Abstract
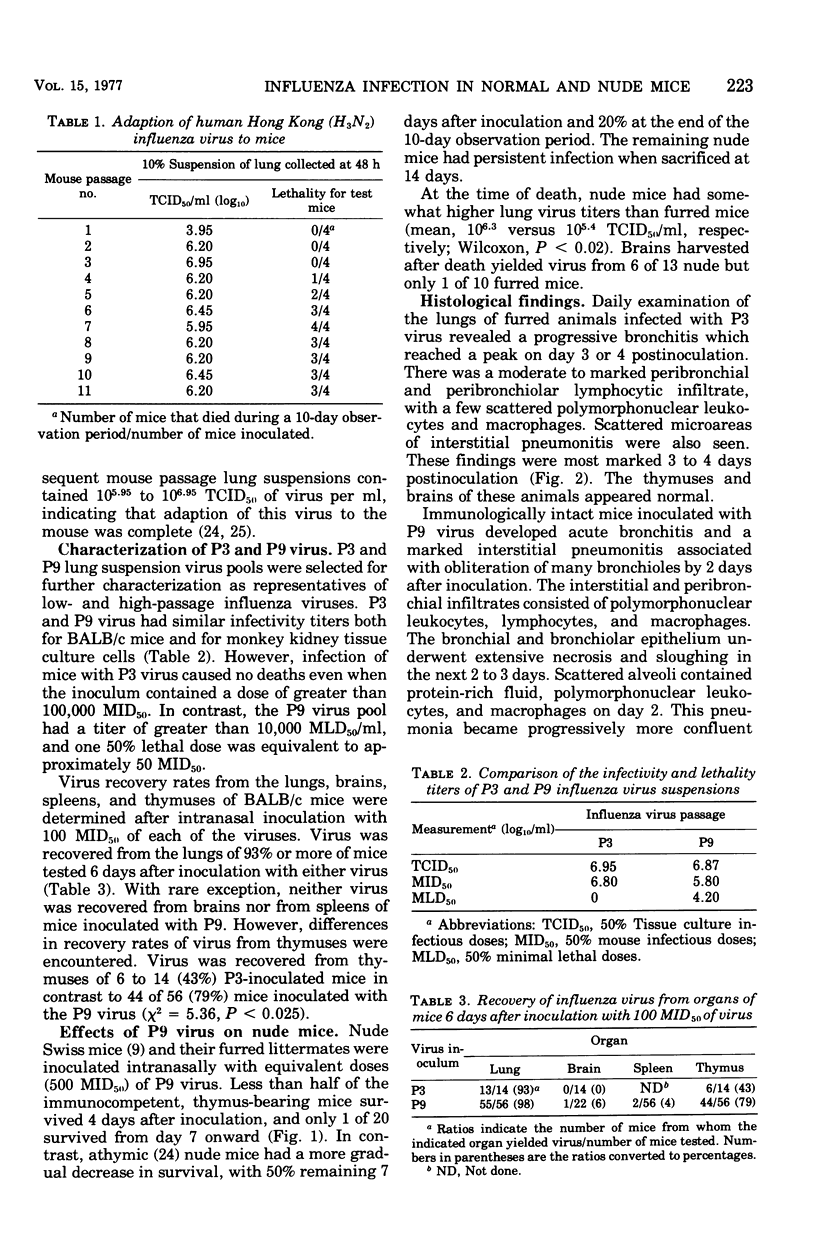
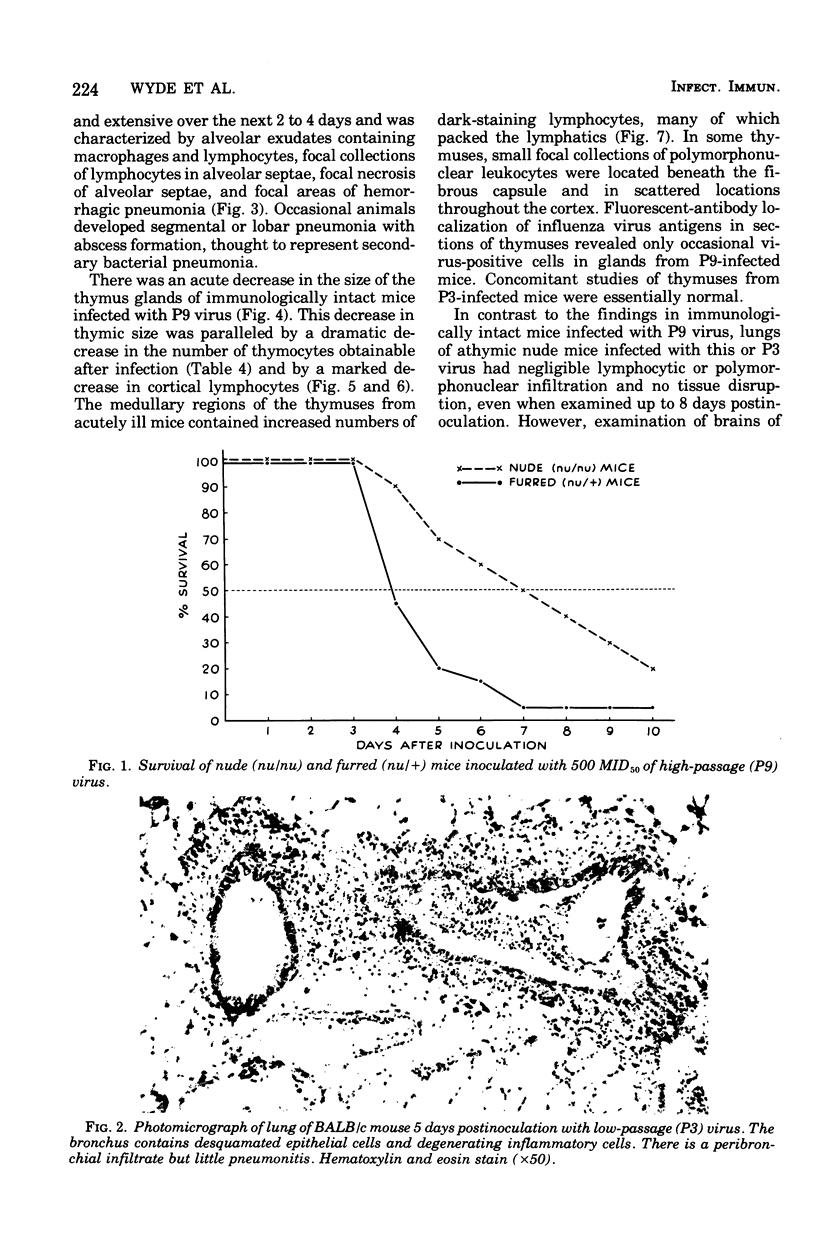
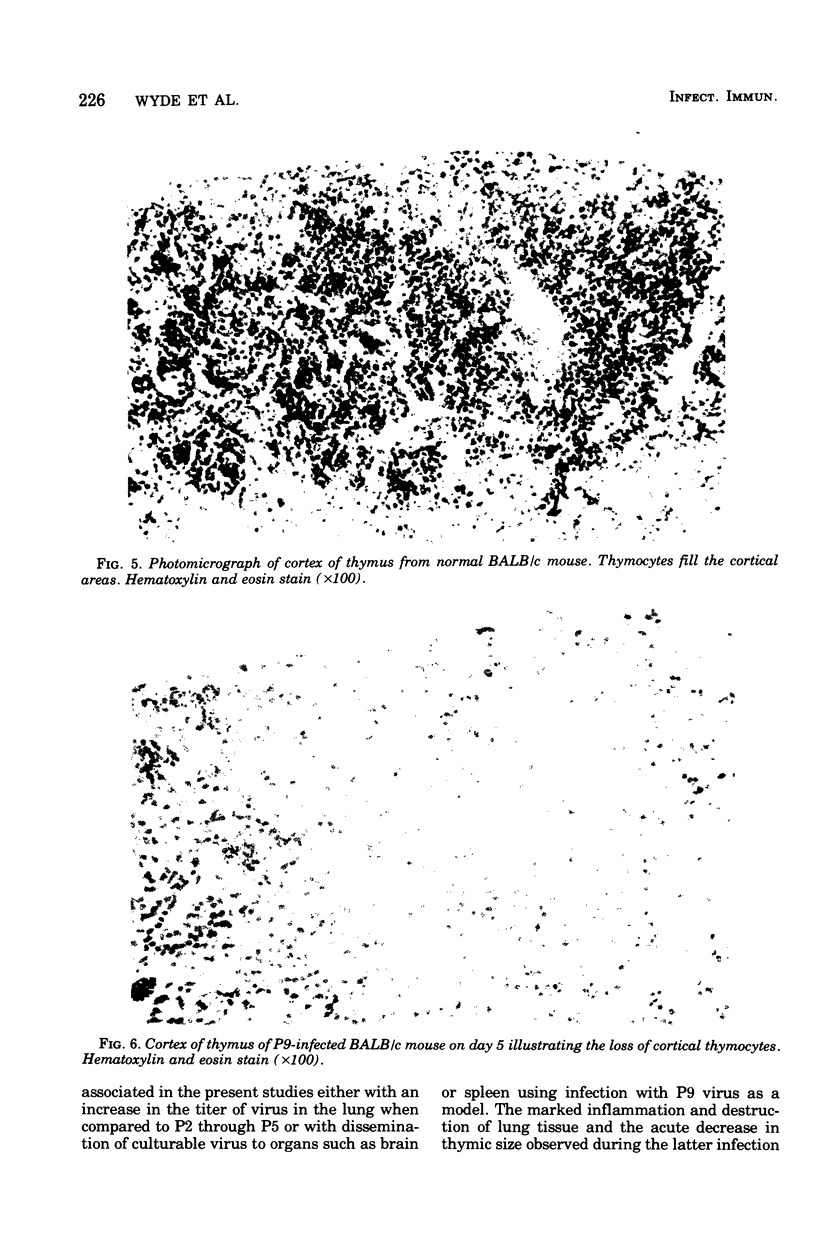
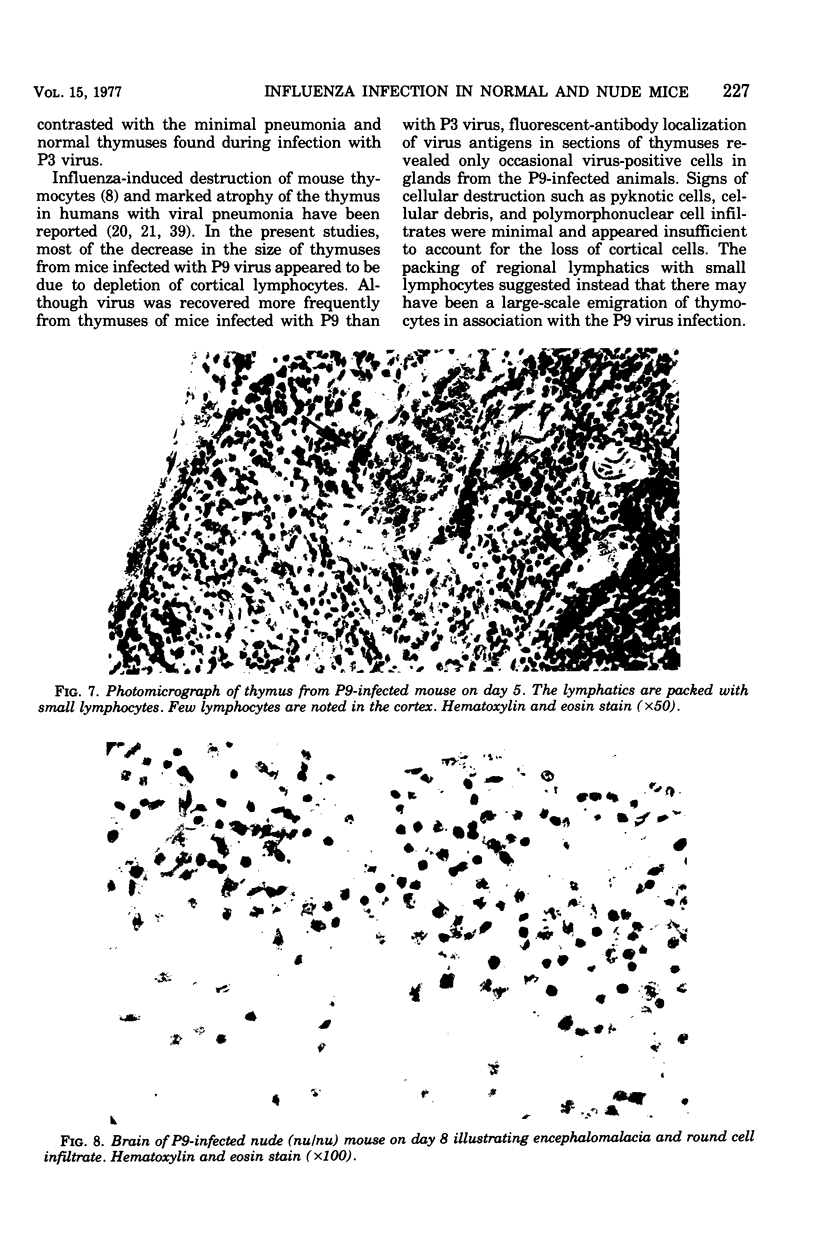
A human isolate of type A Hong Kong influenza virus (H3N2) was adapted to mice by serial passage. Lung homogenates from mice who received low passage levels contained about the same quantity of virus (106.2−6.95 50% tissue culture infective doses/ml) as those from mice who received high passage levels (105.95−6.45 50% tissue culture infective doses/ml); however, death occurred only in animals given high-passage virus. Passage 3 (P3) and passage 9 (P9) viruses were selected as representative of low-passage and high-passage viruses, respectively. Although minimal differences were detected in infectivity for rhesus monkey kidney tissue cultures and mice, P9 virus was at least 10,000 times more lethal for mice (mean lethal dose = 104.2). Infection with P3 virus was accompanied by minimal bronchitis and bronchiolitis only, whereas P9-infected animals exhibited marked bronchitis, bronchiolitis, and pneumonia. Striking thymic cortical atrophy was also demonstrable in the P9-infected animals and, although virus was more commonly recovered from thymuses from these animals, immunofluorescent studies revealed only a few cells containing influenza virus antigens. To further explore the participation of thymus-derived lymphocytes in influenza, athymic nude mice and furred immunocompetent littermates were given 500 50% mouse infectious doses of P9 virus. Nude mice exhibited an increased survival time and, in contrast to the extensive lung pathology seen in furred littermates, manifested minimal cellular infiltration and no tissue destruction in lungs. Brains from nude mice exhibited encephalomalacia with lymphocytic perivascular cuffing, which was not seen in furred animals. Virus was recovered from brains of 6 of 13 nude mice and 1 of 10 furred animals. The contrasting models suggest that thymus-dependent cells play a significant role in the inflammatory response to influenza virus infection and should prove useful for probing host-virus interactions which characterize influenza virus virulence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cambridge G., Mackenzie J. S., Keast D. Cell-mediated immune response to influenza virus infections in mice. Infect Immun. 1976 Jan;13(1):36–43. doi: 10.1128/iai.13.1.36-43.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate T. R., Mold N. G. Increased influenza pneumonia mortality of mice adoptively immunized with node and spleen cells sensitized by inactivated but not live virus. Infect Immun. 1975 May;11(5):908–914. doi: 10.1128/iai.11.5.908-914.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAZEKAS DE ST GROTH S., DONNELLEY M., GRAHAM D. M. Studies in experimental immunology of influenza. VIII. Pathotopic adjuvants. Aust J Exp Biol Med Sci. 1951 Sep;29(5):323–327. doi: 10.1038/icb.1951.39. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Beachey E. H., Rytel M. W. Induction of delayed hypersensitivity to influenza and mumps viruses in mice. J Immunol. 1969 Oct;103(4):844–849. [PubMed] [Google Scholar]
- Garaci E., Caliò R., Djaczenko W. Effect of influenza virus PR8 infection on thymus in intact and adrenalectomized mice. Experientia. 1974 Apr 15;30(4):358–360. doi: 10.1007/BF01921662. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Merchant B., Gelfand M. C., Vickers J., Steinberg A. D., Hansen C. T. The natural history and immunopathology of outbred athymic (nude) mice. Clin Immunol Immunopathol. 1975 Sep;4(3):324–340. doi: 10.1016/0090-1229(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Greenberg S. B., Criswell B. S., Couch R. B. Lymohocyte-mediated cytotoxicity against influenza virus-infected cells: an in vitro method. J Immunol. 1975 Aug;115(2):601–603. [PubMed] [Google Scholar]
- HERS J. F., MUDLER J., MASUREL N., vd KUIP L., TYRRELL D. A. Studies on the pathogenesis of influenza virus pneumonia in mice. J Pathol Bacteriol. 1962 Jan;83:207–217. [PubMed] [Google Scholar]
- Habershon R. B., Molyneux M. E., Slavin G., Loewi G., Tyrrell D. A. Skin tests with influenza virus. J Hyg (Lond) 1973 Dec;71(4):755–760. doi: 10.1017/s0022172400023019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman A., Fowler A. K., Steinman H. G., Buzzerd P. M. Studies of the blastogenic response of murine lymphocyte. 3. Specific viral transformation. Proc Soc Exp Biol Med. 1972 Oct;141(1):106–109. doi: 10.3181/00379727-141-36726. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Nahmias A. J., Murphy F. A., Kramer J. H. Cellular immunity in vaccinia infection of mice. Anti-thymocyte serum effects on primary and secondary responsiveness. J Exp Med. 1968 Jul 1;128(1):121–132. doi: 10.1084/jem.128.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimura T., Hirano A., Okuno Y. The nature of the immunity evoked by infection of mice with avirulent influenza virus. Biken J. 1972 Mar;15(1):31–37. [PubMed] [Google Scholar]
- Loosli C. G., Buckley R. D., Hardy J. D., Hertweck M. S., Kow S. Y., Serebrin R., Ryan D. P., Stinson S. F. The pathogenesis of postinfluenzal collapse of the lungs of mice. Trans Assoc Am Physicians. 1971;84:182–189. [PubMed] [Google Scholar]
- Loosli C. G., Hertweck M. S., Hockwald R. S. Airborne influenza PR8-A virus infections in actively immunized mice. Arch Environ Health. 1970 Sep;21(3):332–346. doi: 10.1080/00039896.1970.10667248. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Kasel J. A., Saglam M., Knight V., Loda F. A. Immunity to influenza to antibody levels. N Engl J Med. 1966 Mar 10;274(10):527–535. doi: 10.1056/NEJM196603102741001. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Pathogenicity and immunogenicity of various strains of influenza virus for mice. Biken J. 1965 Sep;8(3):155–165. [PubMed] [Google Scholar]
- Pantelouris E. M. Absence of thymus in a mouse mutant. Nature. 1968 Jan 27;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- Ruben F. L., Jackson G. G., Gotoff S. P. Humoral and cellular response in humans after immunization with influenza vaccine. Infect Immun. 1973 Apr;7(4):594–596. doi: 10.1128/iai.7.4.594-596.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk E. A., Churukian C. J. Immunofluorescence counterstains. J Histochem Cytochem. 1974 Oct;22(10):962–966. doi: 10.1177/22.10.962. [DOI] [PubMed] [Google Scholar]
- Singer S. H., Noguchi P., Kirschstein R. L. Respiratory diseases in cyclophosphamide-treated mice. II. Decreased virulence of PR8 influenza virus. Infect Immun. 1972 Jun;5(6):957–960. doi: 10.1128/iai.5.6.957-960.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Mayner R. E., Barry D. W., Ennis F. A. Influenza virus infection in nude mice. J Infect Dis. 1976 Jan;133(1):91–94. doi: 10.1093/infdis/133.1.91. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Oya J., Ishida N. Effect of antilymphocyte serum on influenza virus infection in mice. Proc Soc Exp Biol Med. 1974 May;146(1):78–84. doi: 10.3181/00379727-146-38047. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L. Host defenses against influenza virus: the role of anti-hemagglutinin antibody. J Immunol. 1975 Aug;115(2):434–439. [PubMed] [Google Scholar]
- Virelizier J. L., Postlethwaite R., Schild G. C., Allison A. C. Antibody responses to antigenic determinants of influenza virus hemagglutinin. I. Thymus dependence of antibody formation and thymus independence of immunological memory. J Exp Med. 1974 Dec 1;140(6):1559–1570. doi: 10.1084/jem.140.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]
- Webster R. G. The immune response to influenza virus. I. Effect of the route and schedule of vaccination on the time course of the immune response, as measured by three serological methods. Immunology. 1965 Dec;9(6):501–519. [PMC free article] [PubMed] [Google Scholar]
- Wetherbee R. E. Induction of systemic delayed hypersensitivity during experimental viral infection of the respiratory tract with a myxovirus or paramyxovirus. J Immunol. 1973 Jul;111(1):157–163. [PubMed] [Google Scholar]
- White R. G., Boyd J. F. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973 Mar;13(3):343–357. [PMC free article] [PubMed] [Google Scholar]










