Abstract
Background and Purpose
Expert consensus guidelines recommend low-density lipoprotein cholesterol (LDL-C) as the primary serum lipid target for recurrent stroke risk reduction. However, mounting evidence suggests that other lipid parameters might be additional therapeutic targets or at least also predict cardiovascular risk. Little is known about the effects of non-traditional lipid variables on recurrent stroke risk.
Methods
We analyzed the Vitamin Intervention for Stroke Prevention study database comprising 3680 recent (<120 days) ischemic stroke patients followed up for 2 years. Independent associations of baseline serum lipid variables with recurrent ischemic stroke (primary outcome) and the composite endpoint of ischemic stroke/coronary heart disease (CHD)/vascular death (secondary outcomes) were assessed.
Results
Of all variables evaluated, only triglycerides (TG)/high-density lipoprotein cholesterol (HDL-C) ratio was consistently and independently related to both outcomes: compared with the lowest quintile, the highest TG/HDL-C ratio quintile was associated with stroke (adjusted hazard ratio, 1.56; 95% CI, 1.05−2.32) and stroke/CHD/vascular death (1.39; 1.05−1.83), including adjustment for lipid modifier use. Compared with the lowest quintile, the highest total cholesterol/HDL-C ratio quintile was associated with stroke/CHD/vascular death (1.45; 1.03−2.03). LDL-C/HDL-C ratio, non-HDL-C, elevated TG alone, and low HDL-C alone were not independently linked to either outcome.
Conclusions
Of various non-traditional lipid variables, elevated baseline TG/HDL-C and TC/HDL-C ratios predict future vascular risk after a stroke, but only elevated TG/HDL-C ratio is related to risk of recurrent stroke. Future studies should assess the role of TG/HDL as a potential therapeutic target for global vascular risk reduction after stroke.
Keywords: Lipids, Stroke, Cardiovascular Events
INTRODUCTION
A reduction in serum low-density lipoprotein cholesterol (LDL-C) concentrations can substantially decrease stroke incidence,1 but even with aggressive LDL lowering, there remains a substantial residual risk of recurrent vascular events after stroke.2–4 Indeed, emerging evidence suggests that non-traditional serum lipid variables may be better predictors of vascular risk than LDL-C.5–7 For instance, triglycerides (TG)/high-density lipoprotein cholesterol (HDL-C) ratio is a marker of deleterious small-dense LDL particles,7 patients with elevated TG and low HDL-C plus high LDL-C are more likely than those with isolated LDL-C elevation to have increased risk for coronary heart disease (CHD) events,8, 9 and TG/HDL-C ratio is a powerful independent predictor of all-cause mortality and cardiovascular events.6 Furthermore, total cholesterol (TC)/HDL-C ratio9, 10, LDL-C/HDL-C ratio11, and non-HDL-C5, 9 have all been independently linked with greater CHD risk.
The few published studies that have explored the link between non-traditional serum lipid variables and stroke risk largely focused on incident stroke in people who were generally free of stroke or CHD at baseline.12–15 Little is known about the effects non-traditional serum lipid variables on recurrent vascular risk after a recent stroke. The objective of this study was to assess the relationships of 4 baseline non-traditional serum lipid measures (TG/HDL-C, TC/HDL-C, LDL-C/HDL-C ratios, and non-HDL-C) to recurrent vascular risk after a recent non-cardioembolic stroke.
METHODS
Patients and Study
We reviewed data from the Vitamin Intervention for Stroke Prevention (VISP) trial.16 The VISP trial was a multicenter, double-blind, randomized, controlled trial performed at centers across the United States, Canada, and Scotland, which enrolled 3680 patients to determine whether high doses of multivitamin (folic acid, pyridoxine, and cobalamin) given to lower total homocysteine levels would reduce the risk of recurrent stroke and major vascular events in patients with a non-disabling non-cardioembolic ischemic stroke.16 Details of methods and main results of the trial have been reported previously.16 Briefly, the VISP population included patients who experienced a non-disabling ischemic stroke within the preceding 120 days. Demographic, clinical, and laboratory data were collected at baseline, with subsequent clinical and laboratory information obtained at follow-up visits of 6, 12, and 24 months. Serum lipid samples were designed to be obtained after an overnight 12-hour fast, which was suitable for this study because TC, LDL-C, and HDL-C are reduced up to 3 to 5 hours after the last meal while TG levels were increased up to 6 hours after the last meal.17 Based on the routine serum lipid profiles obtained, we computed 4 non-traditional lipid variables as follows: TG/HDL-C, TC/HDL-C, LDL-C/HDL-C ratios, and non-HDL-C (TC – HDL-C), all of which have at least once been reported as a predictor of cardiovascular risk in the literature.5, 6, 8–11 If an LDL-C value was missing, it was calculated using the Friedewald formula (LDL-C = TC – HDL-C − TG/5).18 Otherwise, subjects with other missing serum lipid values or those with serum levels of TG ≥400 mg/dL were excluded because LDL-C could not be calculated using the Friedewald equation.18 For each eligible patient, further medication information including lipid modifying therapy (i.e. statin, ezetimibe, fenofibrate, niacin, or omega-3 fatty acids), antihypertensive, and antithrombotic medication was obtained at the last follow-up visit. Last follow-up visit was defined as the last documented study encounter that preceded either a vascular outcome event or end of the trial. We assessed a last follow-up visit approach (vs. baseline visit), because 1) the number and type of prescriptions for secondary stroke prevention can vary during the post-discharge follow-up setting;19 and 2) our prelim evaluation of the VISP data indicated that several hundred patients had their baseline prescriptions modified to include more therapeutic drug classes (i.e. lipid modifier) at the time of their 2nd or 3rd (and further) follow-up visits. All participants provided written informed consent before enrolment.16
Outcomes
The primary outcome for this analysis was ischemic stroke. Secondary outcome was a composite of stroke, CHD including myocardial infarction (MI), coronary revascularization, cardiac resuscitation, and fatal CHD, or vascular death as major vascular events.
Statistical Analysis
Data were summarized as mean ± standard deviation or number of subjects (percentage), as appropriate. Comparisons across the groups were examined using the χ2 test for categorical variables and one-way analysis of variance (ANOVA), followed by the Dunnett test for multiple comparisons, for continuous variables. For the purpose of this analysis, subjects were analyzed according to the 4 serum lipid variable groups (TG/HDL-C, TC/HDL-C, LDL/HDL-C ratios, and non-HDL-C) and each of the 4 groups was stratified into quintiles according to the distribution of lipid variables. The resulting ranges of quintile were ≤1.93 (first quintile), 1.94–2.86 (second quintile), 2.87–4.05 (third quintile), 4.06–6.21 (forth quintile), and ≥6.22 (fifth quintile) for TG/HDL-C ratio; ≤3.50 (first quintile), 3.51–4.20 (second quintile), 4.21–4.98 (third quintile), 4.99–5.97 (forth quintile), and ≥5.98 (fifth quintile) for TC/HDL-C ratio; ≤1.92 (first quintile), 1.93–2.48 (second quintile), 2.49–3.04 (third quintile), 3.05–3.80 (forth quintile), and ≥3.81 (fifth quintile) for LDL-C/HDL-C ratio; and ≤120 (first quintile), 121–143 (second quintile), 144–164 (third quintile), 165–190 (forth quintile), and ≥191 (fifth quintile) for non-HDL-C. The lowest quintile was the referent group for purposes of comparison. Cox proportional hazard regression analyses were performed to estimate the risk of outcome events. Patients not having these events were censored at the date of nonvascular death, last follow-up examination, or last contact. All the analyses were performed first adjusting for age and sex, then for baseline covariates that were associated with the higher quintile (P<0.10) in each of 4 lipid variables groups. Results are given by hazard ratio (HR) and its 95% confidence interval (CI). A linear trend of adjusted HRs across increasing quintile of each lipid variable was examined using a likelihood ratio test. All analyses were conducted using IBM SPSS Version 22.0 (IBM SPSS Inc, Chicago, IL) and a probability value of <0.05 was considered statistically significant.
RESULTS
Subjects Characteristics by Each Lipid Variable Quintile
Baseline demographic and clinical characteristics according to TG/HDL-C ratio quintile and TC/HDL-C ratio quintile are provided in Supplemental Table I. Of 3680 subjects enrolled in VISP, TG/HDL-C ratio measurements were available in 3385 (92.0%) subjects and TC/HDL-C ratio in 3440 (93.5%) subjects. In TG/HDL-C ratio group with complete data, mean age was 66.3 ± 10.8 years, 37.8% were women and 79.5% were white, all of them were similar in the TC/HDL-C ratio group (66.2 ± 10.8 years, 37.8% and 79.7%, respectively). During an average of 20 months of follow-up, a total of 272 (8.0%) incident strokes and 564 (16.7%) stroke/CHD/vascular death were recorded in the TG/HDL-C ratio group and 279 (8.1%) incident strokes and 572 (16.6%) stroke/CHD/vascular death in the TC/HDL-C ratio group. For TG/HDL-C ratio group, subjects in the higher quintile were more likely to have higher levels of body mass index (BMI), and LDL-C, have higher frequencies of male, hypertension, diabetes, smoking, antihypertensive use, and lipid modifier use as well as history of prior stroke (before VISP qualifying stroke) and history of any cardiac disease (MI, congestive heart failure, coronary angioplasty, or coronary artery bypass graft surgery), whereas age was more like to be younger and non-white was lower. For TC/HDL-C ratio group, subjects in the higher quintile were more likely to have higher levels of BMI, LDL-C and National Institutes of Health Stroke Scale (NIHSS) score, have higher frequencies of male, diabetes, smoking and lipid modifier use, whereas age was more like to be younger.
Supplemental Table II shows baseline demographic and clinical characteristics according to LDL-C/HDL-C ratio quintile and non-HDL-C quintile. LDL-C/HDL-C ratio measurements were available in 3383 (91.9%) subjects and non-HDL-C in 3440 (93.5%) subjects. In LDL-C/HDL-C ratio group, mean age was 66.3 ± 10.8 years, 37.8% were women and 79.5% were white, all were comparable to the non-HDL-C group (66.2 ± 10.8 years, 37.8% and 79.7%, respectively). A total of 272 (8.0%) incident strokes and 562 (16.6%) stroke/CHD/vascular death were recorded in the LDL-C/HDL-C ratio group and 279 (8.1%) incident strokes and 572 (16.6%) stroke/CHD/vascular death in the non-HDL group. For LDL-C/HDL-C ratio group, subjects in the highest quintile were more likely to have higher levels of BMI, diastolic blood pressure, TG and NIHSS score, have higher frequencies of male, smoking and lipid modifier use, whereas age was younger and days from stroke to randomization was more like to be shorter. For non-HDL-C group, subjects in the highest quintile were more likely to have higher levels of BMI and systolic/diastolic blood pressure, have higher frequencies of smoking and lipid modifier use, whereas age was more like to be younger, HDL-C level was lower, and frequencies of male, any cardiac disease and antihypertensive use were lower.
Age and Sex Adjusted Hazard Ratios for Vascular Events by Each Lipid Variable
Results of the age and sex adjusted associations of each lipid variavle with vascular outcomes appear in Table 1. Occurrence of stroke was significantly higher in the highest TG/HDL-C quintile group (HR, 1.68; 95% CI, 1.16−2.45). Occurrence of stroke/CHD/vascular death was higher in the highest TG/HDL-C quintile group (1.57, 1.21−2.03), in the highest TC/HDL-C quintile group (1.51, 1.17−1.95), and in the highest LDL-C/HDL-C quintile group (1.31, 1.01−1.70).
Table 1.
Age and Sex Adjusted Hazard Ratios for Vascular Events by Increasing Strata of Each Lipid Variable
| Quintile of TG/HDL-C ratio |
|||||
| 1 (≤1.93; n=677) | 2 (1.94–2.86; n=678) | 3 (2.87–4.05; n=676) | 4 (4.06−6.21; n=677) | 5 (≥6.22; n=677) | |
| Stroke | 1 [Reference] | 1.23 (0.83−1.81) | 1.22 (0.82−1.80) | 0.98 (0.64−1.48) | 1.68 (1.16−2.45)† |
| Stroke/CHD/vascular death | 1 [Reference] | 1.06 (0.80−1.39) | 1.16 (0.86−1.51) | 1.01 (0.76−1.33) | 1.57 (1.21−2.03)† |
| Quintile of TC/HDL-C ratio |
|||||
| 1 (≤3.50; n=689) | 2 (3.51–4.20; n=688) | 3 (4.21–4.98; n=689) | 4 (4.99−5.97; n=686) | 5 (≥5.98; n=688) | |
| Stroke | 1 [Reference] | 0.90 (0.61−1.33) | 1.20 (0.83−1.74) | 1.04 (0.70−1.53) | 1.35 (0.93−1.95) |
| Stroke/CHD/vascular death | 1 [Reference] | 0.95 (0.72−1.25) | 1.02 (0.78−1.34) | 1.19 (0.91−1.55) | 1.51 (1.17−1.95)† |
| Quintile of LDL-C/HDL-C ratio |
|||||
| 1 (≤1.92; n=686) | 2 (1.93–2.48; n=668) | 3 (2.49–3.04; n=677) | 4 (3.05−3.80; n=677) | 5 (≥3.81; n=675) | |
| Stroke | 1 [Reference] | 0.98 (0.67−1.44) | 1.11 (0.76−1.62) | 1.05 (0.72−1.55) | 1.20 (0.82−1.74) |
| Stroke/CHD/vascular death | 1 [Reference] | 1.06 (0.81−1.38) | 0.94 (0.72−1.24) | 1.12 (0.86−1.47) | 1.31 (1.01−1.70)* |
| Quintile of non-HDL-C |
|||||
| 1 (≤120; n=709) | 2 (121–143; n=682) | 3 (144–164; n=695) | 4 (165−190; n=675) | 5 (≥191; n=679) | |
| Stroke | 1 [Reference] | 0.94 (0.65−1.36) | 0.87 (0.60−1.26) | 1.00 (0.70−1.44) | 1.01 (0.70−1.45) |
| Stroke/CHD/vascular death | 1 [Reference] | 0.98 (0.76−1.27) | 0.86 (0.66−1.12) | 0.99 (0.76−1.28) | 1.28 (0.99−1.64) |
P <0.05,
P <0.01.
TG indicates triglycerides; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; CHD, coronary heart disease.
Effects of Each Lipid Variable on Risk of Vascular Events
For TG/HDL-C ratio, after adjusting for multiple confounders, compared with the lowest quintile at baseline, the highest quintile was associated with increased risk of stroke (1.56, 1.05−2.32; Ptrend=0.002) and stroke/CHD/vascular death (1.39, 1.05−1.83; Ptrend<0.001) (Figure 1). We investigated independent effect of TG and HDL-C, respectively on vascular outcomes, but neither the highest TG quintile nor the lowest HDL-C quintile itself was associated with primary or secondary outcome after multivariable Cox analyses (data not shown). For TC/HDL-C ratio, the highest quintile was associated with increased risk of stroke/CHD/vascular death (1.45, 1.03−2.03; Ptrend=0.002), when referenced to the first quintile (Figure 2) but not of stroke (1.35, 0.83−2.20, data not shown). For LDL-C/HDL-C ratio and non-HDL-C, none of them showed significant association with primary or secondary outcome event (data not shown). The adjusted HRs of covariates included in the adjusted risk models of vascular outcomes by TG/HDL-C or TC/HDL-C ratio appear in Supplemental Table III. In the TG/HDL-C ratio group, age, diabetes, prior stroke history, and lipid modifier use were significant predictors of both the primary and secondary outcomes. In the TC/HDL-C ratio group, age, male, diabetes, smoking, and stroke severity (NIHSS) were significantly associated with stroke/CHD/vascular death.
Figure 1. Adjusted Hazard Ratios (AHRs) for Vascular Events by Increasing Strata of TG/HDL-C Ratio.
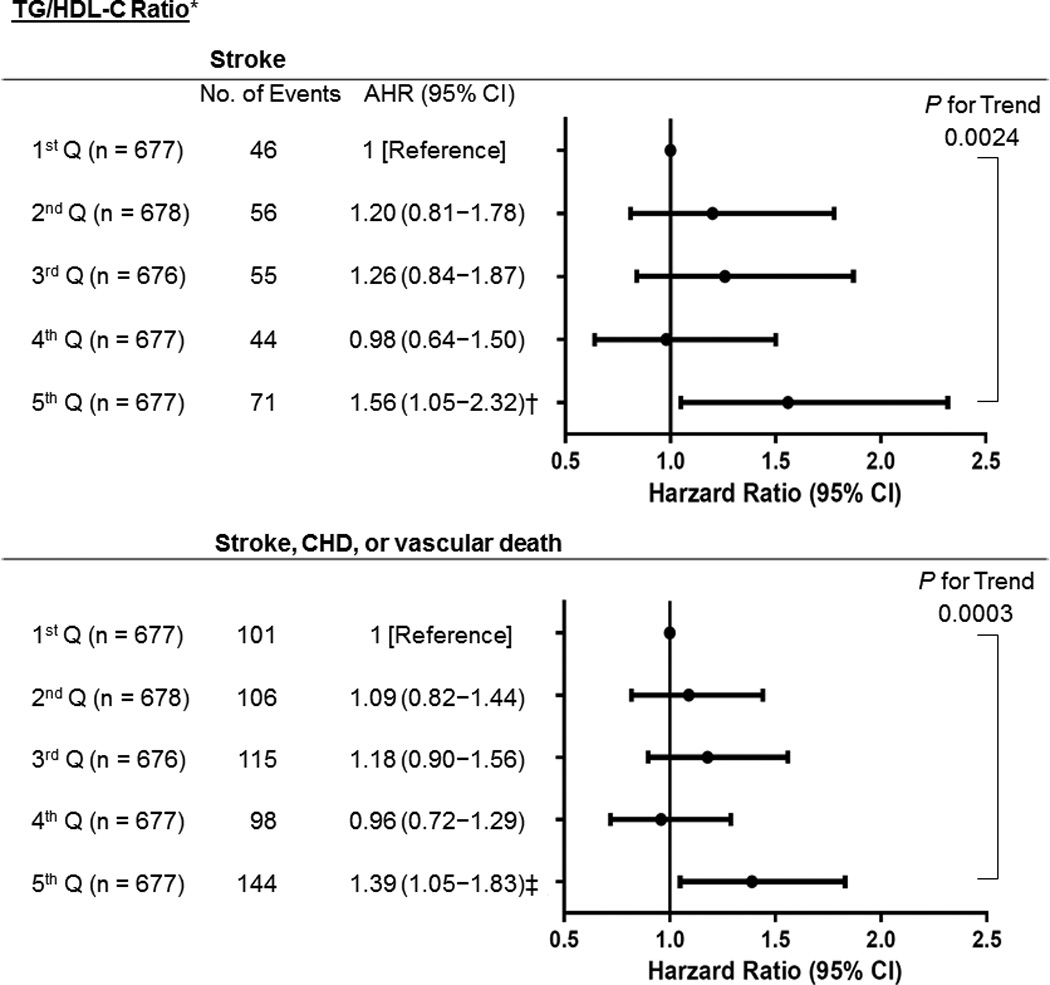
Ranges for TG/HDL-C quintile (Q) were 1st; ≤1.93, 2nd; 1.94–2.86, 3rd; 2.87–4.05, 4th; 4.06−6.21, and 5th: ≥6.22. *Adjusted for age, sex, ethnicity (white vs. non-white), body mass index, hypertension, diabetes, LDL-C, smoking, prior stroke history (before index stroke), any cardiac disease (history of myocardial infarction, congestive heart failure, coronary angioplasty, or coronary artery bypass graft surgery), antihypertensive use, and lipid modifier use. † P=0.028 (vs 1st Q). ‡ P=0.021 (vs 1st Q). TG indicates triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CHD, coronary heart disease.
Figure 2. Adjusted Hazard Ratios (AHRs) Vascular Events by Increasing Strata of TC/HDL-C Ratio.
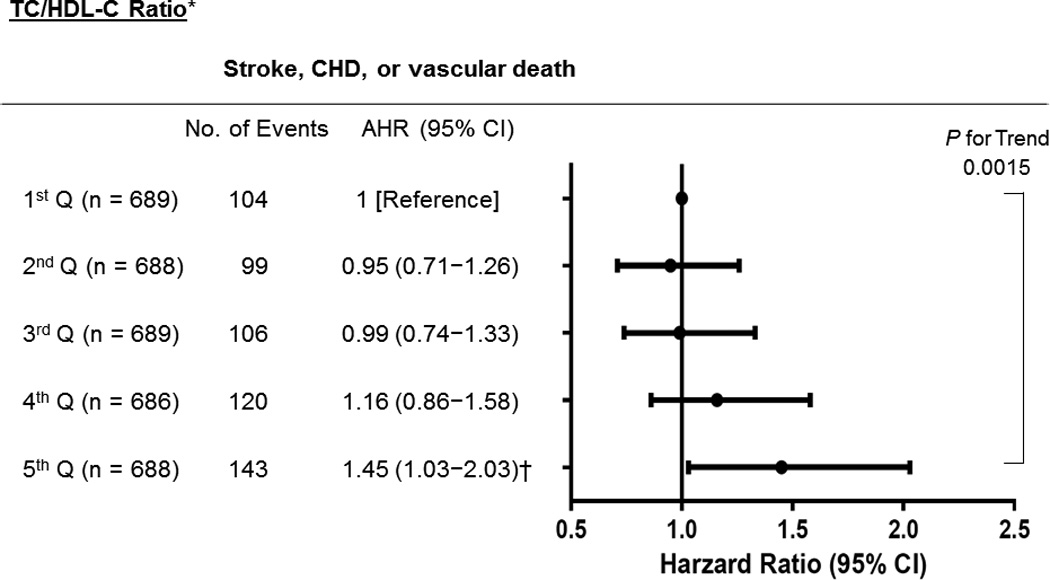
Ranges for TC/HDL quintile (Q) range were 1st; ≤3.50, 2nd; 3.51–4.20, 3rd; 4.21–4.98, 4th; 4.99−5.97, and 5th: ≥5.98. *Adjusted for age, sex, body mass index, diabetes, TG, LDL-C, NIHSS score, smoking, and lipid modifier use. † P=0.032 (vs 1st Q). TC indicates total cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; CHD, coronary heart disease; NIHSS, National Institutes of Health Stroke Scale.
DISCUSSION
We observed that in this analysis of prospectively collected data on >3300 patients with a recent ischemic stroke, despite higher rate of lipid modifier use, elevated TG/HDL-C ratio was significantly associated with higher risk of recurrent stroke and major vascular events during 2-year follow-up period. While another non-traditional lipid variable, TC/HDL-C ratio was also independently linked with major vascular events, only TG/HDL-C ratio was related to recurrent stroke risk. Interestingly, we also noted that history of a stroke before the VISP-qualifying stroke occurred more frequently among those with higher TG/HDL-C quintile (a finding not seen with the other lipid variable) perhaps indirectly supporting the notion that patients with elevated TG/HDL-C ratio are especially stroke-prone. Overall, these results suggest that elevated TG/HDL-C ratio may be of prognostic value for identifying high-risk subjects predisposed to stroke among recent non-cardioembolic stroke populations already receiving secondary prevention.
A population-based prospective cohort study found no relation of stroke risk with higher levels of TG/HDL-C ratio.14 This discrepant result may be explained that all the participants in that study were healthy people free of stroke or CHD at baseline. However, atherogenic dyslipidemia as defined by HDL-C ≤ 40 mg/dL and TG ≥150 mg/dL has been associated with greater risk of recurrent stroke 90 days after a transient ischemic attack (TIA),4 and with higher residual cardiovascular risk among ischemic stroke and TIA patients receiving statin therapy.20
High TG constitutes an independent risk factor for first-ever ischemic stroke in patients with CHD21 and in general population.22 Higher HDL-C is protective on ischemic stroke risk15 and lower HDL-C is associated with CHD and stroke risk.23, 24 In this study, TG or HDL-C by itself was not significantly associated with primary or secondary outcome after a recent stroke in multivariable Cox analyses. Very few studies have specifically examined the independent predictive value of either higher TG or lower HDL-C alone on recurrent vascular risk after an ischemic stroke, but an analysis of symptomatic intracranial atherosclerotic disease patients also did not find a link between either higher TG or lower HDL-C and recurrent vascular risk.25
The highest TC/HDL-C quintile showed significantly increased risk of the composite vascular endpoint but not of stroke, which is a roughly similar pattern to findings from Zhang et al study.15
We did not observe LDL-C/HDL-C ratio to be associated with vascular outcomes, which may be due to the more heterogeneous nature of ischemic stroke, compared to CHD and our recurrent vascular event outcomes. Apolipoprotein (apo) B and apoAI is the major apolipoprotein in LDL-C and HDL-C, respectively. ApoB/apoAI ratio predicts incident stroke among patients with preexisting CHD26 and has been recognized as a better predictor of cardiovascular risk than traditional cholesterol measures.27
Association between non-HDL-C and ischemic stroke remains uncertain. Our result is consistent with the studies28, 29 that non-HDL-C is not associated with stroke risk in either men or women. However, a recent large prospective Chinese cohort study showed positive association of non-HDL-C with stroke risk.30 Since a cross-sectional study showed that large-artery atherosclerotic stroke was associated with elevated levels of non-HDL-C,31 prospective studies are warranted to determine if non-HDL-C has predictive value for atherosclerotic stroke (vs. lacunar infarction).
HDL-raising fibrate (gemfibrozil) was associated with a relative reduction by 25% and 24% in the rate of stroke and major vascular events, respectively32 but the vascular-protective benefits of HDL-boosting has been questioned because another HDL-raising agent (niacin) did not reduce risk of major vascular events in two distinct trials.33, 34 These latter results may cast doubt on the clinical relevance of therapeutic interventions for modulating higher TG/HDL-C or TC/HDL-C ratio. It is also conceivable that the residual risk noted among stroke patients can be ascribed to delayed initiation of lipid-lowering therapies during the period of highest risk35 or an inability to achieve/maintain an intensive LDL-C level (<70 or <100 mg/dL) over the longer term.36 Of note, proprotein convertase subtilisin-kexin type 9 (PCSK9) is a newly established prime modulator of plasma LDL-C and TG.37 Fenofibrate significantly decreased levels of serum PCSK9 and TG in statin-treated patients with type 2 diabetes.38 In a recent phase III trial, PCSK9 inhibitor (evolocumab 420 mg) revealed promising results that showed significantly reduced levels of LDL-C (57% reduction vs. statin) and TG, whilst elevating HDL-C over 12-month follow-up period.39 For now, our results simply suggest that TG/HDL-C ratio could be an ‘indicator of residual vascular risk’, and not necessarily as a therapeutic/preventive target until clear evidence of such clinical benefit is proven.
This study has several limitations. First, it is a post hoc analysis of a completed randomized trial, and so VISP population may not be representative of the overall stroke patient population thereby limiting generalizability. Second, only baseline and no follow-up serum lipid data were available. Third, although we adjusted for these factors and numerous other covariates in our multivariable models, there is a possibility of unmeasured confounding, particularly metabolic syndrome (MetS), which is associated with low HDL-C and elevated TG levels40, or higher apoB/apoAI ratio.41 We could not adjust for MetS or apoB/apoAI ratio because of non-availability of serum glucose and apolipoprotein levels in the VISP database. Finally, the study comprised mostly white subjects (80%), and thus its results cannot be readily extrapolated to the patients of other races and ethnicities. However, this study was strengthened by the rigorous procedures of the prospective VISP trial design, inclusion of non-cardioembolic stroke subjects with large sample size.16
In conclusion, among 4 atherogenic non-traditional serum lipid variables, higher TG/HDL-C ratio after a recent ischemic stroke appears to be the better risk prognosticator of recurrent vascular events including stroke. Consequently, it is possible that the addition of fenofibrate to statin therapy may especially benefit diabetic patients with higher TG and lower HDL-C,42 and since stroke patients with higher TG/HDL-C (and TC/HDL-C) ratio were more likely to have diabetes in the VISP population, the potential role of combination lipid modifier therapy for ischemic stroke patients with residual atherogenic dyslipidemia needs to be further elucidated.
Supplementary Material
Acknowledgments
Source of Funding. Dr. Ovbiagele is supported by Award Number U01 NS079179 from the National Institute of Neurological Disorders And Stroke.
Footnotes
Disclosure: None.
References
- 1.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: A quantitative modeling study. Stroke. 2007;38:1881–1885. doi: 10.1161/STROKEAHA.106.475525. [DOI] [PubMed] [Google Scholar]
- 3.Amarenco P, Labreuche J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. doi: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- 4.Sirimarco G, Deplanque D, Lavallee PC, Labreuche J, Meseguer E, Cabrejo L, et al. Atherogenic dyslipidemia in patients with transient ischemic attack. Stroke. 2011;42:2131–2137. doi: 10.1161/STROKEAHA.110.609727. [DOI] [PubMed] [Google Scholar]
- 5.Bittner V, Hardison R, Kelsey SF, Weiner BH, Jacobs AK, Sopko G. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2537–2542. doi: 10.1161/01.cir.0000038496.57570.06. [DOI] [PubMed] [Google Scholar]
- 6.Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, et al. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: A report from the Women's Ischemia Syndrome Evaluation (WISE) Am Heart J. 2009;157:548–555. doi: 10.1016/j.ahj.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 8.Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046–3051. doi: 10.1161/hc5001.100624. [DOI] [PubMed] [Google Scholar]
- 9.Barzi F, Patel A, Woodward M, Lawes CM, Ohkubo T, Gu D, et al. A comparison of lipid variables as predictors of cardiovascular disease in the asia pacific region. Ann Epidemiol. 2005;15:405–413. doi: 10.1016/j.annepidem.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: The quebec cardiovascular study. Arch Inttern Med. 2001;161:2685–2692. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 11.Assmann G, Schulte H, Funke H, von Eckardstein A. The emergence of triglycerides as a significant independent risk factor in coronary artery disease. Eur Heart J. 1998;19(Suppl M):M8–M14. [PubMed] [Google Scholar]
- 12.Bowman TS, Sesso HD, Ma J, Kurth T, Kase CS, Stampfer MJ, et al. Cholesterol and the risk of ischemic stroke. Stroke. 2003;34:2930–2934. doi: 10.1161/01.STR.0000102171.91292.DC. [DOI] [PubMed] [Google Scholar]
- 13.Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, et al. Plasma lipid profile and incident ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2003;34:623–631. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 14.Willey JZ, Xu Q, Boden-Albala B, Paik MC, Moon YP, Sacco RL, et al. Lipid profile components and risk of ischemic stroke: The Northern Manhattan Study (NOMAS) Arch Neurol. 2009;66:1400–1406. doi: 10.1001/archneurol.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Total and high-density lipoprotein cholesterol and stroke risk. Stroke. 2012;43:1768–1774. doi: 10.1161/STROKEAHA.111.646778. [DOI] [PubMed] [Google Scholar]
- 16.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 17.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: Influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Bushnell CD, Olson DM, Zhao X, Pan W, Zimmer LO, Goldstein LB, et al. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology. 2011;77:1182–1190. doi: 10.1212/WNL.0b013e31822f0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirimarco G, Labreuche J, Bruckert E, Goldstein LB, Fox KM, Rothwell PM, et al. Atherogenic dyslipidemia and residual cardiovascular risk in statin-treated patients. Stroke. 2014;45:1429–1436. doi: 10.1161/STROKEAHA.113.004229. [DOI] [PubMed] [Google Scholar]
- 21.Tanne D, Koren-Morag N, Graff E, Goldbourt U. Blood lipids and first-ever ischemic stroke/transient ischemic attack in the Bezafibrate Infarction Prevention (BIP) registry: High triglycerides constitute an independent risk factor. Circulation. 2001;104:2892–2897. doi: 10.1161/hc4901.100384. [DOI] [PubMed] [Google Scholar]
- 22.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142–2152. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 23.Toth PP. High-density lipoprotein and cardiovascular risk. Circulation. 2004;109:1809–1812. doi: 10.1161/01.CIR.0000126889.97626.B8. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Kawashima S, Itoh H, Yamada N, Sone H, Watanabe H, et al. Low HDL cholesterol is associated with the risk of stroke in elderly diabetic individuals: Changes in the risk for atherosclerotic diseases at various ages. Diabetes Care. 2009;32:1221–1223. doi: 10.2337/dc08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ovbiagele B, Saver JL, Lynn MJ, Chimowitz M. Impact of metabolic syndrome on prognosis of symptomatic intracranial atherostenosis. Neurology. 2006;66:1344–1349. doi: 10.1212/01.wnl.0000210530.46058.5c. [DOI] [PubMed] [Google Scholar]
- 26.Koren-Morag N, Goldbourt U, Graff E, Tanne D. Apolipoproteins B and AI and the risk of ischemic cerebrovascular events in patients with pre-existing atherothrombotic disease. J Neurol Sci. 2008;270:82–87. doi: 10.1016/j.jns.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Jungner I, et al. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment. Lancet. 2003;361:777–780. doi: 10.1016/s0140-6736(03)12663-3. [DOI] [PubMed] [Google Scholar]
- 28.Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Miyamoto Y, Yoshimasa Y, et al. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban japanese cohort study: The suita study. Atherosclerosis. 2009;203:587–592. doi: 10.1016/j.atherosclerosis.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, et al. Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events - the JALS-ECC. Circ J. 2010;74:1346–1356. doi: 10.1253/circj.cj-09-0861. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Chen S, Zhou Y, Wang C, Wang A, Zhang Q, et al. Non-high-density lipoprotein cholesterol on the risks of stroke: A result from the Kailuan study. PLoS One. 2013;8:e74634. doi: 10.1371/journal.pone.0074634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bang OY, Saver JL, Liebeskind DS, Pineda S, Ovbiagele B. Association of serum lipid indices with large artery atherosclerotic stroke. Neurology. 2008;70:841–847. doi: 10.1212/01.wnl.0000294323.48661.a9. [DOI] [PubMed] [Google Scholar]
- 32.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans affairs high-density lipoprotein cholesterol intervention trial study group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 33.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low hdl cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 34.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 35.Sanossian N, Saver JL, Liebeskind DS, Kim D, Razinia T, Ovbiagele B. Achieving target cholesterol goals after stroke: Is in-hospital statin initiation the key? Arch Neurol. 2006;63:1081–1083. doi: 10.1001/archneur.63.8.1081. [DOI] [PubMed] [Google Scholar]
- 36.Ovbiagele B, Saver JL, Bang H, Chambless LE, Nassief A, Minuk J, et al. Statin treatment and adherence to national cholesterol guidelines after ischemic stroke. Neurology. 2006;66:1164–1170. doi: 10.1212/01.wnl.0000208403.18885.0e. [DOI] [PubMed] [Google Scholar]
- 37.Akram ON, Bernier A, Petrides F, Wong G, Lambert G. Beyond LDL cholesterol, a new role for pcsk9. Arterioscler Thromb Vasc Biol. 2010;30:1279–1281. doi: 10.1161/ATVBAHA.110.209007. [DOI] [PubMed] [Google Scholar]
- 38.Chan DC, Hamilton SJ, Rye KA, Chew GT, Jenkins AJ, Lambert G, et al. Fenofibrate concomitantly decreases serum proprotein convertase subtilisin/kexin type 9 and very-low-density lipoprotein particle concentrations in statin-treated type 2 diabetic patients. Diabetes Obes Metab. 2010;12:752–756. doi: 10.1111/j.1463-1326.2010.01229.x. [DOI] [PubMed] [Google Scholar]
- 39.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 40.Wilson PW, Grundy SM. The metabolic syndrome: A practical guide to origins and treatment: Part II. Circulation. 2003;108:1537–1540. doi: 10.1161/01.CIR.0000089506.12223.F1. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Hong KS, Lee EJ, Lee J, Kim DE. High levels of apolipoprotein b/ai ratio are associated with intracranial atherosclerotic stenosis. Stroke. 2011;42:3040–3046. doi: 10.1161/STROKEAHA.111.620104. [DOI] [PubMed] [Google Scholar]
- 42.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


