Abstract
Objective
To characterize and compare the MRI morphological features of the cervical vertebral column of Great Danes with and without clinical signs of cervical spondylomyelopathy (CSM).
Design
Prospective cohort study.
Animals
30 Great Danes (15 clinically normal and 15 CSM-affected).
Procedures
All dogs underwent MRI of the cervical vertebral column (C2–3 through T1–2). Features evaluated included sites of subarachnoid space compression, spinal cord compression, or both; degree, cause, and direction of compression; MRI signal changes of the spinal cord; articular process (facet) joint characteristics; internal vertebral venous plexus visibility; and presence of extradural synovial cysts as well as presence and degree of intervertebral disk degeneration and foraminal stenosis.
Results
Clinically normal and CSM-affected dogs had 11 and 61 compressive sites, respectively, detected with MRI. All CSM-affected dogs had ≥ 1 site of spinal cord compression. No signal changes were observed in spinal cords of normal dogs, whereas 14 sites of hyperintensity were found in 9 CSM-affected dogs. Foraminal stenosis was present in 11 clinically normal and all CSM-affected dogs. The number of stenotic foraminal sites was significantly greater in the CSM-affected group, and severe stenosis appeared to be more common in this group than in the clinically normal group. Significant differences were identified between clinically normal and CSM-affected dogs with regard to amount of synovial fluid evident, regularity of articular surfaces, degree of articular process joint proliferation, and internal vertebral venous plexus visibility.
Conclusions and Clinical Relevance
Abnormalities were detected with MRI in several clinically normal Great Danes. Severe spinal cord compression, number of stenotic foraminal sites, and signal changes within the spinal cord distinguished CSM-affected from clinically normal Great Danes.
Magnetic resonance imaging is the imaging modality of choice for dogs with suspected CSM.1–3 Magnetic resonance imaging is a very sensitive technique for determination of the extent of vertebral canal stenosis and spinal cord compression and yields the most information in regard to the neural tissue.4–7 However, a well-recognized disadvantage of MRI in the human neurology field is the potential for overinterpretation.4,6,7 Several studies4,7–10 have found a high prevalence of abnormal MRI findings in the cervical vertebral column of asymptomatic people, indicating that MRI can display detailed anatomic and pathological changes but cannot directly determine their clinical importance. As such, imaging of clinically normal subjects is important so that clinicians making treatment recommendations can recognize the frequency and spectrum of MRI abnormalities that may be seen in individuals without clinical signs.4,7,9,11 In the veterinary literature, only a few studies3,12 have investigated the presence of cervical vertebral column MRI abnormalities in clinically normal dogs. These studies3,12 focused mostly on Doberman Pinschers, a breed in which disk-associated CSM is common, and revealed that neurologically normal Doberman Pinschers have a high prevalence of abnormalities evident on MRI, including intervertebral disk degeneration, spinal cord compression, and foraminal stenosis, alone or in combination. To our knowledge, no equivalent studies have investigated whether MRI abnormalities are also present in the cervical vertebral columns of clinically normal giant-breed dogs. Giant-breed dogs such as Great Danes commonly have osseous-associated CSM.13 The pathophysiology of osseous-associated CSM differs from that of disk-associated CSM.13 Consequently, results from studies of Doberman Pinschers cannot be extrapolated to Great Danes. In addition, 1 study3 that included 20 clinically normal Doberman Pinschers found that the severity of disk degeneration and disk-associated compression in clinically normal dogs of this breed was significantly associated with increased age. The osseous-associated form of CSM predominantly affects young adult giant dog breeds,13,14 in which an influence of age on MRI findings would not be expected.
To our knowledge, no study has investigated morphological features of the cervical vertebral column via MRI of clinically normal Great Danes or compared morphological features of the cervical vertebral column detectable with MRI between neurologically normal and CSM-affected dogs of this breed. The purpose of the study reported here was to prospectively characterize and compare the morphological features of the cervical vertebral column in Great Danes with and without clinical signs of CSM by means of MRI. We hypothesized that the morphological features of cervical vertebral canal structures would differ between these groups and that abnormalities of these structures would be found by MRI of clinically normal Great Danes.
Materials and Methods
Animals
Thirty client-owned Great Danes were prospectively enrolled in the study between April 4, 2011, and October 4, 2012. The investigation was conducted in accordance with the guidelines and with approval of the Clinical Research Advisory Committee and the Institutional Animal Care and Use Committee of The Ohio State University. Informed consent was obtained from each owner in writing prior to study enrollment.
Fifteen Great Danes that were determined to be clinically normal on the basis of results of physical examination and neurologic examination comprised the clinically normal group. Dogs were required to be ≥ 1 year of age and have no prior history of neurologic disease to be eligible for enrollment in the clinically normal group. Fifteen Great Danes with clinical signs and neurologic examination findings consistent with CSM were enrolled in the CSM-affected group. Neurologic examination findings, age at onset of clinical signs, and administration of any medications at the time of study enrollment were recorded. The 2 investigators performed all neurologic examinations together. All study dogs underwent CBC, serum biochemical analysis, and MRI of the cervical vertebral column.
MRI examination
Magnetic resonance imaging of the cervical vertebral column was performed in all dogs under general anesthesia with a 3.0-T magneta and a surface coil. Dogs were positioned in dorsal recumbency with the head and neck initially in neutral position. Images were acquired with a turbo spin-echo technique. First, T1- and T2-weighted images were obtained in the dorsal, sagittal, and transverse planes. After acquisition of all image sequences with the cervical region in a neutral position, T2-weighted sagittal images were acquired after linear traction was applied by use of a neck harness and weight equal to 20% of the dog’s body weight. The MRI protocol for each dog included the following: a sagittal T1-weighted sequence (TR, 700 milliseconds; TE, 8 milliseconds), transverse and dorsal T1-weighted sequences (TR, 650 milliseconds; TE, 8 milliseconds), sagittal T2-weighted sequences in neutral position and with traction (TR, 5,000 milliseconds; TE, 110 milliseconds), and transverse and dorsal T2-weighted sequences (TR, 4,000 milliseconds; TE, 120 milliseconds). The field of view was 30 cm in the sagittal and dorsal planes and 20 cm in the transverse plane. Slice thickness was set at 3 mm with no interslice interval. Seven intervertebral spaces from C2–3 through T1–2 were imaged in each dog. Five transverse slices were obtained for every intervertebral space. The transverse slices were aligned parallel to the intervertebral disk and arranged to pass through the center of each intervertebral disk as well as the cranial and caudal end plates of the adjacent vertebral bodies, as has been previously described.12
Morphological features
All MRI images were evaluated by 1 investigator (PMV) who was not blinded to clinical status of the dogs. Images were reviewed with dedicated software.b Transverse and sagittal T2-weighted images were used to evaluate dogs for compression of the subarachnoid space, spinal cord, or both. The main site and any additional sites of compression were recorded. Each intervertebral space was graded as previously described3,12: 0, no compression; 1, partial subarachnoid space compression with no spinal cord compression; 2, complete subarachnoid space compression with no spinal cord compression; or 3, spinal cord compression. Spinal cord compression was further categorized on the basis of percentage reduction in spinal cord diameter as previously described15: mild (< 25%), moderate (25% to 50%), or severe (> 50%). The direction of compression was recorded as dorsal, ventral, or lateral. Dorsolateral and ventrolateral compressions were included as lateral compressions. Lateral compressions were classified as unilateral or bilateral; if > 1 direction of compression was observed, all types were recorded, and the direction of the most severe compression was used for statistical analysis. The cause of compression was classified as intervertebral disk associated, osseous associated (related to articular process [facet] joint, pedicle, or lamina proliferative changes), or soft tissue hypertrophy associated (caused by ligamentous hypertrophy).13,16 All available transverse plane images through each intervertebral space were evaluated.
Signal changes of the spinal cord were evaluated on transverse T2- and T1-weighted images. If an area of spinal cord hyperintensity was seen on T2-weighted images, then the corresponding T1-weighted images were evaluated and the spinal cord parenchyma classified as iso- or hypointense. The areas of abnormal spinal cord signal changes were compared with the areas of normal spinal cord intensity cranial and caudal to the abnormality. Spinal cord signal changes were further classified (subjectively) as marked or mild.
The characteristics of articular process joints were evaluated on transverse T1- and T2-weighted images according to 4 criteria: the presence of synovial fluid, regularity of the articular surface and presence of sub chondral bone sclerosis, degree of articular process joint proliferation, and degree of hypertrophy of tissues dorsal to the dorsal lamina. These features were chosen to enable comparison with previously published findings.16 Transverse T2-weighted images obtained at the center of the intervertebral disk were used to assess and grade the presence of hyperintense synovial fluid as follows: grade 0 if the amount of synovial fluid was judged to be normal, 1 if there was a reduced amount of fluid but it was still visible, and 2 if the synovial fluid signal was absent. Transverse T1- and T2-weighted images were both used to assess the remaining 3 criteria. Regularity of the articular surface and presence of subchondral bone sclerosis was graded 0 if the articular surface was smooth with no evidence of subchondral bone sclerosis, 1 if it was smooth but had evidence of sclerosis, and 2 if it was irregular and sclerotic. The degree of articular process joint proliferation was graded as 0 if the articular process joint was considered to have a normal size, 1 if there was mild proliferation consistent with a joint < 25% larger than the (expected) normal size, 2 if there was moderate proliferation consistent with a joint 25% to 50% larger than normal, and 3 if there was severe proliferation consistent with a joint > 50% larger than the expected normal size. The degree of tissue hypertrophy dorsal to the dorsal lamina was graded as 0 is there was a uniform, thin band of hypertrophic tissue over the lamina; 1 if there was a dense and irregular layer of hypertrophic tissue covering < 50% of the dorsal lamina; and 2 if there was a dense and irregular layer of hypertrophic tissue covering ≥ 50% of the lamina.
Transverse T1- and T2-weighted images obtained at the center of the intervertebral disk were used to assess the presence of dorsoventral foraminal stenosis. The condition was subjectively classified as absent, mild (< 25% stenosis), moderate (25% to 50% stenosis), or severe (> 50% stenosis).12,15
Each intervertebral disk was classified as normal (uniform hyperintense signal), partially degenerated (≥ 50% loss of hyperintense signal), or completely degenerated (complete loss of hyperintense signal) on sagittal T2-weighted images. The intervertebral disk was assessed for protrusion on sagittal and transverse T1- and T2-weighted images and subjectively classified as normal or as having mild, moderate, or severe protrusion.3,12
Transverse T1- and T2-weighted images were used to assess visibility of the ventral internal vertebral venous plexus (classified as visible or not visible). The presence of extradural synovial cysts was assessed by examination of transverse and dorsal T2-weighted images. Sagittal T2-weighted images were compared before and after the application of traction. A compressive site was classified as dynamic if the subarachnoid space compression, spinal cord compression, or both observed on neutral images resolved after traction was applied. The compressive site was classified as static if it remained unchanged after applying traction. If the compressive site improved after application of traction but remained present, it was classified as static with a dynamic component.2
Statistical analysis
Comparison between groups for morphological features evaluated via MRI was performed by use of a random-effects logistic regression model with commercially available software.c For those MRI morphological features containing low numbers of observations (< 5) in any of the subcategories assessed within the morphological feature and for which a random- effects logistic regression model could not be used, data were summarized for clinically normal and CSM-affected Great Danes without statistical comparison. For features evaluated with a random-effects logistic regression model, significance was set at a value of P < 0.05.
Results
Clinical data
The clinically normal group included 7 female (6 spayed and 1 sexually intact) and 8 male (7 neutered and 1 sexually intact) Great Danes; median age at the time of study enrollment was 2.3 years (range, 1 to 6.4 years), and median weight was 52 kg (114.4 lb; range, 40.5 to 73 kg [89.1 to 160.6 lb]). The CSM-affected group included 2 female (both spayed) and 13 male (12 neutered and 1 sexually intact) Great Danes. Median age for this group at study enrollment was 4 years (range, 1 to 7.2 years), and median weight was 56.8 kg (125 lb; range, 42 to 79.3 kg [92.4 to 174.5 lb]).
Median reported age at the onset of signs for the CSM-affected dogs was 1.7 years (range, 0.4 to 4.2 years). Clinical signs had been present for a mean of 1.9 years (range, 0 to 5 years) before enrollment in the study. Fourteen of 15 CSM-affected dogs had ambulatory tetraparesis with general proprioceptive ataxia of all 4 limbs. One dog in this group had a spastic thoracic limb gait with ambulatory paraparesis and general proprioceptive ataxia of the pelvic limbs. All CSM-affected dogs had delayed postural reactions involving all 4 limbs. Mild signs of neck pain were elicited in 6 affected dogs at the time of examination for study enrollment.
All clinically normal dogs had normal results for physical and neurologic examinations and hematologic analysis. Eleven of 15 CSM-affected dogs were receiving orally administered anti-inflammatory medications at the time of study enrollment. Seven CSM-affected dogs were receiving prednisone (doses ranged from 0.34 mg/kg [0.15 mg/lb], q 72 h, to 0.6 mg/kg [0.27 mg/lb], q 24 h), 1 was receiving dexamethasone (0.064 mg/kg [0.029 mg/lb], q 24 h), 2 were receiving carprofend (2.3 mg/kg [1.0 mg/lb], q 12 h), and 1 was receiving meloxicame (0.11 mg/kg [0.05 mg/lb], q 24 h). Hematologic results outside of the reference ranges for dogs in the CSM-affected group were interpreted as consistent with the administration of anti-inflammatory medication.
Morphological features
A total of 210 intervertebral spaces and 420 foraminal sites were evaluated in the 30 Great Danes by means of MRI. The number and characteristics of sites of subarachnoid space compression, spinal cord compression, and foraminal stenosis were summarized (Table 1). Representative MRI images showing selected morphological features of clinically normal and CSM-affected Great Danes are shown (Figure 1).
Table 1.
Summary of morphological features of the cervical vertebral column in 30 Great Danes deemed clinically normal (n = 15) or CSM affected (15).
| Variable | Clinically normal |
CSM affected |
|---|---|---|
| Intervertebral sites evaluated (all) | 105 | 105 |
| Compressive sites (all) | 11 | 61 |
| Sites of subarachnoid space compression | 9 | 17 |
| Partial | 8 | 17 |
| Complete | 1 | 0 |
| Sites of spinal cord compression | 2 | 44 |
| Mild | 1 | 17 |
| Moderate | 1 | 11 |
| Severe | 0 | 16 |
| Direction of compression | ||
| Dorsal | 2 | 2 |
| Ventral | 1 | 0 |
| Lateral | 8 | 59 |
| Unilateral | 4 | 6 |
| Bilateral | 4 | 53 |
| Cause of compression | ||
| Soft tissue | 2 | 1 |
| Disk | 1 | 0 |
| Osseous | 8 | 60 |
| Foramina evaluated (all) | 210 | 210 |
| Normal foramina | 154 | 58 |
| Sites of foraminal stenosis (all) | 56 | 152 |
| Mild | 22 | 54 |
| Moderate | 26 | 26 |
| Severe | 8 | 72 |
Data represent number of sites.
Figure 1.
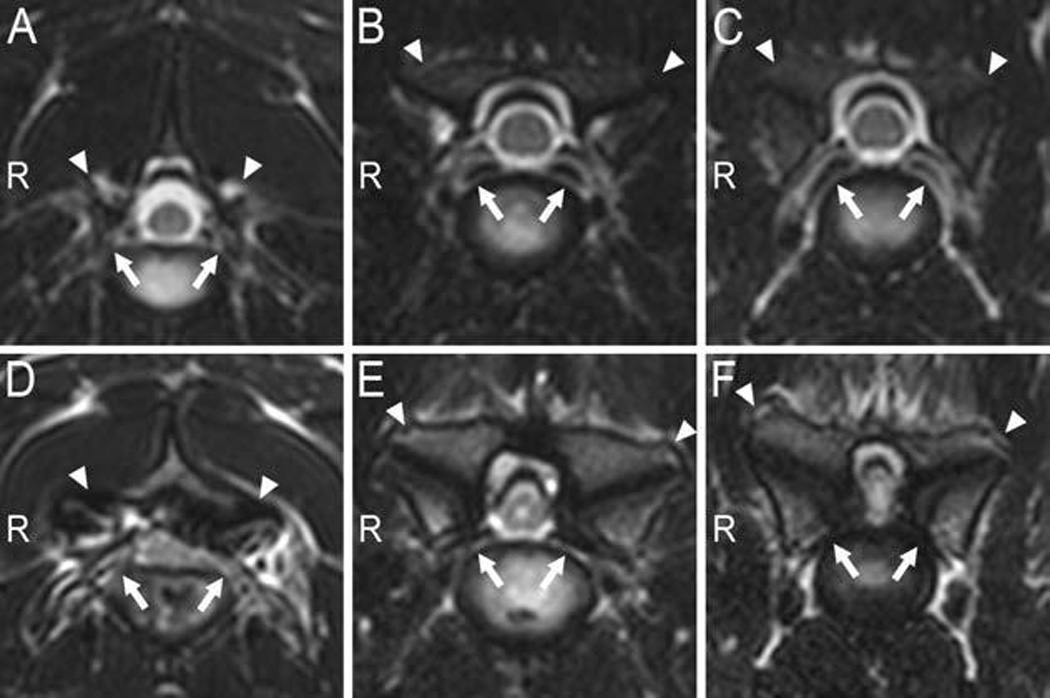
Transverse T2-weighted MRI images obtained at the center of 3 intervertebral disks (from left to right: C2–3, C5–6, and C6–7) in a clinically normal Great Dane (A–C), and the corresponding intervertebral sites in a Great Dane with CSM (D–F). In the CSM-affected dog, notice severe spinal cord compression in the lateral direction at all 3 sites secondary to severe osseous proliferation of the articular (facet) joints (arrowheads), most severe at C6–7. There is also loss of synovial fluid identifiable by loss of the hyperintense signal between the cranial and caudal articular joints, as well as irregular and sclerotic articular surfaces and bilateral foraminal stenosis (arrows) at the 3 sites. In the CSM-affected dog, hyperintensity of the spinal cord signal is evident at C5–6 and C6–7. The ventral internal venous plexus is visible in all 3 images from the clinically normal dog at the level of the intervertebral foramina (arrows) but is not evident in images from the CSM-affected dog. R = Right side.
Evaluation of the MRI images revealed 9 sites of subarachnoid space compression and 2 sites of spinal cord compression in 6 of 15 clinically normal dogs (Table 1). The sites of subarachnoid space compression were recorded at C3–4 (2 dogs), C4–5 (2 dogs), and C6–7 (5 dogs). Three dogs each had 2 sites of subarachnoid space compression, and 3 dogs had 1 affected site each. The 2 sites of spinal cord compression were observed in 1 clinically normal dog at C4–5 and C5–6, with C5–6 being the main site of compression (Figure 2).
Figure 2.
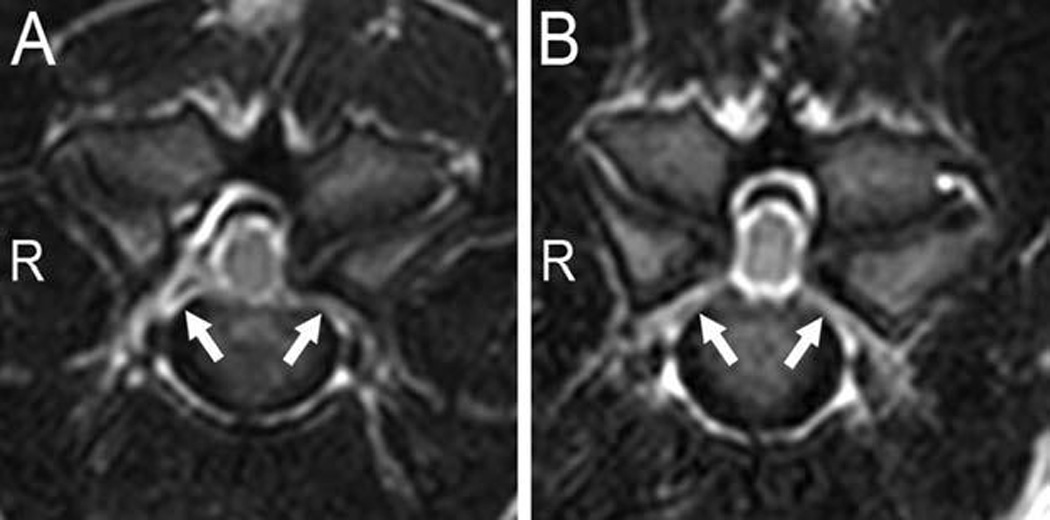
Transverse T2-weighted images at the center of the C4–5 (A) and C5–6 (B) intervertebral disks from a clinically normal Great Dane that had spinal cord compression. The lateral compression was secondary to articular process joint proliferation classified as mild and unilateral (left sided) at C4–5 and moderate and bilateral at C5–6. Bilateral foraminal stenosis was also evident (arrows). R = Right side.
Sixty-one compressive sites were recorded for dogs in the CSM-affected group, including 17 sites of subarachnoid space compression and 44 sites of spinal cord compression (Table 1). The total number of compressive sites recorded was significantly higher in the CSM-affected group (P < 0.001), compared with the clinically normal group. All CSM-affected dogs had ≥ 1 compressive site, and all had > 1 site affected when both subarachnoid space and spinal cord compression were considered. Multiple sites of subarachnoid space compression were recorded in 11 of the 15 CSM-affected dogs; 1 dog had 3 affected sites, 4 dogs each had 2 affected sites, and 6 dogs each had 1 affected site. Among dogs with spinal cord compression, 5 dogs each had 4 affected sites, 6 dogs each had 3 affected sites, 2 dogs each had 2 affected sites, and 2 dogs had 1 affected site each. Sites affected by subarachnoid space compression in CSM-affected dogs included C3–4 (5 dogs), C7-T1 (4 dogs), C5–6, C6–7, and T1–2 (2 dogs each), and C2–3 and C4–5 (1 dog each), and sites affected by spinal cord compression included C4–5 and C6–7 (12 dogs each), C5–6 (10 dogs), C2–3 (5 dogs), C3–4 (3 dogs), and C7-T1 (2 dogs). In CSM-affected dogs, the main site of compression was C6–7 (8 dogs), C4–5 (3 dogs), C5–6 (2 dogs), C2–3 (1 dog), and C3–4 (1 dog).
The direction of compression for most compressive sites (8/11 and 59/61 in clinically normal and CSM-affected dogs, respectively) was lateral, and all of these lesions were secondary to osseous proliferation of articular processes, lamina, or pedicles (Table 1). One clinically normal dog (6.3 years old) had partial ventral subarachnoid space compression at C6–7, which was secondary to a mild intervertebral disk protrusion. Two sites of dorsal compression were noted in dogs of each group; 3 were secondary to dorsal soft tissue hypertrophy and 1 was the result of dorsal lamina proliferation.
None of the clinically normal dogs had detectable signal changes within the spinal cord. There were 14 sites of spinal cord hyperintensity on T2-weighted images recorded for 9 CSM-affected dogs. All the areas of spinal cord hyperintensity were observed at sites of spinal cord compression. One dog had 3 sites of spinal cord hyperintensity, 3 dogs had 2 affected sites each, and 5 dogs had 1 affected site each. The hyperintense spinal cord signal changes in CSM-affected dogs were recorded at C6–7 (8 dogs), C5–6 (3 dogs), C2–3 (2 dogs), and C4–5 (1 dog). Eight of the hyperintensities were classified as marked and 4 as mild. The corresponding T1-weighted images revealed 11 isointense and 3 hypointense sites. The isointense sites were at C6–7 (5 dogs), C5–6 (3 dogs), C2–3 (2 dogs), and C4–5 (1 dog). The hypointense sites were recorded at C6–7 in 3 dogs and corresponded to the main site of compression for those CSM-affected dogs. All hypointense spinal cord signal sites recorded on T1-weighted images corresponded with areas of marked spinal cord hyperintensity on T2-weighted images, and this combination was only recorded at sites of severe spinal cord compression (Figure 3).
Figure 3.
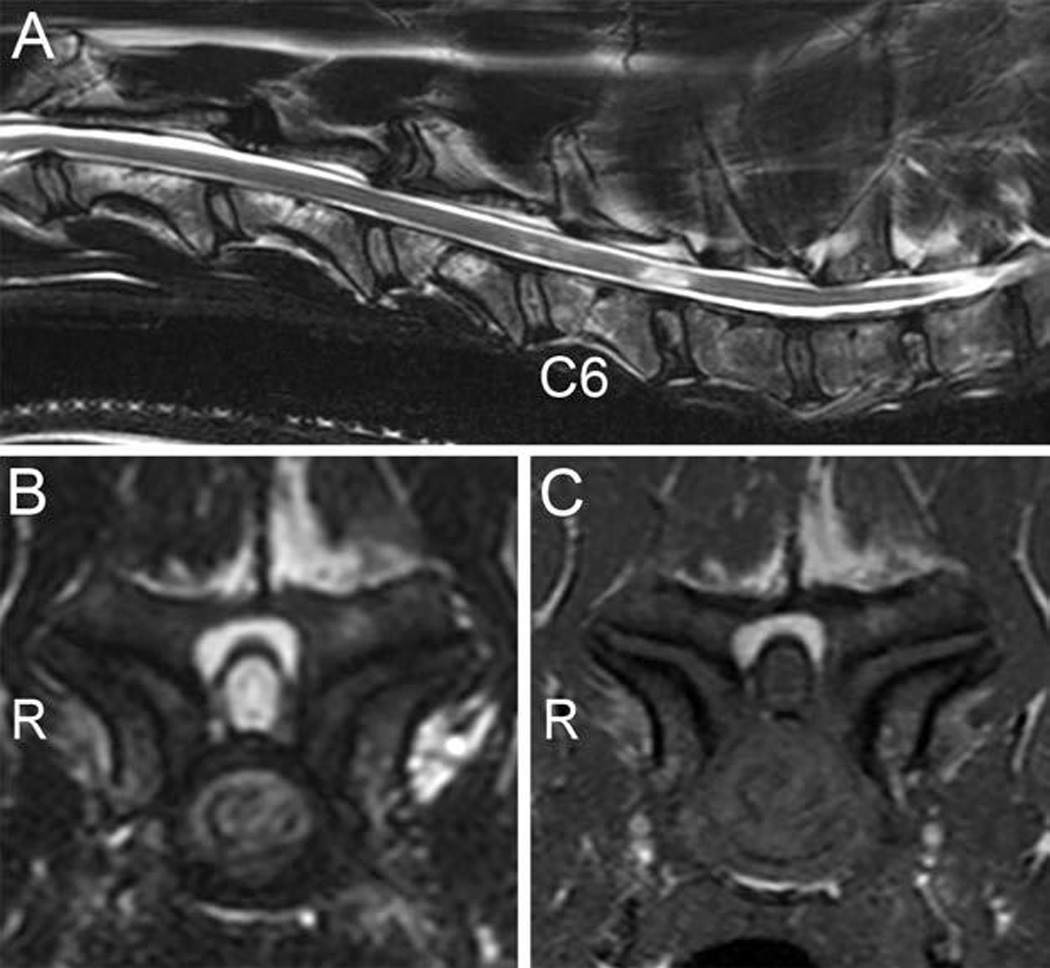
Midsagittal T2-weighted image (A), transverse T2-weighted image at C6–7 (B), and transverse T1-weighted image at C6–7 (C) of a CSM-affected Great Dane with spinal cord compression. The midsagittal image reveals spinal cord signal changes at C5–6 (mild) and C6–7 (marked). Marked spinal cord signal hyperintensity is evident on the transverse T2-weighted image, with hypointensity on the T1-weighted image, attributable to severe lateral compression secondary to bilateral articular process joint proliferation with irregular articular surfaces and subchondral bone sclerosis. The vertebral canal is severely stenotic in the affected region, and the spinal cord has an elongated (narrowed) appearance. R = Right side.
Articular process joint characteristics were summarized (Table 2). The number of sites with abnormal synovial joint fluid signal was significantly (P < 0.039) greater in CSM-affected than in the clinically normal Great Danes. Regularity of the articular surfaces and the presence of subchondral bone sclerosis were also significantly (P < 0.001) different between groups; CSM-affected dogs had a greater number of articular process joints with sclerosis and irregular articular surfaces than did clinically normal dogs. The degree of articular process joint proliferation also differed significantly (P < 0.001) between groups, with CSM-affected dogs having a greater number of articular process joints with proliferative changes.
Table 2.
Summary of articular process joint characteristics evaluated by MRI of 30 Great Danes deemed clinically normal (n = 15) or CSM affected (15).
| Variable | Clinically normal |
CSM affected |
|---|---|---|
| Intervertebral sites evaluated (all) | 105 | 105 |
| Synovial joint fluid signal | ||
| Normal intensity (grade 0) | 32 | 16 |
| Reduced intensity, but visible (grade 1) | 36 | 32 |
| Absent (grade 2) | 37 | 57 |
| Regularity of the articular surface and presence of sclerosis | ||
| Smooth surface, no sclerosis (grade 0) | 97 | 38 |
| Smooth surface with sclerosis (grade 1) | 6 | 13 |
| Irregular surface with sclerosis (grade 2) | 2 | 54 |
| Degree of articular process joint proliferation | ||
| Normal size (grade 0) | 80 | 5 |
| Mild proliferation, < 25% larger than expected size (grade 1) | 22 | 42 |
| Moderate proliferation, 25%–50% larger than expected size (grade 2) | 3 | 41 |
| Severe proliferation, > 50% larger than expected size (grade 3) | 0 | 17 |
| Degree of tissue hypertrophy dorsal to the dorsal lamina | ||
| Normal (grade 0) | 103 | 96 |
| Hypertrophic tissue covering < 50% of the dorsal lamina (grade 1) | 1 | 3 |
| Hypertrophic tissue covering > 50% of the dorsal lamina (grade 2) | 1 | 6 |
Data represent number of sites.
Foraminal stenosis was present in 11 of 15 clinically normal (Figure 4) and 15 of 15 CSM-affected Great Danes (Figure 1). However, the number of stenotic foraminal sites recorded was significantly (P < 0.001) greater in the CSM-affected group than in the clinically normal group. In addition, foraminal stenosis was classified as severe in 72 of 152 sites in CSM-affected dogs (with all dogs having ≥ 1 severely affected site), whereas 8 of 56 stenotic foraminal sites were classified as severe in 6 clinically normal dogs (Table 1). Visibility of the ventral internal venous plexus was significantly (P < 0.001) different between groups. Of 105 intervertebral sites evaluated in each group, 82 venous plexuses were classified as visible in clinically normal dogs, and only 50 were visible in CSM-affected dogs.
Figure 4.
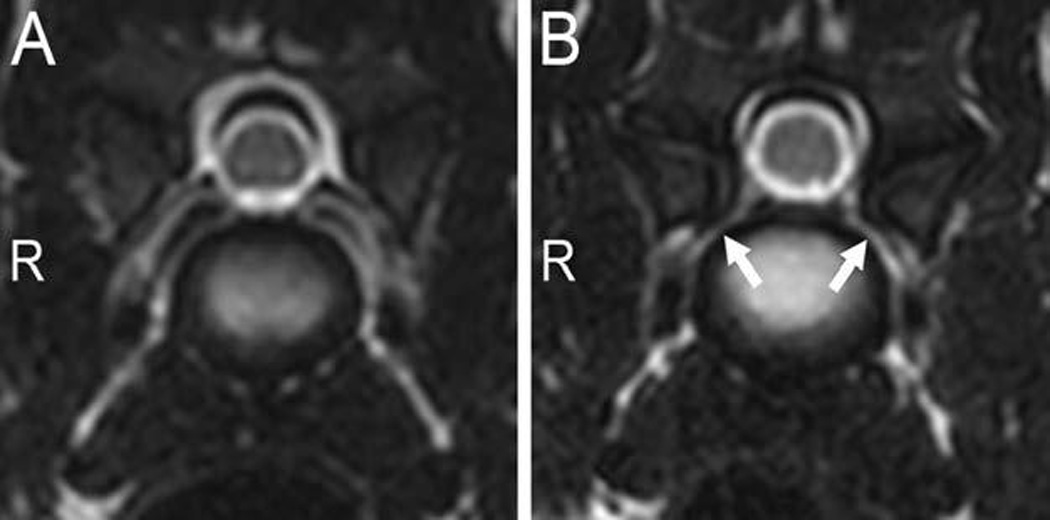
Transverse T2-weighted images obtained at the center of the C6–7 intervertebral disks in 2 clinically normal Great Danes (A and B). In panel B, notice the differences in foraminal size and the presence of bilateral foraminal stenosis (arrows), compared with that in panel A. R = Right side.
Of 105 intervertebral disks evaluated/group, 95 and 76 were classified as normal for the clinically normal and the CSM-affected groups, respectively. Partial intervertebral disk degeneration was recorded at 9 sites in clinically normal dogs and 13 sites in CSM-affected dogs. In addition, complete intervertebral disk degeneration was identified at 1 site in the clinically normal group and 16 sites in the CSM-affected group. The number of sites with intervertebral disk degeneration was significantly (P < 0.039) greater in CSM-affected dogs. Mild intervertebral disk protrusion was recorded in only 2 dogs (1/group) and was found at C6–7 in both.
Three extradural synovial cysts were detected in 3 CSM-affected dogs, at C4–5, C5–6, and C6–7. All cysts were unilateral and present at sites of subarachnoid space or spinal cord compression secondary to osseous-associated proliferative changes. No extradural synovial cysts were found in clinically normal dogs.
After traction was applied, all but 2 of the compressive sites in 2 CSM-affected dogs remained unchanged (static); at these 2 sites, the application of traction did not resolve the compression but allowed an improved visualization of the subarachnoid space (static with a dynamic component).
Discussion
In this study, morphological features of the cervical vertebral column of clinically normal and CSM-affected Great Danes were investigated by means of MRI. The proportion of clinically normal Great Danes in our study that had spinal cord compression (1/15 dogs) was lower than that reported12 for clinically normal Doberman Pinschers evaluated via MRI (4/16), although the proportions of clinically normal dogs with foraminal stenosis were similar (11/15 in the present study vs 11/16 in the previous investigation12). Results of the present study confirmed that clinically normal Great Danes can have cervical vertebral column abnormalities detectable with MRI, although considerable differences were found between MRI findings in clinically normal and CSM-affected dogs. The 15 CSM-affected dogs in our study all had clear indications of spinal cord compression, which was rare (1/15) among clinically normal dogs; in addition, 9 of 15 CSM-affected dogs had ≥ 1 spinal cord signal intensity abnormality, which was not detected in any normal dogs. All CSM-affected dogs had severe foraminal stenosis at ≥ 1 intervertebral site with a total of 72 severely stenotic foramina recorded in this group, whereas severe stenosis was recorded at only 8 foraminal sites in 6 of 11 clinically normal dogs that had foraminal stenosis.
The MRI findings in CSM-affected Great Danes of this study are similar to those in previously reported retrospective studies13,16 in which all CSM-affected Great Danes had osseous-associated CSM secondary to bilateral proliferative changes of the articular process joints, lamina, or pedicles, and these changes were often present at multiple sites. We recorded sites of subarachnoid space or spinal cord compression from C2–3 through T1–2, which highlights the fact that osseous-associated CSM can affect all cervical as well as cranial thoracic vertebrae and not just the caudal cervical region.13 These findings should be taken into consideration when planning imaging studies in breeds predisposed to osseous-associated CSM.
We found spinal cord compression in 1 clinically normal dog. The importance of detecting spinal cord compression on MRI of a clinically normal dog is difficult to determine. Several studies4,7,9,17 have shown that spinal cord compression is not uncommon in asymptomatic people; furthermore, hyperintense signal changes of the spinal cord on T2-weighted images in asymptomatic individuals have also been described. Results of a longitudinal MRI study11 of the cervical vertebral column in asymptomatic people over a 10-year period revealed progression of degenerative changes and development of symptoms in 76 of 223 (34%) subjects, with age being the main factor related to progression. In the present study, the only clinically normal Great Dane with spinal cord compression was 1.4 years old. Great Danes with osseous-associated CSM usually develop clinical signs before 4 years of age,16,18,19 and although this dog was determined to be neurologically normal at the time of enrollment, the 2 sites of spinal cord compression recorded in this dog were secondary to osseous proliferative changes and the possibility exists that it could develop clinical signs of osseous-associated CSM in the future. Prospective, longitudinal clinical and imaging studies that include long-term follow- up of clinically normal dogs with MRI abnormalities would be needed to determine whether these dogs eventually develop associated clinical signs or if they develop other abnormalities detectable via MRI.
Nine of 15 CSM-affected Great Danes had spinal cord signal changes, which were all observed at sites of spinal cord compression. An overall prevalence of 57 of 102 (55.9%) was reported for spinal cord signal changes in dogs with CSM,f which is similar to our findings. We used T2-weighted images and the corresponding T1-weighted images to assess spinal cord signal changes, similar to methods routinely used for evaluation of humans.6,20,21 In people with cervical spondylotic myelopathy, which is a chronic compressive myelopathy similar to CSM of dogs, hyperintense T2-weighted spinal cord signal changes can reflect a spectrum of pathological changes ranging from reversible edema, when the spinal cord signal change is ill-defined, to irreversible injury, when it is intense and well-defined.6,20 However, the presence of intramedullary hypointensity on T1-weighted images generally implies irreversible spinal cord injury.20 Both findings in combination as well as the occurrence of spinal cord signal changes at multiple sites are related to poor surgical outcomes.6,21 Three CSM-affected Great Danes had intense, marked spinal cord hyperintensities on T2-weighted images at C6–7 with corresponding spinal cord hypointensities on T1-weighted images. This combination of spinal cord signal changes was only recorded at severe sites of spinal cord compression and corresponded to the main site of spinal cord compression in these 3 dogs. Each of these 3 dogs had had clinical signs for > 3 years. Similarly, in a retrospective studyf of giant- and large-breed dogs with CSM, dogs with spinal cord signal changes had more severe neurologic deficits and more commonly had severe or moderate spinal cord compression and a chronic history of clinical signs consistent with CSM than did dogs without this finding.
The features of cervical articular process joints observed via MRI in CSM-affected dogs of the present study were consistent with the results of a retrospective study,16 in which most of the articular processes of CSM-affected Great Danes had indications of degenerative changes. In addition, our study evaluated the articular process joint characteristics of clinically normal Great Danes and found that this group had a lower degree of articular process proliferative changes, with more joints considered to be of normal size, and more commonly had smooth articular surfaces with no signs of subchondral bone sclerosis, compared with CSM-affected Great Danes.
Foraminal stenosis was commonly found in both groups of Great Danes in our study, although the number of sites affected was significantly greater in CSM-affected dogs and severe lesions appeared to be more common in this group. In people with cervical spondylotic myelopathy, foraminal stenosis is common and has been identified as a key factor contributing to ischemic insult in pathogenesis of the disease.22 However, asymptomatic foraminal stenosis is also frequently recognized in people.4,11,17 The clinical importance of foraminal stenosis in subclinically affected dogs is currently unknown.
Intervertebral disk degeneration and protrusion were uncommon in the Great Danes of this study. Only one 6.3-year-old clinically normal Great Dane had mild ventral subarachnoid space compression due to an intervertebral disk protrusion. This contrasts with the high prevalence of these abnormalities in Doberman Pinschers with disk-associated CSM,3,12 supporting the different pathogenesis between osseous-associated and disk-associated CSM.13
A limitation of the present study was that, although 1 investigator performed all MRI evaluations, this individual was not blinded to the clinical status of dogs. To help minimize bias, all the morphological features were graded by use of predetermined grading schemes. Whenever possible, the authors followed grading schemes for MRI investigation of cervical vertebral column morphological features in clinically normal large-breed dogs and CSM-affected large- and giant-breed dogs used in previous studies.2,3,12,15,16 This also allowed for subjective comparison between the results of this study and findings in other reports.
The present study characterized several abnormalities on MRI of Great Danes with and without CSM and identified differences in MRI results between these groups. The results reiterate the importance of interpreting MRI findings in conjunction with a thorough neurologic examination before making treatment decisions.
Acknowledgments
Supported by the Great Dane Club of America, an Intramural Canine grant from The Ohio State University College of Veterinary Medicine, and The Ohio State University Center for Clinical and Translational Science Award (grant No. UL1TR000090) from the National Center For Advancing Translational Sciences.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
The authors thank Marc Hardman for assistance with illustrations and Gary Phillips for assistance with statistical analysis.
ABBREVIATIONS
- CSM
Cervical spondylomyelopathy
- TE
Echo time
- TR
Repetition time
Footnotes
The authors report no conflict of interest.
Presented in abstract form at the American College of Veterinary Internal Medicine Forum, Seattle, June 2013.
Achieva 3.0 T, Philips Healthcare, Best, The Netherlands.
E-Film Merge Healthcare, Milwaukee, Wis.
Stata, version 12.1, Stata Corp, College Station, Tex.
Rimadyl, Pfizer Animal Health, Exton, Pa.
Metacam, Boehringer Ingelheim Vetmedica Inc, St Joseph, Mo.
da Costa RC. Relationship between spinal cord signal changes and clinical and MRI findings in dogs with cervical spondylomyelopathy—102 cases (abstr). J Vet Intern Med 2012;26:807.
References
- 1.Lipsitz D, Levitski RE, Chauvet AE, et al. Magnetic resonance imaging features of cervical stenotic myelopathy in 21 dogs. Vet Radiol Ultrasound. 2001;42:20–27. doi: 10.1111/j.1740-8261.2001.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 2.da Costa RC, Parent JP, Dobson H, et al. Comparison of magnetic resonance imaging and myelography in 18 Doberman Pinscher dogs with cervical spondylomyelopathy. Vet Radiol Ultrasound. 2006;47:523–531. doi: 10.1111/j.1740-8261.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 3.De Decker S, Gielen IM, Duchateau L, et al. Low-field magnetic resonance imaging findings of the caudal portion of the cervical region in clinically normal Doberman Pinschers and Foxhounds. Am J Vet Res. 2010;71:428–434. doi: 10.2460/ajvr.71.4.428. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. J Bone Joint Surg Am. 1990;72:1178–1184. [PubMed] [Google Scholar]
- 5.Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16:176–187. doi: 10.1097/NRL.0b013e3181da3a29. [DOI] [PubMed] [Google Scholar]
- 6.Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19:409–421. doi: 10.1177/1073858412467377. [DOI] [PubMed] [Google Scholar]
- 7.Kato F, Yukawa Y, Suda K, et al. Normal morphology, age-related changes and abnormal findings in the cervical spine. Part II: magnetic resonance imaging of over 1,200 asymptomatic subjects. Eur Spine J. 2012;21:1499–1507. doi: 10.1007/s00586-012-2176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bednarik J, Kadanka K, Dusek L, et al. Presymptomatic spondylotic cervical cord compression. Spine. 2004;29:2260–2269. doi: 10.1097/01.brs.0000142434.02579.84. [DOI] [PubMed] [Google Scholar]
- 9.Teresi LM, Lufkin RB, Reicher MA, et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology. 1987;164:83–88. doi: 10.1148/radiology.164.1.3588931. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Kim KT, Suk KS, et al. Asymptomatic cervical cord compression in lumbar spinal stenosis patients: a whole spine magnetic resonance imaging study. Spine. 2010;35:2057–2063. doi: 10.1097/BRS.0b013e3181f4588a. [DOI] [PubMed] [Google Scholar]
- 11.Okada E, Matsumoto M, Ichihara D, et al. Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine. 2009;34:706–712. doi: 10.1097/BRS.0b013e31819c2003. [DOI] [PubMed] [Google Scholar]
- 12.da Costa RC, Parent JM, Partlow G, et al. Morphologic and morphometric magnetic resonance imaging features of Doberman Pinschers with and without clinical signs of cervical spondylomyelopathy. Am J Vet Res. 2006;67:1601–1612. doi: 10.2460/ajvr.67.9.1601. [DOI] [PubMed] [Google Scholar]
- 13.da Costa RC. Cervical spondylomyelopathy (wobbler syndrome) in dogs. Vet Clin North Am Small Anim Pract. 2010;40:881–913. doi: 10.1016/j.cvsm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Lewis M, Olby NJ, Sharp NJH, et al. Long-term effect of cervical distraction and stabilization on neurological status and imaging findings in giant breed dogs with cervical stenotic myelopathy. Vet Surg. 2013;42:701–709. doi: 10.1111/j.1532-950X.2013.12034.x. [DOI] [PubMed] [Google Scholar]
- 15.da Costa RC, Echandi RL, Beauchamp D. Computed tomography myelographic findings in dogs with cervical spondylomyelopathy. Vet Radiol Ultrasound. 2012;53:64–70. doi: 10.1111/j.1740-8261.2011.01869.x. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez-Quintana R, Penderis J. MRI features of cervical articular process degenerative joint disease in Great Dane dogs with cervical spondylomyelopathy. Vet Radiol Ultrasound. 2012;53:304–311. doi: 10.1111/j.1740-8261.2011.01912.x. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of the cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80:19–24. doi: 10.1302/0301-620x.80b1.7929. [DOI] [PubMed] [Google Scholar]
- 18.Trotter EJ, de Lahunta A, Geary JC, et al. Caudal cervical vertebral malformation-malarticulation in Great Danes and Doberman Pinschers. J Am Vet Med Assoc. 1976;168:917–930. [PubMed] [Google Scholar]
- 19.Olsson SE, Stavenborn M, Hoppe F. Dynamic compression of the cervical spinal cord. A myelographic and pathologic investigation in Great Dane dogs. Acta Vet Scand. 1982;23:65–78. doi: 10.1186/BF03546823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maus TP. Imaging of spinal stenosis. Neurogenic intermittent claudication and cervical spondylotic myelopathy. Radiol Clin North Am. 2012;50:651–679. doi: 10.1016/j.rcl.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Avadhani A, Rajasekaran S, Shetty AP. Comparison of prognostic value of different MRI classifications of signal intensity change in cervical spondylotic myelopathy. Spine J. 2010;10:475–485. doi: 10.1016/j.spinee.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine. 1998;23:2730–2737. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]


