Abstract
Introduction
Temperature changes are common in patients in a neurosurgical intensive care unit (NICU): fever is frequent among severe cases and hypothermia is used after cardiac arrest and is currently being tested in clinical trials to lower intracranial pressure (ICP). This study investigated cerebral hemodynamics when body temperature varies in acute brain injured patients.
Methods
We enrolled 26 patients, 14 with acute brain injury who developed fever and were given antipyretic therapy (defervescence group) and 12 who underwent an intracranial neurosurgical procedure and developed hypothermia in the operating room; once admitted to the NICU, still under anesthesia, they were re-warmed before waking (re-warming group). We measured cerebral blood flow velocity (CBF-V) and pulsatility index (PI) at the middle cerebral artery using transcranial color-coded duplex sonography (TCCDS).
Results
In the defervescence group mean CBF-V decreased from 75 ± 26 (95% CI 65 to 85) to 70 ± 22 cm/s (95% CI 61 to 79) (P = 0.04); the PI also fell, from 1.36 ± 0.33 (95% CI 1.23 to 1.50) to 1.16 ± 0.26 (95% CI 1.05 to 1.26) (P = 0.0005). In the subset of patients with ICP monitoring, ICP dropped from 16 ± 8 to 12 ± 6 mmHg (P = 0.003). In the re-warming group mean CBF-V increased from 36 ± 10 (95% CI 31 to 41) to 39 ± 13 (95% CI 33 to 45) cm/s (P = 0.04); the PI rose from 0.98 ± 0.14 (95% CI 0.91 to 1.04) to 1.09 ± 0.22 (95% CI 0.98 to 1.19) (P = 0.02).
Conclusions
Body temperature affects cerebral hemodynamics as evaluated by TCCDS; when temperature rises, CBF-V increases in parallel, and viceversa when temperature decreases. When cerebral compliance is reduced and compensation mechanisms are exhausted, even modest temperature changes can greatly affect ICP.
Introduction
Temperature changes are common in patients in a neurosurgical intensive care unit (NICU) where fever is frequent, especially in the most severe patients [1]. In fact, hypothermia is applied for survivors of cardiac arrest and is currently being tested in clinical trials to lower intracranial pressure (ICP) after traumatic brain injury [2].
In experimental studies fever has negative effects on the brain, because it increases metabolism, glutamate release, free radical production and blood-brain barrier permeability, and worsening edema [3]. Of additional concern is the effect on ICP. Our group [4] simultaneously measured brain temperature (Tbrain) and ICP, showing that ICP rises with fever onset and declines after defervescence; others subsequently confirmed this relationship [5,6]. Conversely, when hypothermia is applied the cerebral metabolism is slowed, and cerebral blood flow (CBF) falls. These effects both contribute to lower ICP [7]. The relationships between temperature and CBF are therefore of clinical interest, in view of the parallel changes in ICP.
Ideally, CBF at various Tbrain should be explored but unfortunately there are obvious limitations to this: Tbrain requires special probes, and some techniques marketed for CBF monitoring induce Tbrain changes themselves in order to quantify local CBF, so they do not seem appropriate for assessing CBF in response to spontaneous changes. A different technique, transcranial color-coded duplex sonography (TCCDS), measures CBF velocity (CBF-V) in a particular tract of a specific vessel. From this measurement, CBF is estimated according to a mathematical equation [8].
Core body temperature (Tcore) is the most widely available proxy of brain temperature itself [4]. In this study we investigated cerebral hemodynamics using TCCDS, at different body temperatures in patients with acute brain injury. We explored two conditions: (1) defervescence, when febrile patients were treated with antipyretic drugs and (2) re-warming, after intracranial neurosurgical procedures.
Materials and methods
The study involved 26 patients admitted to the NICU at the Ospedale Maggiore Policlinico of Milan. Of these patients, 14 (8 male, 6 female, age 54 ± 16 years) with acute brain injury developed fever and were given antipyretic therapy (defervescence group), and 12 patients (7 male, 5 female, age 56 ± 14) underwent an intracranial neurosurgical procedure under general anesthesia and suffered unexpected hypothermia; these patients were re-warmed once admitted to the NICU, before waking (re-warming group). Their main epidemiological data are shown in Tables 1 and 2.
Table 1.
Main epidemiological data: defervescence group
| Patient | Pathology | ICP catheter | GCS m | Starting T core (°C) | Final T core (°C) | Antipyretic drug | Physical therapy |
|---|---|---|---|---|---|---|---|
| 1 | TBI | + | 1 | 38.9 | 38.3 | + | + |
| 2 | ICH | + | 6 | 38.5 | 37.5 | + | + |
| 3 | SAH | + | 6 | 38.5 | 37.2 | + | + |
| 4 | TBI | + | 2 | 39.1 | 37.9 | + | - |
| 5 | SAH | - | 6 | 39.0 | 37.7 | + | - |
| 6 | TBI | + | 3 | 38.8 | 38.0 | + | - |
| 7 | TBI | - | 6 | 39.3 | 38.2 | + | - |
| 8 | TBI | - | 5 | 39.9 | 38.4 | + | - |
| 9 | TBI | - | 6 | 38.8 | 37.3 | + | - |
| 10 | SAH | - | 6 | 39.5 | 38.5 | + | + |
| 11 | TBI | + | 5 | 39.1 | 37.9 | + | - |
| 12 | SAH | + | 5 | 38.9 | 37.9 | + | - |
| 13 | SAH | + | 6 | 39.3 | 38.2 | + | - |
| 14 | ICH | - | 5 | 39.8 | 38.8 | + | - |
TBI, traumatic brain injury; ICH, intraparenchymal hemorrhage; SAH subarachnoid hemorrhage, ICP, intracranial pressure; GCSm Glasgow coma scale motor response; Tcore core temperature.
Table 2.
Main epidemiological data: re-warming group
| Patient | Pathology | Starting T core (°C) | Final T core (°C) |
|---|---|---|---|
| 1 | Intra-axial neoplasm | 33.7 | 34.7 |
| 2 | Intra-axial neoplasm | 35.5 | 36.2 |
| 3 | Intra-axial neoplasm + aneurysm | 35.5 | 36.5 |
| 4 | Intra-axial neoplasm | 35.9 | 36.6 |
| 5 | Extra-axial neoplasm | 36.5 | 37.2 |
| 6 | Intra-axial neoplasm | 35.5 | 36.5 |
| 7 | Intra-axial neoplasm | 36.3 | 37.0 |
| 8 | Aneurysm | 36.3 | 37.1 |
| 9 | Extra-axial neoplasm | 35.4 | 36.3 |
| 10 | Aneurysm | 36.1 | 36.7 |
| 11 | Intra-axial neoplasm | 35.0 | 35.7 |
| 12 | Extra-axial neoplasm | 34.5 | 35.5 |
Tcore core temperature.
Tcore was measured in the bladder or the pharynx. None of the other parameters, such as the ventilatory setting and drug infusions (particularly sedatives and analgesics), were modified during the study.
Defervescence group
Data were collected at two times: with fever (defined as a Tcore ≥38.4°C [9]) and after defervescence (a temperature reduction of at least 0.7°C) induced by antipyretic drugs, acetaminophen or diclofenac (Table 1). Eight patients had ICP monitoring and we calculated the cerebral perfusion pressure (CPP) as:
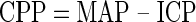 |
where MAP is mean arterial pressure. In six patients the internal jugular bulb was cannulated for intermittent determination of oxygen saturation in the jugular vein (SjO2).
Re-warming group
Patients in this group suffered an unexpected drop in Tcore during general anesthesia. Data were collected immediately when they entered the NICU and when the temperature target, a Tcore increase ≥0.7°C, was achieved by active warming. We used a convective air warming system connected to a full-body blanket (WarmTouch™ Convective Air Warming System, Covidien, Boulder, CO, USA). ICP and SjO2 were not monitored in this group. All patients had uneventful post-surgery recovery.
TCCDS measurements
We used the iE33 xMATRIX Echography System (Koninklijke Philips Electronics NV, Amsterdam, The Netherlands) for TCCDS, measuring CBF-V and pulsatility index (PI) in the middle cerebral artery (MCA), at the M1 tract, the most proximal to the internal carotid artery bifurcation. The measurements were always taken at the same depth and insonation angle, with very accurate angle correction, in order to ensure they were comparable. We insonated the MCA bilaterally in 21 patients, and on the right side only in 3 patients, and on the left side in 2 patients, because there was no adequate controlateral insonation window. We insonated the MCA at a mean depth of 54 ± 3 mm on the right and 54 ± 7 mm on the left; the angle was 22 ± 19° on the right and 27 ± 13° on the left.
Statistical analysis
We used two statistical software packages: Prism 5.00 (GraphPad Software, San Diego, CA USA) and SPSS 7.0 (IBM Software, Armonk, NY, USA). All data were normally distributed (checked with D’Agostino-Pearson omnibus normality test) so they are all summarized as mean ± standard deviation.
Our analysis consisted of two steps: (1) first, the paired t-test was used to compare TCCDS measurements before and after defervescence or warming; (2) then, we built a general linear model for repeated measures, in order to verify the effect of three independent variables on CBF-V changes: insonation side (right or left), method used to modify temperature (defervescence or re-warming) and temperature itself (Tcore). A probability (P-value) <0.05 was accepted as significant.
Ethics
The interventions described (antipyretic treatment in the case of fever and re-warming after neurosurgery) are part of routine management. Monitoring, including continuous invasive ICP and intermittent TCCDS recording, is also part of the standard of care. No informed consent was sought for this specific study. Relatives of unconscious patients were informed in writing that monitoring data, once anonymized, might be used for research. Our hospital institutional ethics committee (Comitato Etico of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico) was notified of the study, and approved it, waiving the need for consent.
Results
Defervescence group
We studied patients on average 7 ± 6 days after brain injury. The mean Tcore fell from 39.1 ± 0.4 to 37.9 ± 0.5°C. Cerebral monitoring showed a reduction in ICP from 16 ± 8 to 12 ± 6 mmHg (P = 0.003) (Figure 1A) although CPP did not change (Figure 1B). CBF-V decreased: mean velocity from 75 ± 26 (95% CI 65, 85) to 70 ± 22 cm/s (95% CI 61, 79) (P = 0.04) and systolic peak velocity from 144 ± 54 (95% CI 123, 166) to 126 ± 41 (95% CI 110, 142) cm/s (P = 0.002). The PI dropped from 1.36 ± 0.33 (95% CI 1.23, 1.50) to 1.16 ± 0.26 (95% CI 1.05, 1.26) (P = 0.0005) (Figure 2A).
Figure 1.
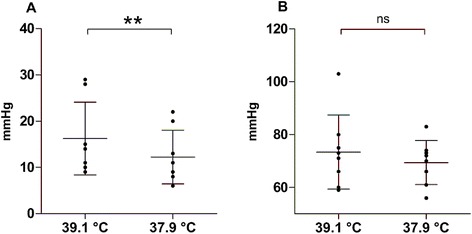
Data are presented as mean ± standard deviation; every dot represents the value of the considered parameter in one patient. (A) Mean difference in intracranial pressure (ICP) between the two time points: before (mean core temperature 39.1°C) and after defervescence (mean core temperature 37.9°C) (8 patients). (B) corresponding mean difference in cerebral perfusion pressure (CPP). Paired t-test: ns, not significant, **P <0.01.
Figure 2.
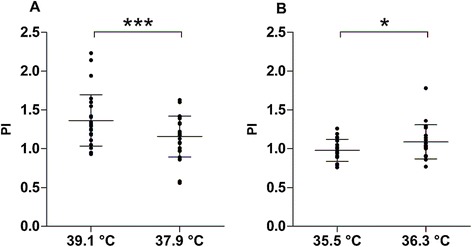
Data are presented as mean ± standard deviation; every dot represents the value of the considered parameter in one artery. (A) Mean difference in pulsatility index (PI) between the two time points in the defervescence group: during fever (mean core temperature 39.1°C) and after defervescence (mean core temperature 37.9°C) (14 patients). (B) Mean difference in PI between the two time points in the re-warming group: before (mean core temperature 35.5°C) and after re-warming (mean core temperature 36.3°C) (12 patients). Paired t-test. *P <0.05, ***P <0.001.
Systemic monitoring showed a reduction in HR from 86 ± 17 to 80 ± 16 bpm (P = 0.006) and MAP from 91 ± 14 to 85 ± 15 mmHg (P = 0.02); other parameters were stable (Table 3).
Table 3.
Blood gases and systemic monitoring
| Parameter | Defervescence group | Re-warming group | ||||
|---|---|---|---|---|---|---|
| (n = 14) | (n = 12) | |||||
| Before | After | P-value | Before | After | P-value | |
| T core (°C) | 39.1 ± 0.4 | 37.9 ± 0.5 | <0.0001 | 35.5 ± 0.8 | 36.3 ± 0.7 | <0.0001 |
| P a CO 2 (mmHg) | 33 ± 5 | 34 ± 5 | 0.08 | 33 ± 4 | 34 ± 5 | 0.43 |
| pH a | 7.48 ± 0.04 | 7.48 ± 0.04 | 0.15 | 7.45 ± 0.05 | 7.44 ± 0.07 | 0.80 |
| lactate (mmol/L) | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.00 | 1.3 ± 0.8 | 1.1 ± 0.7 | 0.01 |
| MAP (mmHg) | 91 ± 14 | 85 ± 15 | 0.02 | 82 ± 9 | 91 ± 15 | 0.01 |
| HR (bpm) | 86 ± 17 | 80 ± 16 | 0.006 | 64 ± 12 | 67 ± 13 | 0.10 |
Tcore, core temperature; PaCO2, arterial carbon dioxide partial pressure; pHa, arterial pH; MAP, mean arterial pressure; HR, heart rate. The P-value is reported for each parameter.
Re-warming group
On average, the Tcore rose from 35.5 ± 0.8 to 36.3 ± 0.7°C. CBF-V increased: mean velocity from 36 ± 10 (95% CI 31, 41) to 39 ± 13 (95% CI 33, 45) cm/s (P = 0.04) and systolic peak velocity from 59 ± 18 (95% CI 51, 67) to 68 ± 20 (95% CI 58, 77) cm/s (P = 0.0004). The PI rose from 0.98 ± 0.14 (95% CI 0.91, 1.04) to 1.09 ± 0.22 (95% CI 0.98, 1.19) (P = 0.02) (Figure 2B).
Systemic monitoring showed an increase in MAP from 82 ± 9 to 91 ± 15 mmHg (P = 0.01) and a reduction in arterial lactate from 1.3 ± 0.8 to 1.1 ± 0.7 (P = 0.01); other parameters were stable (Table 3).
The general linear model for repeated measures analysis confirmed that the only independent variable that explained CBF-V changes was Tcore (P = 0.01); the other two independent variables (insonation side and method used to modify temperature) could not predict changes in CBF-V, by Greenhouse-Geisser, Huynh-Feldt or lower-bound analysis (Figure 3).
Figure 3.
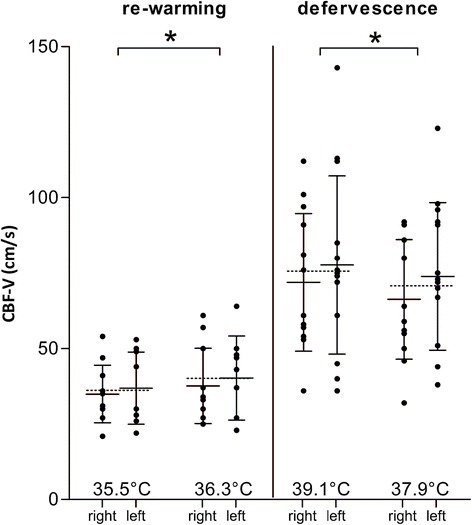
General linear model for repeated measures. Data are presented as mean ± standard deviation; every dot represents the value of the considered parameter in one artery. On the left, results for the re-warming group (12 patients); on the right, results for the defervescence group (14 patients). Mean cerebral blood flow velocity (CBF-V) is presented in separate columns, for insonation of the right and left middle cerebral artery. The dotted lines indicate the mean for each pair of columns. Temperature changes had an effect on CBF-V (P = 0.01). Insonation side and method used to modify temperature (defervescence or re-warming) did not significantly affect mean CBF-V changes.
Discussion
Main findings
CBF-V decreased during defervescence in patients with acute brain damage, and rose during re-warming in a Tcore range from 33.7 to 39.9°C. The PI also dropped during defervescence and rose during re-warming. CBF-V varied despite constant CPP, which remained stable because the MAP reduction was concurrent to the decrease in ICP during defervescence. SjO2, which reflects the balance between cerebral metabolic rate and delivery of oxygen, did not change during defervescence, suggesting that the relationship between metabolism and CBF was preserved. A more complex but similar explanation can be proposed for the changes in PI. There are reports of a linear relationship between the PI and ICP [10,11] but from our data we could not distinguish whether changes in PI correlated more with Tcore or ICP.
What was already known
There is little information about how temperature affects cerebral hemodynamics in the clinical setting. Published data mainly focus on hypothermia and the most widely investigated setting is cardiac surgery, where a reduction in CBF-V during cooling and a parallel increase when temperature returned to baseline has been observed [12,13]. In one study 19 patients with chronic hepatitis C virus infection were subjected to experimental therapy with extracorporeal whole-body hyperthermia at 41.8°C for 120 minutes under propofol anesthesia; there was a decrease in cerebral oxygen extraction and an increase in CBF-V [14]. These findings take the same direction as our results, but our brain-injured patients had spontaneous episodes of fever, not passive hyperthermia. Hyperthermia and fever follow different pathways, so studies on hyperthermia cannot be directly translated into the fever setting.
Findings in healthy volunteers seem to contrast with our findings. CBF-V rose when Tcore was lowered by an external system in healthy volunteers; this might be explained by concomitant changes in several other parameters, like arterial carbon dioxide partial pressure, which is the most widely investigated factor that influences CBF-V [15]. Some authors [16] also investigated the effect of exercise-induced hyperthermia on CBF-V and their results too appear to contrast with ours: CBF-V fell as temperature rose. Prolonged exercise causes hyperventilation, hence hypocapnia, which can reduce CBF and consequently CBF-V. We do not know how the other parameters change.
Clinical implications
Changes in temperature affect cerebral hemodynamics, and potentially ICP. CBF-V, which estimates CBF, increases when temperature rises. Cerebral blood volume (CBV) is likely to increase as well. In patients with reduced intracranial compliance, small changes in CBV can be very important in terms of the effect on ICP. As temperature affects CBF-V, it must be taken into consideration for the interpretation of TCCDS data, as well as arterial partial pressure of carbon dioxide (PaCO2) [17-22], sedatives/analgesics [23,24] and induced hypertension [25], which are already known to change CBF-V. Moreover, changes in the PI parallel temperature and ICP; if this is the case, it can hardly serve as a useful basis for non-invasively estimating ICP, as previously proposed [10,11].
Limitations
Our study has some limitations. First of all TCCDS does not directly quantify CBF but only estimates it. Autoregulation changes, which are of obvious importance, could not be studied. In addition, our analysis involved a limited number of patients. Even if we tried to change nothing other than the temperature, in some cases there were small changes in certain parameters. We also could not measure Tbrain but only Tcore, which generally underestimates Tbrain.
Conclusions
Body temperature affects cerebral hemodynamics, as estimated with TCCDS. CBF-V changes, consistent both after re-warming and after defervescence, may be interpreted as physiological CBF adaptations with potential impact on ICP. TCCDS offers an important insight into cerebral physiology, and is better understood when evaluated together with other measurements, such as temperature, ICP, CPP and SjO2.
Key messages
Body temperature affects cerebral hemodynamics
Fever, a recognized entity that worsens brain injury, increases cerebral blood flow velocity and ICP
Hypothermia decreases cerebral blood flow velocity
We have to consider the effects of body temperature on cerebral blood flow velocity and pulsatility index when we use TCCDS as a diagnostic tool.
Abbreviations
- CBF
cerebral blood flow
- CBF-V
cerebral blood flow velocity
- CPP
cerebral perfusion pressure
- ICP
intracranial pressure
- MAP
mean arterial pressure
- MCA
middle cerebral artery
- NICU
neurosurgical intensive care unit
- PI
pulsatility index
- SjO2
oxygen saturation in the jugular vein
- Tbrain
brain temperature
- TCCDS
transcranial color-coded duplex sonography
- Tcore
core body temperature
Footnotes
Competing interests
Federica Stretti, Miriam Gotti, Silvia Pifferi, Giovanna Brandi, Federico Annoni and Nino Stocchetti have no competing interests to declare.
Authors’ contributions
FS, MG, GB and NS all contributed to conception and design of the present study. Data were collected and analyzed by FS, MG, SP, and GB. The results were critically interpreted with the help of FA and NS. FS and MG drafted the manuscript. All the authors read and approved the final manuscript.
Contributor Information
Federica Stretti, Email: federica.stretti@unimi.it.
Miriam Gotti, Email: miriam.gotti@unimi.it.
Silvia Pifferi, Email: silviapifferi@libero.it.
Giovanna Brandi, Email: giovanna.brandi@gmail.com.
Federico Annoni, Email: federico.annoni@unimi.it.
Nino Stocchetti, Email: stocchet@policlinico.mi.it.
References
- 1.Stocchetti N, Rossi S, Roncati Zanier E, Colombo A, Beretta L, Citerio G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28:1555–1562. doi: 10.1007/s00134-002-1513-1. [DOI] [PubMed] [Google Scholar]
- 2.Andrews PJ, Sinclair HL, Battison CG, Polderman KH, Citerio G, Mascia L, Harris BA, Murray GD, Stocchetti N, Menon DK, Shakur H, De Backer D, Eurotherm3235Trial collaborators European society of intensive care medicine study of therapeutic hypothermia (32-35°C) for intracranial pressure reduction after traumatic brain injury (the Eurotherm3235Trial) Trials. 2011;12:8. doi: 10.1186/1745-6215-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37:S250–S257. doi: 10.1097/CCM.0b013e3181aa5e8d. [DOI] [PubMed] [Google Scholar]
- 4.Rossi S, Roncati Zanier E, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001;71:448–454. doi: 10.1136/jnnp.71.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oddo M, Frangos S, Milby A, Chen I, Maloney-Wilensky E, Murtrie EM, Stiefel M, Kofke WA, Le Roux PD, Levine JM. Induced normothermia attenuates cerebral metabolic distress in patients with aneurysmal subarachnoid hemorrhage and refractory fever. Stroke. 2009;40:1913–1916. doi: 10.1161/STROKEAHA.108.534115. [DOI] [PubMed] [Google Scholar]
- 6.Puccio AM, Fischer MR, Jankowitz BT, Yonas H, Darby JM, Okonkwo DO. Induced normothermia attenuates intracranial hypertension and reduces fever burden after severe traumatic brain injury. Neurocrit Care. 2009;11:82–87. doi: 10.1007/s12028-009-9213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marion DW, Obrist WD, Carlier PM, Penrod LE, Darby JM. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg. 1993;79:354–362. doi: 10.3171/jns.1993.79.3.0354. [DOI] [PubMed] [Google Scholar]
- 8.Schatlo B, Glasker S, Zauner A, Thompson BG, Oldfield EH, Pluta RM. Continuous neuromonitoring using Transcranial Doppler reflects blood flow during carbon dioxide challenge in primates with global cerebral ischemia. Neurosurgery. 2009;64:1148–1154. doi: 10.1227/01.NEU.0000343542.61238.DF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Grady NP, Barie PS, Bartlett J, Bleck T, Garvey G, Jacobi J, Linden P, Maki DG, Nam M, Pasculle W, Pasquale MD, Tribett DL, Masur H. Practice parameters for evaluating new fever in critically ill adult patients. Crit Care Med. 1998;26:392–408. doi: 10.1097/00003246-199802000-00046. [DOI] [PubMed] [Google Scholar]
- 10.Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP) Surg Neurol. 2004;62:45–51. doi: 10.1016/j.surneu.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Brandi G, Béchir M, Sailer S, Haberthür C, Stocker R, Stover JF. Transcranial color-coded duplex sonography allows to assess cerebral perfusion pressure noninvasively following severe traumatic brain injury. Acta Neurochir (Wien) 2010;152:965–972. doi: 10.1007/s00701-010-0643-4. [DOI] [PubMed] [Google Scholar]
- 12.Joshi B, Brady K, Lee J, Easley B, Panigrahi R, Smielewski P, Czosnyka M, Hogue CW., Jr Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110:321–328. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendjelid K, Poblete B, Baenziger O, Romand JA. The effects of hypothermic cardiopulmonary bypass on Doppler cerebral blood flow during the first 24 postoperative hours. Interact Cardiovasc Thorac Surg. 2003;2:46–52. doi: 10.1016/S1569-9293(02)00098-1. [DOI] [PubMed] [Google Scholar]
- 14.Crèmer OL, Diephuis JC, van Soest H, Vaessen PHB, Bruens MGJ, Hennis PJ, Kalkman CJ. Cerebral oxygen extraction and autoregulation during extracorporeal whole body hyperthermia in humans. Anesthesiology. 2004;100:1101–1107. doi: 10.1097/00000542-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Mahmood AM, Voorhees ME, Parnell M, Zweifler RM. Transcranial Doppler ultrasonographic evaluation of middle cerebral artery hemodynamics during mild hypothermia. J Neuroimaging. 2005;15:336–340. doi: 10.1111/j.1552-6569.2005.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 16.Nybo L, Møller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- 17.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, McConnel Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. XIV. Hyperventilation. J Neurotrauma. 2007;24:S87–S90. doi: 10.1089/neu.2006.0058. [DOI] [PubMed] [Google Scholar]
- 18.Curley GMB, Kavanagh BP, Laffey JG. Hypocapnia and the injured brain: more harm than benefit. Crit Care Med. 2010;38:1348–1359. doi: 10.1097/CCM.0b013e3181d8cf2b. [DOI] [PubMed] [Google Scholar]
- 19.Conte V, Paternò R, Citerio G, Stocchetti N. Comment on: ‘Hypocapnia and the injured brain: more harm than benefit’. Crit Care Med. 2010;38:1923. doi: 10.1097/CCM.0b013e3181e61a51. [DOI] [PubMed] [Google Scholar]
- 20.Stocchetti N, Maas AIR, Chieregato A, Van der Plas AA. Hyperventilation in head injury: a review. Chest. 2005;127:1812–1827. doi: 10.1378/chest.127.5.1812. [DOI] [PubMed] [Google Scholar]
- 21.Ide K, Eliasziw M, Poulin MJ. Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol. 2003;95:129–137. doi: 10.1152/japplphysiol.01186.2002. [DOI] [PubMed] [Google Scholar]
- 22.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17:913–915. doi: 10.1161/01.STR.17.5.913. [DOI] [PubMed] [Google Scholar]
- 23.Ederberg S, Westerlind A, Houltz E, Svensson SE, Elam M, Ricksten SE. The effects of propofol on cerebral blood flow velocity and cerebral oxygen extraction during cardiopulmonary bypass. Anesth Analg. 1998;86:1201–1206. doi: 10.1097/00000539-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Trindle MR, Dodson BA, Rampil IJ. Effects of fentanyl versus sufentanil in equianesthetic doses on middle cerebral artery blood flow velocity. Anesthesiology. 1993;78:454–460. doi: 10.1097/00000542-199303000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Manno ED, Gress DR, Schwamm LH, Diringer MN, Ogilvy CS. Effects of induced hypertension on transcranial Doppler ultrasound velocities in patients after subarachnoid hemorrhage. Stroke. 1998;29:422–428. doi: 10.1161/01.STR.29.2.422. [DOI] [PubMed] [Google Scholar]


