Prion-like features and possible hazards of amyloid-β-, tau- and α-synuclein aggregates
Increasing evidence suggests that the misfolding and aggregation of different disease-associated proteins such as amyloid-β (Aβ) and tau in Alzheimer’s disease (AD), α-synuclein in Parkinson’s Disease (PD) and dementia with Lewy bodies (DLB), or prion protein (PrP) in prion diseases is based on a common molecular mechanism of nucleation-dependent protein polymerization [1-3]. Consistent with this concept it has been recently demonstrated that the aggregation and deposition of Aβ, tau, and α-synuclein in the brain can be stimulated in animal models by injection of inocula that contain aggregated forms of these proteins (for a review see: [4]). Additionally, intracerebral implantation of stainless steel wires previously contaminated with Aβ-containing brain extract was found to stimulate cerebral beta-amyloidosis in APP23 transgenic mice [5], and Aβ aggregates resisted inactivation of nucleating (“seeding”) activity by boiling [5] or formaldehyde [6]. Taken together, these findings raised concerns that the transmission of pathological protein particles from common neurodegenerative diseases may possibly pose a risk to patient safety, e.g. in transfusion medicine or surgery. However, so far neither experimental nor epidemiological studies provided evidence for a transmission of severe or even fatal disease by Aβ-, tau- or α-synuclein aggregates [4]. Yet, stimulation of cerebral protein aggregation by iatrogenically transmitted Aβ-, tau- or α-synuclein particles could possibly have harmful effects below full-blown disease transmission. For α-synuclein such scenarios have been experimentally exemplified. Intracerebrally or intramuscularly injected samples containing aggregated human α-synuclein led to both earlier onset of severe motor dysfunction in and premature death of transgenic mice expressing mutated human α-synuclein [7-9]. In addition, intracerebral injection of similar inocula caused neurotoxic effects and neurological impairments in transmission experiments with wild-type mice [10]. Whether those harmful effects can be also caused by transmitted protein particles in humans who express mutated or normal α-synuclein, Aβ or tau is still unknown.
Testing the depletion of aggregated amyloid-β, tau and α-synuclein in carrier assays
Thus, the ability to decontaminate medical instruments from aggregated Aβ, tau and α-synuclein may potentially add to patient safety. When discussing this question, data on the efficacy of routinely applicable reprocessing procedures for medical instruments against Aβ-, tau- and α-synuclein aggregates can provide helpful guidance. For this reason, we assessed the activity of different reprocessing procedures against those contaminations in depletion assays that used stainless steel wire grids as surrogates for medical instruments. These assays were developed by adapting a method previously used to test the decontamination of medical devices from contaminations of infectious prion protein (for details see [11]). In brief: Two stainless steel wire grids (100 × 5 mm; DIN 1.4301, Spörl) each were contaminated with 20% (w/v) brain tissue homogenates from patients with AD or demential α-synuclein aggregation disease (SD) and jointly used for the analysis of the respective decontamination treatment. Grids were air-dried for 2 days at room temperature (RT) and subsequently incubated in the formulations for the time-periods and at the temperatures indicated in Figure 1. After incubation in the specified formulations, some grids were additionally steam sterilized at 134°C for the indicated time periods. Residual protein contaminations were eluted from pairs of jointly coiled up wire grids by boiling in 300 μl double-concentrated electrophoresis loading buffer and analysed by Western blotting. 12% Tris-Glycine gels were used for sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (SDS-PAGE), and after SDS-PAGE the whole gels (including the stacking gels) were blotted onto polyvinylidene difluoride membranes. The blots were incubated in the antibody- and blocking solutions specified in Table 1, and labelled proteins were visualized using CDP-star and Amersham Hyperfilm ECL.
Figure 1.
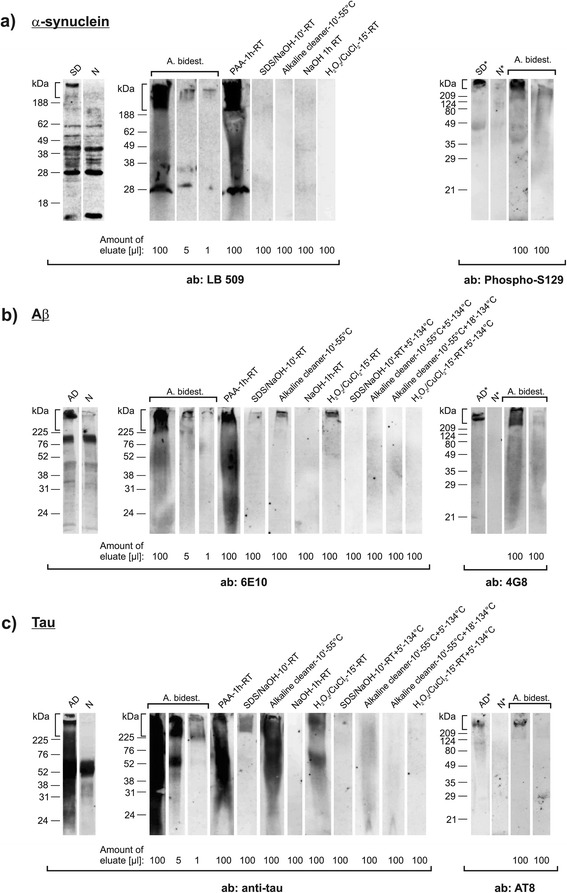
In vitro carrier assay for testing the depletion of aggregated human α-synuclein, amyloid-β and tau. Western blot detection of aggregated human α-synuclein (a), Aβ (b), and tau (c) by the indicated antibodies (Table 1) in protein eluates from steel wire grids that had been contaminated with 20% (w/v) brain tissue homogenates (BTH) from donors with SD (a) or AD (b and c). Lanes “SD” (a), “AD” (b and c) and “N” (a-c) (“SD*” [a], “AD*” [b and c] and “N*” [a-c]) represent 5 μl (or 20 μl in lanes marked with an asterisk) 20% (w/v) BTH from SD- and AD patients and control donors (without SD or AD), respectively. The identity of α-synuclein, Aβ, and tau aggregates in BTH and on steel wire grids washed with bi-distilled water was confirmed by two different antibodies each (most right lanes in a-c represent steel wire grids that had been contaminated with BTH from control donors). For testing the presence and depletion of aggregated α-synuclein, Aβ, and tau contaminated wires were processed by I) washing with bi-distilled water (a-c, lanes “A. bidest.”), or exposure to II) 0.25% (v/v) peracetic acid for 1 hour at room temperature (RT) (a-c, lanes “PAA-1 h-RT”), III) a mixture of 0.2% (w/v) SDS and 0.3% (w/v) NaOH (pH 12.7 - 12.9, non-adjusted) for 10 min. at RT (a-c, lanes “SDS/NaOH-10'-RT”), IV) an alkaline cleaner (0.5%, pH 11.6 - 12.0 [non-adjusted]) [13] for 10 min. at 55°C (a-c, lanes “Alkaline cleaner-10'-55°C”), V) 1 M NaOH (pH 13.5 - 13.8, non-adjusted) for 1 h at RT (a-c, lanes “NaOH-1 h-RT”), VI) a solution of 7.5% (w/v) H2O2 containing Cu2+ ions [14] (prepared in 100 mM NaCO3 [pH 9.5, adjusted] by adding CuCl2 to a final concentration of 500 μM) for 15 min. at RT (a-c, lanes “ H2O2/CuCl2-15′-RT”), VII) treatment as in III) with subsequent PVSS for 5 min. at 134°C (b and c, lanes “SDS/NaOH-10′-RT + 5′-134°C”), VIII) treatment as in IV) with subsequent PVSS for 5 min. (b and c, lanes “Alkaline cleaner-10′-55°C + 5′-134°C”) or 18 min. (b and c, lanes “Alkaline cleaner-10′-55°C + 18′-134°C”), or IX) treatment as in VI) with subsequent PVSS for 5 min. (b and c, lanes “H2O2/CuCl2-15′- RT + 5′-134°C”). PVSS was always performed at 3 bar. ab: antibody. Vertical brackets indicate insoluble α-synuclein-, Aβ- and tau aggregates retained in stacking gels.
Table 1.
Antibodies and blocking reagents for Western blot detection of aggregated human α-synuclein, amyloid-β and tau
| 1 st Antibody | Provider and product code | Epitope | Dilution | Blocking solution | 2 nd Antibody | Dilution |
|---|---|---|---|---|---|---|
| LB 509 (monoclonal) | Abcam, Cambridge, UK ab27766 | aa 115-122 of α-synuclein | 1:5,000 | 0.03% (w/v) Casein in TBS with 0.05% (w/v) Tween 20 | Alkaline-phosphatase-conjugated goat anti-mouse IgG (DAKO) | 1:5,000 |
| Phospho-S129 (monoclonal) | Abcam, Cambridge, UK ab51253 | α-synuclein phosphorylated at Ser129 | 1:2,000 | 3% (w/v) Low-fat milk powder in TBS with 0.05% (w/v) Tween 20 | Alkaline-phosphatase-conjugated goat anti-rabbit IgG (DAKO) | 1:10,000 |
| Anti-tau (monoclonal) | Merck Millipore, Darmstadt, Germany MAB2239 | C-terminal region of tau | 1:500 | 3% (w/v) Low-fat milk powder in TBS with 0.05% (w/v) Tween 20 | Alkaline-phosphatase-conjugated goat anti-mouse IgG (DAKO) | 1:10,000 |
| AT8 (monoclonal) | Thermo Scientific, Rockford, USA MN1020 | Phospho-PHF-tau (Ser202/Thr205) | 1:500 | 0.03% (w/v) Casein in TBS with 0.05% (w/v) Tween 20 | Alkaline-phosphatase-conjugated goat anti-mouse IgG (DAKO) | 1:10,000 |
| 6E10 (monoclonal) | Covance, Emeryville, USA SIG-39320 | aa 3-8 of Aβ | 1:2,000 | 5% (w/v) Low-fat choco powder in TBS with 0.05% (w/v) Tween 20a | Alkaline-phosphatase-conjugated goat anti-mouse IgG (DAKO) | 1:10,000 |
| 4G8 (monoclonal) | Covance, Emeryville, USA SIG-39220 | aa 17-24 of Aβ | 1:1,000 | 5% (w/v) Low-fat choco powder in TBS with 0.05% (w/v) Tween 20a | Alkaline-phosphatase-conjugated goat anti-mouse IgG (DAKO) | 1:10,000 |
aTina Zimmermann, personal communication and diploma thesis (2012, Establishment of the organotypic slice culture assay as a model for neurodegenerative diseases. Free University of Berlin, Berlin, Germany).
In our study we focused on large aggregates of pathological Aβ, tau and α-synuclein. These aggregates were largely retained in the stacking gel (indicated in Figure 1 by vertical brackets) and constituted the molecular species which seemed to be most difficult to wash off from the grids below the threshold of detection (Figure 1a-c, lanes “A. bidest. [100 μl], [5 μl], [1 μl]”). Furthermore, our rationale was based on the general assumption that depletion of such protein aggregates from medical instruments concomitantly reduces potentially harmful seeding effects. Recent findings by Duran-Aniotz and colleagues experimentally confirmed this rationale for Aβ aggregates from human AD brain extracts [12].
Substantial depletion of amyloid-β-, tau- and α-synuclein aggregates on test carriers by exposure to cleaners and steam sterilization
When we assessed the activity of prion-effective reprocessing procedures against Aβ-, tau and α-synuclein aggregates we found that these were simultaneously reduced up to 100-fold, and below the threshold of detection, by alkaline formulations, applied at RT, such as 1 M NaOH (Figure 1a-c, lanes “NaOH-1 h-RT”) or a mixture of 0.2% SDS and 0.3% NaOH without or with subsequent pre-vacuum steam sterilization (PVSS), respectively, for 5 minutes at 134°C (Figure 1a, lane “SDS/NaOH-10'-RT“ and Figure 1b and c, lanes ”SDS/NaOH-10′-RT + 5′-134°C”).
The same depletion effects were observed for α-synuclein aggregates after exposure to more material-friendly formulations such as a commercially available alkaline cleaner [13] for 10 minutes at 55°C (Figure 1a, lane “Alkaline cleaner-10′-55°C”), or a 7.5% H2O2 solution containing low concentrations of Cu2+ ions [14] for 15 minutes at RT (Figure 1a, lane “H2O2/CuCl2-15′-RT”). Similarly effective depletion of Aβ- and tau aggregates was achieved when treatments with the H2O2/CuCl2 formulation or the alkaline cleaner were followed by PVSS at 134°C for 5 or 18 minutes, respectively (Figure 1b and c, lanes “H2O2/CuCl2-15′-RT + 5′-134°C” and “Alkaline cleaner-10′-55°C + 18′-134°C”). These findings of our pilot study suggest that a simultaneous and quantifiably depletion of pathological Aβ-, tau-and α-synuclein aggregates is basically feasible by using prion-effective formulations in routine procedures for the reprocessing of selected medical devices. Peracetic acid (PAA), a commonly used disinfectant being not effective against prions, failed to achieve a detectable depletion of aggregated Aβ, tau and α-synuclein in our assays (Figure 1a-c, lanes “PAA-1 h-RT”).
How to deal with transmissible protein seeding in the reprocessing of medical devices?
It is open to discussion whether the findings from transmission studies in animals give reason to include specific preventive measures against pathological amyloid-β-, tau- and α-synuclein aggregates in the routine decontamination of certain medical devices. At the same time, studies specifically pursuing the identification and validation of such measures are still scarce. Therefore, available or newly developed assays should be used to experimentally assess on a broader scale the efficacy of reprocessing procedures for medical devices against AD-, PD- or DLB-associated protein aggregates. Ideally, such assessments would also include measurements of the reduction of seeding activity in vitro and/or in vivo. Broadening the data basis from both transmission and decontamination studies could greatly help to answer the question of how to reprocess medical devices in view of transmissible protein seeding.
Acknowledgements
We are grateful to Patrizia Reckwald for technical assistance. The German Federal Ministry of Health financially supported this work by sponsoring a study at the Robert Koch-Institute that addresses the safety of blood and medical devices in view of prion-like phenomena possibly associated with Alzheimer’s disease, Parkinson’s disease and other non-prion protein aggregation diseases (IIA5-2512NIK004//321-4471-02).
Abbreviations
- ab
Antibody
- Aβ
Amyloid-β
- AD
Alzheimer’s disease
- DLB
Dementia with Lewy bodies
- PAA
Peracetic acid
- PD
Parkinson’s disease
- PVSS
Pre-vacuum steam sterilization
- PrP
Prion protein
- RT
Room temperature
- SD
Demential α-synuclein aggregation disease
- SDS
Sodium dodecyl sulfate
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AT, conceived parts of the study and participated in its design, performed experiments, analysed data and designed the figure and table; KW, participated in the design of the study, performed experiments, and analysed data; MJ, performed experiments and analysed data; MLD, participated in the design of the study and analysed data; WJSS, participated in the design of the study, provided materials and analysed data; MM, participated in the design of the study and analysed data; MT, performed experiments and analysed data; MB, conceived parts of the study and participated in its design, analysed data and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Achim Thomzig, Email: ThomzigA@rki.de.
Katja Wagenführ, Email: WagenfuehrK@rki.de.
Martin L Daus, Email: DausM@rki.de.
Marion Joncic, Email: JoncicM@rki.de.
Walter J Schulz-Schaeffer, Email: wjschulz@med.uni-goettingen.de.
Marc Thanheiser, Email: ThanheiserM@rki.de.
Martin Mielke, Email: MielkeM@rki.de.
Michael Beekes, Email: BeekesM@rki.de.
References
- 1.Come JH, Fraser PE, Lansbury PT. A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci U S A. 1993;90:5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 4.Beekes M, Thomzig A, Schulz-Schaeffer WJ, Burger R. Is there a risk of prion-like disease transmission by Alzheimer- or Parkinson-associated protein particles? Acta Neuropathol. 2014;128:463–476. doi: 10.1007/s00401-014-1324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker LC, Jucker M. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritschi SK, Cintron A, Ye L, Mahler J, Bühler A, Baumann F, Neumann M, Nilsson KP, Hammarström P, Walker LC, Jucker M. Abeta seeds resist inactivation by formaldehyde. Acta Neuropathol. 2014;128:477–484. doi: 10.1007/s00401-014-1339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A. 2013;110:19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, Golde TE, Giasson BI. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A. 2014;111:10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, Fariñas I, Obeso JA, Bezard E, Vila M. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 11.Beekes M, Lemmer K, Thomzig A, Joncic M, Tintelnot K, Mielke M. Fast, broad-range disinfection of bacteria, fungi, viruses and prions. J Gen Virol. 2010;91:580–589. doi: 10.1099/vir.0.016337-0. [DOI] [PubMed] [Google Scholar]
- 12.Duran-Aniotz C, Morales R, Moreno-Gonzalez I, Hu PP, Fedynyshyn J, Soto C. Aggregate-depleted brain fails to induce Abeta deposition in a mouse model of Alzheimer’s disease. PLoS One. 2014;9:e89014. doi: 10.1371/journal.pone.0089014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baier M, Schwarz A, Mielke M. Activity of an alkaline ‘cleaner’ in the inactivation of the scrapie agent. J Hosp Infect. 2004;57:80–84. doi: 10.1016/j.jhin.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann S, Rauwel GPM, Rogez-Kreuz C, Richard M, Belondrade M, Rauwel G, Durand F, Yousfi R, Criquelion J, Clayette P, Perret-Liaudet A. New hospital disinfection processes for both conventional and prion infectious agents compatible with thermosensitive equipment. J Hosp Infect. 2009;72:342–350. doi: 10.1016/j.jhin.2009.03.024. [DOI] [PubMed] [Google Scholar]


