Abstract
Veterinary use of the non-steroidal anti-inflammatory drug diclofenac on domesticated ungulates caused populations of resident Gyps vultures in the Indian sub-continent to collapse. The birds died when they fed on carrion from treated animals. Veterinary diclofenac was banned in 2006 and meloxicam was advocated as a ‘vulture-safe’ alternative. We examine the effectiveness of the 2006 ban, whether meloxicam has replaced diclofenac, and the impact of these changes on vultures. Drug residue data from liver samples collected from ungulate carcasses in India since 2004 demonstrate that the prevalence of diclofenac in carcasses in 2009 was half of that before the ban and meloxicam prevalence increased by 44%. The expected vulture death rate from diclofenac per meal in 2009 was one-third of that before the ban. Surveys at veterinary clinics show that diclofenac use in India began in 1994, coinciding with the onset of rapid Gyps declines ascertained from measured rates of declines. Our study shows that one pharmaceutical product has had a devastating impact on Asia's vultures. Large-scale research and survey were needed to detect, diagnose and quantify the problem and measure the response to remedial actions. Given these difficulties, other effects of pharmaceuticals in the environment may remain undetected.
Keywords: non-steroidal anti-inflammatory drugs, Gyps vultures, diclofenac, meloxicam, scavengers, pharmaceuticals
1. Introduction
Until recently, the documented effects of pharmaceuticals in the environment (PIE) on non-target biota were largely from freshwater systems [1]. Studies have highlighted the chronic impacts that certain drugs can have on aquatic organisms, especially fish [1–6]. In terrestrial systems, PIE has received less attention, yet one drug has had an acute impact on global population sizes of at least three bird species. Across the Indian sub-continent, veterinary use of diclofenac, a non-steroidal anti-inflammatory drug (NSAID), has resulted in the loss of more than 99% of this region's vultures [7]. Prior to the veterinary use of this drug in India, Pakistan, Nepal and Bangladesh, there were vast populations of Gyps vultures. Numbers were so high in the 1980s that the birds were considered a risk to aircraft [8], and the Oriental white-backed vulture Gyps bengalensis (OWBV) was thought to be the most abundant large bird of prey in the world [9]. Populations of OWBV, long-billed vulture Gyps indicus (LBV) and slender-billed vulture Gyps tenuirostris (SBV) numbered tens of millions and were supported by a large cattle population whose slaughter for human consumption is constrained by religious rules in most parts of the sub-continent: either prohibited (Hinduism) or done according to strict rules (Islam). India has approximately 400 million cattle [10], of which around 65 million head die each year [10] producing vast amounts of carrion because most of the meat is not acceptable to humans as food. Prior to the impact of diclofenac, vultures consumed and disposed of this biohazardous waste. However, this vital ecosystem service is now almost entirely lost.
The situation for vultures on the Indian sub-continent changed drastically when cheap off-patent diclofenac was introduced as a veterinary drug, probably sometime in the 1990s. Cattle are considered valuable assets as draught animals and to provide milk and dung for fuel, and are kept until they die naturally. Old animals, or those that were sick or near death, were likely to be treated with diclofenac and other common, inexpensive drugs like analgesics or antibiotics. Legal restrictions on euthanasia as a means of reducing the pain and suffering of dying animals also encouraged palliative use of diclofenac. The extent to which diclofenac was used on livestock in India between 2004 and 2006, within a few days of death, was estimated from ungulate carcass surveys undertaken across India and revealed that about 10% of carcasses tested contained detectable residues in liver tissue [11,12].
Several strands of research have now established the link between veterinary use of diclofenac in livestock and the collapse of vulture populations in the Indian sub-continent. Evidence has demonstrated (i) the presence of extensive visceral gout (deposits of the excretory product uric acid) in tissues of the majority of wild Gyps vulture carcasses recovered from Pakistan, India and Nepal [13,14]; (ii) the proportion of dead wild vultures with visceral gout is consistent with its cause being sufficient to produce the observed rapid rates of population decline [15]; (iii) a perfect association between the presence of gout and diclofenac residues in liver and kidney samples from dead wild vultures [13,14]; (iv) that experimental dosing of captive vultures with diclofenac quickly leads to the death of the treated birds with visceral gout [13,16]; (v) that residues of diclofenac sufficient to kill vultures remain in the tissues of cattle dying within a few days of receiving a standard veterinary dose of diclofenac [13,17]; and (vi) that the prevalence and concentration of diclofenac in available ungulate carcasses was sufficient to cause population declines at the rates observed [18]. This evidence indicates that ‘diclofenac is the main, if not the only cause of the widespread declines in vulture populations’ seen on the Indian sub-continent [15]. Moreover, research on other potential causal agents, such as other pollutants (heavy metals, pesticides, etc.), disease, food availability and persecution, has failed to show that such factors are acting to a sufficient degree or over a large enough area to account for the rapid and widespread declines observed [13] or that any of these factors are strongly associated with the widespread occurrence of visceral gout in dead vultures.
The impact of diclofenac on Asia's vulture populations has been profound, with numbers of OWBV in India declining by more than 99.9% in 15 years (1992–2007). The annual rate of decline after 2000 was 44–50% [7]. In 2000, all three formerly abundant resident Gyps species (OWBV, SBV and LBV) were classified as Critically Endangered [19]. When the cause of the vulture decline was still unknown, moribund vultures were taken into captivity for veterinary investigations. After the discovery of the role of veterinary diclofenac, national and international conservation organizations raced to capture more vultures for a conservation breeding programme to safeguard the survival of these species, in case action to remove diclofenac from their food supply came too late. An international team of researchers also searched for an alternative NSAID without a toxic effect on vultures, but with a similar therapeutic value to diclofenac when used to treat cattle. A survey of the use of NSAIDs to treat raptors in zoos and rehabilitation centres identified meloxicam as a drug that did not appear to cause death or visceral gout in vultures and raised concerns about the potential toxic impacts of other veterinary NSAIDs [20]. Safety testing of meloxicam on African white-backed vultures Gyps africanus in Southern Africa [21] and on two of the critically endangered Asian vulture species in India [22] established that meloxicam was of low toxicity to Gyps vultures at dosages likely to be encountered by them in the wild when feeding on carcasses of meloxicam-dosed ungulates. The veterinary use of meloxicam to replace diclofenac was therefore advocated to veterinarians, pharmacists and farmers from 2006 onwards [23].
After reviewing the evidence about the role of diclofenac and the safety of meloxicam to vultures, the Indian, Nepalese and Pakistan governments banned the manufacture and importation of veterinary diclofenac, with reinforcement of the regulations over the next few years. Bangladesh introduced a ban in 2010. Given that the toxicity of diclofenac to Gyps was reported in 2004 [13], this rapid positive action to halt the decline of Asia's vultures was commendable. However, diclofenac remains a serious threat to vultures in the Indian sub-continent because enforcement of the regulations has been incomplete [24,25] and formulations for humans, in vials that contain a larger quantity of the drug than a human dose, are on sale widely and misused to treat ungulates [26].
The collapse of Gyps vulture populations in Asia, the discovery of the role diclofenac was playing, and the research and conservation actions outlined above, demonstrate the impact that this drug has had on three keystone species. While a large body of research on this topic now exists, many questions remain. In this paper, we set out to answer several of them: (i) when did diclofenac enter the veterinary market and become widely used in the region, (ii) what attitudes do vets have to the use of alternative NSAIDs compared with diclofenac, (iii) to what extent are conservation actions producing changes in the use of diclofenac and its impact on vulture populations and (iv) how are changes in the use of diclofenac related to changes in the use of other veterinary drugs?
2. Material and methods
(a). Veterinary surveys, the timing of non-steroidal, anti-inflammatory drug use and vulture declines
Standardized interviews were conducted with veterinary professionals at 29 clinics in India between May and September 2004. These surveys were undertaken after the discovery of the toxicity of diclofenac [13], but before meloxicam had been identified and tested as an alternative [21,22]. The 29 clinics, considered typical of those in the regions surveyed, were located in Gujarat (n = 12), Rajasthan (12), Maharashtra (3) and Uttar Pradesh (2). Caseloads varied from 15 to 1350 cases per month, all clinics routinely treated livestock, and clients included commercial dairy farmers, moderately wealthy farmers, poor farmers, and cattle and animal welfare charities. Surveys collected information regarding the NSAIDs used for treatment, the year each NSAID was first used in the clinic, the clinical conditions that were treated, any side effects and which alternative NSAIDs to diclofenac were recommended. The year of introduction of each NSAID was then compared with the timing of the decrease in the OWBV population in India. We estimated the year that declines began in India by backwards extrapolation of the observed decline rates from counts of OWBV nesting at Keoladeo National Park [27,28] and from nationwide road transects of this species [7]. Rates of decline were estimated using log-linear Poisson regression models for both the road transect and Keoladeo data, using previously published methods [7,29].
(b). Prevalence of diclofenac and meloxicam in the food supply of vultures
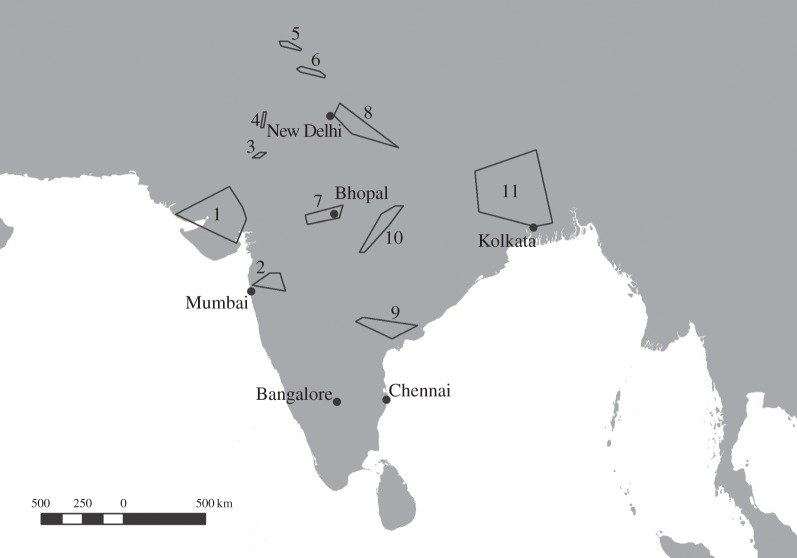
Liver samples were collected from carcasses of dead mammals, principally domesticated ungulates, available to scavengers in India between May 2004 and July 2010 (n = 6207). Samples were taken opportunistically when/where it was possible to obtain access and permission, with most samples collected from carcass dumps managed by local government corporations, cooperatives, private companies and individuals and cattle welfare charities. Sampling locations were typical of sites used by vultures before their populations declined and were distributed across India (figure 1). Carcasses at dumps were sampled according to availability and over the whole study period were predominantly taken from cattle (Bos indicus, Bos taurus and hybrids, 61.6%) and water buffalo (Bubalus bubalis, 33.5%), with smaller numbers from sheep (Ovis aries, 2.0%), goats (Capra hircus, 2.5%) and other mammals (camel Camelus sp., horse Equus caballus, dog Canis familiaris and unidentified; 0.5% combined). Sample collection and storage protocols were reported previously [12,18,30]. GPS coordinates were collected at all sites and each site was assigned to one of 11 clusters based on the site being nearer to the geodesic centroid of a particular cluster than to that of any other cluster (figure 1). Cluster boundaries were selected so that samples from the cluster were available over a period of at least 3 and preferably 5 years to allow estimates of change over time in prevalence and concentration of diclofenac and meloxicam for each cluster. Site-cluster assignments differed from those used previously [18,24] when only the geographical proximity of sampling sites to one another was considered. Details of cluster locations and collection dates are given in the electronic supplementary material, table S1.
Figure 1.
Map of the Indian sub-continent showing convex polygons around the outermost locations of each of 11 site-clusters where liver samples were collected from domesticated ungulates. Numbers denote the site-cluster codes used in tables 2 and 3 and the electronic supplementary material, table S1.
Sub-samples of collected liver tissue were weighed and then homogenized and extracted using acetonitrile [11,12]. Diclofenac and meloxicam concentrations in extracts were determined by liquid chromatography with electrospray ionization mass spectrometry (LC-ESI/MS). The limit of quantification (LOQ), back-calculated to concentration in wet tissue, was 0.01 mg kg−1. Detailed protocols for extraction and analysis of these NSAIDs were reported previously [11,12].
(c). Estimating change in diclofenac and meloxicam prevalence and concentration over time
Statistical analyses of diclofenac and meloxicam concentrations were based on previous methods [16,24] that estimated the scale parameter a and shape parameter b of a Weibull distribution of diclofenac concentrations left-censored at the LOQ. The cumulative distribution function (cdf) assumed for the distribution of diclofenac concentrations in liver dliver in the diclofenac-contaminated liver samples was 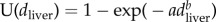 . This ignores a proportion (1 − f) of samples that had no trace of the drug, with f being the true prevalence of diclofenac in liver samples. Hence, the cdf of diclofenac concentration in all samples is given by
. This ignores a proportion (1 − f) of samples that had no trace of the drug, with f being the true prevalence of diclofenac in liver samples. Hence, the cdf of diclofenac concentration in all samples is given by 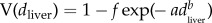 . As previously [18,24], we estimated f, a and b by determining numerically the parameter values that maximized the log-likelihood of the observed data, assuming that the observed concentrations were truncated at the LOQ. Hence, the modelled proportion of samples with no detectable diclofenac was the sum of the proportion with no trace of the drug and the proportion with concentrations below the LOQ: this sum being 1 + f (U(dLOQ) − 1).
. As previously [18,24], we estimated f, a and b by determining numerically the parameter values that maximized the log-likelihood of the observed data, assuming that the observed concentrations were truncated at the LOQ. Hence, the modelled proportion of samples with no detectable diclofenac was the sum of the proportion with no trace of the drug and the proportion with concentrations below the LOQ: this sum being 1 + f (U(dLOQ) − 1).
In contrast to earlier work, we used smoothing to describe changes in f and a over time. We regard this as an improvement because effects of time are now modelled as a continuous variable, whereas data were previously assigned to a few arbitrarily defined time periods. Our previous approach also assumed that differences in diclofenac prevalence and concentration among time periods were the same for all site-clusters, whereas the smoothing method allows the form of changes over time to differ geographically. For simplicity, we fixed the shape parameter b to be constant and modelled site-cluster differences and changes over time in prevalence and concentration by allowing changes in f and a only. To perform smoothing, we assumed that the required smoothed values of f and a on a focal date t* could be estimated by obtaining those values that maximized the weighted log-likelihood calculated across all the data for a site-cluster, obtained at various sampling dates ti, using weights w = exp(−(ti − t*)2/s2). Hence, w = 1 when ti = t*, but diminished with increasing difference between the focal t* and sampling date ti. For a given focal date and for specified values of the smoothing parameter s and the shape parameter b, products of the contribution to the log-likelihood of the result for a given liver sample and w were summed across all samples for a site-cluster and divided by the sum of the weights, to give the weighted log-likelihood. Values of f and a that maximized the weighted log-likelihood were obtained for each focal date upon which samples were collected in a site-cluster. The maximum-likelihood value of b for all dates and site-clusters combined was determined by a bisection search [31], with the above procedure for estimating the smoothed values of f and a performed at each step of the search. We determined the optimal value of the smoothing parameter s by leave-one-out cross-validation (LOOCV) [32]. The sampling dates for each site-cluster were divided into successive time-blocks. For diclofenac, boundaries of time-blocks were chosen so there were at least 25 liver samples taken within each time-block and there were at least three (maximum six) time-blocks for each site-cluster and 45 time-blocks overall. For a given value of s, we repeated the procedure described above for estimating the shape parameter b and the smoothed values of f and a, on all of the sample collection dates, except that smoothed values of f and a for sample collection dates within a given time-block were calculated by only using observations from outside that time-block and excluding those within it. These parameter estimates were used to calculate the log-likelihood of the observations within the focal time-block using the smoothed f and a values obtained from data outside the time-block. This procedure was repeated for each time-block in turn and the log-likelihoods summed. The value of s at which this summed LOOCV log-likelihood was maximized was determined by bisection search [31].
Once the optimal value of the smoothing parameter s had been determined, it was used to obtain smoothed values for the true prevalence and arithmetic mean concentration of diclofenac in those samples in which diclofenac was present (including those with concentrations less than LOQ) at daily intervals from the beginning of 2006 to the end of 2010. For graphical purposes, results are only shown for the period spanning the central 80% of the sample collection dates for a site-cluster to avoid extrapolation beyond the periods within which the smoothed values are likely to be reliable. We used smoothed values of model parameters to calculate true prevalence and arithmetic mean concentration in samples on 30 June 2005 (mid-2005), 31 December 2006 (end-2006) and 30 June 2009 (mid-2009). These dates were chosen to represent points in time (i) before the ban on veterinary diclofenac (mid-2005), (ii) just after the ban was implemented (end-2006) and (iii) a date after ban implementation (mid-2009). These dates also overlapped with the central 80% of sample collection dates for most site-clusters.
The procedures for estimating changes in meloxicam were as described for diclofenac, but, since meloxicam measurements began in 2006, we defined 33 time-blocks for the meloxicam LOOCV procedure. There were between two and five time-blocks per site-cluster. We used smoothed values of model parameters to calculate the true prevalence and arithmetic mean concentration of meloxicam on 31 December 2006 (end-2006) and 30 June 2009 (mid-2009), following the same logic as described above.
We calculated ratios and differences of estimates on the above dates and used bootstrap resampling of results for site-clusters and Wilcoxon matched-pairs tests to assess the statistical significance of changes.
(d). Estimating expected vulture death rates caused by diclofenac contamination of carcasses
We estimated the proportion of OWBV killed by diclofenac per meal from the observed level of contamination using Steps 3–7 from a previous analysis [18]. The average proportion of vultures killed per meal, averaged across all meals taken by the vulture population, was then obtained from the probability density function of the dose of diclofenac per unit vulture body weight per meal and the dose–response relationship between diclofenac dose and the proportion of vultures killed. Integration under the curve given by the product of these two functions gives the average proportion of vultures killed per meal [18,24].
3. Results
(a). Timing of the introduction of veterinary diclofenac and veterinary attitudes in 2004
When questioned in 2004, Indian veterinary professionals reported a median year for first use of diclofenac as 1994. Other NSAIDs including metamizole (analgin), acetylsalicylic acid (aspirin) and phenylbutazone became widely used during the late-1970s to mid-1980s, whereas ketoprofen and meloxicam were not reported as widely used until 2003 and 2004, respectively (figure 2). Backwards extrapolation of the observed decline rate of OWBV at Keoladeo National Park and from road transect counts both indicated that population declines began in 1994 (figure 2). Hence, the OWBV population decline is therefore coincident with the reported introduction of diclofenac and not with the introduction of other NSAIDs (figure 2). Survey results also indicated that diclofenac was the most commonly used NSAID in India in 2004, accounting for more than half (52%) of all treatments. Other NSAIDs used were: metamizole (17% of treatments), phenylbutazone (12%), meloxicam (11%), ketoprofen (3%), acetylsalicylic acid (3%) and others (pitofenone, ibuprofen and flunixin, 2% combined). When asked which veterinary treatments should be used to replace diclofenac, veterinarians most frequently suggested meloxicam (54% of respondents), followed by metamizole (43%), phenylbutazone (18%), ibuprofen (7%), paracetamol (7%) and herbal formulations (4%). Common conditions treated with NSAIDs were pneumonia, mastitis, ephemeral fever, lameness, prolapsed uterus, respiratory infections, colic, arthritis, heat stroke, rheumatoid pain, gynaecological treatment, bronchitis and dengue fever. Veterinarians reported side effects following treatment with a number of NSAIDs (table 1), with problems most frequently associated with diclofenac (73% of respondents). We would expect that drugs that had been in use for the longest time prior to interviews in 2004 would be those for which the most clinics reported side effects, if the true incidence of side effects did not vary among drugs. This expectation was fulfilled by the small proportion of clinics reporting side effects for meloxicam, which was introduced just before the interviews, but it is notable that a higher proportion of clinics reported side effects for diclofenac than for metamizole, acetylsalicylic acid and phenylbutazone, which had been in use for far longer. The most common side effects were gastro-intestinal problems, including gastritis, gastro-intestinal bleeding, constipation and peptic ulcers. Other side effects reported for diclofenac were reduced milk production, animals going off their feed and cases of abortion and allergic reaction (table 1).
Figure 2.
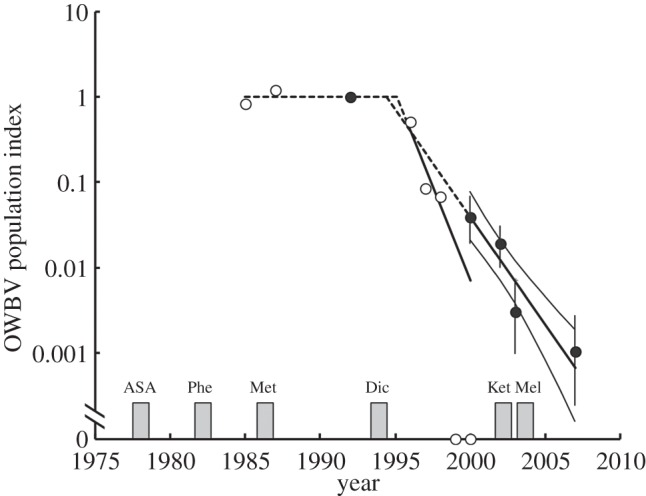
Median year of first veterinary use of metamizole (Met, analgin), acetylsalicylic acid (ASA, aspirin), diclofenac (Dic), ketoprofen (Ket), meloxicam (Mel) and phenylbutazone (Phe) (bars). Also shown are population indices for OWBV at Keoladeo National Park (unfilled circles) and from nationwide surveys (filled circles), and the log-linear Poisson regression models fitted to the data for the period of exponential population decline. Thick lines show the regression models with the period for which the model was fitted being indicated by the continuous part of the line. The dashed part of the line indicates the backwards extrapolations. Data from both surveys were standardized to have a mean value of 1.0 prior to the start of the declines. Error bars (vertical lines) around road transect point estimates and the thin curves on either side of the road transect regression are 95% bootstrap CIs.
Table 1.
Number of clinics reporting each of several categories of side effects for metamizole (analgin), acetylsalicylic acid (aspirin), diclofenac, meloxicam and phenylbutazone and the overall proportion of clinics reporting any side effects based upon interviews at 29 veterinary clinics in 2004.
| metamizole | acetylsalicylic acid | diclofenac | meloxicam | phenylbutazone | |
|---|---|---|---|---|---|
| % clinics noting side effects | 14 | 10 | 73 | 9 | 29 |
| number with side effect: | |||||
| gastro-intestinala | 1 | 0 | 11 | 0 | 0 |
| off feed | 0 | 0 | 3 | 0 | 1 |
| drop in milk prod. | 0 | 0 | 3 | 0 | 1 |
| injection siteb | 0 | 1 | 4 | 1 | 1 |
| abortions | 0 | 0 | 1 | 0 | 0 |
| allergy/death | 0 | 0 | 1 | 0 | 0 |
| recumbency/death | 1 | 0 | 0 | 0 | 0 |
aGastritis, gastro-intestinal bleeding, constipation and peptic ulcers.
bIrritation at site, abscess and swelling, and gangrene at site.
(b). Trends in the prevalence and mean concentration of diclofenac in carcasses
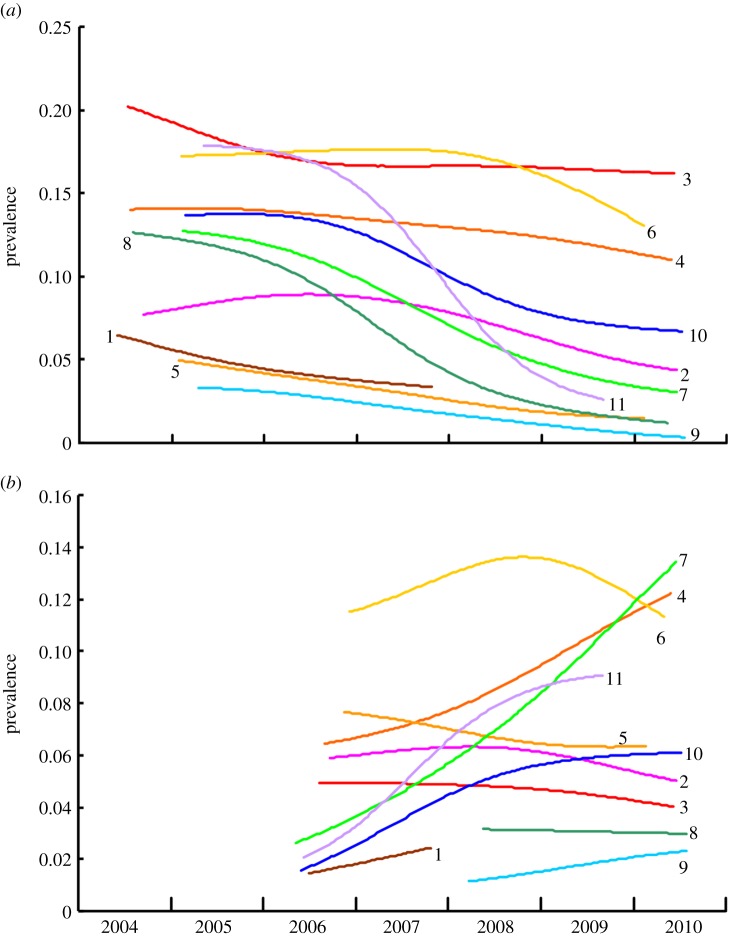
The LOOCV estimate of the smoothing parameter for diclofenac was 737 and the Weibull shape parameter was 0.609. Graphs of smoothed true diclofenac prevalence showed a tendency for decline in all site-clusters, but with large differences in average level, rate and timing (figure 3a). The smoothed mean true prevalence of diclofenac in liver samples in mid-2009, 3 years after the ban on veterinary use, was about 50% of the prevalence in mid-2005, before the ban, for the 10 site-clusters with eligible estimates for both dates (table 2). Prevalence declined over time in all of these site-clusters. The bootstrap 95% CI for the mid-2009 to mid-2005 ratio of prevalence did not overlap 1 and the Wilcoxon matched-pairs test indicated a highly significant decline in prevalence. Figure 3a shows that declines in diclofenac prevalence continued well into 2010 for the nine site-clusters sampled beyond that time. Arithmetic mean diclofenac concentration in contaminated liver samples declined in some site-clusters, but increased or showed no clear pattern in others (electronic supplementary material, figure S1). The smoothed arithmetic mean concentration of diclofenac in mid-2009 was 85% of the concentration in mid-2005 (table 2). However, the bootstrap 95% CI for the mid-2009 to mid-2005 ratio of concentration overlapped 1 and the Wilcoxon matched-pairs test indicated only a marginally significant decline in diclofenac concentration.
Figure 3.
Smoothed curves representing changes over time in the corrected prevalence of (a) diclofenac and (b) meloxicam in liver samples from domesticated ungulates for 11 site-clusters, indicated by numerals adjacent to the curves. Curves are shown for periods that include the central 80% of sample collection dates for each site-cluster.
Table 2.
Corrected prevalence and arithmetic mean concentration (mg kg−1 wet weight) of diclofenac in liver samples from domesticated ungulates in mid-2005 and mid-2009 by site-cluster. Estimated proportions of G. bengalensis dying from diclofenac poisoning per meal are shown for assumed intervals between meals F of 2 days and 3 days. Summary statistics in the lower part of the table were obtained after excluding the results for site-cluster 1, which was not monitored for a sufficiently long period.
| site-cluster | prevalence |
mean concentration |
death rate per meal F = 2 |
death rate per meal F = 3 |
||||
|---|---|---|---|---|---|---|---|---|
| 2005 | 2009 | 2005 | 2009 | 2005 | 2009 | 2005 | 2009 | |
| 1 | 0.050 | — | 1.210 | — | 0.0051 | — | 0.0077 | — |
| 2 | 0.085 | 0.048 | 0.782 | 0.323 | 0.0052 | 0.0008 | 0.0085 | 0.0015 |
| 3 | 0.183 | 0.163 | 1.131 | 0.753 | 0.0174 | 0.0094 | 0.0266 | 0.0156 |
| 4 | 0.141 | 0.114 | 1.146 | 1.032 | 0.0136 | 0.0098 | 0.0208 | 0.0152 |
| 5 | 0.046 | 0.015 | 0.475 | 1.556 | 0.0014 | 0.0020 | 0.0025 | 0.0029 |
| 6 | 0.173 | 0.134 | 0.820 | 0.209 | 0.0112 | 0.0010 | 0.0182 | 0.0022 |
| 7 | 0.125 | 0.034 | 0.643 | 0.267 | 0.0059 | 0.0004 | 0.0100 | 0.0008 |
| 8 | 0.118 | 0.014 | 1.277 | 1.401 | 0.0128 | 0.0017 | 0.0192 | 0.0025 |
| 9 | 0.033 | 0.006 | 0.109 | 0.131 | 0.0001 | 0.0000 | 0.0002 | 0.0000 |
| 10 | 0.137 | 0.069 | 1.519 | 1.203 | 0.0179 | 0.0071 | 0.0260 | 0.0107 |
| 11 | 0.178 | 0.023 | 0.782 | 0.476 | 0.0109 | 0.0007 | 0.0178 | 0.0013 |
| mean | 0.122 | 0.062 | 0.868 | 0.735 | 0.0097 | 0.0033 | 0.0150 | 0.0053 |
| 95% CL of mean | 0.087–0.152 | 0.029–0.097 | 0.624–1.112 | 0.443–1.056 | 0.0059–0.0132 | 0.0012–0.0056 | 0.0087–0.0203 | 0.0019–0.0091 |
| ratio of means 2009 : 2005 | 0.509 | 0.847 | 0.342 | 0.352 | ||||
| 95% CL of ratio | 0.290–0.687 | 0.573–1.243 | 0.158–0.506 | 0.162–0.529 | ||||
| Wilcoxon T+ | 55 | 41 | 53 | 53 | ||||
| two-tailed p | 0.002 | 0.193 | 0.006 | 0.006 | ||||
| clusters with decrease | 10/10 | 7/10 | 9/10 | 9/10 | ||||
(c). Trends in the prevalence and mean concentration of meloxicam in carcasses
The LOOCV estimate of the smoothing parameter for meloxicam was 714 and the Weibull shape parameter was 0.723. Graphs of smoothed true meloxicam prevalence showed a tendency for increase at most site-clusters, but some showed declines or complex patterns (figure 3b). The smoothed mean true prevalence of meloxicam in liver samples in mid-2009, 3 years after the ban on veterinary use of diclofenac, was 44% higher than the prevalence at end-2006, just after the ban had been implemented, for the eight site-clusters with eligible estimates for both dates (table 3). Prevalence increased over time in six of these site-clusters. The bootstrap 95% CI for the mid-2009 to end-2006 ratio of prevalence did not overlap 1 and the Wilcoxon matched-pairs test indicated a marginally significant increase in meloxicam prevalence. Arithmetic mean meloxicam concentration in contaminated liver samples showed no consistent trend over time (electronic supplementary material, figure S2). The smoothed arithmetic mean concentration of meloxicam in mid-2009 was 4% higher than that at the end of 2006, with increases in half of the eight site-clusters with eligible estimates at both times and decreases in the other half (table 3). Bootstrap 95% CIs for the mid-2009 to end-2006 ratio of concentration overlapped 1 and the Wilcoxon matched-pairs test indicated no significant change in meloxicam concentration.
Table 3.
Corrected prevalence and arithmetic mean concentration (mg kg−1 wet weight) of meloxicam in liver samples from domesticated ungulates at the end of 2006 and in mid-2009 by site-cluster. Summary statistics in the lower part of the table were obtained after excluding the results for site-clusters 1, 8 and 9.
| site-cluster | prevalence |
mean concentration |
||
|---|---|---|---|---|
| 2006 | 2009 | 2006 | 2009 | |
| 1 | 0.018 | — | 0.586 | — |
| 2 | 0.060 | 0.058 | 0.683 | 0.692 |
| 3 | 0.049 | 0.058 | 0.777 | 0.692 |
| 4 | 0.067 | 0.105 | 0.576 | 0.561 |
| 5 | 0.076 | 0.063 | 1.020 | 0.363 |
| 6 | 0.116 | 0.130 | 0.769 | 0.898 |
| 7 | 0.036 | 0.100 | 0.797 | 0.779 |
| 8 | — | 0.031 | — | 1.142 |
| 9 | — | 0.018 | — | 0.523 |
| 10 | 0.025 | 0.059 | 0.655 | 1.398 |
| 11 | 0.033 | 0.090 | 0.728 | 0.877 |
| mean | 0.058 | 0.083 | 0.751 | 0.783 |
| 95% CL of mean | 0.042–0.078 | 0.067–0.102 | 0.669–0.838 | 0.613–1.006 |
| ratio of means 2009 : 2006 | 1.436 | 1.042 | ||
| 95% CL of ratio | 1.114–1.949 | 0.738–1.435 | ||
| Wilcoxon T+ | 32 | 20 | ||
| two-tailed p | 0.0546 | 0.8438 | ||
| site-clusters with increase | 6/8 | 4/8 | ||
(d). Trends in the proportion of vultures expected to be killed by diclofenac per meal
Graphs of the smoothed expected proportion of OWBV killed per meal for an assumed interval F = 3 days between meals declined in all except one of the 11 site-clusters, but with large differences in average level, rate and timing (figure 4). The expected death rate per meal in mid-2009 was 34–35% of the rate in mid-2005, before the ban (table 2), depending upon whether a value of F = 2 or F = 3 was used. Death rate declined over time in nine of the 10 site-clusters with eligible estimates at both times. The bootstrap 95% CI for the mid-2009 to mid-2005 ratio of mean death rate did not overlap 1 and the Wilcoxon matched-pairs test indicated a highly significant decline in expected death rate per meal. Inspection of figure 4 shows that declines in expected death rate continued well into 2010 for most of the site-clusters sampled in that year.
Figure 4.
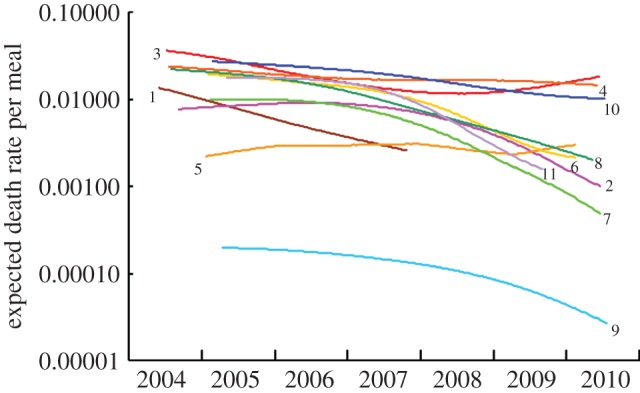
Smoothed curves showing trends in the expected death rate per meal caused by diclofenac poisoning of oriental white-backed vultures exposed to the prevalence and concentration of the drug measured in carcasses of domesticated ungulates between 2004 and 2010. Results are shown for 11 site-clusters, indicated by numerals adjacent to the curves. The interval between meals F is assumed to be 3 days. Note the logarithmic scale. Curves are shown for periods that include the central 80% of sample collection dates for each cluster.
(e). Relationship between diclofenac and meloxicam prevalence
Meloxicam has been advocated to veterinarians, pharmacists and livestock owners as an alternative NSAID that is safe for vultures when used in place of diclofenac since 2006 [23]. Consequently, if the observed decline in diclofenac prevalence in carcasses was due to substitution with meloxicam, we would expect site-clusters with the largest decrease over time in diclofenac prevalence to be those with the largest increase in meloxicam. We tested this by calculating the absolute difference in smoothed true prevalence between mid-2009 and end-2006 for each site-cluster for both drugs. Clusters where the largest decrease in diclofenac prevalence occurred tended to be those with a large increase in meloxicam (figure 5). However, the diclofenac decrease tended to be consistently larger than the meloxicam increase (figure 5). The positive relationship between the magnitude of decrease in diclofenac and increase in meloxicam prevalence was significant when the analysis was restricted to the eight site-clusters with eligible estimates of prevalence for both drugs on both dates (r6 = 0.675, one-tailed p = 0.033) and remained significant when extrapolated smoothed prevalence values were added for the other three site-clusters (r9 = 0.591, one-tailed p = 0.028).
Figure 5.
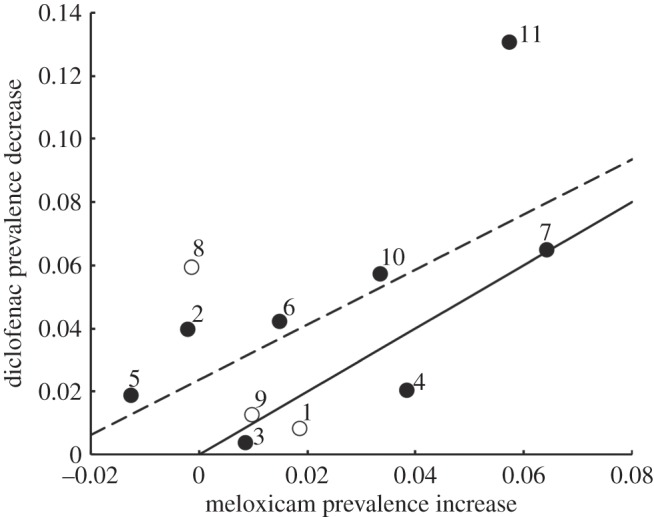
Decrease in the corrected prevalence of diclofenac between end-2006 and mid-2009 in relation to the increase in the corrected prevalence of meloxicam. Points represent site-clusters as indicated by numerals adjacent to points. Clusters for which the central 80% of sample collection dates completely overlapped the period from the end-2006 to mid-2009 are shown by solid points. For three clusters, shown by open circles, the central 80% of sample collection dates did not overlap the period of interest and an extrapolation was made using smoothing. The solid line represents equality between the decrease in prevalence of diclofenac and the increase in prevalence of meloxicam. The dashed line shows the ordinary least-squares regression fitted to data for all 11 site-clusters: diclofenac decrease = 0.0237 + 0.8731 × meloxicam increase.
4. Discussion
It was reported in interviews conducted at clinics treating livestock in 2004 that veterinary diclofenac started to be used in India in 1994. This date coincides exactly with two independent estimates of the onset of the decline of the OWBV population in India obtained by backwards extrapolation of regression models fitted to vulture counts (figure 2). By the time of the interviews in 2004, diclofenac was the NSAID of choice within veterinary clinics in India, accounting for over half of all NSAID treatments administered, probably due to its low cost [26] and efficacy. Although meloxicam accounted for only about 10% of all treatments administered in 2004, more than 50% of respondents considered this drug to be the most suitable replacement for diclofenac, even though results of research establishing the safety of this drug in Gyps vultures were not published until 2006–2007 [21,22].
There is no indication of a match between the timing of the beginning of veterinary use and the onset of vulture declines for any of the other veterinary NSAIDs in widespread use before diclofenac was introduced (figure 2). However, this lack of coincidence should not be regarded as evidence that use of these drugs is safe for vultures. At present, meloxicam is the only drug to be demonstrated as such by safety testing on Gyps vultures. Evidence for the potential toxicity of various NSAIDs to 79 species of birds suggests that compounds other than diclofenac, in particular carprofen and flunixin, may be associated with mortality with renal failure and/or gout in raptors [20]. However, to the best of our knowledge, carprofen is not used in India and flunixin is administered infrequently [26]. The only other NSAID thought to have a high potential to cause mortality in Gyps vultures in India is ketoprofen [33]. However, the emergence of veterinary use of this drug in India came considerably later than the onset of the vulture declines (figure 2). Ketoprofen is known to have a relatively short post-administration half-life in cattle [34] and is, perhaps as a result, rarely detected in livestock carcasses in India. It was detected in 0.8% of 1488 carcasses sampled in 2006 [12], although a few of these contained concentrations that might have been lethal [12,33].
Although diclofenac was clearly a popular drug with veterinary professionals in 2004, it seems from our survey that they recognized that more negative side effects from administration of this drug can occur than for other drugs (table 1). Hence, despite its perceived efficacy for treating livestock, its popularity and low cost [26], there were significant perceived drug safety drawbacks to the use of diclofenac as a veterinary NSAID. By contrast, veterinary professionals reported few side effects from meloxicam (table 1), though this might partly be because it had been in use for a short time. Meloxicam is a second generation NSAID with preferential cyclooxygenase-2 (COX-2) inhibition and reduced risk of adverse side effects [35], including gastro-intestinal problems [36], than non-selective COX inhibiting drugs like diclofenac. Advocating the pharmacological and drug safety benefits of properly formulated versions of meloxicam to veterinarians and farmers in the Indian sub-continent may be beneficial for vulture conservation and animal welfare, and hasten the switch from diclofenac to ‘vulture-safe’ meloxicam [37]. Although veterinary formulation, sale and veterinary use of diclofenac has been illegal across much of the Indian sub-continent since bans were issued in mid-2006, large volume injectable human formulations remain widely available in the loosely regulated pharmaceutical marketplace in India [26]. The analysis presented here, and in previous work [11,12,17,18,24,30], regarding the level of diclofenac contamination in livestock carcasses sampled in India demonstrates that illegal diclofenac use on domesticated ungulates continued after the ban at least up to 2010, though it had declined significantly. In India, there has been a two-thirds reduction in the vulture death rate per meal from diclofenac poisoning expected from declines in the prevalence and concentration of diclofenac in ungulate carcasses after the ban. Vulture counts indicate that the rate of vulture population change in India and Nepal has become less negative since about 2006, with approximate population stability recently for LBV and SBV in India [38], and recent slight increases for OWBV in India and Nepal [38] and LBV in Pakistan [39]. These are encouraging signs, but vulture populations are still at a critically depleted level.
Geographical variation within India in recent changes in the prevalence of diclofenac and meloxicam suggest that a large part of the decrease in diclofenac use has been accompanied by, and may well have been due to, an increase in meloxicam use. This is the most likely explanation for the correlation we found across regions of India between the magnitude of the diclofenac decrease since 2006 and the meloxicam increase over the same period (figure 5). This is an encouraging indication that the substantial efforts made to encourage veterinary professionals and livestock keepers to switch to meloxicam from diclofenac have had some success. There is every prospect that continuation and enhancement of these efforts will eventually remove the threat to vulture populations from diclofenac. However, there is also cause for concern in these results. The use of other NSAIDs is evident from analyses of liver samples from domesticated ungulates in India and from surveys of veterinary pharmacies [12,26], and we consider it likely that the difference between the increase in meloxicam and the reduction in diclofenac is due to the availability of these other compounds for veterinary use. Among these compounds are several with unknown toxicity to vultures and one (ketoprofen) which has been shown to be toxic to Gyps vultures at concentrations likely to be encountered by wild vultures feeding from the carcass of an ungulate given a standard veterinary dose of the drug [33]. Despite this information, the veterinary use of ketoprofen has not yet been banned in India. Avoidance of similar problems in future to those caused to Asian Gyps vulture populations by diclofenac will require substantial improvements in systems for education, monitoring, drug testing and regulation of use.
5. Conclusion
The introduction of diclofenac as a veterinary product in the Indian sub-continent and its subsequent impact on the region's formerly abundant vulture populations is the most dramatic example to date of the unforeseen consequences of pharmaceuticals in the environment. This ‘PIE in the sky’ represents a shift in our understanding of the potential impacts of pharmaceuticals on wildlife populations. The impacts of the veterinary use of NSAIDs have, to date, only been observed in the Indian sub-continent, and only for diclofenac. However, the route of exposure from veterinary-treated livestock to avian scavengers may now be present more widely. In 2013, 9 years after evidence of the toxicity of diclofenac to vultures was published, the veterinary use of diclofenac was approved in Spain and Italy. These countries hold over 80% of the European breeding population of Eurasian griffon vulture Gyps fulvus, one of the species in which the toxicity of diclofenac has been demonstrated experimentally [16], as well as three other vulture species. Other regions of concern include Southern Africa and the Middle East where feeding stations or ‘vulture restaurants’ using carcasses of farm animals are a frequent conservation initiative. Carcasses of domesticated ungulates in these areas might contain NSAIDs and other pharmaceuticals, and while diclofenac is not licensed as a veterinary drug in these areas, concerns about the toxicity and lack of safety testing of other NSAIDs [20] highlight the potential impact of other pharmaceutical products. Gyps vultures are not the only scavengers at carcasses and recent evidence suggests that a wider range of scavenging raptors may be at risk from NSAID poisoning [40]. While the impact of the veterinary use of diclofenac on vultures has occurred at a speed and scale that is unprecedented, the results of this paper demonstrate that conservation efforts linking research, advocacy and government actions have had a major influence on human behaviour and may have begun to allow vulture populations to recover. The impacts of diclofenac on vultures in the Indian sub-continent show that careful evaluation of the impacts of pharmaceuticals in the environment is needed and also provide hope that seemingly lost causes can be recovered by concerted conservation initiatives.
Supplementary Material
Acknowledgements
We thank the Indian Veterinary Research Institute (Izatnagar), Wildlife Institute of India (Dehradun), Bombay Natural History Society (India), University of Aberdeen (UK) and University of Castilla-la-Mancha (Spain) for providing the staff, logistical support and facilities necessary to carry out this work over the last decade. Thanks to all of those involved in liver sampling and laboratory analysis since carcass surveys began in 2004 and to those veterinary clinics in India that provided the questionnaire responses reported here.
Funding statement
We thank the UK Government's Darwin Initiative and the Royal Society for the Protection of Birds (UK) for funds.
References
- 1.Arnold KE, Brown AR, Ankley GT, Sumpter JP. 2014. Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems. Phil. Trans. R. Soc. B 369, 20130569 ( 10.1098/rstb.2013.0569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corcoran J, Winter MJ, Tyler CR. 2010. Pharmaceuticals in the aquatic environment: a critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 40, 287–304. ( 10.3109/10408440903373590) [DOI] [PubMed] [Google Scholar]
- 3.Ankley GT, Brooks BW, Huggett DB, Sumpter JP. 2007. Repeating history: pharmaceuticals in the environment. Environ. Sci. Technol. 41, 8211–8217. ( 10.1021/es072658j) [DOI] [PubMed] [Google Scholar]
- 4.Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. 2007. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl Acad. Sci. USA 104, 8897–8901. ( 10.1073/pnas.0609568104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown AR, Gunnarsson L, Kristiansson E, Tyler CR. 2014. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Phil. Trans. R. Soc. B 369, 20130576 ( 10.1098/rstb.2013.0576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd KA, Paterson MJ, Rennie MD, Podemski CL, Findlay DL, Blanchfield PJ, Liber K. 2014. Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen. Phil. Trans. R. Soc. B 369, 20130578 ( 10.1098/rstb.2013.0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakash V, et al. 2007. Recent changes in populations of resident Gyps vultures in India. J. Bombay Nat. Hist. Soc. 104, 129–135. [Google Scholar]
- 8.Grubh RB, Narayan G, Satheesan SM. 1990. Conservation of vultures in (developing) India. In Conservation in developing countries (eds Daniel JC, Serrao JS.), pp. 360–363. Bombay, India: Bombay Natural History Society and Oxford University Press. [Google Scholar]
- 9.Houston D. 1985. Indian white-backed vulture Gyps bengalensis. In Conservation studies on raptors (eds Newton I, Chancellor RD.), pp. 465–466. Cambridge, UK: International Council for Bird Preservation Technical Publication No. 5. [Google Scholar]
- 10.Wright T. 2013. India – livestock and products semi-annual report 2013. Global Agricultural Information Network report no. IN3017. Washington, DC: USDA Foreign Agricultural Service. [Google Scholar]
- 11.Taggart MA, Senacha KR, Green RE, Jhala YV, Raghavan B, Rahmani AR, Cuthbert R, Pain DJ, Meharg AA. 2007. Diclofenac residues in carcasses of domestic ungulates available to vultures in India. Environ. Int. 33, 759–765. ( 10.1016/j.envint.2007.02.010) [DOI] [PubMed] [Google Scholar]
- 12.Taggart MA, Senacha KR, Green RE, Cuthbert R, Jhala YV, Meharg AA, Mateo R, Pain DJ. 2009. Analysis of nine NSAIDs in ungulate tissues available to critically endangered vultures in India. Environ. Sci. Technol. 43, 4561–4566. ( 10.1021/es9002026) [DOI] [PubMed] [Google Scholar]
- 13.Oaks JL, et al. 2004. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427, 630–633. ( 10.1038/nature02317) [DOI] [PubMed] [Google Scholar]
- 14.Shultz S, et al. 2004. Diclofenac poisoning is widespread in declining vulture populations across the Indian subcontinent. Phil. Trans. R. Soc. B 271, S458–S460. ( 10.1098/rsbl.2004.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green RE, Newton I, Shultz S, Cunningham AA, Gilbert M, Pain DJ, Prakash V. 2004. Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent. J. Appl. Ecol. 41, 793–800. ( 10.1111/j.0021-8901.2004.00954.x) [DOI] [Google Scholar]
- 16.Swan GE, et al. 2006. Toxicity of diclofenac to Gyps vultures. Biol. Lett. 2, 279–282. ( 10.1098/rsbl.2005.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green RE, Taggart MA, Das D, Pain DJ, Sashikumar C, Cunningham AA, Cuthbert R. 2006. Collapse of Asian vulture populations: risk of mortality from residues of the veterinary drug diclofenac in carcasses of treated cattle. J. Appl. Ecol. 43, 949–956. ( 10.1111/j.1365-2664.2006.01225.x) [DOI] [Google Scholar]
- 18.Green RE, Taggart MA, Senacha KR, Raghavan B, Pain DJ, Jhala Y, Cuthbert R. 2007. Rate of decline of the oriental white-backed vulture population in India estimated from a survey of diclofenac residues in carcasses of ungulates. PLoS ONE 2, e686 ( 10.1371/journal.pone.0000686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BirdLife International. 2013. Gyps bengalensis. In IUCN 2013. IUCN Red List of Threatened Species, v. 2013.2 See www.iucnredlist.org (accessed on 7 March 2014).
- 20.Cuthbert R, Parry-Jones J, Green RE, Pain DJ. 2007. NSAIDs and scavenging birds: potential impacts beyond Asia's critically endangered vultures. Biol. Lett. 3, 90–93. ( 10.1098/rsbl.2006.0554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swan G, et al. 2006. Removing the threat of diclofenac to critically endangered Asian vultures. PLoS Biol. 4, 0395–0402. ( 10.1371/journal.pbio.0040066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swarup D, et al. 2007. Safety of meloxicam to critically endangered Gyps vultures and other scavenging birds in India. Anim. Conserv. 10, 192–198. ( 10.1111/j.1469-1795.2006.00086.x) [DOI] [Google Scholar]
- 23.Pain DJ, et al. 2008. The race to prevent the extinction of South Asian vultures. Bird Conserv. Int. 18, S30–S48. [Google Scholar]
- 24.Cuthbert R, et al. 2011. Effectiveness of action in India to reduce exposure of Gyps vultures to the toxic veterinary drug diclofenac. PLoS ONE 6, e19069 ( 10.1371/journal.pone.0019069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuthbert RJ, Prakash V, Saini M, Upreti S, Swarup D, Das A, Green RE, Taggart MA. 2011. Are conservation actions reducing the threat to India's vulture populations? Curr. Sci. 101, 1480–1484. [Google Scholar]
- 26.Cuthbert RJ, Dave R, Chakraborty SS, Kumar S, Prakash S, Ranade SP, Prakash V. 2011. Assessing the ongoing threat from veterinary non-steroidal anti-inflammatory drugs to critically endangered Gyps vultures in India. Oryx 45, 420–426. ( 10.1017/S0030605311000135) [DOI] [Google Scholar]
- 27.Prakash V, Pain DJ, Cunningham AA, Donald PF, Prakash N, Verma A, Gargi R, Sivakumar S, Rahmani AR. 2003. Catastrophic collapse of Indian white-backed Gyps bengalensis and long-billed Gyps indicus vulture populations. Biol. Conserv. 109, 381–390. ( 10.1016/S0006-3207(02)00164-7) [DOI] [Google Scholar]
- 28.Prakash V. 1999. Status of vultures in Keoladeo National Park, Bharatpur, Rajasthan, with special reference to population crash in Gyps species. J. Bombay Nat. Hist. Soc. 96, 365–378. [Google Scholar]
- 29.Cuthbert R, Green RE, Ranade S, Saravanan S, Pain DJ, Prakash V, Cunningham AA. 2006. Rapid population declines of Egyptian vulture (Neophron percnopterus) and red-headed vulture (Sarcogyps calvus) in India. Anim. Conserv. 9, 349–354. ( 10.1111/j.1469-1795.2006.00041.x) [DOI] [Google Scholar]
- 30.Senacha KR, Taggart MA, Rahmani AR, Jhala YV, Cuthbert R, Pain DJ, Green RE. 2008. Diclofenac levels in livestock carcasses in India before the 2006 ‘ban’. J. Bombay Nat. Hist. Soc. 105, 148–161. [Google Scholar]
- 31.Kalbfleisch JG. 1985. Probability and statistical inference, vol. II New York, NY: Springer. [Google Scholar]
- 32.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 33.Naidoo V, Wolter K, Cromarty D, Diekman M, Duncan N, Meharg AA, Taggart MA, Venter L, Cuthbert R. 2010. Toxicity of non-steroidal anti-inflammatory drugs to Gyps vultures: a new threat from ketoprofen. Biol. Lett. 6, 339–341. ( 10.1098/rsbl.2009.0818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.EMEA. 1995. Committee for Veterinary Medicinal Products: Ketoprofen Summary Report. EMEA/MRL/020/95 See http://www.emea.europa.eu/pdfs/vet/mrls/002095en.pdf.
- 35.Brater DC. 2002. Renal effects of cyclooxygyenase-2-selective inhibitors. J. Pain Symptom Manage. 23, S15–S20. ( 10.1016/S0885-3924(02)00370-6) [DOI] [PubMed] [Google Scholar]
- 36.Engelhard G, Homma D, Schlegel K, Utzmann R, Schnitzler C. 1995. Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new nonsteroidal anti-inflammatory agent with favourable gastrointestinal tolerance. Inflamm. Res. 44, 422–433. [DOI] [PubMed] [Google Scholar]
- 37.Cuthbert RJ, Paudel S, Chaudhary A, Kaphle K, Thapa J, Subedi TR, Taggart MA. 2014. Aligning species conservation with animal welfare: formulation of vulture-safe meloxicam manufactured in South Asia and the reaction of goats to its administration. RSPB Research Report No. 52. Sandy, UK: Royal Society for the Protection of Birds.
- 38.Prakash V, et al. 2012. The population decline of Gyps vultures in India and Nepal has slowed since veterinary use of diclofenac was banned. PLoS ONE 7, e49118 ( 10.1371/journal.pone.0049118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhry MJI, Ogada DL, Malik RN, Virani MV, Giovanni MD. 2012. First evidence that populations of the critically endangered long-billed vulture Gyps indicus in Pakistan have increased following the ban of the toxic veterinary drug diclofenac in South Asia. Bird Conserv. Int. 22, 389–397. ( 10.1017/S0959270912000445) [DOI] [Google Scholar]
- 40.Sharma AK, et al. 2014. Diclofenac is toxic to the steppe eagle (Aquila nipalensis): widening the diversity of raptors threatened by NSAID misuse in South Asia. Bird Conserv. Int. 24, 1–5. ( 10.1017/S0959270913000609) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.