Abstract
The chemotaxis of neutrophils has been shown to be modulated by serum factors, tissue factors, bacterial products, and a host of other substances. In vivo, these factors may act in concert with each other to modify neutrophil movement. We examined the effect of aggregated gamma globulin-activated serum (AS), bacterial factors, and endotoxin either alone or in combination with each other, on human neutrophil chemotaxis. Exposure of neutrophils to AS resulted in deactivation to AS but not to Escherichial coli or Staphylococcus epidermis culture filtrate. Exposure of neutrophils to S. epidermis or E. coli CF or E. coli endotoxin resulted in deactivation to AS or C5a but not to E. coli or S. epidermis culture filtrate. Addition of endotoxin to AS or C5a resulted in inhibition of chemotaxis by untreated neutrophils toward this combination as compared with AS alone. These results suggest that separate mechanisms may be involved when serum or bacterial chemotactic factors initiate human neutrophil chemotaxis. Furthermore, the potent but specific inhibitory effect of endotoxin on chemotaxis toward AS may be of clinical significance.
Full text
PDF

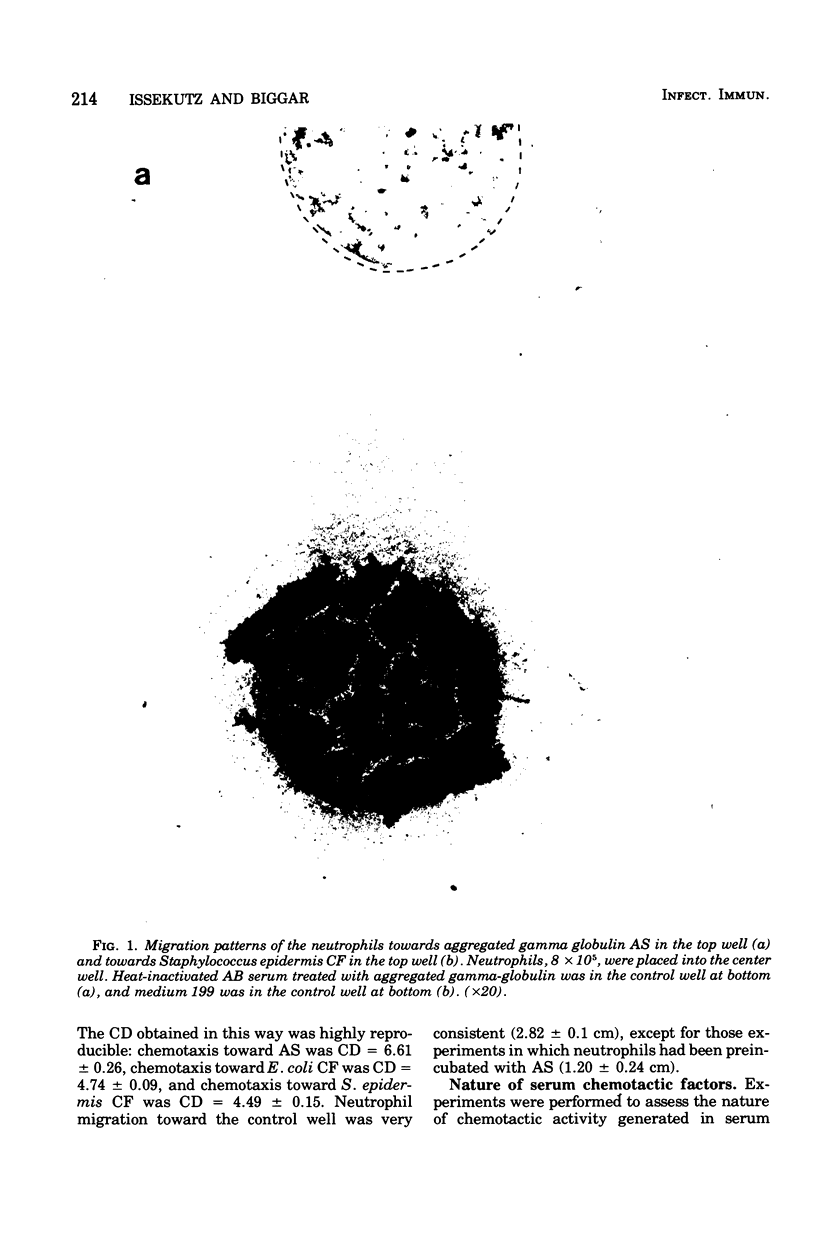
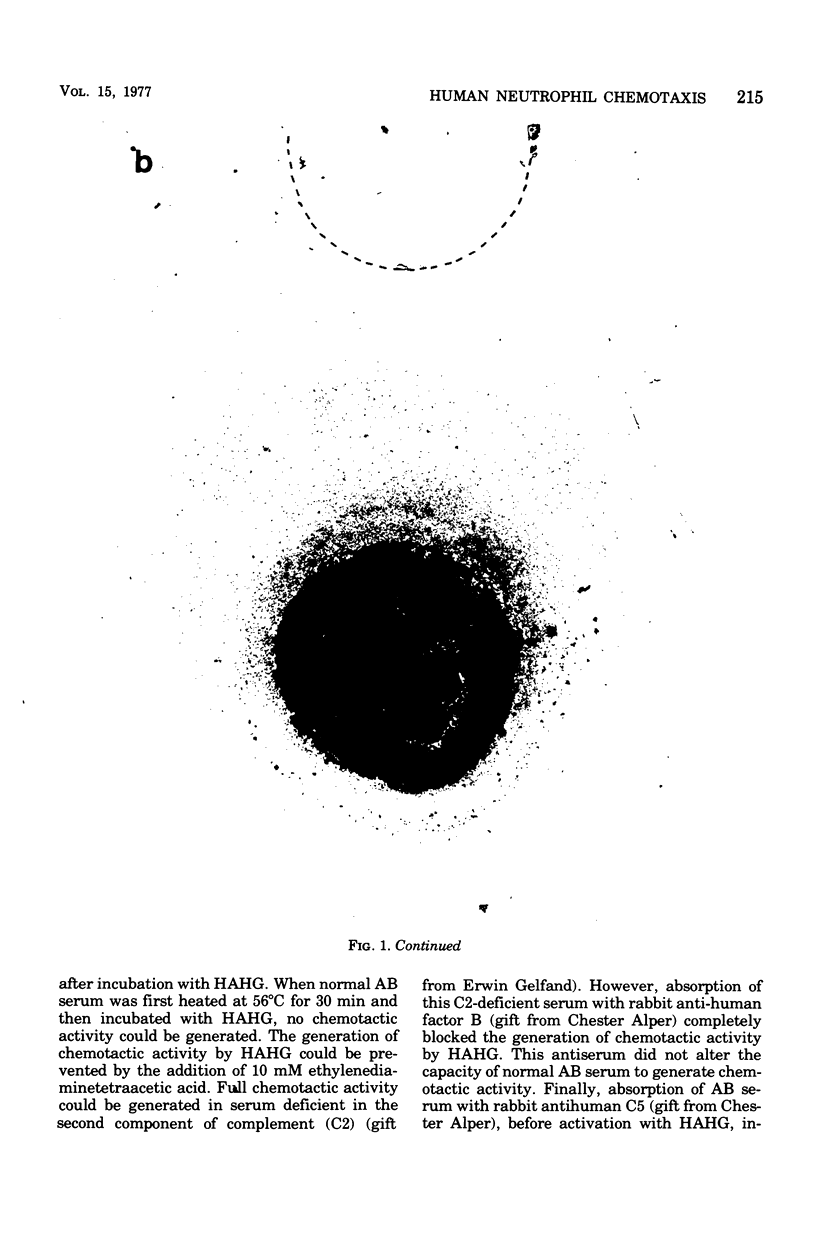




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L. The relationship of the chemotactic behavior of the complement-derived factors, C3a, C5a, and C567, and a bacterial chemotactic factor to their ability to activate the proesterase 1 of rabbit polymorphonuclear leukocytes. J Exp Med. 1972 Feb 1;135(2):376–387. doi: 10.1084/jem.135.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Caner J. E. Colchicine inhibition of chemotaxis. Arthritis Rheum. 1965 Oct;8(5):757–764. doi: 10.1002/art.1780080438. [DOI] [PubMed] [Google Scholar]
- Chang Y. H. Mechanism of action of colchicine. II. Effects of colchicine and its analogs on phagocytosis and chemotaxis in vitro. J Pharmacol Exp Ther. 1975 Jul;194(1):159–164. [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Jacob H. S. Complement-mediated granulocyte dysfunction in paroxysmal nocturnal hemoglobinuria. Blood. 1976 Jun;47(6):931–939. [PubMed] [Google Scholar]
- Cutler J. E., Munoz J. J. A simple in vitro method for studies on chemotaxis. Proc Soc Exp Biol Med. 1974 Nov;147(2):471–474. doi: 10.3181/00379727-147-38367. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimber P. E., Rafter G. W. The interaction of Escherichia coli endotoxin with leukocytes. Arch Biochem Biophys. 1969 Dec;135(1):14–20. doi: 10.1016/0003-9861(69)90510-4. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T. J., Sieber O. F., Jr Chemotactic migration of neutrophils under agarose. Life Sci. 1976 Jan 15;18(2):177–181. doi: 10.1016/0024-3205(76)90022-9. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Kaplan A. P. Chemotaxis and haemostasis. Br J Haematol. 1975 Dec;31(4):417–422. doi: 10.1111/j.1365-2141.1975.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Kay A. B., Thompson R. A. The chemotactic activity for neutrophil and eosinophil leucocytes of the trimolecular complex of the fifth, sixth and seventh components of human complement (C567) prepared in free solution by the 'reactive lysis' procedure. Immunology. 1970 Dec;19(6):895–899. [PMC free article] [PubMed] [Google Scholar]
- Miller M. E. Pathology of chemotaxis and random mobility. Semin Hematol. 1975 Jan;12(1):59–82. [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Inhibition of lipopolysaccharide-initiated activation of serum complement by polymyxin B. Infect Immun. 1976 Jan;13(1):298–301. doi: 10.1128/iai.13.1.298-301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Phelps P., McCarty D. J., Jr Crystal-induced arthritis. Postgrad Med. 1969 Jan;45(1):87–93. doi: 10.1080/00325481.1969.11696985. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Showell H. V., Corcoran B. A., Ward P. A., Smith E., Becker E. L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975 Jun;114(6):1831–1837. [PubMed] [Google Scholar]
- Snyderman R., Phillips J., Mergenhagen S. E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and Guinea pig serum treated with immune complexes: evidence for c5a as the major chemotactic factor. Infect Immun. 1970 Jun;1(6):521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer G. F., Adye J. C. Endotoxin-binding substances from human leukocytes and platelets. Infect Immun. 1975 Nov;12(5):978–986. doi: 10.1128/iai.12.5.978-986.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo M. C., Golde D. W. Granulocyte function in experimental human endotoxemia. Blood. 1976 Apr;47(4):539–544. [PubMed] [Google Scholar]
- Van Epps D. E., Andersen B. R. Streptolysin O inhibition of neutrophil chemotaxis and mobility: nonimmune phenomenon with species specificity. Infect Immun. 1974 Jan;9(1):27–33. doi: 10.1128/iai.9.1.27-33.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G., MUELLER-EBERHARD H. J. THE ROLE OF SERUM COMPLEMENT IN CHEMOTAXIS OF LEUKOCYTES IN VITRO. J Exp Med. 1965 Aug 1;122:327–346. doi: 10.1084/jem.122.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. Biochemical demonstration of the activatable esterase of the rabbit netrophil involved in the chemotactic response. J Immunol. 1970 Nov;105(5):1057–1067. [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. Neutrophil chemotactic factors and related clinical disorders. Arthritis Rheum. 1970 Mar-Apr;13(2):181–186. doi: 10.1002/art.1780130210. [DOI] [PubMed] [Google Scholar]