2 catalysts by a thermal decomposition process. Chemosphere. 2009;75(9):1206–1209. doi: 10.1016/j.chemosphere.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 173.Inomata M., Miyamoto A., Murakami Y. Determination of the number of vanadium = oxygen species on the surface of vanadium oxide catalysts. 2. Vanadium pentoxide /titanium dioxide catalysts. Journal of Physical Chemistry. 1981;85(16):2372–2377. doi: 10.1021/j150616a016. [DOI] [Google Scholar]
- 174.Chin S., Park E., Kim M., Bae G. N., Jurng J. Effect of the support material (TiO2) synthesis conditions in chemical vapor condensation on the catalytic oxidation for 1,2-dichlorobenzene over V2O5/TiO2 . Powder Technology. 2012;217:388–393. doi: 10.1016/j.powtec.2011.10.055. [DOI] [Google Scholar]
- 175.Zhang F., Chen F., Xiao Q., Zhong Y., Zhu W. Ammoxidation of 3-picoline over V2O5/TiO2 catalysts: effects of TiO2 supports on the catalytic performance. Advanced Materials Research. 2012;396-398:791–797. doi: 10.4028/www.scientific.net/AMR.396-398.791. [DOI] [Google Scholar]
- 176.Fievez T., de Proft F., Geerlings P., Weckhuysen B. M., Havenith R. W. A. Conceptual chemistry approach towards the support effect in supported vanadium oxides: valence bond calculations on the ionicity of vanadium catalysts. Catalysis Today. 2011;177(1):3–11. doi: 10.1016/j.cattod.2011.06.026. [DOI] [Google Scholar]
- 177.Löfberg A., Giornelli T., Paul S., Bordes-Richard E. Catalytic coatings for structured supports and reactors: /TiO2 catalyst coated on stainless steel in the oxidative dehydrogenation of propane. Applied Catalysis A: General. 2011;391(1-2):43–51. doi: 10.1016/j.apcata.2010.09.002. [DOI] [Google Scholar]
- 178.Lü G., Song C.-L., Bin F., Zhang Q.-M., Pei Y.-Q. Physicochemical properties and catalytic activity of vanadium contained SCR catalysts with different synthetic methods. Journal of Engineering Thermophysics. 2009;30(12):2157–2160. [Google Scholar]
- 179.Li Y.-T., Zhong Q., Ma W.-H. Preparation and activity of F-doped VOx/TiOy low temperature SCR catalysts. Dongli Gongcheng/Power Engineering. 2009;29(9):854–858. [Google Scholar]
- 180.Choi S. H., Cho S. P., Lee J. Y., Hong S. H., Hong S. C., Hong S.-I. The influence of non-stoichiometric species of V/TiO2 catalysts on selective catalytic reduction at low temperature. Journal of Molecular Catalysis A: Chemical. 2009;304(1-2):166–173. doi: 10.1016/j.molcata.2009.02.008. [DOI] [Google Scholar]
- 181.Carlson T., Griffin G. L. Photooxidation of methanol using V2O5/TiO2 and MoO3/TiO2 surface oxide monolayer catalysts. Journal of Physical Chemistry. 1986;90(22):5896–5900. doi: 10.1021/j100280a087. [DOI] [Google Scholar]
- 182.Susumu O., Masanori K., Jiro Y., Koji K., Kozo T. Effect of sulfate ion on the catalytic activity of molybdenum oxide-titanium dioxide (MoOx-TiO2) for the reduction of nitric oxide with ammonia. Industry Engineering Chemical Production Research Division. 1981;20(2):301–304. [Google Scholar]
- 183.Ramesh K., Chen L., Chen F., Liu Y., Wang Z., Han Y.-F. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts. Catalysis Today. 2008;131(1):477–482. doi: 10.1016/j.cattod.2007.10.061. [DOI] [Google Scholar]
- 184.Cadus L. E., Ferretti O. Characterization of Mo-MnO catalyst for propane oxidative dehydrogenation. Applied Catalysis A: General. 2002;233(1-2):239–253. doi: 10.1016/S0926-860X(02)00193-X. [DOI] [Google Scholar]
- 185.Qi G., Yang R. T., Chang R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Applied Catalysis B: Environmental. 2004;51(2):93–106. doi: 10.1016/j.apcatb.2004.01.023. [DOI] [Google Scholar]
- 186.An Z., Zhuo Y., Xu C., Chen C. Influence of the TiO2 crystalline phase of MnOx/TiO2 catalysts for NO oxidation. Chinese Journal of Catalysis. 2014;35(1):120–126. doi: 10.1016/S1872-2067(12)60726-8. [DOI] [Google Scholar]
- 187.Peng Z., Zhong L., Li H., Zhang S., Zhong Q. Catalytic performance research of N-doped MnOx/TiO2 for low-temperature selective reduction of NO with NH3 . Proceedings of the Chinese Society of Electrical Engineering. 2013;33(35):58–66. [Google Scholar]
- 188.Aboukaïs A., Abi-Aad E., Taouk B. Supported manganese oxide on TiO2 for total oxidation of toluene and polycyclic aromatic hydrocarbons (PAHs): characterization and catalytic activity. Materials Chemistry and Physics. 2013;142(2-3):564–571. doi: 10.1016/j.matchemphys.2013.07.053. [DOI] [Google Scholar]
- 189.Feng S., Gao P., Dong C., Lu Q. The effect of doping Ce and Fe on the Mn/TiO2 catalyst for low temperature NO selective catalytic reduction with NH3 . Applied Mechanics and Materials. 2013;261-262:1041–1046. doi: 10.4028/www.scientific.net/AMM.260-261.1041. [DOI] [Google Scholar]
- 190.Wu Z., Jiang B., Liu Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Applied Catalysis B Environmental. 2008;79(4):347–355. doi: 10.1016/j.apcatb.2007.09.039. [DOI] [Google Scholar]
- 191.Smirniotis P. G., Sreekanth P. M., Peña D. A., Jenkins R. G. Manganese oxide catalysts supported on TiO2, Al 2O3, and SiO2: a comparison for low-temperature SCR of NO with NH3 . Industrial and Engineering Chemistry Research. 2006;45(19):6436–6443. doi: 10.1021/ie060484t. [DOI] [Google Scholar]
- 192.Kim S. S., Hong S. C. Improving the activity of Mn/TiO2 catalysts through control of the pH and valence state of Mn during their preparation. Journal of the Air and Waste Management Association. 2012;62(3):362–369. doi: 10.1080/10473289.2011.653515. [DOI] [PubMed] [Google Scholar]
- 193.Lee S. M., Park K. H., Hong S. C. MnOx/CeO2-TiO2 mixed oxide catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chemical Engineering Journal. 2012;195-196:323–331. doi: 10.1016/j.cej.2012.05.009. [DOI] [Google Scholar]
- 194.Pozan G. S. Effect of support on the catalytic activity of manganese oxide catalyts for toluene combustion. Journal of Hazardous Materials. 2012;221-222:124–130. doi: 10.1016/j.jhazmat.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 195.Li X., Liu H.-Y., Xia Q.-B., Liu Z.-M., Jiang X., Li Z. Preparation of MnCe (y)Ox/TiO2 catalysts and their catalytic activity for catalytic combustion of toluene. Journal of Functional Materials. 2012;43(10):1357–1360. [Google Scholar]
- 196.Wu D. W., Zhang Q. L., Lin T., Gong M. C., Chen Y. Q. Effect of Fe on the selective catalytic reduction of NO by NH3 at low temperature over Mn/CeO2-TiO2 catalyst. Journal of Inorganic Materials. 2012;27(5):495–500. doi: 10.3724/SP.J.1077.2012.00495. [DOI] [Google Scholar]
- 197.Thirupathi B., Smirniotis P. G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: catalytic evaluation and characterizations. Journal of Catalysis. 2012;288:74–83. doi: 10.1016/j.jcat.2012.01.003. [DOI] [Google Scholar]
- 198.Blondeel G., Harriman A., Porter G., Urwin D., Kiwi J. Design, preparation, and characterization of RuO2/TiO2 colloidal catalytic surfaces active in photooxidation of water. Journal of Physical Chemistry. 1983;87(14):2629–2636. doi: 10.1021/j100237a031. [DOI] [Google Scholar]
- 199.Perkas N., Minh D. P., Gallezot P., Gedanken A., Besson M. Platinum and ruthenium catalysts on mesoporous titanium and zirconium oxides for the catalytic wet air oxidation of model compounds. Applied Catalysis B: Environmental. 2005;59(1-2):121–130. doi: 10.1016/j.apcatb.2005.01.009. [DOI] [Google Scholar]
- 200.Swider K. E., Merzbacher C. I., Hagans P. L., Rolison D. R. Synthesis of ruthenium dioxide-titanium dioxide aerogels: redistribution of electrical properties on the nanoscale. Chemistry of Materials. 1997;9(5):1248–1255. doi: 10.1021/cm960622c. [DOI] [Google Scholar]
- 201.Ju J., Shi Y., Wu D. TiO2 nanotube supported PdNi catalyst for methanol electro-oxidation. Powder Technology. 2012;230:252–256. doi: 10.1016/j.powtec.2012.06.046. [DOI] [Google Scholar]
- 202.Bracey C. L., Ellis P. R., Hutchings G. J. Application of copper-gold alloys in catalysis: current status and future perspectives. Chemical Society Reviews. 2009;38(8):2231–2243. doi: 10.1039/b817729p. [DOI] [PubMed] [Google Scholar]
- 203.Ajaikumar S., Ahlkvist J., Larsson W., Shchukarev A., Leino A.-R., Kordas K., Mikkola J.-P. Oxidation of α-pinene over gold containing bimetallic nanoparticles supported on reducible TiO2 by deposition-precipitation method. Applied Catalysis A: General. 2011;392(1-2):11–18. doi: 10.1016/j.apcata.2010.10.015. [DOI] [Google Scholar]
- 204.Kazantzis G. Role of cobalt, iron, lead, manganese, mercury, platinum, selenium, and titanium in carcinogenesis. Environmental Health Perspectives. 1981;40:143–161. doi: 10.1289/ehp.8140143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Morales F., de Smit E., de Groot F. M. F., Visser T., Weckhuysen B. M. Effects of manganese oxide promoter on the CO and H2 adsorption properties of titania-supported cobalt Fischer-Tropsch catalysts. Journal of Catalysis. 2007;246(1):91–99. doi: 10.1016/j.jcat.2006.11.014. [DOI] [Google Scholar]
- 206.Morales F., De Groot F. M. F., Gijzeman O. L. J., Mens A., Stephan O., Weckhuysen B. M. Mn promotion effects in Co/TiO2 Fischer-Tropsch catalysts as investigated by XPS and STEM-EELS. Journal of Catalysis. 2005;230(2):301–308. doi: 10.1016/j.jcat.2004.11.047. [DOI] [Google Scholar]
- 207.Weerachawanasak P., Hutchings G. J., Edwards J. K., et al. Surface functionalized TiO2 supported Pd catalysts for solvent-free selective oxidation of benzyl alcohol. Catalysis Today. 2014 doi: 10.1016/j.cattod.2014.06.005. [DOI] [Google Scholar]
- 208.Arellano U., Wang J. A., Timko M. T., Chen L. F., Paredes Carrera S. P., Asomoza M., González Vargas O. A., Llanos M. E. Oxidative removal of dibenzothiophene in a biphasic system using sol-gel FeTiO2 catalysts and H2O2 promoted with acetic acid. Fuel. 2014;126:16–25. doi: 10.1016/j.fuel.2014.02.028. [DOI] [Google Scholar]