Abstract
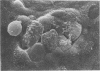
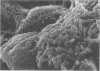
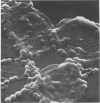
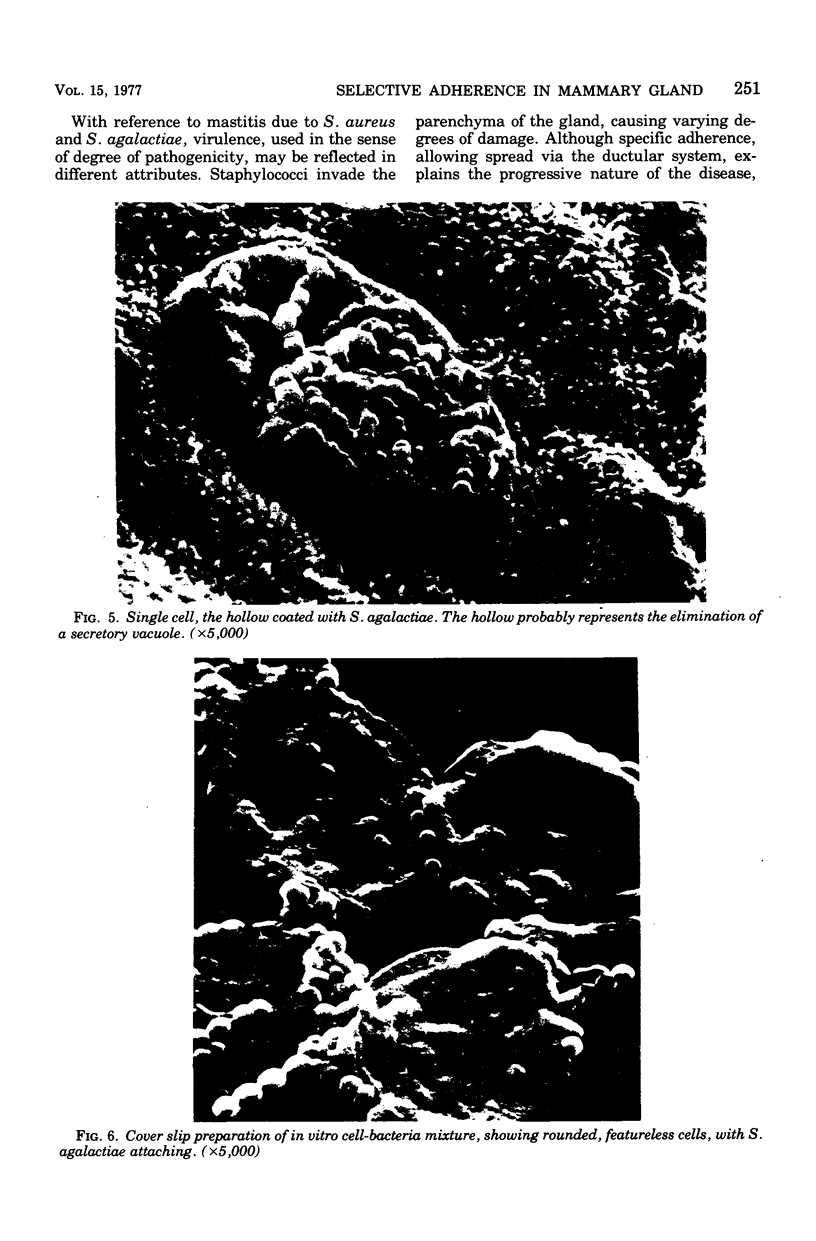
Some parameters affecting the adherence of microbes to the ductular epithelium of the bovine mammary gland were studied. Adherence increased from teat sinus to lactiferous sinus to the large ducts, and cells from the lactiferous sinus to the large ducts, and cells from the lactiferous sinus were used for all other experiments. There was no difference in adherence to cells from different quarters of the same cow, but there were significant differences between cows. Scanning electron microscopy suggested that the cells of the ductular epithelium undergo dynamic changes that probably result in secretion and/or desquamation. Adherence to cells could be demonstrated only at a late stage of these changes. The adherence of organisms associated with mastitis was studied using an in vitro test. Adherence generally paralleled prevalence as cause of disease, with Staphylococcus aureus and Streptococcus agalactiae adhering best. Strain variation suggested that virulence was related to adherence with S. agalactiae and S. dysgalactiae but not with S. aureus. It is proposed that specific adherence is an important aspect of pathogenesis of mastitis due to S. aureus and S. agalactiae.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black R. T., Marshall R. T., Bourland C. T. Locus of mammary gland infections of Corynebacterium bovis. J Dairy Sci. 1972 Apr;55(4):413–416. doi: 10.3168/jds.S0022-0302(72)85508-5. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROST A. J., SANDERSON C. J. CONTROL AND ERADICATION OF STREPTOCOCCUS AGALACTIAE INFECTION IN DAIRY HERDS. Aust Vet J. 1965 Apr;41:97–100. doi: 10.1111/j.1751-0813.1965.tb08819.x. [DOI] [PubMed] [Google Scholar]
- Frost A. J. Selective adhesion of microorganisms to the ductular epithelium of the bovine mammary gland. Infect Immun. 1975 Nov;12(5):1154–1156. doi: 10.1128/iai.12.5.1154-1156.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHALM O. W., CARROLL E. J., LASMANIS J. THE LEUKOCYTE BARRIER AND SEROLOGIC INVESTIGATIONS OF EXPERIMENTAL COLIFORM (AEROBACTER AEROGENES) MASTITIS IN CATTLE. Am J Vet Res. 1964 Jan;25:90–96. [PubMed] [Google Scholar]
- Ward M. E., Watt P. J. Adherence of Neisseria gonorrhoeae to urethral mucosal cells: an electron-microscopic study of human gonorrhea. J Infect Dis. 1972 Dec;126(6):601–605. doi: 10.1093/infdis/126.6.601. [DOI] [PubMed] [Google Scholar]