Abstract
There has been a significant investment in research to define exposures and potential hazards of pharmaceuticals in freshwater and terrestrial ecosystems. A substantial number of integrated environmental risk assessments have been developed in Europe, North America and many other regions for these situations. In contrast, comparatively few empirical studies have been conducted for human and veterinary pharmaceuticals that are likely to enter coastal and marine ecosystems. This is a critical knowledge gap given the significant increase in coastal human populations around the globe and the growth of coastal megacities, together with the increasing importance of coastal aquaculture around the world. There is increasing evidence that pharmaceuticals are present and are impacting on marine and coastal environments. This paper reviews the sources, impacts and concentrations of pharmaceuticals in marine and coastal environments to identify knowledge gaps and suggests focused case studies as a priority for future research.
Keywords: pharmaceuticals, ecotoxicology, risk assessment, water quality, marine, coastal
1. Introduction
Over the last 15 years increasing attention has been paid to understanding the presence and impacts of pharmaceuticals entering or detected in freshwater ecosystems [1]. By contrast, significantly less attention has been paid to understanding releases of pharmaceuticals from sewage and other routes into coastal environments and their potential marine impacts. There is now widespread recognition of the need for a cradle-to-grave stewardship of medicines for minimizing environmental exposure while promoting human and animal health [2]. Large centres of human population are often found in coastal areas and pharmaceutical releases via municipal effluent discharges are probable. For example, Martínez et al. [3] reported that based on 2003 data, over 2.3 billion people live within coastal limits (representing 41% of world global population) and more than 50% of coastal countries have 80–100% of their total population within 100 km of the coastline. Twenty-one of the world's 33 megacities (cities with more than 8 million inhabitants) are on the coast and face a range of environmental management issues [4]. Global demographic trends towards coastal conurbations suggest increasing numbers of people living along coastlines, while waste management from coastal megacities is increasingly recognized as a major challenge [3,5,6]. These trends suggest the potential for increasing inputs of human pharmaceuticals into coastal environments and therefore the need to address potential exposure scenarios and implications for marine risk assessments of drug residues and their transformation products [7–9]. Marine risk assessments for pharmaceuticals are also relevant to veterinary medicines used in aquaculture [10–12].
More broadly, if releases of pharmaceuticals into coastal ecosystems are high enough to induce biological impacts, they may act as additional stressors on marine ecosystems already impacted by climate change, eutrophication and over-fishing [13]. It is estimated that 49% of marine ecosystems worldwide are strongly impacted by anthropogenic stressors with significant economic implications [3,14]. If unmanaged, multiple anthropogenic impacts on marine ecosystems may also affect coastal fisheries and aquaculture. For example, human health concerns linked to aquaculture include exposure to pharmaceuticals through consumption of seafood and the induction and spread of antibiotic resistance [15,16]. This paper reviews the sources, concentrations and potential impacts of human and veterinary pharmaceuticals in coastal environments to support risk assessments and to identify key knowledge gaps as priorities for future research. The scope of the review has been limited to human pharmaceuticals and antibiotics used as veterinary medicines.
2. Sources of pharmaceuticals in marine environments
(a). Sewage
Sewage effluent is recognized as a major source of multiple pharmaceuticals, including their metabolites, entering aquatic environments. Removal rates for pharmaceuticals in wastewater treatment plants (WWTPs) range from less than 10 to almost 100% and depend on the physico-chemical characteristics of the pharmaceutical and type of treatment technology [17]. Sources of human pharmaceuticals in sewage include patient use in the community, discharges from hospitals and, in some cases, wastewater from pharmaceutical manufacturing [18]. Sewage can be discharged into marine environments through coastal and ocean outfalls for WWTPs combined sewer overflows and via rivers receiving WWTP effluents [19,20]. For example, the Yangtze River in China transports sewage from 400 million people out to sea and releases an estimated 152 tonnes of pharmaceuticals annually [21]. Sewage may also be discharged into the marine environment from boats. Ships, including cruise liners, may discharge (under Annex IV of MARPOL 73/78 ships) treated sewage into the sea 4 nautical miles from the nearest land and 12 nautical miles for untreated sewage [22]. The volumes of sewage discharged can be significant as cruise liners can have passenger numbers equivalent to populations found in small towns. Sewage effluents from small boats, on the other hand, may not receive any treatment prior to being discharged. Typhoon shelters for small boats were a point source of antibiotics in Victoria Harbour, Hong Kong [23]. As discussed by Kookana et al. [24] in this issue, many large cities in Asia still rely on septic tanks with poorly managed septage which can contaminate surface and groundwaters with pharmaceuticals and ultimately be discharged into coastal areas.
Sewage impacted groundwater can also be a source of pharmaceuticals entering coastal waters. Pharmaceuticals have been detected in a coastal aquifer on the Yucatan Peninsula, Mexico injected with municipal sewage discharges [25]. Reuse of treated domestic wastewater for irrigation contributed to pharmaceutical contamination in groundwater on Mallorca [26]. Throughout the world rural and peri-urban areas including popular coastal holiday areas are reliant on septic tanks or small decentralized systems for sewage treatment disposal [27]. Depending on their treatment efficiency and the capacity of the local soils, these systems are a potential source of pharmaceuticals in coastal waters via leakage to ground and surface waters [28,29].
(b). Aquaculture
Globally the production of seafood through aquaculture is rapidly increasing with over 90% of aquaculture based in Asia [30]. A range of veterinary medicines including antibiotics, also registered for human use, is used prophylactically and to control disease outbreaks in marine aquaculture. Up to 75% of the administered dietary dose of a veterinary medicine can be lost to the surrounding environment. The loss mechanisms include dispersal of non-ingested pellets, gill and renal excretion of the unprocessed drug, and renal and faecal excretion of drug metabolites [31]. Other marine organisms in the vicinity including wild fish feed on leftover food and faecal material from marine aquaculture potentially further spreading pharmaceuticals and their transformation products. Pond-based farms located in coastal areas are also a source of antibiotics entering coastal waters through leaks and discharge of wastewaters which can contain elevated concentrations of pharmaceuticals. Extremely high antibiotic concentrations of up to 2.5 mg l−1 were measured in water samples from shrimp ponds in Vietnamese mangroves [32]. The ancient practice of wastewater- (human and animal) fed aquaculture, although declining, still occurs in some parts of Asia [30]. Aquaculture practices including the use of antibiotics vary greatly between countries [33].
(c). Animal husbandry and horticulture
Animal husbandry and horticulture along rivers and in coastal areas may also contribute to loadings of pharmaceuticals entering coastal waterways [17,34]. Antibiotics are added to animal feeds and in some cases drinking water to treat disease particularly in feedlots housing large numbers of animals [35]. The use of low doses of antibiotics in feed as growth promoters still occurs in some regions of the world despite being banned in Europe [36]. Some countries permit the use of antibiotics including oxytetracycline and streptomycin on horticultural crops [17]. Application of municipal biosolids to farmland as fertilizer is a further source of pharmaceuticals entering agricultural systems [37].
(d). Waste disposal
Waste disposal in coastal areas is a further source of pharmaceuticals entering the marine environment. Leachate from coastal landfills and seafills may be a pathway for pharmaceuticals disposed of in household and clinical wastes to enter coastal waters. Landfill leachate on the island of Mallorca contained up to 27 000 ng l−1 total concentration of pharmaceuticals [26]. Historically, in some regions drug manufacturing waste, sewage sludge and animal manure were dumped at sea [38,39].
(e). Environmental fate of pharmaceuticals in marine environments
Once discharged into aquatic environments, pharmaceuticals and their metabolites can undergo biotic and abiotic transformation (degradation) and sorb to suspended particulate matter (SPM) and sediments, and in some cases accumulate in the tissues of aquatic organisms [40]. Existing data for the environmental fate of pharmaceuticals generated for freshwater environments may not necessarily be transferable to marine environments. The differences in physico-chemical conditions including salinity, pH and organic matter between freshwater and seawater can impact on the environmental fate of pharmaceuticals [41]. The environmental fate of ionizable pharmaceuticals may be altered by the increased pH of seawater. Photodegradation may be a less important removal mechanism in coastal waters compared with more shallow freshwater environments due to light attenuation. Indirect photodegradation mechanisms may differ to those occurring in freshwater due to differences in water composition [42,43]. There is some evidence to suggest that the environmental fate of pharmaceuticals can differ between fresh and saline environments. The transformation behaviour of ibuprofen differed between freshwater and seawater [44] and prochlorperazine was more stable in seawater than freshwater [43].
3. Current state of knowledge of pharmaceutical concentrations in marine environments
(a). Seawater
The assessment of the concentrations of pharmaceuticals in coastal environments has been limited. Forty-nine studies have reported concentrations for individual pharmaceuticals and metabolites detected in estuarine and coastal waters. Only studies published since 2000 are considered. Seventy per cent of these studies have been published since 2010. The geographical breakdown for the studies is Europe (20), Asia (21), North America (6), South America (1) and Oceania (1). The studies included those investigating the presence of 30 or more pharmaceuticals over a wide spatial area [45] studies targeting specific classes of compounds, for example, sulfonamide antibiotics [34] and method validation studies screening only a limited number of ‘real’ samples [46].
To date, 113 pharmaceuticals and pharmaceutical metabolites have been detected in coastal waters at concentrations ranging from 0.01 to 6800 ng l−1 with the maximum concentrations for 69 of these compounds exceeding the European Medicines Agency threshold for predicted environmental concentrations for surface waters of 0.01 μg l−1 [47] (electronic supplementary material, tables S1–S3). Data were most frequently reported for antibiotics (41 compounds) followed by non-steroidal anti-inflammatories (8) and analgesics (8). Twenty compounds were reported in five or more studies (table 1) including acetaminophen, atenolol, carbamazepine, clarithromycin, diclofenac, 17α-ethinyloestradiol, erythromycin-H2O, gemfibrozil, ibuprofen, ketoprofen, naproxen, norfloxacin, oxafloxacin, propanolol, roxithromycin, sulfadiazine, sulfadimidine, sulfamethoxazole, tetracycline and trimethoprim. The higher frequency of reporting for concentrations of antibiotics and painkillers for the marine environment are consistent with Hughes et al. [1] synthesis of pharmaceutical data for freshwater environments.
Table 1.
Summary of seawater and biota concentrations and marine ecotoxicology data for the human and veterinary pharmaceuticals most frequently detected in seawater (ww, wet weight; dw, dry weight).
| seawater |
marine biota |
marine ecotoxicology data |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| pharmaceutical | classa | no. studies | concentration range [ng L−1] | no. studies | concentration range [ng g−1] |
no. studies | organisms tested | most sensitive endpoint reported [µg L−1] | |
| dw | ww | ||||||||
| acetominophen | analgesic | 7 | 1.9–1952 | 1 | 65–115 | 1 | mussels | feeding rate LOEC = 23 | |
| ibuprofen | analgesic | 18 | 0.01–2370 | 2 | algae mussels | biochemical responses 0.25 | |||
| carbamazepine | anticonvulsant | 18 | 0.4–1400 | 4 | 1.3–11 | 2 | algae amphipods | 21d geotaxisNOEC = 1 | |
| erythromycin-H2O | antibiotic metabolite | 9 | 0.1–1900 | 2 | 0.1–2 | ||||
| clarithromycin | antibiotic | 8 | 0.3–17.6 | ||||||
| norfloxacin | antibiotic | 8 | 2.3–6800 | 3 | 370 | 2.7–255 | |||
| ofloxacin | antibiotic | 7 | 3.5–5100 | 3 | 5–242 | ||||
| roxithromycin | antibiotic | 8 | 0.1–630 | ||||||
| sulfadiazine | antibiotic | 10 | 0.4–71.8 | 3 | 2.7 | 3.0–5.2 | |||
| sulfadimidine | antibiotic | 9 | 0.2–219 | 3 | 29.8–430 | 3.9 | |||
| sulfamethoxazole | antibiotic | 18 | 0.6–765 | 2 | 20.1 | 2.3 | |||
| tetracycline | antibiotic | 7 | 2.4–313 | 1 | 1.9 | 1 | bacteria diatom | growthEC50 = 16000 | |
| trimethoprim | antibiotic | 20 | 0.2–870 | 1 | <4–9 | ||||
| atenolol | anti-hypertensive agent | 5 | 3.8–293 | 2 | 0.3–13 | ||||
| propranolol | anti-hypertensive agent | 5 | 0.3–142 | 1 | 19–52 | 3 | mussels | FeedingrateNOEC = 11 | |
| gemfibrozil | hypolipidemic agent | 11 | 1–758 | ||||||
| diclofenac | NSAID | 11 | 0.6–843 | 7 | algae amphipods copepods decapods diatoms mussels |
biochemical responses 0.25 | |||
| ketoprofen | NSAID | 7 | 0.6–805 | ||||||
| naproxen | NSAID | 8 | 1.1–130 | ||||||
| 17α-ethinylestradiol | SERM | 9 | 0.1–38 | 2 | 7.2–38 | 2.7–3.4b | 2 | copepod echinoderm | developmentEC50 = 30.3 |
aClass descriptors as used in DrugBank [http://www.drugbank.ca/; [48]].
bUnits are ng g−1 lipid.
Current methodologies targeting the dissolved fraction of pharmaceuticals may be underestimating the environmental concentrations and the potential impacts on aquatic ecosystems [49]. The majority of the studies published to date have reported pharmaceutical concentrations in seawater for the dissolved fraction only with filtering being the first step in sample extraction methods. Two studies have investigated pharmaceutical concentrations in SPM. Mean concentrations of pharmaceuticals in SPM (more than 0.7 µm) from the Long Island Sound Estuary ranged from 7 to 44 ng g−1. The pharmaceuticals detected in the SPM were either hydrophobic, for example, tamoxifen, or positively charged, for example, clarithromycin, and up to 47% of the total concentration was sorbed to the SPM [19]. Yang et al. [19] compared concentrations of pharmaceuticals in the sediment, SPM and the colloidal and soluble phases in the Yangtze River Estuary and adjacent coastal areas. SPM concentrations were up to 5 times higher than that in the sediments. The colloidal phase had sorption affinities of 2–4 orders magnitude greater for pharmaceuticals than the SPM and contributed up to 45% of the target pharmaceuticals in the Yangtze system.
Pharmaceutical metabolites and transformation products can be more persistent and more toxic than the parent compound [50]. Twenty-one studies reported data for pharmaceutical transformation products in coastal waters with erythromycin-H2O the most commonly reported transformation product. Transformation products can be present in WWTP effluents and surface waters at concentrations equivalent to or exceeding the parent compound. For example, concentrations of metabolites of carbamazepine (carbamazepine epoxide), diclofenac (4′- and 5-hydroxy diclofenac) and atorvastin (o- and p-hydroxy atorvastin) in wastewater discharged into the Oslofjord were present at higher concentrations than the parent compounds [51]. Similarly the concentrations of sulfonamide metabolites measured in Liaodong Bay, China were comparable to those of the parent compounds [34].
Pharmaceuticals have been detected significant distances from their source(s). Pharmaceuticals were detected at a reference site approximately 9 km downstream from the WWTP outfall in Halifax Estuary [52]. Similarly, pharmaceuticals in the Baltic Sea were detected 17 km downstream of WWTP outfalls [53]. Zhang et al. [54] detected antibiotics including erythromycin-H2O, sulfamethoxazole and trimethoprim (0.1–16.7 ng l−1) 400 km offshore of the coast of China.
(i). Seasonal trends
Identifying seasonal trends for pharmaceutical concentrations in marine and coastal waters is crucial for determining time periods during which sensitive ecosystems may be at greater risk from exposure [55,56]. To date only a handful of studies have investigated seasonal trends for pharmaceutical concentrations in the marine environment. Pharmaceutical concentrations in the Yangtze River and Pearl River Estuary were higher in the dry season than in the wet season [57]. Similarly, heavy rainfall events reduced pharmaceutical concentrations in Jamaica Bay, a wastewater impacted estuary [20]. Conversely, Zheng et al. [58] and Qi et al. [21] reported increased river water concentrations of antibiotics in China during the wet season and attributed the increased concentrations to increased runoff of veterinary medicines and decreased efficiency of WWTPs due to increased wastewater flow. Temporal trends in pharmaceutical concentrations were not observed in Southern California coastal waters with relatively constant year-round temperatures [59]. By contrast, Hedgespeth et al. [55] reported higher probability of detecting acetaminophen in seawater from Charleston Harbor, South Carolina during winter. Pharmaceuticals were transported further downstream when the Aura River (Finland) was covered by snow and ice with the spring snowmelt increasing the speed of transport [60].
Seasonal trends in WWTP effluent pharmaceutical concentrations have also been reported which will in turn influence seawater concentrations. For example, total concentrations of NSAID drugs and bezafibrate were 3–5 times higher in effluent in winter than in summer [60]. Reduced removal rates in WWTPs and in surface seawaters can occur during colder months due to lower temperatures and resulting in lower rates of biological activity enhancing the persistence of pharmaceuticals in marine ecosystems [43]. Reduced sunlight levels during winter can inhibit removal of pharmaceuticals susceptible to photodegradation [60]. Snowmelt reduced pharmaceutical concentrations in effluent in Norway [61]. Seasonal trends in pharmaceutical usage should also be considered [62]. Antibiotic use patterns can be influenced by a number of factors. High antibiotic use in winter can be due to the inappropriate use for treating respiratory tract infections including the common cold and viral infections [63]. Anti-allergenic medicines may also have a seasonal profile. Wastewater concentrations of over-the-counter anti-allergenic cetirizine peaked in summer and followed the pollen season [64]. Certain disease-specific pharmaceuticals, such as antiviral drugs, will peak during disease outbreaks, as has been demonstrated for oseltamivir (Tamiflu) during the recent influenza A(H1N1)pdm09 outbreak in Europe [65].
(b). Sediment data
Sediments are a reservoir for the accumulation of pharmaceuticals in marine ecosystems and can act as a secondary pollution source from which pharmaceuticals can be released by changes in environmental conditions such as salinity and pH [57]. Sediments can be resuspended during tidal changes and during storm events exposing marine biota to sorbed pharmaceuticals. Twenty-two studies reporting sediment concentrations of pharmaceuticals for estuarine and marine environments have been published since 2000. In total, 62 pharmaceuticals and transformation products have been detected in marine sediments at concentrations up to 2 615 000 ng g−1 wet weight (electronic supplementary material, tables S4 and S5). Excluding the extremely high concentrations of antimicrobials measured in marine shrimp aquaculture pond sediments [32], 17α-ethinyloestradiol was the pharmaceutical measured in sediment at the highest concentration (129.8 ng g−1). 17α-ethinyloestradiol was also the pharmaceutical most frequently detected with sediment concentrations reported for nine studies. All other pharmaceutical compounds were reported in a maximum of three studies. Data were most frequently reported for antibiotics (26 compounds) followed by anti-hypertensive agents (6). Marine sediment data for pharmaceutical transformation products and metabolites are currently almost non-existent with only four studies reporting concentrations of pharmaceutical metabolites in marine sediments. Langford & Thomas [51] reported concentrations of α-hydroxy metoprolol (1–3 ng g−1) and simvastatin hydroxy carboxylic acid (2–4 ng g−1) in sediments collected from Oslofjord in Norway. Erythromycin-H2O was reported in San Francisco Bay (3.4 ng g−1 dw) [66] and in the Pearl River Estuary, China (0.7–14 ng g−1 dw) [57]. Only nine of the 22 studies analysed both seawater and sediment samples.
(c). Factors influencing pharmaceutical concentrations in seawater and sediment
Factors reported to increase concentrations of pharmaceuticals in seawater and sediment include proximity to WWTP outfalls [67,68], higher effluent outflows [69], size of the urban area and population [11,70,71], the number of rivers discharging into coastal waters [70], the type of wastewater treatment [19], low mixing and dilution rates for WWTP effluents [72], the hydrodynamic flushing and residence time for confined water bodies [61,73,74], the type, scale and density of animal husbandry [34,58] and proximity to aquaculture [74,75]. Higher concentrations of pharmaceuticals have been measured in estuaries during low and incoming tides [76]. Re-suspension of sediments during weather events including monsoons and during incoming tides can increase surface water concentrations of pharmaceuticals. Stratification of pharmaceuticals in the water column with higher concentrations being measured at the surface has been reported in the Long Island Sound Estuary [19] and in Victoria Harbour, Hong Kong [77]. Local conditions may inhibit wastewater treatment resulting in higher surface water concentrations. For example, Arctic permafrost conditions reduce the efficiency of WWTPs [78].
(d). Marine biota
Data for accumulation of pharmaceuticals in marine biota are scant most probably because of the lack of reliable analytical methods for these challenging analytical matrices [66]. Fourteen studies were identified reporting data for concentrations of pharmaceuticals in finfish, crustaceans and shellfish (electronic supplementary material, table S6). Ten of these studies reported results for filter-feeding marine shellfish and five for marine finfish. Tissue concentrations of 60 pharmaceuticals and seven metabolites have been reported with antibiotics being the most frequent class reported (38) followed by anti-hypertensive agents (6). Carbamazepine, ciprofloxacin and enrofloxacin were the most frequently reported compounds each being reported in four studies. Only three studies reported concentrations for pharmaceutical transformation products including erythromycin-H2O, salicylic acid and metabolites of venlafaxine [62,66,79]. Higher concentrations of venlafaxine metabolites than parent compound were detected in mussels (Mytilus galloprovincialis). As some marine organisms also metabolize pharmaceuticals [80], a wide range of metabolites could potentially be present.
Marine organisms can be exposed to pharmaceuticals over widespread geographical areas. The anti-depressant sertraline was detected at 43 of 68 mussels sampling stations along the California Coast [81]. Antibiotics were detected in 142 out of 190 mollusc samples collected from nine cities along the Bohai Sea in China [82]. Detectable concentrations of pharmaceuticals were measured in wild seafood samples purchased from Czech supermarkets including squid caught in the Eastern Central Pacific, herring from the Atlantic Northeast and shark from the Eastern Central Atlantic [83].
Pharmaceuticals have been detected in marine organisms despite not being detected in water or sediment. Ranitidine, sertraline and enlapril were detected in mussels from San Francisco Bay but not in seawater [66]. Diazepam was detected in all liver samples of hornyhead turbot but only infrequently detected in sediments near wastewater outfalls in the Southern Californian Bight [84]. Fluoroquinolone antibiotics were detected less frequently in water than in fish from six sampling sites in two marine aquaculture regions of the Pearl River Delta, China [74].
Pharmaceutical uptake in marine organisms is compound, species and body-tissue specific. Oxytetracycline preferentially accumulated in the viscera and oxolinic acid in the gills of Mytilus edulis [85]. Concentrations of fluoroquinolones antibiotics in fish from marine aquaculture regions of the Pearl River Delta were higher in liver tissue than in muscle tissue [74]. Fluoxetine tissue concentrations in Mytilus gallioprovincialis followed the order digestive gland>gills>mantle/gonads [86]. Li et al. [82] reported differences in uptake of antibiotics between mollusc species harvested from the Bohai Sea, China. In some situations, gender may also influence uptake of pharmaceuticals by marine organisms. Higher concentrations of diazepam were measured in male than female Pleuronichthys verticalis (hornyhead turbot) [84]. These preferential uptakes have implications for ecotoxicological impacts and human exposure to pharmaceuticals via consumption of seafood.
Field data for bioaccumulation of pharmaceuticals in marine organisms is limited. Field-derived bioaccumulation factors (BAFs) for pharmaceuticals in mussels from San Francisco Bay included dehydropnifedipine (290–764), carbamezepine (90–322), diphenhydramine (118–218), triamterene (57–71) and erythromycin-H2O (11–54). The BAFs varied between sites by up to a factor of 7 [66]. Bioconcentration factors (BCFs) ranged from 1300 to 1500 for uptake of 17α-ethinyloestradiol by mussels (M. galloprovincialis) harvested from Venice Lagoon, Italy [87]. Field-derived BAFs for antibiotics ranged from 0 to 11 000 in shellfish collected from the coastal environment of Dalian in China. Based on the average BAFs, the authors concluded that sulfamethazine, sulfamethiazole, sulfamonomethoxine and doxycycline are potentially bioaccumulative and that sulfadiazine, sulfameter, sulfamethoxypyridazine and chloramphenicol are bioaccumulative in shellfish [88].
The uptake of pharmaceuticals by marine bivalves has been investigated using laboratory assays. Maximum BCF values in mussels of 100 were reported for tetrazepam and 51 for diazepam [89] and ranged from 200 to 800 for fluoxetine [86]. BAFs for diclofenac and propanolol in mussels (Mytilus edulis) ranged between 10 and 180 [90] and from 0.12 to 2 for oxytetracycline and from 0.27 to 0.55 for oxolinic acid [85]. No studies could be found reporting BCFs or BAFs for the uptake of pharmaceuticals by marine finfish.
Only one study has reported pharmaceutical concentrations in higher tropic level marine organisms. Federova et al. [83] reported a flumequine concentration of 2.9 ng g−1 in an Eastern Central Atlantic shark sample. It is probable that trophic transfer of pharmaceuticals to top level predators including sharks, dolphins and whales is occurring in coastal ecosystems. Six anti-depressants and ethinyloestradiol were measured at trace concentrations (below quantitative limit to 4 ng ml−1) in plasma from bull sharks (Carcharhinus leucas) caught in the Caloosahatchee River, a wastewater impacted freshwater tributary of Florida's Charlotte Harbour [91]. The personal care product triclosan has been detected in plasma from wild Atlantic bottlenose dolphins (Tursiops truncatus) [92] and UV filters have been detected in Franciscana dolphins (Pontoporia blainvillei) [93]. Coastal avian species that feed on fish and shellfish may also be chronically exposed to pharmaceuticals.
4. Biological impacts in marine organisms
(a). Marine ecotoxicology studies
While the body of work on the aquatic ecotoxicology of both human and veterinary pharmaceuticals is steadily growing, there is currently minimal data on the toxicity of pharmaceuticals to marine organisms. Only one study reporting field ecotoxicity data for marine organisms could be found. Exposure of benthic microalgal communities in the North Inlet Estuary (USA) to the antimicrobial tylosin in sediments resulted in reduction of microalgal biomass and primary productivity and retarded diatom growth [94]. Laboratory ecotoxicity data could be found for 22 compounds and for the majority of compounds only one or two studies have been undertaken using marine organisms (electronic supplementary material, table S7). Fluoxetine was the exception, with marine ecotoxicity data reported in seven studies. Marine ecotoxicity laboratory data could be found for only seven of the 20 pharmaceuticals most frequently reported in seawater highlighting the current gap between researchers focusing on environmental presence and researchers focusing on ecotoxicity (table 1). Only one of these studies investigated the toxicity to sediment dwelling organisms [95]. A limited range of marine organisms have been tested to date including primary producers (e.g. microalgae and diatoms), primary consumers (e.g. bivalve molluscs and copepods) and consumers (e.g. crustaceans and fish). It is of great concern that in most studies nominal rather than measured pharmaceutical exposure concentrations were used.
Despite the limited number of studies, a wide variety of adverse effects have been reported for marine organisms with the effects being both test species and pharmaceutical specific. Examples of reported adverse effects for analgesics include reduced feeding rates [96], impacts on survival [97], reduced mussel byssus strength [90] and changes in immune response [96] and biochemical markers [98]. Studies have tended to focus on endpoints related to the therapeutic mode of action of the pharmaceutical. For example, reduced survival and developmental effects have been reported for anti-cancer drugs whereas studies on anti-depressant drugs have focused on neurobehavioural endpoints and spawning [99,100]. The reported no observable effect concentrations (NOECs) and lowest observable effect concentrations (LOECs) ranged from several orders of magnitude above environmental concentrations to comparable to reported environmental concentrations. For example, despite the NOECs for diclofenac for effects on bysuss strength and oxidative stress in mussels of 1000 µg l−1[101], transient tissue-specific changes were reported after a 7 day exposure to 0.25 µg l−1 diclofenac [98], a concentration well within the range reported in seawater (table 1).
Pharmaceuticals are present in marine ecosystems as mixtures complicating risk assessments. These complex mixtures may contain a wide variety of pharmaceuticals and other contaminants as well as a number of compounds from the same class (e.g. quinolone antibiotics) or with similar modes of action (e.g. non-steroidal anti-inflammatories) [102]. Additive effects have been reported for mixtures of pharmaceuticals on marine organisms. DeLorenzo & Fleming [103] investigated the toxicity of six pharmaceuticals and personal care products to the marine phytoplankton species Dunaliella tertiolecta both singly and in binary mixtures and reported additive toxicity for a mixture containing simvastatin and clofibric acid. As mixture toxicity effects including synergistic effects have also been reported for freshwater organisms and cell lines [104,105], NOECs and LOECs derived from single substance testing may not be sufficient for deriving environmental quality standards [106].
There is a need to assess the impacts of pharmaceuticals on marine food webs. Marine food webs could either be directly affected through bioaccumulation of pharmaceuticals in the food chain to toxic levels or indirectly through the loss of a key species particularly sensitive to pharmaceuticals. The impacts of pharmaceuticals on primary producers such as phytoplankton is a key concern for marine ecosystems due to the potential follow on effects on nutrient cycling and availability of food for other organisms [103]. Similarly, endocrine disrupting compounds which impact growth and reproduction in fish have the potential to affect predator and prey species [107].
(b). Antibiotic resistance
Exposure of microorganisms to sub-lethal concentrations of antimicrobial compounds including antibiotics can induce antibiotic resistance. The rapid development of antibiotic resistance in bacteria is considered to be a global health security emergency and attention is being focused on mechanisms of transfer of antibiotic-resistant bacteria between species and identifying aquatic environmental reservoirs [108]. As high rates of horizontal gene transfer have been reported for marine bacteria [109], the contribution of contaminants in the marine environment to induction of antibiotic resistance and pathways for dispersal of clinically relevant antibiotic-resistant pathogens warrant further investigation. The development of antibiotic resistance in marine bacteria has been linked with wastewater discharges and the use of antibiotics in aquaculture [33,58]. Widespread antibiotic resistance has been reported in fish, marine mammals and seabirds living in coastal waters including in the North Eastern United States [110]. Higher prevalence of antibiotic-resistant strains of bacteria has been reported for marine wildlife populations exposed to sewage [111] and there is evidence to suggest that the antibiotic-resistant bacteria present in seabirds are of human origin [112]. The presence of antibiotic resistance genes in marine ecosystems may be an indicator of ecological shifts occurring due to the presence of pharmaceuticals [113].
5. Data gaps and priorities for future research
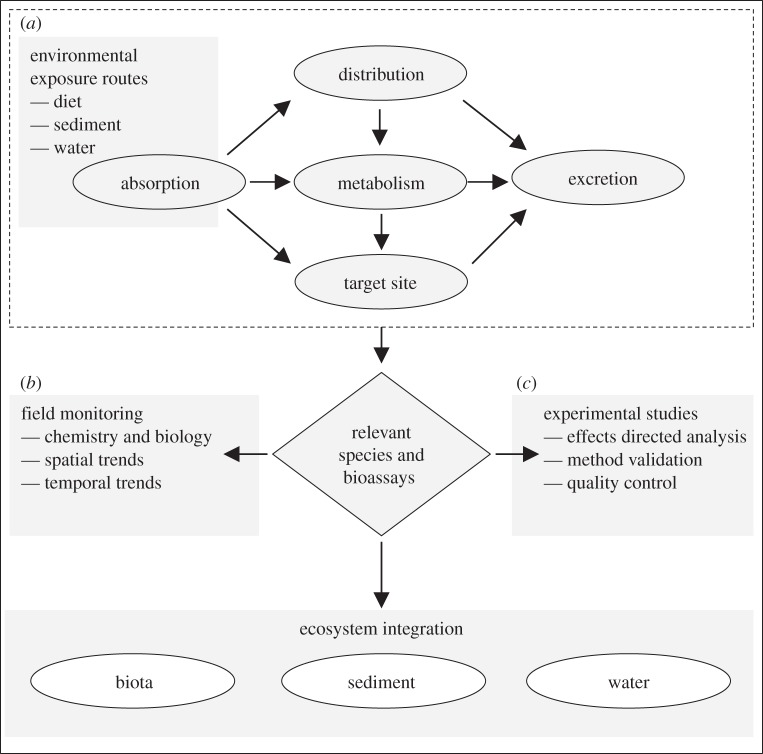
This review has highlighted that human and veterinary pharmaceuticals and their transformation products (including metabolites) are present in coastal ecosystems. Occurrence data for the marine environment are only available for a tiny fraction of the large number of pharmaceuticals currently in global use. There are extremely limited laboratory ecotoxicology data for the impacts of pharmaceuticals on marine organisms and a marked lack of field data. As for other ecosystems, a forward-looking prioritization approach is needed for the marine risk assessment of both generic and novel prescription pharmaceuticals. For example, such an approach has been successfully used for Tamiflu that involved defining both the predicted exposure concentration (PECmarine) and predicted no-effect concentrations (PNECmarine) to provide a prospective risk assessment [8]. For the PNECmarine to be reliable, it is important to consider the mode of action of the pharmaceutical, for instance, through the evaluation of Adverse Outcome Pathways in freshwater organisms and to extrapolate this to marine species [114] (figure 1). An Adverse Outcome Pathway is a conceptual framework for the link between exposure, the interaction of a contaminant at the molecular level within a cell and an adverse outcome or toxicological endpoint at the individual or community level.
Figure 1.
Adverse outcome pathways of chemicals fundamentally reflect patterns of absorption, distribution, metabolism, excretion and target sites in either acute or chronic exposure scenarios pertinent to marine contaminant monitoring. (Adapted from Hutchinson et al. [113].)
Mechanisms for the increased sharing of data also need to be developed and a number of schemes have been developed in Europe (see the Swedish scheme www.fass.se and http://www.lif.se/default.aspx?id=29916 and the Norman network's EMPODAT Database www.norman-network.net/empodat) and by individual companies through their Material Safety Data Sheets for specific pharmaceuticals. More widely, Daughton [56] recently proposed the development of a database on pharmaceutical occurrence in the environment, contributed to and curated by the wider science community.
The monitoring of prioritized pharmaceuticals and relevant metabolites in coastal environments should be considered as complementary to prospective risk assessments and include both dissolved and particulate fractions. In Europe, the Water Framework Directive (WFD; Directive 2000/60/EC) covers both freshwaters and transitional waters (the estuarine and coastal area up to one nautical mile, or 1.85 km, from the shore). Two hormones (17α-ethinyloestradiol and 17β-oestradiol) and diclofenac have been placed on a watch list for emerging pollutants under the WFD. In a global context, it would be prudent to develop a monitoring suite of priority pharmaceuticals and transformation products that can be used in conjunction with biological assays to identify marine environments at risk from major centres of pharmaceutical inputs (e.g. WWTPs from megacities, intensive areas of aquaculture and pharmaceutical manufacturing industries).
As highlighted in reviews for pharmaceutical concentrations in freshwater [1] there is a marked absence of data for pharmaceuticals in marine environments in many regions (notably Africa, South America and small island nations in Oceania). These data gaps could easily be overcome by collaboration between well-resourced groups, with access to appropriate technology and validated analytical methods in developed countries, and local scientists in developing countries, at the same time providing valuable scientific and technical training.
The majority of data reported to date for pharmaceutical concentrations in marine organisms are for antibiotics used in aquaculture. In contrast, there are limited data for the accumulation of other classes of pharmaceuticals, their metabolites and transformation products in marine organisms. Further research is required to identify appropriate analytical methods for risk assessments for fish and shellfish to ensure that potentially reversible pharmaceutical metabolite conjugates are accounted for.
There are insufficient data on the potential for impacts on higher trophic levels, either through trophic transfer of pharmaceuticals or indirect effects, such as limited availability of food, due to impacts on lower trophic levels including algae. For high priority pharmaceuticals, it would be desirable to extend the environmental assessment to include fish-eating birds and mammals as recently illustrated by Murray Smith et al. [115].
Supplementary Material
References
- 1.Hughes SR, Kay P, Brown LE. 2012. Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ. Sci. Technol. 47, 661–677. ( 10.1021/es3030148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daughton CG. 2003. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future directions. Environ. Health Perspect. 111, 775 ( 10.1289/ehp.5948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez M, Intralawan A, Vázquez G, Pérez-Maqueo O, Sutton P, Landgrave R. 2007. The coasts of our world: ecological, economic and social importance. Ecol. Econ. 63, 254–272. ( 10.1016/j.ecolecon.2006.10.022) [DOI] [Google Scholar]
- 4.Li H. 2003. Management of coastal mega-cities—a new challenge in the 21st century. Mar. Policy 27, 333–337. ( 10.1016/S0308-597X(03)00045-9) [DOI] [Google Scholar]
- 5.Small C, Nicholls RJ. 2003. A global analysis of human settlement in coastal zones. J. Coastal Res. 19, 584–599. [Google Scholar]
- 6.Sekovski I, Newton A, Dennison WC. 2012. Megacities in the coastal zone: using a driver-pressure-state-impact-response framework to address complex environmental problems. Estuarine Coastal Shelf Sci. 96, 48–59. ( 10.1016/j.ecss.2011.07.011) [DOI] [Google Scholar]
- 7.Richardson BJ, Lam PK, Martin M. 2005. Emerging chemicals of concern: pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to Southern China. Mar. Pollut. Bull. 50, 913–920. ( 10.1016/j.marpolbul.2005.06.034) [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson TH, Beesley A, Frickers PE, Readman JW, Shaw JP, Straub JO. 2009. Extending the environmental risk assessment for oseltamivir (Tamiflu) under pandemic use conditions to the coastal marine compartment. Environ. Int. 35, 931–936. ( 10.1016/j.envint.2009.04.001) [DOI] [PubMed] [Google Scholar]
- 9.Claessens M, Vanhaecke L, Wille K, Janssen CR. 2013. Emerging contaminants in Belgian marine waters: single toxicant and mixture risks of pharmaceuticals. Mar. Pollut. Bull. 71, 41–50. ( 10.1016/j.marpolbul.2013.03.039) [DOI] [PubMed] [Google Scholar]
- 10.Burridge L, Weis JS, Cabello F, Pizarro J, Bostick K. 2010. Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture 306, 7–23. ( 10.1016/j.aquaculture.2010.05.020) [DOI] [Google Scholar]
- 11.Zou S, Xu W, Zhang R, Tang J, Chen Y, Zhang G. 2011. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: impacts of river discharge and aquaculture activities. Environ. Pollut. 159, 2913–2920. ( 10.1016/j.envpol.2011.04.037) [DOI] [PubMed] [Google Scholar]
- 12.Rico A, et al. 2013. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 412, 231–243. ( 10.1016/j.aquaculture.2013.07.028) [DOI] [Google Scholar]
- 13.Crain CM, Halpern BS, Beck MW, Kappel CV. 2009. Understanding and managing human threats to the coastal marine environment. Ann. NY Acad. Sci. 1162, 39–62. ( 10.1111/j.1749-6632.2009.04496.x) [DOI] [PubMed] [Google Scholar]
- 14.Alexander M. 2000. Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ. Sci. Technol. 34, 4259–4265. ( 10.1021/es001069+) [DOI] [Google Scholar]
- 15.Love DC, Rodman S, Neff RA, Nachman KE. 2011. Veterinary drug residues in seafood inspected by the European Union, United States, Canada, and Japan from 2000 to 2009. Environ. Sci. Technol. 45, 7232–7240. ( 10.1021/es201608q) [DOI] [PubMed] [Google Scholar]
- 16.Le TX, Munekage Y, Kato S-I. 2005. Antibiotic resistance in bacteria from shrimp farming in mangrove areas. Sci. Total Environ. 349, 95–105. ( 10.1016/j.scitotenv.2005.01.006) [DOI] [PubMed] [Google Scholar]
- 17.Kümmerer K. 2009. Antibiotics in the aquatic environment: a review. I. Chemosphere 75, 417–434. ( 10.1016/j.chemosphere.2008.11.086) [DOI] [PubMed] [Google Scholar]
- 18.Daughton CG, Ternes TA. 1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 107(Suppl. 6), 907 ( 10.1289/ehp.99107s6907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lara-Martín PA, González-Mazo E, Petrovic M, Barceló D, Brownawell BJ. 2014. Occurrence, distribution and partitioning of nonionic surfactants and pharmaceuticals in the urbanized Long Island Sound Estuary (NY). Mar. Pollut. Bull. 85, 710–719. ( 10.1016/j.marpolbul.2014.01.022) [DOI] [PubMed] [Google Scholar]
- 20.Benotti MJ, Brownawell BJ. 2007. Distributions of pharmaceuticals in an urban estuary during both dry- and wet-weather conditions. Environ. Sci. Technol. 41, 5795–5802. ( 10.1021/es0629965) [DOI] [PubMed] [Google Scholar]
- 21.Qi W, Müller B, Pernet-Coudrier B, Singer H, Liu H, Qu J, Berg M. 2014. Organic micropollutants in the Yangtze River: seasonal occurrence and annual loads. Sci. Total Environ. 472, 789–799. ( 10.1016/j.scitotenv.2013.11.019) [DOI] [PubMed] [Google Scholar]
- 22.Organisation IM. 2003. International Convention for the Prevention of Pollution from Ships (MARPOL), Annex IV Prevention of Pollution by Sewage from Ships (entered into force 27 September 2003). http://www.imo.org/About/Conventions/ListOfConventions/Pages/International-Convention-for-the-Prevention-of-Pollution-from-Ships-(MARPOL).aspx (accessed 23 March 2014).
- 23.Minh TB, et al. 2009. Antibiotics in the Hong Kong metropolitan area: ubiquitous distribution and fate in Victoria Harbour. Mar. Pollut. Bull. 58, 1052–1062. ( 10.1016/j.marpolbul.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 24.Kookana RS, et al. 2014. Potential ecological footprints of active pharmaceutical ingredients: an examination of risk factors in low-, middle- and high-income countries. Phil. Trans. R. Soc. B 369, 20130586 ( 10.1098/rstb.2013.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metcalfe CD, Beddows PA, Bouchot GG, Metcalfe TL, Li H, Van Lavieren H. 2011. Contaminants in the coastal karst aquifer system along the Caribbean coast of the Yucatan Peninsula, Mexico. Environ. Pollut. 159, 991–997. ( 10.1016/j.envpol.2010.11.031) [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Navas C, Björklund E, Bak SA, Hansen M, Krogh KA, Maya F, Forteza R, Cerdà V. 2013. Pollution pathways of pharmaceutical residues in the aquatic environment on the island of Mallorca, Spain. Arch. Environ. Contam. Toxicol. 65, 56–66. ( 10.1007/s00244-013-9880-x) [DOI] [PubMed] [Google Scholar]
- 27.Withers PJ, Jordan P, May L, Jarvie HP, Deal NE. 2013. Do septic tank systems pose a hidden threat to water quality? Front. Ecol. Environ. 12, 123–130. ( 10.1890/130131) [DOI] [Google Scholar]
- 28.Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, Wang L, Yang XB, Stauber JL, Warne MS. 2010. Occurrence and a screening-level risk assessment of human pharmaceuticals in the Pearl River system, South China. Environ. Toxicol. Chem. 29, 1377–1384. ( 10.1002/etc.161) [DOI] [PubMed] [Google Scholar]
- 29.Dougherty JA, Swarzenski PW, Dinicola RS, Reinhard M. 2010. Occurrence of herbicides and pharmaceutical and personal care products in surface water and groundwater around Liberty Bay, Puget Sound, Washington. J. Environ. Qual. 39, 1173–1180. ( 10.2134/jeq2009.0189) [DOI] [PubMed] [Google Scholar]
- 30.Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, Lawrence R. 2008. Aquaculture practices and potential human health risks: current knowledge and future priorities. Environ. Int. 34, 1215–1226. ( 10.1016/j.envint.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 31.Grigorakis K, Rigos G. 2011. Aquaculture effects on environmental and public welfare—the case of Mediterranean mariculture. Chemosphere 85, 899–919. ( 10.1016/j.chemosphere.2011.07.015) [DOI] [PubMed] [Google Scholar]
- 32.Le TX, Munekage Y. 2004. Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam. Mar. Pollut. Bull. 49, 922–929. ( 10.1016/j.marpolbul.2004.06.016) [DOI] [PubMed] [Google Scholar]
- 33.Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A, Buschmann AH. 2013. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 15, 1917–1942. ( 10.1111/1462-2920.12134) [DOI] [PubMed] [Google Scholar]
- 34.Jia A, Hu J, Wu X, Peng H, Wu S, Dong Z. 2011. Occurrence and source apportionment of sulfonamides and their metabolites in Liaodong Bay and the adjacent Liao River Basin, North China. Environ. Toxicol. Chem. 30, 1252–1260. ( 10.1002/etc.508) [DOI] [PubMed] [Google Scholar]
- 35.Kemper N. 2008. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indicators 8, 1–13. ( 10.1016/j.ecolind.2007.06.002) [DOI] [Google Scholar]
- 36.Du L, Liu W. 2012. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 32, 309–327. ( 10.1007/s13593-011-0062-9) [DOI] [Google Scholar]
- 37.Kümmerer K. 2009. The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. J. Environ. Manage. 90, 2354–2366. ( 10.1016/j.jenvman.2009.01.023) [DOI] [PubMed] [Google Scholar]
- 38.Lee WY, Arnold C. 1983. Chronic toxicity of ocean-dumped pharmaceutical wastes to the marine amphipod Amphithoe valida. Mar. Pollut. Bull. 14, 150–153. ( 10.1016/0025-326X(83)90070-X) [DOI] [Google Scholar]
- 39.Son S, Wang M, Shon J-K. 2011. Satellite observations of optical and biological properties in the Korean dump site of the Yellow Sea. Remote Sensing Environ. 115, 562–572. ( 10.1016/j.rse.2010.10.002) [DOI] [Google Scholar]
- 40.Ramirez AJ, et al. 2009. Occurrence of pharmaceuticals and personal care products in fish: results of a national pilot study in the United States. Environ. Toxicol. Chem. 28, 2587–2597. ( 10.1897/08-561.1) [DOI] [PubMed] [Google Scholar]
- 41.Weigel S, Kuhlmann J, Hühnerfuss H. 2002. Drugs and personal care products as ubiquitous pollutants: occurrence and distribution of clofibric acid, caffeine and DEET in the North Sea. Sci. Total Environ. 295, 131–141. ( 10.1016/S0048-9697(02)00064-5) [DOI] [PubMed] [Google Scholar]
- 42.Ge L, Chen J, Wei X, Zhang S, Qiao X, Cai X, Xie Q. 2010. Aquatic photochemistry of fluoroquinolone antibiotics: kinetics, pathways, and multivariate effects of main water constituents. Environ. Sci. Technol. 44, 2400–2405. ( 10.1021/es902852v) [DOI] [PubMed] [Google Scholar]
- 43.Mei Fun Choong A, Lay-Ming Teo S, Lene Leow J, Ling Koh H, Chi Lui Ho P. 2006. A preliminary ecotoxicity study of pharmaceuticals in the marine environment. J. Toxicol. Environ. Health, Part A 69, 1959–1970. ( 10.1080/15287390600751371) [DOI] [PubMed] [Google Scholar]
- 44.Weigel S, Berger U, Jensen E, Kallenborn R, Thoresen H, Hühnerfuss H. 2004. Determination of selected pharmaceuticals and caffeine in sewage and seawater from Tromsø/Norway with emphasis on ibuprofen and its metabolites. Chemosphere 56, 583–592. ( 10.1016/j.chemosphere.2004.04.015) [DOI] [PubMed] [Google Scholar]
- 45.Spongberg AL, Witter JD, Acuña J, Vargas J, Murillo M, Umaña G, Gómez E, Perez G. 2011. Reconnaissance of selected PPCP compounds in Costa Rican surface waters. Water Res. 45, 6709–6717. ( 10.1016/j.watres.2011.10.004) [DOI] [PubMed] [Google Scholar]
- 46.Pintado-Herrera MG, González-Mazo E, Lara-Martín PA. 2013. Environmentally friendly analysis of emerging contaminants by pressurized hot water extraction–stir bar sorptive extraction–derivatization and gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 405, 401–411. ( 10.1007/s00216-012-6453-1) [DOI] [PubMed] [Google Scholar]
- 47.Committee for Medicinal Products for Human Use (CHMP). 2006. European Medicines Agency Guideline on the environmental risk assessment of medicinal products for human use. Doc. Ref. EMEA/CHMP/SWP/4447/00.
- 48.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. 2008. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 36(Suppl. 1), D901–D906. ( 10.1093/nar/gkm958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lahti M, Oikari A. 2011. Pharmaceuticals in settleable particulate material in urban and non-urban waters. Chemosphere 85, 826–831. ( 10.1016/j.chemosphere.2011.06.084) [DOI] [PubMed] [Google Scholar]
- 50.Celiz MD, Tso J, Aga DS. 2009. Pharmaceutical metabolites in the environment: analytical challenges and ecological risks. Environ. Toxicol. Chem. 28, 2473–2484. ( 10.1897/09-173.1) [DOI] [PubMed] [Google Scholar]
- 51.Langford K, Thomas KV. 2011. Input of selected human pharmaceutical metabolites into the Norwegian aquatic environment. J. Environ. Monitor. 13, 416–421. ( 10.1039/c0em00342e) [DOI] [PubMed] [Google Scholar]
- 52.Yang Y, Fu J, Peng H, Hou L, Liu M, Zhou J. 2011. Occurrence and phase distribution of selected pharmaceuticals in the Yangtze Estuary and its coastal zone. J. Hazard. Mater. 190, 588–596. ( 10.1016/j.jhazmat.2011.03.092) [DOI] [PubMed] [Google Scholar]
- 53.Wahlberg C, Björlenius B, Paxéus N. 2011. Fluxes of 13 selected pharmaceuticals in the water cycle of Stockholm, Sweden. Water Sci. Technol. 63, 1772–1780. ( 10.2166/wst.2011.124) [DOI] [PubMed] [Google Scholar]
- 54.Zhang R, et al. 2013. Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China: occurrence, distribution and ecological risks. Environ. Pollut. 174, 71–77. ( 10.1016/j.envpol.2012.11.008) [DOI] [PubMed] [Google Scholar]
- 55.Hedgespeth ML, Sapozhnikova Y, Pennington P, Clum A, Fairey A, Wirth E. 2012. Pharmaceuticals and personal care products (PPCPs) in treated wastewater discharges into Charleston Harbor, South Carolina. Sci. Total Environ. 437, 1–9. ( 10.1016/j.scitotenv.2012.07.076) [DOI] [PubMed] [Google Scholar]
- 56.Daughton CG. 2014. The Matthew Effect and widely prescribed pharmaceuticals lacking environmental monitoring: case study of an exposure-assessment vulnerability. Sci. Total Environ. 466, 315–325. ( 10.1016/j.scitotenv.2013.06.111) [DOI] [PubMed] [Google Scholar]
- 57.Liang X, Chen B, Nie X, Shi Z, Huang X, Li X. 2013. The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 92, 1410–1416. ( 10.1016/j.chemosphere.2013.03.044) [DOI] [PubMed] [Google Scholar]
- 58.Zheng S, et al. 2011. Antibiotics pollution in Jiulong River estuary: source, distribution and bacterial resistance. Chemosphere 84, 1677–1685. ( 10.1016/j.chemosphere.2011.04.076) [DOI] [PubMed] [Google Scholar]
- 59.Vidal-Dorsch DE, Bay SM, Maruya K, Snyder SA, Trenholm RA, Vanderford BJ. 2012. Contaminants of emerging concern in municipal wastewater effluents and marine receiving water. Environ. Toxicol. Chem. 31, 2674–2682. ( 10.1002/etc.2004) [DOI] [PubMed] [Google Scholar]
- 60.Vieno NM, Tuhkanen T, Kronberg L. 2005. Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ. Sci. Technol. 39, 8220–8226. ( 10.1021/es051124k) [DOI] [PubMed] [Google Scholar]
- 61.Vasskog T, Anderssen T, Pedersen-Bjergaard S, Kallenborn R, Jensen E. 2008. Occurrence of selective serotonin reuptake inhibitors in sewage and receiving waters at Spitsbergen and in Norway. J. Chromatogr. A 1185, 194–205. ( 10.1016/j.chroma.2008.01.063) [DOI] [PubMed] [Google Scholar]
- 62.Martinez Bueno MJ, Boillot C, Munaron D, Fenet H, Casellas C, Gomez E. 2014. Occurrence of venlafaxine residues and its metabolites in marine mussels at trace levels: development of analytical method and a biomonitoring program. Anal. Bioanal. Chem. 406, 601–610. ( 10.1007/s00216-013-7477-x) [DOI] [PubMed] [Google Scholar]
- 63.Ferech M, Coenen S, Dvorakova K, Hendrickx E, Suetens C, Goossens H. 2006. European Surveillance of Antimicrobial Consumption (ESAC): outpatient penicillin use in Europe. J. Antimicrob. Chemotherapy 58, 408–412. ( 10.1093/jac/dkl186) [DOI] [PubMed] [Google Scholar]
- 64.Harman C, Reid M, Thomas KV. 2011. In situ calibration of a passive sampling device for selected illicit drugs and their metabolites in wastewater, and subsequent year-long assessment of community drug usage. Environ. Sci. Technol. 45, 5676–5682. ( 10.1021/es201124j) [DOI] [PubMed] [Google Scholar]
- 65.Fussell RJ, Garcia Lopez M, Mortimer DN, Wright S, Sehnalova M, Sinclair CJ, Fernandes A, Sharman M. 2014. An investigation into the occurrence in food of veterinary medicines, pharmaceuticals, and chemicals used in personal care products. J. Agric. Food Chem. 62, 3651–3659. ( 10.1021/jf4052418) [DOI] [PubMed] [Google Scholar]
- 66.Klosterhaus SL, Grace R, Hamilton MC, Yee D. 2013. Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ. Int. 54, 92–99. ( 10.1016/j.envint.2013.01.009) [DOI] [PubMed] [Google Scholar]
- 67.Gulkowska A, He Y, So M, Yeung LW, Leung H, Giesy J, Lam PKS, Martin M, Richardson BJ. 2007. The occurrence of selected antibiotics in Hong Kong coastal waters. Mar. Pollut. Bull. 54, 1287–1293. ( 10.1016/j.marpolbul.2007.04.008) [DOI] [PubMed] [Google Scholar]
- 68.Madureira TV, Barreiro JC, Rocha MJ, Rocha E, Cass QB, Tiritan ME. 2010. Spatiotemporal distribution of pharmaceuticals in the Douro River estuary (Portugal). Sci. Total Environ. 408, 5513–5520. ( 10.1016/j.scitotenv.2010.07.069) [DOI] [PubMed] [Google Scholar]
- 69.McEneff G, Barron L, Kelleher B, Paull B, Quinn B. 2014. A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Sci. Total Environ. 476, 317–326. ( 10.1016/j.scitotenv.2013.12.123) [DOI] [PubMed] [Google Scholar]
- 70.Jiang J-J, Lee C-L, Fang M-D. 2014. Emerging organic contaminants in coastal waters: anthropogenic impact, environmental release and ecological risk. Mar. Pollut. Bull. 85, 391–399. ( 10.1016/j.marpolbul.2013.12.045) [DOI] [PubMed] [Google Scholar]
- 71.Jindal T, Singh DK, Agarwal HC. 2000. Persistence, degradation and leaching of coumaphos in soil. J. Environ. Sci. Health B 35, 309–320. ( 10.1080/03601230009373272) [DOI] [PubMed] [Google Scholar]
- 72.Comeau F, Surette C, Brun G, Losier R. 2008. The occurrence of acidic drugs and caffeine in sewage effluents and receiving waters from three coastal watersheds in Atlantic Canada. Sci. Total Environ. 396, 132–146. ( 10.1016/j.scitotenv.2008.02.031) [DOI] [PubMed] [Google Scholar]
- 73.Bayen S, Zhang H, Desai MM, Ooi SK, Kelly BC. 2013. Occurrence and distribution of pharmaceutically active and endocrine disrupting compounds in Singapore's marine environment: influence of hydrodynamics and physical–chemical properties. Environ. Pollut. 182, 1–8. ( 10.1016/j.envpol.2013.06.028) [DOI] [PubMed] [Google Scholar]
- 74.He X, et al. 2012. Residues of fluoroquinolones in marine aquaculture environment of the Pearl River Delta, South China. Environ. Geochem. Health 34, 323–335. ( 10.1007/s10653-011-9420-4) [DOI] [PubMed] [Google Scholar]
- 75.Zheng Q, Zhang R, Wang Y, Pan X, Tang J, Zhang G. 2012. Occurrence and distribution of antibiotics in the Beibu Gulf, China: impacts of river discharge and aquaculture activities. Mar. Environ. Res. 78, 26–33. ( 10.1016/j.marenvres.2012.03.007) [DOI] [PubMed] [Google Scholar]
- 76.Ferguson EM, Allinson M, Allinson G, Swearer SE, Hassell KL. 2013. Fluctuations in natural and synthetic estrogen concentrations in a tidal estuary in south-eastern Australia. Water Res. 47, 1604–1615. ( 10.1016/j.watres.2012.12.020) [DOI] [PubMed] [Google Scholar]
- 77.Xu W, Zhang G, Zou S, Li X, Liu Y. 2007. Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ. Pollut. 145, 672–679. ( 10.1016/j.envpol.2006.05.038) [DOI] [PubMed] [Google Scholar]
- 78.Gunnarsdóttir R, Jenssen PD, Erland Jensen P, Villumsen A, Kallenborn R. 2013. A review of wastewater handling in the Arctic with special reference to pharmaceuticals and personal care products (PPCPs) and microbial pollution. Ecol. Eng. 50, 76–85. ( 10.1016/j.ecoleng.2012.04.025) [DOI] [Google Scholar]
- 79.Wille K, et al. 2011. Development of analytical strategies using U-HPLC-MS/MS and LC-ToF-MS for the quantification of micropollutants in marine organisms. Anal. Bioanal. Chem. 400, 1459–1472. ( 10.1007/s00216-011-4878-6) [DOI] [PubMed] [Google Scholar]
- 80.McEneff G, Barron L, Kelleher B, Paull B, Quinn B. 2013. The determination of pharmaceutical residues in cooked and uncooked marine bivalves using pressurised liquid extraction, solid-phase extraction and liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 405, 9509–9521. ( 10.1007/s00216-013-7371-6) [DOI] [PubMed] [Google Scholar]
- 81.Dodder NG, Maruya KA, Ferguson PL, Grace R, Klosterhaus S, La Guardia MJ, Lauenstein GG, Ramirez J. 2013. Occurrence of contaminants of emerging concern in mussels (Mytilus spp.) along the California coast and the influence of land use, storm water discharge, and treated wastewater effluent. Mar. Pollut. Bull. 81, 340–346. ( 10.1016/j.marpolbul.2013.06.041) [DOI] [PubMed] [Google Scholar]
- 82.Li W, Shi Y, Gao L, Liu J, Cai Y. 2012. Investigation of antibiotics in mollusks from coastal waters in the Bohai Sea of China. Environ. Pollut. 162, 56–62. ( 10.1016/j.envpol.2011.10.022) [DOI] [PubMed] [Google Scholar]
- 83.Fedorova G, Nebesky V, Randak T, Grabic R. 2014. Simultaneous determination of 32 antibiotics in aquaculture products using LC-MS/MS. Chem. Pap. 68, 29–36. ( 10.2478/s11696-013-0428-3) [DOI] [Google Scholar]
- 84.Maruya KA, Vidal-Dorsch DE, Bay SM, Kwon JW, Xia K, Armbrust KL. 2012. Organic contaminants of emerging concern in sediments and flatfish collected near outfalls discharging treated wastewater effluent to the Southern California Bight. Environ. Toxicol. Chem. 31, 2683–2688. ( 10.1002/etc.2003) [DOI] [PubMed] [Google Scholar]
- 85.Le Bris H, Pouliquen H. 2004. Experimental study on the bioaccumulation of oxytetracycline and oxolinic acid by the blue mussel (Mytilus edulis). An evaluation of its ability to bio-monitor antibiotics in the marine environment. Mar. Pollut. Bull. 48, 434–440. ( 10.1016/j.marpolbul.2003.08.018) [DOI] [PubMed] [Google Scholar]
- 86.Franzellitti S, Buratti S, Capolupo M, Du B, Haddad SP, Chambliss CK, Brooks BW, Fabbri E. 2013. An exploratory investigation of various modes of action and potential adverse outcomes of fluoxetine in marine mussels. Aquat. Toxicol. 151, 14–26. ( 10.1016/j.aquatox.2013.11.016) [DOI] [PubMed] [Google Scholar]
- 87.Pojana G, Gomiero A, Jonkers N, Marcomini A. 2007. Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ. Int. 33, 929–936. ( 10.1016/j.envint.2007.05.003) [DOI] [PubMed] [Google Scholar]
- 88.Na G, Fang X, Cai Y, Ge L, Zong H, Yuan X, Yao Z, Zhang Z. 2013. Occurrence, distribution, and bioaccumulation of antibiotics in coastal environment of Dalian, China. Mar. Pollut. Bull. 69, 233–237. ( 10.1016/j.marpolbul.2012.12.028) [DOI] [PubMed] [Google Scholar]
- 89.Gomez E, Bachelot M, Boillot C, Munaron D, Chiron S, Casellas C, Fenet H. 2012. Bioconcentration of two pharmaceuticals (benzodiazepines) and two personal care products (UV filters) in marine mussels (Mytilus galloprovincialis) under controlled laboratory conditions. Environ. Sci. Pollut. Res. 19, 2561–2569. ( 10.1007/s11356-012-0964-3) [DOI] [PubMed] [Google Scholar]
- 90.Ericson H, Thorsén G, Kumblad L. 2010. Physiological effects of diclofenac, ibuprofen and propranolol on Baltic Sea blue mussels. Aquat. Toxicol. 99, 223–231. ( 10.1016/j.aquatox.2010.04.017) [DOI] [PubMed] [Google Scholar]
- 91.Gelsleichter J, Szabo NJ. 2013. Uptake of human pharmaceuticals in bull sharks (Carcharhinus leucas) inhabiting a wastewater-impacted river. Sci. Total Environ. 456, 196–201. ( 10.1016/j.scitotenv.2013.03.078) [DOI] [PubMed] [Google Scholar]
- 92.Fair PA, Lee H-B, Adams J, Darling C, Pacepavicius G, Alaee M, Bossart GD, Henry N, Muir D. 2009. Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environ. Pollut. 157, 2248–2254. ( 10.1016/j.envpol.2009.04.002) [DOI] [PubMed] [Google Scholar]
- 93.Gago-Ferrero P, et al. 2013. First determination of UV filters in marine mammals. Octocrylene levels in Franciscana dolphins. Environ. Sci. Technol. 47, 5619–5625. ( 10.1021/es400675y) [DOI] [PubMed] [Google Scholar]
- 94.Pinckney JL, Hagenbuch IM, Long RA, Lovell CR. 2013. Sublethal effects of the antibiotic tylosin on estuarine benthic microalgal communities. Mar. Pollut. Bull. 68, 8–12. ( 10.1016/j.marpolbul.2013.01.006) [DOI] [PubMed] [Google Scholar]
- 95.Méndez N, Lacorte S, Barata C. 2013. Effects of the pharmaceutical fluoxetine in spiked-sediments on feeding activity and growth of the polychaete Capitella teleta. Mar. Environ. Res. 89, 76–82. ( 10.1016/j.marenvres.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 96.Solé M, Shaw JP, Frickers PE, Readman JW, Hutchinson TH. 2010. Effects on feeding rate and biomarker responses of marine mussels experimentally exposed to propranolol and acetaminophen. Anal. Bioanal. Chem. 396, 649–656. ( 10.1007/s00216-009-3182-1) [DOI] [PubMed] [Google Scholar]
- 97.Guler Y, Ford AT. 2010. Anti-depressants make amphipods see the light. Aquat. Toxicol. 99, 397–404. ( 10.1016/j.aquatox.2010.05.019) [DOI] [PubMed] [Google Scholar]
- 98.Gonzalez-Rey M, Bebianno MJ. 2014. Effects of non-steroidal anti-inflammatory drug (NSAID) diclofenac exposure in mussel Mytilus galloprovincialis. Aquat. Toxicol. 148, 221–230. ( 10.1016/j.aquatox.2014.01.011) [DOI] [PubMed] [Google Scholar]
- 99.Bossus MC, Guler YZ, Short SJ, Morrison ER, Ford AT. 2013. Behavioural and transcriptional changes in the amphipod Echinogammarus marinus exposed to two antidepressants, fluoxetine and sertraline. Aquat. Toxicol. 151, 46–56. ( 10.1016/j.aquatox.2013.11.025) [DOI] [PubMed] [Google Scholar]
- 100.Di Poi C, Darmaillacq A-S, Dickel L, Boulouard M, Bellanger C. 2013. Effects of perinatal exposure to waterborne fluoxetine on memory processing in the cuttlefish Sepia officinalis. Aquat. Toxicol. 132, 84–91. ( 10.1016/j.aquatox.2013.02.004) [DOI] [PubMed] [Google Scholar]
- 101.Schmidt W, O'Rourke K, Hernan R, Quinn B. 2011. Effects of the pharmaceuticals gemfibrozil and diclofenac on the marine mussel (Mytilus spp.) and their comparison with standardized toxicity tests. Mar. Pollut. Bull. 62, 1389–1395. ( 10.1016/j.marpolbul.2011.04.043) [DOI] [PubMed] [Google Scholar]
- 102.Backhaus T. 2014. Medicines, shaken and stirred: a critical review on the ecotoxicology of pharmaceutical mixtures. Phil. Trans. R. Soc. B 369, 20130585 ( 10.1098/rstb.2013.0585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.DeLorenzo ME, Fleming J. 2008. Individual and mixture effects of selected pharmaceuticals and personal care products on the marine phytoplankton species Dunaliella tertiolecta. Arch. Environ. Contam. Toxicol. 54, 203–210. ( 10.1007/s00244-007-9032-2) [DOI] [PubMed] [Google Scholar]
- 104.Cleuvers M. 2003. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 142, 185–194. ( 10.1016/S0378-4274(03)00068-7) [DOI] [PubMed] [Google Scholar]
- 105.Schnell S, Bols NC, Barata C, Porte C. 2009. Single and combined toxicity of pharmaceuticals and personal care products (PPCPs) on the rainbow trout liver cell line RTL-W1. Aquat. Toxicol. 93, 244–252. ( 10.1016/j.aquatox.2009.05.007) [DOI] [PubMed] [Google Scholar]
- 106.Backhaus T, Scholze M, Grimme LH. 2000. The single substance and mixture toxicity of quinolones to the bioluminescent bacterium Vibrio fischeri. Aquat. Toxicol. 49, 49–61. ( 10.1016/S0166-445X(99)00069-7) [DOI] [PubMed] [Google Scholar]
- 107.Kidd KA, Paterson MJ, Rennie MD, Podemski CL, Findlay DL, Blanchfield PJ, Liber K. 2014. Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen. Phil. Trans. R. Soc. B 369, 20130578 ( 10.1098/rstb.2013.0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taylor NG, Verner-Jeffreys DW, Baker-Austin C. 2011. Aquatic systems: maintaining, mixing and mobilising antimicrobial resistance? Trends Ecol. Evol. 26, 278–284. ( 10.1016/j.tree.2011.03.004) [DOI] [PubMed] [Google Scholar]
- 109.McDaniel LD, Young E, Delaney J, Ruhnau F, Ritchie KB, Paul JH. 2010. High frequency of horizontal gene transfer in the oceans. Science 330, 50 ( 10.1126/science.1192243) [DOI] [PubMed] [Google Scholar]
- 110.Rose JM, Gast RJ, Bogomolni A, Ellis JC, Lentell BJ, Touhey K, Moore M. 2009. Occurrence and patterns of antibiotic resistance in vertebrates off the Northeastern United States coast. FEMS Microbiol. Ecol. 67, 421–431. ( 10.1111/j.1574-6941.2009.00648.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blackburn JK, Mitchell MA, Blackburn M-CH, Curtis A, Thompson BA. 2010. Evidence of antibiotic resistance in free-swimming, top-level marine predatory fishes. J. Zoo Wildlife Med. 41, 7–16. ( 10.1638/2007-0061.1) [DOI] [PubMed] [Google Scholar]
- 112.Bonnedahl J, et al. 2009. Dissemination of Escherichia coli with CTX-M type ESBL between humans and yellow-legged gulls in the south of France. PLoS ONE 4, e5958 ( 10.1371/journal.pone.0005958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moore JE, Rao JR, Moore PJ, Millar BC, Goldsmith CE, Loughrey A, Rooney PJ. 2010. Determination of total antibiotic resistance in waterborne bacteria in rivers and streams in Northern Ireland: can antibiotic-resistant bacteria be an indicator of ecological change? Aquat. Ecol. 44, 349–358. ( 10.1007/s10452-009-9294-z) [DOI] [Google Scholar]
- 114.Hutchinson TH, Lyons BP, Thain JE, Law RJ. 2013. Evaluating legacy contaminants and emerging chemicals in marine environments using adverse outcome pathways and biological effects-directed analysis. Mar. Pollut. Bull. 74, 517–525. ( 10.1016/j.marpolbul.2013.06.012) [DOI] [PubMed] [Google Scholar]
- 115.Murray-Smith RJ, Coombe VT, Grönlund MH, Waern F, Baird JA. 2012. Managing emissions of active pharmaceutical ingredients from manufacturing facilities: an environmental quality standard approach. Integr. Environ. Assess. Manage. 8, 320–330. ( 10.1002/ieam.1268) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.