Abstract
Global pharmaceutical consumption is rising with the growing and ageing human population and more intensive food production. Recent studies have revealed pharmaceutical residues in a wide range of ecosystems and organisms. Environmental concentrations are often low, but pharmaceuticals typically are designed to have biological effects at low doses, acting on physiological systems that can be evolutionarily conserved across taxa. This Theme Issue introduces the latest research investigating the risks of environmentally relevant concentrations of pharmaceuticals to vertebrate wildlife. We take a holistic, global view of environmental exposure to pharmaceuticals encompassing terrestrial, freshwater and marine ecosystems in high- and low-income countries. Based on both field and laboratory data, the evidence for and relevance of changes to physiology and behaviour, in addition to mortality and reproductive effects, are examined in terms of the population- and community-level consequences of pharmaceutical exposure on wildlife. Studies on uptake, trophic transfer and indirect effects of pharmaceuticals acting via food webs are presented. Given the logistical and ethical complexities of research in this area, several papers focus on techniques for prioritizing which compounds are most likely to harm wildlife and how modelling approaches can make predictions about the bioavailability, metabolism and toxicity of pharmaceuticals in non-target species. This Theme Issue aims to help clarify the uncertainties, highlight opportunities and inform ongoing scientific and policy debates on the impacts of pharmaceuticals in the environment.
Keywords: pharmaceuticals, ecotoxicology, sewage, population ecology, contaminants, environmental risk assessment
1. Introduction
The number and density of humans and livestock requiring healthcare is escalating. This problem is further exacerbated, particularly in high-income countries, by expanding cohorts of obese and elderly people with chronic health problems [1]. With this comes an increase in the quantity and diversity of pharmaceuticals consumed and subsequently excreted. Significant quantities of pharmaceuticals can be emitted to the environment from manufacturing sites (particularly, but not exclusively, in lower income countries, reviewed by Larsson [2]), in addition to those released via inadequately treated sewage [3]. Different types of pharmaceuticals also are commonly used to enhance livestock production in both high- and low-income countries, sometimes without strong regulatory controls for use or discharge. While the health benefits of medication are important, it is only in the past 10–15 years that the potential environmental risks of these substances have been considered in any detail [4,5]. Currently, there are a number of uncertainties associated with the environmental risk assessment of pharmaceuticals due to lack of knowledge concerning their fate in wastes and the environment, their uptake, metabolism and excretion (pharmacokinetics) in wildlife, and their target affinity and functional effects (pharmacodynamics) in non-target species [6]. This Theme Issue focuses on risks posed by pharmaceuticals (principally hydrocarbon-based small molecules, as opposed to larger biopharmaceuticals) in the environment to vertebrate wildlife which share the greatest level of orthology for mammalian drug targets important to human and veterinary medicine [7–9]. Both laboratory and field-based evidence of pharmaceutical exposure and effects are considered [10–15]. Some of the papers and ideas developed here derive from a Royal Society International Scientific Seminar held in April 2013, with the title ‘Assessing the exposure risk and impacts of pharmaceuticals in the environment on individuals and ecosystems’ [16].
A number of key questions and uncertainties will be investigated in this Theme Issue: first, we explore exposures to wildlife occupying a range of ecosystems; most pharmaceuticals are emitted continuously to the environment (figure 1), dispersing within aquatic and terrestrial habitats and in some cases bioaccumulating in ecological food chains, potentially affecting organisms at higher trophic levels [17–19]. Second, several papers explore the nature of pharmaceuticals as contaminants and how we can prioritize research based on the potential risk they pose. Pharmaceuticals can be more potent than many historical environmental contaminants because they are designed to elicit specific biological effects at relatively low concentrations. These specific effects can be expressed as behavioural, physiological and histological alterations, which potentially can be triggered at environmentally relevant concentrations of pharmaceuticals. Importantly, some alterations caused by pharmaceuticals in non-target species have not been commonly used or interpreted in environmental risk assessments; therefore, their reliability as predictors of adverse effects on non-target species needs to be explored. Moreover, pharmaceutical effects can act indirectly on populations through the food chain, for example, if a key prey species is negatively affected [13]. Finally, we examine the prospective interactions of pharmaceuticals in environmental mixtures [20] and begin to explore their relative impacts on wildlife compared with the other environmental stressors, both natural and anthropogenic [21].
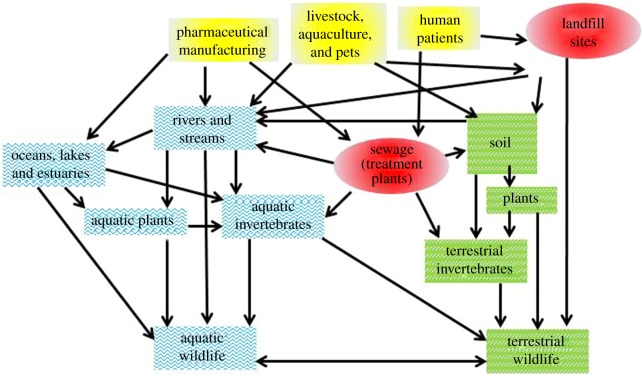
Figure 1.
Pharmaceuticals can disperse through the environment via multiple and potentially complex pathways some of which are shown here. Sources of pharmaceuticals in the environment (yellow boxes) include pharmaceutical manufacturing, livestock, aquaculture and pets, and human patients. Pharmaceuticals can then disperse directly into the environment or via sewage treatment plants and landfill (red ovals). The fate of pharmaceuticals in aquatic (boxes with blue waves) and terrestrial (green boxes with white dashes) environments can result in uptake of pharmaceuticals into wildlife. Simple food webs are shown to illustrate the potential for pharmaceuticals to bioaccumulate. (Online version in colour.)
2. How we are medicating the environment
Over 4000 pharmaceuticals are used across the globe for medical and veterinary healthcare, as well as growth promotion of livestock [6]. When most medications are consumed, parent compounds and metabolites are excreted into the wastewater system or directly into the environment [22] (figure 1). Depending on their physico-chemical properties, compounds can be degraded, partition to water or solid phases including biosolids (such as sewage sludge), enter the aquatic or terrestrial environment, or all of the above [22]. Ironically, some policies designed to improve the quality of aquatic environments and sustainability of water resources might contribute to the contamination of terrestrial ecosystems. For example, biosolids previously dumped in the ocean are now sent to landfills, incinerated or applied to agricultural land as fertilizer. In the USA, 5–7 Mt of dry sewage biosolids are estimated to be produced annually [23] with approximately 60% being applied to land [24]. There is a very similar situation in parts of Europe [25]. Aqueous sewage effluent is also being used increasingly for irrigation, particularly in water-stressed regions of the world [26]. Agricultural applications of sewage-derived biosolids containing human pharmaceuticals are currently dwarfed by the use of livestock manure (dung and urine) containing veterinary drugs, as fertilizer [27]. Veterinary drugs also may be released into the environment both directly (e.g. anthelmintic ‘worming’ treatments excreted in dung and antibiotics from the aquaculture industry) and indirectly via predation or scavenging of medicated animals [12,22,28–32] (figure 1). Recycled raw sewage, municipal wastewater and biosolids are also being applied increasingly to urban green spaces such as parks and golf courses, which is likely to result in increased environmental exposure to organisms occurring across a range of habitats [14,33,34]. Pharmaceuticals also have been detected at high concentrations near drug production facilities, particularly in countries with developing economies [2].
Using modern analytical techniques pioneered by some of the authors featured in this Theme Issue, a range of pharmaceuticals, including synthetic hormones, anti-inflammatories and antidepressants have recently been detected in soils, surface waters, sediments, groundwaters and in marine ecosystems [12,22,32]. A number of pharmaceuticals have been shown to be persistent in the environment [22,35]. The anticonvulsant drug carbamazepine, for example, can persist in soil unchanged for at least 40 days and be taken up into crop plants, accumulating particularly into leaves [36]. Similarly, the antidepressant fluoxetine (the active ingredient in Prozac) excreted by humans is only partially metabolized, incompletely removed by current wastewater treatment plant (WWTP) processes, and exhibits minimal degradation or transformation in sewage or soil over many months [35,37]. While such compounds truly are persistent, most human- and veterinary-use pharmaceuticals released to surface waters are regarded as ‘pseudo-persistent’ in that they are used continuously and released to the environment, resulting in constant exposures of organisms, even to relatively degradable compounds [22].
3. How can we predict environmental risk?
Risk is a concept common across many disciplines and depends both on exposure (e.g. frequency, timing and level of contact with a stressor) and effects (e.g. the nature and magnitude of the response to the stressor). The often relatively low concentrations of pharmaceuticals in many ecosystems have led some researchers, regulators and industry stakeholders to debate whether or not they pose significant risk to humans or wildlife [7] (also see [38]). However, there are large numbers of drugs with little or no environmental data and, due to their biological potency, even comparatively low concentrations of some pharmaceuticals could cause adverse effects. A number of prioritization schemes have been proposed based on the intrinsic properties of drugs (hazard) and their scale of use (exposure) [39–41] (also see [42]), but environmental risk assessors still face several uncertainties.
Predicting the exposure of organisms to pharmaceuticals depends on a number of environmental and ecological factors, including exposure pathway (aquatic or terrestrial) and uptake route (direct or indirect, internal dietary or uptake via external ‘exchange’ surfaces, e.g. gills and cuticles). There is evidence that some pharmaceuticals can bioconcentrate and bioaccumulate in aquatic food chains; for example, the antidepressant fluoxetine has a bioconcentration factor (BCF) of over 1000 in freshwater mussels Elliptio complanata, which are consumed by many vertebrate predators [43]. However, predicting uptake can be complex: by assessing the relative importance of uptake route for drugs with a range of lipophilicities (log Kow) including carbamazepine (1.5), diphenhydramine (3.4) and sertraline (5.3), Du et al. [44] demonstrated that direct uptake in fish via the gills was more important than dietary exposure, due to trophic dilution (i.e. lower bioaccumulation in fish compared with their invertebrate prey) [44]. Substantial direct uptake (bioconcentration) from water into vertebrates is also indicated for other pharmaceuticals. For example, in effluent contaminated waterways in North America diltiazem (used to treat hypertension) concentrations in fish plasma were 21.6 times greater than those in water, and osprey plasma concentrations were 4.71 times higher than those of fish. However, these figures varied between sites, fish species and also individuals within species [45]. Such studies illustrate the uncertainties associated with predicting exposure and uptake in ‘the real world’.
In the terrestrial environment, pharmaceuticals can also be taken up into plants [36,46] or remain bound to the external surfaces of leaves following biosolids or waste water application, thus potentially exposing herbivores to associated contaminants [47]. Predicting bioavailability of drugs in soils and also sediments is complicated by the fact that many compounds are ionizable and those that form cations may become bound to negatively charged clay particles [35,36]. Nevertheless, some pharmaceuticals might still be available to soil- or sediment-ingesting organisms and could potentially accumulate in terrestrial and benthic food webs (reviewed by Shore et al. [14]). Consequently, wildlife across diverse ecosystems are likely to be exposed to pharmaceuticals in the environment either directly or indirectly. However, the extent of exposure remains unknown for most taxa and ecosystems.
Following their uptake, many medical compounds are metabolized to Phase I metabolites that can sometimes be more reactive or more toxic than the parent pharmaceutical [48]. There are fewer data concerning subsequent Phase II and III metabolism of drugs in non-target organisms, but zero-order metabolism and consequent increased susceptibility to harmful pharmaceutical effects, or side effects, have been shown for the non-steroidal anti-inflammatory drug (NSAID) diclofenac in raptors [12,49]).
A key assumption in the environmental risk assessment of pharmaceuticals is that effects and associated blood plasma concentrations in non-target organisms will be comparable to therapeutic effects and concentrations in patients. Furthermore, this ‘read-across’ hypothesis predicts that pharmacological (therapeutic) effects will occur before adverse toxicological effects [50], potentially providing sensitive biomarkers of exposure or an early warning of possible adverse effects [42]. However, it should also be noted that adverse effects in non-target organisms can differ from therapeutic effects or even the side effects in humans (table 1). Moreover, it is also quite possible that the therapeutic effects of some pharmaceuticals themselves could be harmful [11,50,51]. For example, environmental exposure to painkillers could lead animals to over-exert themselves during chases and fights, thus risking exhaustion or injury.
Table 1.
Five of the most heavily prescribed and used human pharmaceuticals in high-income countries that are known or predicted to disperse into the environment. For each pharmaceutical (UK trade name in brackets), the class and intended action of the pharmaceutical in humans are listed. Some of the common side effects in humans of these compounds are described along with suggested potential analogues of behavioural and physiological responses that could be measured in wildlife.
| compound | class | common side effects in humans | analogue traits to measure in non-model animals |
|---|---|---|---|
| fluoxetinea (Prozac) |
SSRI antidepressant | sexual dysfunction | — reproductive success |
| anxiety and suicidal thoughts | — behavioural and hormonal stress responsiveness | ||
| weight loss | — mass and body condition | ||
| feeling restless | — activity levels | ||
| fenofibrateb | lipid regulator (Fibrate) | diarrhoea | — defaecation rate and mass loss |
| muscle pain or weakness | — locomotory performance | ||
| orlistatc (Xenical) |
anti-obesity | infection of the upper respiratory tract | — immune function |
| lowered blood sugar | — blood glucose concentration | ||
| oily discharge from the rectum | — fat scores | ||
| — fur/feather soiling | |||
| irregular menstrual periods | — timing of breeding | ||
| diclofenacd (Voltarol) |
NSAID | gastrointestinal problems | — food intake |
| — body condition | |||
| tiredness | — activity levels | ||
| — escape speed | |||
| skin problems | — colour and quality of sexual ornaments | ||
| loratadinee (Clarityn) |
antihistamine | feeling nervous | — risk-responsiveness |
| appetite gain | — body mass changes | ||
| hair loss | — moult rate and timing |
Predicting the effectiveness of a molecule as a therapeutic medicine in patients, let alone its effect on non-target organisms, is not straightforward [53]. First of all, it is necessary to assess the potential for the active pharmaceutical ingredient (API) to reach its protein target (e.g. an enzyme or receptor), taking into account its adsorption, distribution, metabolism and excretion. Of course, this assumes that we know the identity of the target receptor, that the wildlife species has the target receptor and that the pharmaceutical binds to it rather than a non-target receptor. Then one needs to determine how well the molecule's active site (ligand) binds and interacts with the target. Some useful predictors of uptake and distribution in the body are molecular size and lipophilicity [54]. However, API binding and potency may be more difficult to gauge, depending on the shape and size of its ligand [8], and the structure and biochemistry of the drug target's ligand-binding domain, as well as the physiological function of the target [7,9]. In this Theme Issue, several papers discuss in more detail the uncertainties associated with risk assessment [20,38,42].
4. Pharmaceutical exposure and dose in non-target species
To assess uptake and thus exposure for wildlife, we need to integrate data on the physico-chemical properties and behaviour of pharmaceuticals and their metabolites with existing ecological and life-history data. An emerging topic discussed in this issue is how the probability of exposure of wildlife to environmental contaminants can be influenced by fitness-related traits such as foraging range, feeding behaviour and reproductive strategy, including spawning and dispersal patterns [9,14].
Freshwater taxa have been relatively well studied in terms of exposure to pharmaceuticals [9,10,45,52,55] compared with terrestrial [14,15,56–58] or marine [9,32] species. In many aquatic taxa uptake of soluble, hydrophilic compounds occurs mainly via the gills or dermally (particularly in small organisms such as larvae), whereas uptake of less soluble, hydrophobic compounds more often occurs via a dietary route [59]. Regardless of uptake route, it is necessary to consider internal processes such as metabolism, distribution and excretion when assessing effective pharmaceutical dose (blood plasma or tissue concentrations) [49] and bioaccumulation potential [45,59]. Both external exposure and internal dose can be used to derive indices of the comparative vulnerability of different wildlife species to environmental contaminants [42,49,60,61]. The majority of exposure and uptake data are derived from laboratory organisms, while there are only a limited number of studies that quantify variation in the environmental uptake of pharmaceuticals in the wild [44]. With the aid of established environmental surveillance schemes focusing on well-characterized classes of persistent or bioaccumulative historical contaminants, Shore et al. [14] examine the sources, pathways and food webs in terrestrial and freshwater systems that potentially result in the greatest exposure. Using data extrapolation, they identify the likely factors that should also mediate the degree of uptake of pharmaceuticals. A key observation is that exposure studies would in general be enhanced by more routine measurement of pharmaceutical concentrations in blood plasma, which can provide an indication of possible effects in wildlife, based on pharmacologically active, i.e. therapeutic, doses in humans [44,45,50].
Pharmaceutical exposure and uptake within marine and estuarine ecosystems remains relatively understudied, but a number of chemical and biological markers suggest that marine and estuarine biota have been exposed to human and livestock waste, from which we might extrapolate that they also are being exposed to pharmaceuticals (reviewed by Gaw et al. [32]). There are also data from laboratory studies indicating the potential for pharmaceuticals to move through marine food chains; for example, at environmentally relevant concentrations in the laboratory, diclofenac (an NSAID) and propranolol (a beta-blocker) have BCFs of 10–180 in blue mussels Mytilus edulis trossulus [62].
Exposure of terrestrial vertebrates to pharmaceuticals has also been less well studied than for freshwater species. In one study, it was demonstrated that some WWTPs provide rich sources of emergent insects that attract bats at numbers comparable to favoured riparian foraging habitats [56]. Thus, exposure of bats, birds and other insectivores [63] to human pharmaceuticals derived from WWTPs could be unexpectedly large. In general, however, more studies of uniquely marked or tagged individuals are required in order to monitor foraging and dispersal behaviours to better estimate terrestrial exposures to pharmaceuticals via dung, biosolids or WWTPs. This would also enable an assessment of possible age or sex biases in exposure if, for example, subordinates or breeding females are particularly drawn to contaminated food sources at stressful times of year (e.g. during winter) [64]. In turn, this could result in differential selection pressures acting on exposed individuals or sub-populations, for example higher predation rates on emboldened fish exposed to antidepressants [11].
Specific, and unexpected, exposure routes also need to be explored. Scavengers, for example, can be reliant on carcases of livestock or companion animals for food, often from landfills or provided as a conservation measure. This brings with it the potential for exposure to veterinary drugs used to treat domesticated animals [14,65]. For example, residues of barbiturates in carrion of euthanized pets have been found to exceed the lethal dose for a spectrum of scavengers and there have been reports of secondary barbiturate poisoning [14]. The foraging mode of Asian vultures and their sensitivity to certain NSAIDs resulted in the near extinction of three keystone species [12,49]. The management of both species important for conservation (e.g. supplementary feeding) and the waste from rural and urban communities (e.g. carcases from livestock, feral animals and pets) needs to be considered when assessing exposure risk to scavenging animals [12,14].
5. Pharmaceuticals as environmental contaminants of concern
Pharmaceuticals differ from other bioactive chemical pollutants, such as pesticides or biocides, because they are generally not intended to kill organisms or regulate populations (with some exceptions, e.g. anthelmintics, antibiotics and fungicides). Instead, many pharmaceuticals are intended as modifiers of physiology and, in some cases, also behaviour. One widely studied physiological response likely to impact individual fitness in wildlife is vitellogenin induction. This has been observed in fish at environmental concentrations of the birth control and hormone replacement drug 17α-ethinylestradiol (EE2) as low as 1 ng l−1 (reviewed in [66]). Vitellogenin is required for egg-yolk provisioning essential for developing embryos and larvae, but overproduction in females can lead to impaired liver and reproductive function, and in males reduces kidney function and survival [66–68]. However, there is still some argument as to whether it is more applicable as a biomarker of oestrogen exposure rather than effect [69]. There is also a growing concern over psychoactive drugs because they are designed to alter (human) behaviour and have side effects that could also influence fitness-related traits in free-living animals [11,15,55,70] (table 1 and §6).
Recalling the ‘read-across’ hypothesis (§3), there are a number of reasons why compounds designed to have therapeutic effects in humans and livestock could impact non-target animals and ecosystems: there is strong evolutionary conservation of drug targets across phyla; pharmaceuticals are generally highly potent, and long term, low-level exposure due to their continual release to the environment is likely to lead to chronic effects; for some pharmaceuticals, mode of action is associated with a potentially harmful effect (e.g. cytostatic or endocrine modulating); taxonomic variation may lead to inadequacy in metabolic, excretory or detoxification systems in some species; age, sex, population and species-specific differences may affect susceptibility; direct and indirect effects may occur (the latter via the food chain); there may be additive or synergistic ‘cocktail’ effects from mixtures of pharmaceutical and other stressors.
Molecular predictors offer the potential for read-across between compounds and drug targets in different species, as reviewed in Madden et al. [8]. Assessing the downstream effects of target activation on physiological function in non-target species via adverse outcome pathways (AOPs) is also an important area of research that will help further validate species read-across, as highlighted by several papers in this issue [8,9,42,44]. Interestingly, Brown et al. [9] conclude that while most drug targets are conserved in fish, evolutionary divergence in drug target activation, physiology, behaviour and ecological life history make it extremely difficult to predict population-level effects from one fish species to another [9].
Another reason why some non-target species might be particularly susceptible to pharmaceuticals is if they do not possess the metabolic, excretory or detoxification systems present in the, primarily mammalian, target species, including humans; the physiology and anatomy of renal systems in Gyps vultures and cats, for example, appear to be particularly susceptible to accumulation of uric acid and occurrence of visceral gout, following exposure to diclofenac [12,49]. Genetic variation among populations of the same species might also incorporate the loss of polymorphisms or allozymes associated with altered drug target sensitivity or increased uptake versus loss rate of environmental chemicals, including pharmaceuticals, in a range of wildlife, humans and model species (reviewed in [71]). This presents a potential challenge for read-across which is based on the (often true) premise that related species react similarly to pharmaceuticals [66], as it appears that certain species can be susceptible to pharmaceuticals, even when closely related taxa are not [9]. Returning to vultures, some species appear to be able to tolerate the NSAID diclofenac while others succumb at relatively low doses, which suggests that differential sensitivity among avian species is a hallmark of cyclooxygenase-2 inhibitors [72] (table 1). Thus, predictive models based solely on evolutionary relationships may not be sufficient to assess risk.
Finally, wildlife can be exposed to pharmaceuticals throughout their whole lives, and at any stage of development. Life stage can also determine susceptibility to pharmaceutical exposure and effects, as ecological niche and feeding strategy often differ between juveniles and adults, as does metabolic capacity for coping with chemical or pharmaceutical exposure [9,14,49]. Just as many medicinal compounds are contraindicated for human infants, pregnant and lactating women, or people with certain health conditions, combinations of drugs are also known to have additive or synergistic effects that might exacerbate the likelihood of adverse health outcomes [20]. In the environment, individuals will rarely be exposed to a single compound but a cocktail of pharmaceuticals and other contaminants, which can have interactive effects, as reviewed by Backhaus in this Theme Issue [20]: for example, quinolone antibiotics can cause effects on the central nervous system which are exacerbated by NSAIDs, resulting in seizures in humans [73]. This Theme Issue will review and extend our current understanding of pharmaceuticals as environmental contaminants and highlight the complex, multifaceted approaches required to investigate the ecological and evolutionary impacts of pharmaceuticals in the environment.
6. Pharmaceutical effects at individual and population levels
With exception of the few studies noted below (including some in this Theme Issue), little research has been conducted on wildlife species under natural conditions in the receiving environment or simulated environmental exposures to pharmaceuticals. Significant milestones have recently been reached in some key studies and they are reviewed or in some cases reported here for the first time.
One of the clearest cases of pharmaceuticals causing population-level effects occurred on the Indian subcontinent, where the consumption of carcases of livestock that were medicated with diclofenac resulted in over 95% of Gyps vultures dying of kidney failure [28]. Cuthbert et al. [12] present data on the effectiveness of mitigation measures mediated by conservation organizations and the Indian government designed to reverse the dramatic declines in populations of scavenging raptors. Fundamental to this has been to understand why the LD50 of diclofenac to vultures is 0.1 mg kg−1 [74] compared with 9.8 mg kg−1 for the domestic chicken [75] and up to 1500 mg kg−1 for some mammals [76] (which also highlights the taxonomic diversity in responses to pharmaceuticals). In this issue, Hutchinson et al. [49] investigate how some taxa, such as Gyps vultures, possess low metabolic competency, which can lead to zero-order metabolic (pharmacokinetic) profiles and thus high pharmaceutical toxicity.
Probably the most well-publicized example of pharmaceuticals affecting free-living animals involves the feminization of male fish exposed to effluent containing the synthetic oestrogen EE2 discharged by WWTPs (reviewed in [59]). Signs of feminization can vary from the presence of vitellogenin in blood plasma to the presence of developing oocytes and/or oviducts in the testes of otherwise male fish (the intersex condition). Such effects have been demonstrated both in the wild and laboratory (reviewed in [77]). Knowledge in this area has been greatly enhanced by the large-scale lake experiment in Canada [78] in which EE2 levels were experimentally elevated (5–6 ng l−1), leading to the collapse of the lake's population of fathead minnows, Pimephales promelas [78]. In this issue, Kidd et al. [13] present, for the first time, long-term data on responses of the lake's foodweb which showed strong indirect effects from EE2 acting though the foodweb. For example, although not previously reported to be particularly sensitive to EE2, the lake's top predator declined significantly in the years following the experimental dosing, most probably due to the loss of its primary prey species [13]. Although Kidd et al.'s work demonstrates the potential for EE2 to cause changes to populations, and many studies have shown individual-level effects, it is unknown whether hormonally active pharmaceuticals have caused significant population declines in the wild.
Intersex frogs have recently been found in urban ponds contaminated with wastewater; however, so far the exact mechanism through which this occurs remains unknown [79]. Laboratory experiments reviewed and reported in this Theme Issue by Säfholm et al. [10] suggest that, as with fish, exposures to environmentally relevant concentrations of synthetic steroid hormones, such as oestrogens and progestagens, can impair the reproductive functions of amphibians, for example, through their effects on vitellogenesis and reproductive behaviour. Given the catastrophic declines in amphibian populations globally, this is clearly an area for further research.
Death and severe reproductive malfunction are not the only population-relevant endpoints from an ecological perspective. Animals that fail to forage efficiently, avoid predators or attract mates will have low or zero relative fitness [5,11,15,30,57,59]. Such ‘subtle’ effects of pharmaceuticals on fitness-related traits will often be less dramatic and therefore less obvious than death, but could have similar, if somewhat delayed impacts on populations. For example, manipulative experiments on wild starlings (Sturnus vulgaris) showed that consumption of a mixture of endocrine-active chemicals, including EE2, resulted in immunosuppression in adults and nestlings, as well as changes in behaviour and brain structure in adult males [57,58]. In this Theme Issue, Bean et al. [15] simulated the exposure of starlings to the antidepressant fluoxetine (Prozac) via foraging directly on invertebrates living in the trickling filters of WWTPs. Such chronic exposure to fluoxetine in captivity induced changes in birds' foraging behaviour and physiological responses [15]. While populations of starlings and other avian species have declined across much of Europe [80], it is unclear whether exposure to pharmaceuticals is involved, for example, via foraging directly on WWTPs or fields amended with sewage sludge or ‘grey water’.
Antidepressants and other psychoactive drugs have also been the focus of a burgeoning number of research studies of aquatic ecosystems (reviewed in [11,70]). Such compounds are designed not only to alter behaviour as well as physiology but also have a range of side effects in humans (table 1), are heavily prescribed, and in many cases slow to degrade in the environment [22]. Thus, they are interesting and potentially significant in terms of their capacity to affect changes to behaviour and other ecologically important traits in wildlife (table 1; e.g. [44,81–83]). Carbamazepine, for example, which is used to treat epilepsy and bipolar disorder, seems to be ubiquitous in sewage-contaminated ecosystems, dominating samples taken from different matrices, species and at all trophic levels examined [44,45]. In this Theme Issue, Brodin et al. [11] explore how behavioural modification in predators and prey exposed to psychoactive medication can result in alterations to aquatic food chains and ecosystems under different environmental scenarios.
The possible ecological fitness consequences of physiological or behavioural effects of pharmaceuticals are explored in this issue by several authors [10–15,49]. Some of the endpoints discussed by these authors include responses not typically assessed in routine tests use for regulatory ecotoxicology. In addition to these types of physiological and behavioural endpoints, there might be other relevant (possibly very subtle and sensitive) endpoints in wildlife that are considered tolerable side effects in humans, e.g. lethargy or loss of libido (table 1). The relevance of these effects may be revealed by systematic analysis of AOPs, which can be applied to pharmaceuticals and other environmental chemicals [42].
7. Prioritizing research effort
Thousands of compounds are used in human medicine [6], translating into a worldwide consumption of active compounds that amounts to over 100 000 tonnes. It is, therefore, essential to identify the priorities to be addressed in order to increase the effectiveness of research [84] and regulation [38]. Environmental regulation of pharmaceuticals is recognized as being important in China [85], Europe [86,87], North America [88], Canada [37] and Japan [89]. Despite environmental risk assessment procedures being established as part of the registration and approval of pharmaceuticals, there is a legacy of existing, untested products in use globally. There is also a lack of environmental monitoring and regulation, in both developed and developing countries, with regard to the release of APIs from manufacturing and also via effluent and sewage sludge from WWTPs [2]. This Theme Issue has the potential to provide much needed evidence for improving or tailoring the environmental risk assessment of pharmaceuticals by considering the vulnerability of wildlife to exposure and adverse effects, based on their ecology and physiology [16]. Studies of non-model and free-living species, such as those included here, can help guide both prospective (pre-registration testing) and retrospective risk assessment (i.e. ecopharmacovigilance) [16]. Of course this is not a straight-forward process. For example, the growing evidence for the persistence of steroids in the environment and their ability to induce biological effects at low concentrations in a wide range of taxa, including humans, have made them one of the first classes of pharmaceuticals in the environment to attract attention from legislators [30]. However, in the absence of clear population-level effects, the case for identifying EE2 and E2 (17β-oestradiol) as priority substances and setting statutory environmental quality standards is challenging. The paper by Küster & Adler [38] from the German Federal Environment Agency discusses the links between scientific evidence of pharmaceuticals in the environment and the regulation of pharmaceuticals. Our hope is that the science presented here will help to inform future policy debates on the risk posed by pharmaceuticals in the environment.
Given that several thousand legacy pharmaceuticals lack environmental testing data, some form of prioritization is required to deal with them, as well as identify optimal testing options for new compounds. Several ways to approach this have been proposed [39,40]. Options could include identification of potentially sensitive non-target wildlife species based on cross-species conservation of molecular drug targets (e.g. receptors and enzymes), in conjunction with estimates of exposures, which could be indexed to predicted internal blood plasma concentration, and compared via read-across to known human therapeutic concentrations [39,90]. From a practical standpoint, public databases such as Drugbank and NCBI contain much of the necessary data for applying read-across to generic, legacy compounds. In this Theme Issue, a paper by LaLone et al. [42] discusses the use of these types of databases in the context of an integrated framework to prioritize pharmaceuticals for testing and monitoring, based both on considerations related to dose (absorption, distribution, metabolism and excretion) and potential effects in non-target species.
The number and diversity of pharmaceuticals in existence is exceeded by the number and diversity of animals and ecosystems. For most species of wildlife, empirical toxicity and effects data are unlikely ever to become available. In this context, Madden et al. [8] discuss how the AOP concept can be employed to allow evidence from both in silico and in vitro studies to be rationally combined to fill gaps in knowledge concerning toxicological events [8]. Fundamental to this paradigm is a greater understanding of the mechanisms of toxicity and their potential conservation across taxa, such as between model animals and related wild species [8]. Thus, we have the opportunity to make predictions across diverse species and potentially identify taxa at risk from pharmaceuticals in the environment, but need to be aware of the uncertainties and exceptions prevalent in applying such approaches in the ‘real world’ [42].
8. Medicating the environment: future challenges
Given that populations of many species living in human-altered landscapes are declining for reasons that cannot be fully explained, we believe that it is time to explore emerging challenges to individual fitness, population dynamics and ultimately ecosystems. As with other environmental stressors, pharmaceuticals in the environment should be investigated in a holistic fashion.
Pharmaceutical impacts, where they occur, need to be teased apart from variation in fitness-related traits due to natural and also anthropogenically-mediated fluctuations in food and habitat availability, competition and parasitism or disease, as discussed by Johnson & Sumpter [21]. All these ecological factors, as well as prescribing patterns for pharmaceuticals, may also be influenced by climate change.
One advantage in studying pharmaceuticals over other contaminants is that there are comparatively large amounts of information available on pharmaceuticals, at least in terms of their intended actions in target organisms. Using preclinical and clinical safety data, read-across to vertebrates should be more straightforward than for invertebrates due to phylogenetic conservation of drug targets, but there is still considerable variation in susceptibility between species [9,49]. Moreover, pharmaceuticals can break down into numerous metabolites within patients and transformation products in WWTPs and the environment, but their risks remain poorly understood. For most wildlife, exposure to pharmaceuticals in the environment could be long term, potentially occurring via multiple exposure routes (figure 1) and involving mixtures of compounds [20]. Making predictions based on such complex scenarios poses substantial and multi-disciplinary challenges to researchers.
While there is evidence of ecosystems and free-living animals being exposed to pharmaceuticals and laboratory studies showing potential effects on individuals, there are few studies showing effects in the wild (but see [12,13]). Ideally, ecotoxicological studies on the field impacts of chemical contaminants would (but generally do not) incorporate data from marked individuals over ecologically relevant time periods [91]. While they can provide important information, population- or group-level studies will often struggle to identify the mechanistic cause of any alteration in population size unless the effects are sudden and catastrophic [28,91]. For example, laboratory studies have shown that embryonic or larval stages can be particularly sensitive to environmentally relevant concentrations of pharmaceuticals [92]. However, there are still some regulatory concerns that long-term chronic impacts on fitness-related traits in adults may not be detected in these early life-stage laboratory studies [93]. Subtle chronic effects are also difficult to detect in the wild unless known-age individuals can be compared. Similarly, by following identifiable individuals that might already be compromised by disease, inadequate resources, and so on, we can test whether species already of conservation interest are particularly vulnerable to pharmaceutical-mediated impacts. In reality, this will only be practical for a few relatively large, long-lived species for which there is considerable economic interest. In the future, approaches that can integrate new technologies, which are getting ever smaller and cheaper, for following individuals in time and space with sensors revealing exposure to focal contaminants could allow us to realize the goal of linking exposure with effects in the wild.
One limitation in realizing this goal is that much of our current knowledge about pharmaceuticals in the environment, and indeed animal ecology, is based on research in Europe and North America. However, Kookana et al. [3] suggest that a number of factors, such as climate, culture, ecology and also existing regulatory frameworks, mean that the risks in Asia and other developing economies may require different research approaches and management solutions [3]. Transnational collaborations will be immensely profitable in terms of knowledge exchange and development.
As highlighted by this Theme Issue, we need better and more standardized methods for assessing sublethal, e.g. behavioural, effects of contaminants [11,13,15,44]. In addition to its ecological and evolutionary relevance, behaviour can provide a non-lethal biomarker of physiological, molecular and neurological responses to contaminant exposure. Hopefully, ecologically relevant endpoints will be increasingly accepted, but this requires validating them as reliable indices of survival and reproduction that can be interpreted as part of ecological risk assessments. Linked to this is the need to establish the relative importance of pharmaceuticals in causing changes to fitness-related traits, including behaviour, that in turn impact upon wildlife populations [16,21]. This is important not least because risk assessors require evidence of population-level outcomes [38]. Establishing potential exposures and possible effects in individuals are not sufficient to drive regulatory changes restricting product use or environmental effluent release. Although this situation is not unique to pharmaceuticals, the need for adequate risk–benefit analysis seems particularly germane, given the many benefits (both social and financial benefits related to human health) conferred by pharmaceuticals. Ultimately, the challenge is to extrapolate beyond population effects in order to achieve ecosystem-level risk assessments, which should also include evaluations of both direct, e.g. toxic, and indirect, e.g. via trophic webs, effects due to pharmaceutical exposure [11,13,44].
This Theme Issue reviews existing and new data on the potential exposure to and effects of pharmaceuticals on wildlife and ecosystems, clearly demonstrating that better focused research is required (e.g. on priority compounds, ecologically significant exposure pathways, susceptible species and relevant effects endpoints) to inform environmental risk assessment and regulation. In order to achieve this, greater integration between ecologists, toxicologists and environmental chemists is required. Thus, knowledge of the physiology, ecology, life history and behaviour of non-target species, as well as the physico-chemical ‘behaviour’ of pharmaceuticals in the environment, will be vital in developing realistic predictions of environmental risk. A combination of in situ monitoring, manipulative studies and laboratory experiments, supplemented by computational models will be required to discern fully the impacts of existing and future pharmaceuticals, and other stressors, in a rapidly changing and increasingly populated world.
Acknowledgements
Thanks to T. Bean, A. Boxall, D. Raffaelli, P. White and C. Roberts for comments on the manuscript.
Funding statement
K.E.A. was funded by a Royal Society University Research Fellowship. A.R.B. was funded by AstraZeneca's Global Safety, Health and Environment Research Programme.
References
- 1.MEA. 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press. [Google Scholar]
- 2.Larsson DGJ. 2014. Pollution from drug manufacturing: review and perspectives. Phil. Trans. R. Soc. B 369, 20130571 ( 10.1098/rstb.2013.0571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kookana RS, et al. 2014. Potential ecological footprints of active pharmaceutical ingredients: an examination of risk factors in low-, middle- and high-income countries. Phil. Trans. R. Soc. B 369, 20130586 ( 10.1098/rstb.2013.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos LH, Araujo AN, Fachini A, Pena A, Delerue-Matos C, Montenegro MC. 2010. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 175, 45–95. ( 10.1016/j.jhazmat.2009.10.100) [DOI] [PubMed] [Google Scholar]
- 5.Boxall ABA. 2009. Assessing environmental effects of human pharmaceuticals. Toxicol. Lett. 189, S33 ( 10.1016/j.toxlet.2009.06.065) [DOI] [Google Scholar]
- 6.Boxall ABA, et al. 2012. Pharmaceuticals and personal care products in the environment: what are the big questions? Environ. Health Perspect. 120, 1221–1229. ( 10.1289/ehp.1104477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunnarsson L, Jauhiainen A, Kristiansson E, Nerman O, Larsson DGJ. 2008. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 42, 5807–5813. ( 10.1021/es8005173) [DOI] [PubMed] [Google Scholar]
- 8.Madden JC, Rogiers V, Vinken M. 2014. Application of in silico and in vitro methods in the development of adverse outcome pathway constructs in wildlife. Phil. Trans. R. Soc. B 369, 20130584 ( 10.1098/rstb.2013.0584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AR, Gunnarsson L, Kristiansson E, Tyler CR. 2014. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Phil. Trans. R. Soc. B 369, 20130576 ( 10.1098/rstb.2013.0576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Säfholm M, Ribbenstedt A, Fick J, Berg C. 2014. Risks of hormonally active pharmaceuticals to amphibians: a growing concern regarding progestagens. Phil. Trans. R. Soc. B 369, 20130577 ( 10.1098/rstb.2013.0577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodin T, Piovano S, Fick J, Klaminder J, Heynen M, Jonsson M. 2014. Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations. Phil. Trans. R. Soc. B 369, 20130580 ( 10.1098/rstb.2013.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuthbert RJ, et al. 2014. Avian scavengers and the threat from veterinary pharmaceuticals. Phil. Trans. R. Soc. B 369, 20130574 ( 10.1098/rstb.2013.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidd KA, Paterson MJ, Rennie MD, Podemski CL, Findlay DL, Blanchfield PJ, Liber K. 2014. Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen. Phil. Trans. R. Soc. B 369, 20130578 ( 10.1098/rstb.2013.0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shore RF, Taggart MA, Smits J, Mateo R, Richards NL, Fryday S. 2014. Detection and drivers of exposure and effects of pharmaceuticals in higher vertebrates. Phil. Trans. R. Soc. B 369, 20130570 ( 10.1098/rstb.2013.0570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bean TG, Boxall ABA, Lane J, Herborn KA, Pietravalle S, Arnold KE. 2014. Behavioural and physiological responses of birds to environmentally relevant concentrations of an antidepressant. Phil. Trans. R. Soc. B 369, 20130575 ( 10.1098/rstb.2013.0575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold KE, et al. 2013. Assessing the exposure risk and impacts of pharmaceuticals in the environment on individuals and ecosystems. Biol. Lett. 9, 20130492 ( 10.1098/rsbl.2013.0492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson G, Metcalfe CD. 2008. Uptake and depuration of the anti-depressant fluoxetine by the Japanese medaka (Oryzias latipes). Chemosphere 74, 125–130. ( 10.1016/j.chemosphere.2008.08.022) [DOI] [PubMed] [Google Scholar]
- 18.Brown JN, Paxeus N, Forlin L, Larsson DGJ. 2007. Variations in bioconcentration of human pharmaceuticals from sewage effluents into fish blood plasma. Environ. Toxicol. Pharmacol. 24, 267–274. ( 10.1016/j.etap.2007.06.005) [DOI] [PubMed] [Google Scholar]
- 19.Meredith-Williams M, Carter LJ, Fussell R, Raffaelli D, Ashauer R, Boxall ABA. 2012. Uptake and depuration of pharmaceuticals in aquatic invertebrates. Environ. Pollut. 165, 250–258. ( 10.1016/j.envpol.2011.11.029) [DOI] [PubMed] [Google Scholar]
- 20.Backhaus T. 2014. Medicines, shaken and stirred: a critical review on the ecotoxicology of pharmaceutical mixtures. Phil. Trans. R. Soc. B 369, 20130585 ( 10.1098/rstb.2013.0585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AC, Sumpter JP. 2014. Putting pharmaceuticals into the wider context of challenges to fish populations in rivers. Phil. Trans. R. Soc. B 369, 20130581 ( 10.1098/rstb.2013.0581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteiro SC, Boxall ABA. 2010. Occurrence and fate of human pharmaceuticals in the environment. Rev. Environ. Contam. Toxicol. 202, 53–154. ( 10.1007/978-1-4419-1157-5_2) [DOI] [PubMed] [Google Scholar]
- 23.Beecher N, Crawford K, Goldstein N, Kester G, Lono-Batura M, Dziezyk E. 2007. A national biosolids regulation, quality, end use and disposal survey. Tamworth, NH: North East Biosolids and Residuals Association.
- 24.USEPA 2008. Notice of proposed rulemaking—Identification of non-hazardous secondary materials that are solid waste. http://www.regulations.gov/#!Documentdetail;D=EPA-HQ-RCRA-2008-0329-0243 (accessed 4 September 2014).
- 25.EC 2010. Environmental, economic and social impacts of the use of sewage sludge on land. Final Report . (ed. Milieu Ltd W.a.R.), European Commission.
- 26.Gielen G, van den Heuvel MR, Clinton PW, Greenfield LG. 2009. Factors impacting on pharmaceutical leaching following sewage application to land. Chemosphere 74, 537–542. ( 10.1016/j.chemosphere.2008.09.048) [DOI] [PubMed] [Google Scholar]
- 27.Topp E, et al. 2008. Runoff of pharmaceuticals and personal care products following application of biosolids to an agricultural field. Sci. Total Environ. 396, 52–59. ( 10.1016/j.scitotenv.2008.02.011) [DOI] [PubMed] [Google Scholar]
- 28.Oaks JL, et al. 2004. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427, 630–633. ( 10.1038/nature02317) [DOI] [PubMed] [Google Scholar]
- 29.Boxall ABA. 2008. Fate of veterinary medicines applied to soils. In Pharmaceuticals in the environment: sources, fate, effects and risks (ed. Kümmerer K.), pp. 103–119, 3rd edn Berlin, Germany: Springer. [Google Scholar]
- 30.Sumpter JP. 2009. Protecting aquatic organisms from chemicals: the harsh realities. Phil. Trans. R. Soc. A 367, 3877–3894. ( 10.1098/rsta.2009.0106) [DOI] [PubMed] [Google Scholar]
- 31.Park KJ, Muller CT, Markman S, Swinscow-Hall O, Pascoe D, Buchanan KL. 2009. Detection of endocrine disrupting chemicals in aerial invertebrates at sewage treatment works. Chemosphere 77, 1459–1464. ( 10.1016/j.chemosphere.2009.08.063) [DOI] [PubMed] [Google Scholar]
- 32.Gaw S, Thomas KV, Hutchinson TH. 2014. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Phil. Trans. R. Soc. B 369, 20130572 ( 10.1098/rstb.2013.0572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fricker CR, Metcalfe N. 1984. Campylobacters in wading birds (Charadrii)—incidence, biotypes and isolation techniques. Zentralbl. Bakteriol. Mikrobiol. Hyg. B 179, 469–475. [PubMed] [Google Scholar]
- 34.Fatta-Kassinos D, Kalavrouziotis IK, Koukoulakis PN, Vasquez MI. 2011. The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci. Total Environ. 409, 3555–3563. ( 10.1016/j.scitotenv.2010.03.036) [DOI] [PubMed] [Google Scholar]
- 35.Redshaw CH, Cooke MP, Talbot HM, McGrath S, Rowland SJ. 2008. Low biodegradability of fluoxetine HCl, diazepam and their human metabolites in sewage sludge-amended soil. J. Soils Sedim. 8, 217–230. ( 10.1007/s11368-008-0024-2) [DOI] [Google Scholar]
- 36.Carter LJ, Harris E, Williams M, Ryan JJ, Kookana RS, Boxall ABA. 2014. Fate and uptake of pharmaceuticals in soil–plant systems. J. Agric. Food Chem. 62, 816–825. ( 10.1021/jf404282y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metcalfe CD, Chu SG, Judt C, Li HX, Oakes KD, Servos MR, Andrews DM. 2010. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 29, 79–89. ( 10.1002/etc.27) [DOI] [PubMed] [Google Scholar]
- 38.Küster A, Adler N. 2014. Pharmaceuticals in the environment: scientific evidence of risks and its regulation. Phil. Trans. R. Soc. B 369, 20130587 ( 10.1098/rstb.2013.0587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber R, Guendel U, Franz S, Kuester A, Rechenberg B, Altenburger R. 2011. Using the fish plasma model for comparative hazard identification for pharmaceuticals in the environment by extrapolation from human therapeutic data. Regul. Toxicol. Pharmacol. 61, 261–275. ( 10.1016/j.yrtph.2011.08.006) [DOI] [PubMed] [Google Scholar]
- 40.Roos V, Gunnarsson L, Fick J, Larsson DGJ, Ruden C. 2012. Prioritising pharmaceuticals for environmental risk assessment: towards adequate and feasible first-tier selection. Sci. Total Environ. 421, 102–110. ( 10.1016/j.scitotenv.2012.01.039) [DOI] [PubMed] [Google Scholar]
- 41.Caldwell DJ, Mastrocco F, Margiotta-Casaluci L, Brooks BW. In press. An integrated approach for prioritizing pharmaceuticals found in the environment for risk assessment, monitoring and advanced research. Chemosphere. ( 10.1016/j.chemosphere.2014.01.021) [DOI] [PubMed] [Google Scholar]
- 42.LaLone CA, Berninger JP, Villeneuve DL, Ankley GT. 2014. Leveraging existing data for prioritization of the ecological risks of human and veterinary pharmaceuticals to aquatic organisms. Phil. Trans. R. Soc. B 369, 20140022 ( 10.1098/rstb.2014.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bringolf RB, Heltsley RM, Newton TJ, Eads CB, Fraley SJ, Shea D, Cope WG. 2010. Environmental occurrence and reproductive effects of the pharmaceutical fluoxetine in native freshwater mussels. Environ. Toxicol. Chem. 29, 1311–1318. ( 10.1002/etc.157) [DOI] [PubMed] [Google Scholar]
- 44.Du B, et al. 2014. Bioaccumulation and trophic dilution of human pharmaceuticals across trophic positions of an effluent-dependent wadeable stream. Phil. Trans. R. Soc. B 369, 20140058 ( 10.1098/rstb.2014.0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarus RS, Rattner BA, Brooks BW, Du B, McGowan PC, Blazer VS, Ottinger MA. In press. Exposure and food web transfer of pharmaceuticals in ospreys (Pandion haliaetus): Predictive model and empirical data. Integrated Environmental Assessment and Management. ( 10.1002/ieam.1570) [DOI] [PubMed] [Google Scholar]
- 46.Boxall ABA, Johnson P, Smith EJ, Sinclair CJ, Stutt E, Levy LS. 2006. Uptake of veterinary medicines from soils into plants. J. Agric. Food Chem. 54, 2288–2297. ( 10.1021/jf053041t) [DOI] [PubMed] [Google Scholar]
- 47.Fowler PA, et al. 2008. In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol. Hum. Reprod. 14, 269–280. ( 10.1093/molehr/gan020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halling-Sorensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lutzhoft HC, Jorgensen SE. 1998. Occurrence, fate and effects of pharmaceutical substances in the environment—a review. Chemosphere 36, 357–393. ( 10.1016/S0045-6535(97)00354-8) [DOI] [PubMed] [Google Scholar]
- 49.Hutchinson TH, Madden JC, Naidoo V, Walker CH. 2014. Comparative metabolism as a key driver of wildlife species sensitivity to human and veterinary pharmaceuticals. Phil. Trans. R. Soc. B 369, 20130583 ( 10.1098/rstb.2013.0583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rand-Weaver M, Margiotta-Casaluci L, Patel A, Panter GH, Owen SF, Sumpter JP. 2013. The read-across hypothesis and environmental risk assessment of pharmaceuticals. Environ. Sci. Technol. 47, 11 384–11 395. ( 10.1021/es402065a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brodin T, Fick J, Jonsson M, Klaminder J. 2013. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 339, 814–815. ( 10.1126/science.1226850) [DOI] [PubMed] [Google Scholar]
- 52.Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL. 2009. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 28, 2677–2684. ( 10.1897/08-556.1) [DOI] [PubMed] [Google Scholar]
- 53.Madden JC, Hewitt M, Przybylak K, Vandebriel RJ, Piersma AH, Cronin MTD. 2012. Strategies for the optimisation of in vivo experiments in accordance with the 3Rs philosophy. Regul. Toxicol. Pharmacol. 63, 140–154. ( 10.1016/j.yrtph.2012.03.010) [DOI] [PubMed] [Google Scholar]
- 54.Lipinski C, Lombardo F, Dominy B, Feeney PJ. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26. ( 10.1016/S0169-409X(96)00423-1) [DOI] [PubMed] [Google Scholar]
- 55.Fong PP, Ford AT. 2014. The biological effects of antidepressants on the molluscs and crustaceans: a review. Aquat. Toxicol. 151, 4–13. ( 10.1016/j.aquatox.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 56.Park KJ, Cristinacce A. 2006. Use of sewage treatment works as foraging sites by insectivorous bats. Anim. Conserv. 9, 259–268. ( 10.1111/j.1469-1795.2006.00031.x) [DOI] [Google Scholar]
- 57.Markman S, Leitner S, Catchpole C, Barnsley S, Muller CT, Pascoe D, Buchanan KL. 2008. Pollutants increase song complexity and the volume of the brain area HVC in a songbird. PLoS ONE 3, e1674 ( 10.1371/journal.pone.0001674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markman S, Müller CT, Pascoe D, Dawson A, Buchanan KL. 2011. Pollutants affect development in nestling starlings Sturnus vulgaris. J. Appl. Ecol. 48, 391–397. ( 10.1111/j.1365-2664.2010.01931.x) [DOI] [Google Scholar]
- 59.Corcoran J, Winter MJ, Tyler CR. 2010. Pharmaceuticals in the aquatic environment: a critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 40, 287–304. ( 10.3109/10408440903373590) [DOI] [PubMed] [Google Scholar]
- 60.De Lange HJ, Lahr J, Van der Pol JJC, Wessels Y, Faber JH. 2009. Ecological vulnerability in wildlife: an expert judgment and multicriteria analysis tool using ecological traits to assess relative impact of pollutants. Environ. Toxicol. Chem. 28, 2233–2240. ( 10.1897/08-626.s1) [DOI] [PubMed] [Google Scholar]
- 61.Spromberg JA, Birge WJ. 2005. Population survivorship index for fish and amphibians: application to criterion development and risk assessment. Environ. Toxicol. Chem. 24, 1541–1547. ( 10.1897/04-159.1) [DOI] [PubMed] [Google Scholar]
- 62.Ericson H, Thorsen G, Kumblad L. 2010. Physiological effects of diclofenac, ibuprofen and propranolol on Baltic Sea blue mussels. Aquat. Toxicol. 99, 223–231. ( 10.1016/j.aquatox.2010.04.017) [DOI] [PubMed] [Google Scholar]
- 63.Markman S, Guschina IA, Barnsley S, Buchanan KL, Pascoe D, Muller CT. 2007. Endocrine disrupting chemicals accumulate in earthworms exposed to sewage effluent. Chemosphere 70, 119–125. ( 10.1016/j.chemosphere.2007.06.045) [DOI] [PubMed] [Google Scholar]
- 64.Rubach MN, Ashauer R, Buchwalter DB, De Lange HJ, Hamer M, Preuss TG, Töpke K, Maund SJ. 2011. Framework for traits-based assessment in ecotoxicology. Integr. Environ. Assess Manag. 7, 172–186. ( 10.1002/ieam.105) [DOI] [PubMed] [Google Scholar]
- 65.Phipps WL, Willis SG, Wolter K, Naidoo V. 2013. Foraging ranges of immature African white-backed vultures (Gyps africanus) and their use of protected areas in southern Africa. PLoS ONE 8, e52813 ( 10.1371/journal.pone.0052813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caldwell DJ, Mastrocco F, Anderson PD, Länge R, Sumpter JP. 2012. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ. Toxicol. Chem. 31, 1396–1406. ( 10.1002/etc.1825) [DOI] [PubMed] [Google Scholar]
- 67.Thorpe KL, Benstead R, Hutchinson TH, Tyler CR. 2007. Associations between altered vitellogenin concentrations and adverse health effects in fathead minnow (Pimephales promelas). Aquat. Toxicol. 85, 176–183. ( 10.1016/j.aquatox.2007.08.012) [DOI] [PubMed] [Google Scholar]
- 68.Sárria MP, Santos MM, Castro LFC, Vieira NM, Monteiro NM. 2013. Estrogenic chemical effects are independent from the degree of sex role reversal in pipefish. J. Hazard. Mater. 263, 746–753. ( 10.1016/j.jhazmat.2013.10.043) [DOI] [PubMed] [Google Scholar]
- 69.Hutchinson TH, Ankley GT, Segner H, Tyler CR. 2006. Screening and testing for endocrine disruption in fish-biomarkers as ‘signposts,’ not ‘traffic lights,’ in risk assessment. Environ. Health Perspect. 114(Suppl. 1), 106–114. ( 10.1289/ehp.8062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ford AT. 2014. From gender benders to brain benders (and beyond!). Aquat. Toxicol. 151, 1–3. ( 10.1016/j.aquatox.2014.02.005) [DOI] [PubMed] [Google Scholar]
- 71.Brown AR, Hosken DJ, Balloux F, Bickley LK, LePage G, Owen SF, Hetheridge MJ, Tyler CR. 2009. Genetic variation, inbreeding and chemical exposure-combined effects in wildlife and critical considerations for ecotoxicology. Phil. Trans. R. Soc. B 364, 3377–3390. ( 10.1098/rstb.2009.0126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rattner BA, Whitehead MA, Gasper G, Meteyer CU, Link WA, Taggart MA, Meharg AA, Pattee OH, Pain DJ. 2008. Apparent tolerance of turkey vultures (Cathartes aura) to the non-steroidal anti-inflammatory drug diclofenac. Environ. Toxicol. Chem. 27, 2341–2345. ( 10.1897/08-123.1) [DOI] [PubMed] [Google Scholar]
- 73.Kim J, Ohtani H, Tsujimoto M, Sawada Y. 2009. Quantitative comparison of the convulsive activity of combinations of twelve fluoroquinolones with five nonsteroidal antiinflammatory agents. Drug Metab. Pharmacokinet. 24, 167–174. ( 10.2133/dmpk.24.167) [DOI] [PubMed] [Google Scholar]
- 74.Swan GE, et al. 2006. Toxicity of diclofenac to Gyps vultures. Biol. Lett. 2, 279–282. ( 10.1098/rsbl.2005.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naidoo V, Duncan N, Bekker L, Swan G. 2007. Validating the domestic fowl as a model to investigate the pathophysiology of diclofenac in Gyps vultures. Environ. Toxicol. Pharmacol. 24, 260–266. ( 10.1016/j.etap.2007.06.003) [DOI] [PubMed] [Google Scholar]
- 76.EMEA 2003. CVMP summary report—diclofenac . (ed. Products EA.f.t.E.o.M.), London.
- 77.Harris CA, et al. 2011. The consequences of feminization in breeding groups of wild fish. Environ. Health Perspect. 119, 306–311. ( 10.1289/ehp.1002555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. 2007. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl Acad. Sci. USA 104, 8897–8901. ( 10.1073/pnas.0609568104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smits AP, Skelly DK, Bolden SR. 2014. Amphibian intersex in suburban landscapes. Ecosphere 5, art11 ( 10.1890/ES13-00353.1) [DOI] [Google Scholar]
- 80.Smith HG, Ryegård A, Svensson S. 2012. Is the large-scale decline of the starling related to local changes in demography? Ecography 35, 741–748. ( 10.1111/j.1600-0587.2011.06310.x) [DOI] [Google Scholar]
- 81.Schultz MM, Painter MM, Bartell SE, Logue A, Furlong ET, Werner SL, Schoenfuss HL. 2011. Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat. Toxicol. 104, 38–47. ( 10.1016/j.aquatox.2011.03.011) [DOI] [PubMed] [Google Scholar]
- 82.Fong PP, Hoy CM. 2012. Antidepressants (venlafaxine and citalopram) cause foot detachment from the substrate in freshwater snails at environmentally relevant concentrations. Mar. Freshw. Behav. Physiol. 45, 145–153. ( 10.1080/10236244.2012.690579) [DOI] [Google Scholar]
- 83.Di Poi C, Darmaillacq A-S, Dickel L, Boulouard M, Bellanger C. 2013. Effects of perinatal exposure to waterborne fluoxetine on memory processing in the cuttlefish Sepia officinalis. Aquat. Toxicol. 132–133, 84–91. ( 10.1016/j.aquatox.2013.02.004) [DOI] [PubMed] [Google Scholar]
- 84.Touraud E, Roig B. 2008. Knowledge and need assessment on pharmaceutical products in environmental waters In Final report KNAPPE (036864) on specific support action project priority 1163: global change and sustainable development—sub-priority global change and ecosystems. See http://cordis.europa.eu/documents/documentlibrary/124584761EN6.pdf.
- 85.Sui Q, Wang B, Zhao W, Huang J, Yu G, Deng S, Qiu Z, Lu S. 2012. Identification of priority pharmaceuticals in the water environment of China. Chemosphere 89, 280–286. ( 10.1016/j.chemosphere.2012.04.037) [DOI] [PubMed] [Google Scholar]
- 86.EEA 2010. Pharmaceuticals in the environment. Results of an EEA workshop. EEA technical report no. 1. Copenhagen, Denmark: European Environment Agency.
- 87.Kuester A, et al. 2009. Regulatory demands on data quality for the environmental risk assessment of pharmaceuticals. Regulat. Toxicol. Pharmacol. 55, 276–280. ( 10.1016/j.yrtph.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 88.Daughton CG, Ternes TA. 1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 107, 907–938. ( 10.1289/ehp.99107s6907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamamoto H, et al. 2007. Initial ecological risk assessment of eight selected human pharmaceuticals in Japan. Environ. Sci. 14, 177–193. [PubMed] [Google Scholar]
- 90.Huggett DB, Cook JC, Ericson JF, Williams RT. 2003. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk Assess. 9, 1789–1799. ( 10.1080/714044797) [DOI] [Google Scholar]
- 91.Clutton-Brock T, Sheldon BC. 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25, 562–573. ( 10.1016/j.tree.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 92.Wheeler JR, Maynard SK, Crane M. 2014. An evaluation of fish early life stage tests for predicting reproductive and longer term toxicity from plant protection product active substances. Environ. Toxicol. Chem. 33, 1874–1878. ( 10.1002/etc.2630) [DOI] [PubMed] [Google Scholar]
- 93.Bailey GP, Marien D. 2011. The value of juvenile animal studies ‘what have we learned from preclinical juvenile toxicity studies? II’. Birth Defects Res. B Dev. Reprod. Toxicol. 92, 273–291. [DOI] [PubMed] [Google Scholar]