Abstract
Many wildlife species forage on sewage-contaminated food, for example, at wastewater treatment plants and on fields fertilized with sewage sludge. The resultant exposure to human pharmaceuticals remains poorly studied for terrestrial species. On the basis of predicted exposure levels in the wild, we administered the common antidepressant fluoxetine (FLUOX) or control treatment via prey to wild-caught starlings (Sturnus vulgaris) for 22 weeks over winter. To investigate responses to fluoxetine, birds were moved from their group aviaries into individual cages for 2 days. Boldness, exploration and activity levels showed no treatment effects but controls and FLUOX birds habituated differently to isolation in terms of the concentration of corticosterone (CORT) metabolites in faeces. The controls that excreted higher concentrations of CORT metabolites on day 1 lost more body mass by day 2 of isolation than those which excreted lower levels of CORT metabolites. CORT metabolites and mass loss were unrelated in FLUOX birds. When we investigated the movements of birds in their group aviaries, we found the controls made a higher frequency of visits to food trays than FLUOX birds around the important foraging periods of sunrise and sunset, as is optimal for wintering birds. Although individual variability makes interpreting the sub-lethal endpoints measured challenging, our data suggest that fluoxetine at environmentally relevant concentrations can significantly alter behaviour and physiology.
Keywords: fluoxetine, personality traits, corticosterone, Prozac, foraging behaviour
1. Introduction
Worldwide, thousands of different pharmaceuticals are used daily in human and veterinary medicine [1]. Many of these pharmaceuticals are only partially metabolized to inactive compounds and remain in the tissues of carcasses or the waste products excreted to the environment [1] (also see [2]). A significant proportion of the human pharmaceuticals that are excreted enter wastewater treatment plants (WWTPs) [1]. Here, they can be taken up into the invertebrates living on the trickling filters involved in secondary treatment [3]. Thus, there is a potential for animals such as birds [4] and bats [5] that forage on WWTPs to be exposed to pharmaceuticals. Moreover, many of the pharmaceuticals that enter WWTPs are incompletely removed by treatment processes [1,6]. Consequently, effluent discharges to surface waters [7] and the application of sewage sludge [8] to farmland potentially exposes terrestrial and aquatic wildlife beyond those foraging directly on WWTPs [9–11]. This is important because pharmaceuticals in the environment are potentially insidious contaminants, designed to alter physiology and behaviour at low concentrations by interacting with receptors many of which are evolutionarily conserved across vertebrate taxa [12]. To date, studies using environmentally relevant concentrations of pharmaceuticals and non-model animals are rare, particularly for terrestrial species and exposure routes ([13,14]).
Mortality and reproductive failure resulting from exposure to pharmaceuticals in the environment have already been observed in wildlife including birds (e.g. [15,16]). Beyond these severe endpoints, pharmaceuticals may act indirectly on fitness. For example, it has recently been demonstrated in the laboratory that ‘environmentally relevant’ concentrations of the anxiolytic drug, oxazepam, caused changes in activity, feeding rate and social behaviours of wild European perch (Perca fluviatilis) [17]. Wild starling (Sturnus vulgaris) nestlings fed endocrine-disrupting chemicals, including 17α-ethinylestradiol from the contraceptive pill, mimicking exposure via invertebrates from WWTPs, grew more slowly and showed poorer immune function than controls [18]. Interestingly, basal stress hormone levels, an index of chronic stress, were unaffected by the treatment. Changes in behaviour or physiology could be just as important in terms of fitness, and consequently population dynamics, as direct effects on mortality and reproduction ([15,16], also see [19]).
In this study, we first identified pharmaceuticals predicted to pose potential risks to birds that forage on the invertebrates living on WWTPs (see the electronic supplementary material). This prioritization process was based on the characteristics of each pharmaceutical including the human usage rate, degradation rate in sewage, bioaccumulation factor in invertebrates and chemical properties. This process identified fluoxetine (Prozac), a selective serotonin re-uptake inhibitor (SSRI) antidepressant, as a potential risk to birds (electronic supplementary material). Fluoxetine, along with other antidepressants, has been detected in effluent and surface water at concentrations up to the µg l−1 level and in fish tissue at the ng g−1 level [20,21].
Fluoxetine prevents the re-uptake of serotonin by the pre-synaptic cells in the brain, thus increasing the neurotransmission of serotonin [22]. Fluoxetine is commonly used to treat anxiety-related conditions such as depression, obsessive compulsive disorder and bulimia [23]. Moreover, there is growing evidence that SSRI antidepressants not only reduce depression and anxiety, but can also change personality traits, for example, resulting in people becoming less neurotic and more extroverted [24,25]. In animals, personality traits are defined as behavioural differences that are stable within individuals measured repeatedly across a range of situations or contexts [26], and include aggression, activity, exploration and boldness [27,28]. An individual's combination of personality traits essentially determines how it will cope with environmental and social stressors. Thus, exposure to fluoxetine could alter behavioural responses to stimuli and potentially also personality traits in non-target wildlife [15].
Fluoxetine also induces a range of side effects in humans, for example, sexual dysfunction [29], lethargy [23] and weight and appetite changes are all common [30], most probably owing to the connection between the serotonergic system and the neuroendocrine system [31]. If fluoxetine causes some of the changes to behaviour and physiology in free-living animals that are commonly observed in humans, then there could be negative implications for survival chances. In the wild, food resources vary and are inevitably probabilistic, so in a wide range of taxa (from bees, fish, birds to mammals), animals are generally risk averse unless they are at a high risk of starvation [32,33]. Altered activity levels, food intake, mass balance and stress responsiveness are predicted to shift how an individual manages the trade-off between starvation and predation risk. In birds, there is a very finely balanced trade-off between maintaining the fat reserves required to prevent starvation, particularly in cold weather, and retaining the ability to fly away from predators [34–39]. The physiological mechanisms underpinning this trade-off involve corticosterone (CORT), the main glucocorticoid in birds. Levels of CORT are known to affect body mass and behaviour in birds [40,41]. CORT is elevated in response to environmental perturbations to adjust physiology and behaviour (e.g. foraging) appropriately for the prevalent conditions [42]. The release of CORT promotes gluconeogenesis, which can mobilize fat reserves for energetically demanding activities such as escaping predators [42] and responding to thermal conditions. CORT responses to environmental stressors are, therefore, predicted to be affected by exposure to fluoxetine.
Unlike previous studies on fluoxetine in terrestrial species [43], we assessed the effects of exposure to environmentally relevant levels of fluoxetine, administered in prey items over an ecologically relevant period of time, on wildlife using starlings as the test species. Starlings are a good model as they forage on WWTPs throughout the year [44] with up to 50% of their diet coming from trickling filters in the breeding season [45]. To maintain body mass in captivity, starlings must consume at least 60% of their diet on a dry mass basis as invertebrates, this equates to approximately 35 g of larvae per day [46]; higher energetic demands in the wild will inflate this value further [47]. Thus, we assumed that wild starlings would consume 45–50 g of invertebrates per day (wet weight), with 50% coming from trickling filters. The fluoxetine concentration in invertebrates was estimated based on values obtained from the literature [48] and our laboratory. A second reason for selecting starlings is that their behaviours are relatively well characterized [49].
Overall, we aimed to investigate whether exposure to fluoxetine modified behavioural and physiological endpoints. Specifically, we addressed whether experimental exposure to fluoxetine altered: (i) diurnal variations in visits to food trays; (ii) risk-taking behaviours and personality traits (exploration in a novel environment and boldness); (iii) activity levels; (iv) physiological stress responses to isolation; and (v) the relationship between CORT metabolite levels in isolation and change in body mass.
2. Material and methods
We captured 24 wild starlings from a roost site in October 2011 and transported them to outdoor aviaries. Upon arrival, birds were weighed, aged and sexed (adults: five males and five females; first year birds: seven males and seven females). Each bird was colour ringed and the colour ring was fitted with a unique Passive Integrated Transponder tag (PIT tag, less than 0.1 g, Trovan Unique). Twelve birds each were allocated to both the fluoxetine-treated (FLUOX: seven males and five females) and the control group (five males and seven females). Birds from both treatments were placed into each of four outdoor aviaries which were visually but not acoustically isolated. Aviaries contained multiple perches in the roofed and unroofed areas, two food trays, one water hopper and a water bath were available ad libitum. All birds experienced a period of four weeks acclimatization and ‘wash-out’ before pre-treatment ‘baseline’ data were collected. Diurnal variation in foraging was recorded between weeks 8 and 18 of treatment. Behavioural experiments and faecal sampling were repeated at the ‘end’, after 16 weeks of treatment (see the electronic supplementary material for more details of husbandry and experimental set-up). Start and finish date for each aviary was staggered over four weeks.
(a). Experimental treatment
The level of fluoxetine administered to the birds was designed to simulate the exposure that birds would receive from feeding on invertebrates at WWTPs. The dosing calculation, 1.3 µg fluoxetine 5 days per week (0.92 µg d−1), for the fluoxetine-treated birds was calculated from the predicted environmental concentrations of fluoxetine (derived from the mass of fluoxetine prescribed in England in a year, the percentage of active ingredient excreted unchanged, the population of England and the volume of wastewater per capita per year), the bioconcentration factor of fluoxetine from soil and pore water into worms (a value of 133 was used; LJ Carter et al. 2011, unpublished data) and the mass of invertebrates consumed daily by starlings (wet weight; a value of 23.5 g was used [46,50–52]).
Our predicted daily dose for birds was 0.92 µg d−1, which was later confirmed as environmentally relevant based on analysis of worms from four WWTP trickling filters which gave a mean concentration in earthworms (Eisenia fetida) of 26.2 ng g−1 (range 6.9–35.5 ng g−1) which corresponds to a daily dose of 0.62 µg d−1 (range = 0.10–0.83 µg d−1; see the electronic supplementary material for full details of methods).
To administer the treatments to the birds, each day live lesser wax worms (Achroia grisella) were injected with either 1.3 µg fluoxetine solution (Prozit 20 mg/5 ml solution, PineWood Healthcare, Clonmel, Republic of Ireland) dissolved in 2.5 µl carrier medium (deionized water) or 2.5 µl carrier medium (controls). Each day, duplicate treatment and control wax worms were also injected and stored at −20°C. A subset of these additional worms were randomly selected, extracted with methanol and analysed by high performance liquid chromatography as a quality control (mean concentration 1.58 µg worm−1, N = 8, percentage relative standard deviation (%RSD) = 13; see the electronic supplementary material for methods). Each bird was caught in its home aviary and hand-fed one worm per day, 5 days per week (electronic supplementary material). While every care was taken to minimize the stress of capture and handling (the help of an experienced animal technician was used to capture and feed birds), capture and handling are likely to represent stressors to which birds are unlikely to fully habituate [53]. Total capture time was typically less than 20 min and it usually took approximately 10 s to remove a bird from its bird bag, feed it a worm and release it to its home aviary. Both treatment groups experienced the same capture process. Many individuals voluntarily took their treated invertebrate from the forceps.
(b). Diurnal variation in foraging
In order to assess diurnal variation in foraging behaviour of individuals in their home aviary, we used a system of electronic tag readers. Two antennae (8 × 5 cm; Trovan, www.trovan.com) were positioned flat in the two food trays (40 × 20 × 6 cm). The monitoring system was set up to read at 1 s intervals, recording the unique PIT tag code along with a date and time thus enabling us to calculate the total number of feeding visits (a visit was classed as an absence of more than 4 s, based on pilot data) per bird per hour. After 48 h of acclimatization to the recording equipment, visits to feeders were recorded for 48 h. The readers were rotated around the aviaries so that foraging behaviour was recorded twice per aviary for a period of 2 days between 15 February 2012 and 26 April 2012. During this period ambient temperatures ranged from −7°C to 23°C.
(c). Behavioural assays
At the baseline and end, behavioural and physiological responses of individuals to standardized stressors were assayed in isolation over a 2-day period. The test cages (127 × 39 × 36 cm; Kent Cages, Kent, UK) sat within an outdoor aviary so that birds were exposed to natural weather and light conditions but visually occluded from other birds.
Exploratory tendency was assayed over two trials, one each on consecutive days, at baseline and again at the end (adapted from [54]). Each bird moved from its home aviary to one-half of a randomly selected test cage at least 1 h before dusk. By containing the bird within one-half of the cage, a familiar half and a novel half (behind the wooden divider) were created. One-half was lined with white paper and contained two perches wreathed with vines of plastic ‘sycamore’ leaves, whereas the other half had brown paper and plastic ‘ivy’ vines to create two ‘habitats’. In other respects, both halves of the cages were identical. The familiar half of the cage and the ‘habitat’ type were randomly selected prior to the trial. Birds were provided with food ad libitum (usual diet of chick starter crumb, wild bird seed and insectivore mix as well as a few meal worms) and water. The following morning (day 1), food and water were removed (typically between 8.00 and 9.00) an hour before the start of the trial to standardize hunger. All spilt food was removed from the cage bottom, faeces collected and the lining paper replaced (see next section ‘Corticosterone metabolites’).
To start the trial, the wooden divider between the two cage halves was removed and the observer retreated behind a screen with an observation hole (2 × 2 cm). When the bird was perched, a movement was defined as a hop or a flight; when on the ground, any movement of the feet or a flight was defined as a movement with the endpoint of a movement used to define its location (i.e. novel or familiar and ground or perched). When the end of a movement was on the central ridge of the cage (on average 4.8% of total movements at baseline and 7.6% at the end), then the direction in which the bird was facing defined the endpoint of the movement (novel ground or familiar ground). As starlings are ground feeders [55], exploratory tendency was defined as the number of total movements on the ground in the novel half during the 10 min trial as a proportion of the total moves [55]. General activity level was scored as the total number of movements in the 10 min exploration trial. The exploration trial was repeated the next day (‘day 2’) at both baseline and end. For the end trials, after each exploration trial, each bird was captured and immediately given its experimental treatment before returning to the test cage. The bird then had access to the whole cage and was given 1 h to feed and drink undisturbed before the boldness trial.
Forty-five to sixty minutes before the start of the boldness trials, the food (along with any spilt food) and water were removed to induce hunger. To start the trial, the food bowl containing three to four meal worms (Tenebrio molitor) as well as the usual food was returned to the outermost side of the cage. The latency of the first approach to the food bowl (defined as less than one body length) was recorded. Birds that approached before the watch could be started were given a latency of 0.1 s with trials lasting for up to 1800 s. Trials were terminated as soon as the bird had approached for a second time or after 1800 s, whichever came first. The latency to approach rather than to feed was recorded as not all birds fed during the experiment [54], however, in all trials latency to approach and latency to feed were correlated (p < 0.05 in all cases). After the day 1 trials, the birds were switched between cages, the divider was replaced and the arrangements of habitats were changed in preparation for the exploration trial on day 2.
(d). Corticosterone metabolites
We analysed faecal CORT metabolites rather than plasma CORT to avoid additional stress from handling and blood sampling which could have affected the second day's behaviour trials. Also, we wanted to measure how the birds habituated to individual isolation rather than testing their responses to repeated handling stress. At baseline and end trials, each bird was put in an individual test cage on the afternoon of day 0. The following morning (day 1) fresh faeces were removed from the paper lining the cage and placed in a 1.5 ml sealed tube. The lining paper was again replaced prior to dusk on day 1 and the faeces collected the following morning. Faeces were placed into a freezer at −20°C while behavioural measures were completed before being weighed to ±0.001 g later the same day and dried at 40°C until there was no change in mass. Dried faecal samples were stored at −20°C until analysis in September 2012. Analysis took place on consecutive days using the CORT OCTEIA ELISA kit (ID Labs, Boldon, UK). This ELISA has been validated for a range of species (sensitivity 0.55 ng ml−1, mean recovery using faeces = 93%, mean linearity = 100%, intra assay mean = 4.95% CV, inter assay mean = 7.9% CV).
(e). Change in body mass
Body mass was recorded in the late afternoon on day 0 and again at a similar time on day 2 just before birds returned to their home aviaries.
(f). Statistical methods
All data were analysed using R v. 3.0.2 [56]. To assess diurnal variation in feeder visits, we used a zero inflated repeated measures model with a Poisson error structure in R-package glmmADMB. It was important to control for zeroes in the model as they were generated in different ways (e.g. a bird was not feeding or a bird was not in range of the antenna when it fed). For each day's data, we took the first and last 4 h of recordings and took the number of visits per bird per hour as the response variable. We corrected time relative to sunrise and sunset, respectively. Initially, we looked for the linear and the quadratic relationship, however, only the quadratic relationship (indicative of the expected bimodal peaks in feeding effort around sunrise and sunset) was significant and so we dropped the linear relationship from the models. Treatment and day effects were tested using generalized estimation equations (GEEs) which were fitted with R package geepack [57] to account for data from the same individual being repeated (i.e. days 1 and 2). Repeatability between days was quantified using the effect of day in the GEE. The boldness and natural log-transformed activity data were normally distributed and exploratory tendency (number of ground movements in novel half out of total number of movements) showed a binomial distribution. In each case, the difference between the treatment and control groups was tested using the Wald statistic produced by the GEE, by comparing it to a χ2-distribution with 1 d.f. When analysing the changes in body mass in response to CORT, we used a linear model. In all the analyses below, factors were tested against the 5% significance level.
3. Results
(a). Diurnal variation in foraging behaviour
Within their home aviaries, control birds visited the food trays more than the FLUOX birds (Z =−2.20, N = 768, p = 0.028). Time after sunrise and before sunset was also significant in the model (Z =−2.45, N = 768, p = 0.014). Variation in feeding rate was described by quadratic relationships with time which differed between treatment groups (Z = 2.53, N = 768, p = 0.011): controls increased their frequency of visits to food trays in the 3 h after sunrise (figure 1a) compared with FLUOX birds. There was a less pronounced peak in food tray visits just before sunset in controls but not FLUOX birds (figure 1b).
Figure 1.
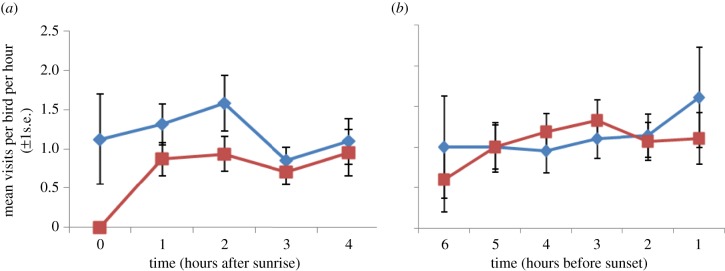
Mean (±1 s.e.) feeder visits per hour per bird for the first and last 4 h of data against time for control (blue diamonds) and fluoxetine-treated birds (red squares). Time is expressed relative to sunrise (a) and sunset (b) and so the 4 h period for which observations were taken depended upon the time when birds first fed in the morning and last fed at night. (Online version in colour.)
(b). Behavioural assays
For both baseline (electronic supplementary material, table S1) and end trials (table 1), exploratory tendency, activity and boldness were repeatable between days 1 and 2. Exploratory tendency (table 1 and figure 2a for end, and electronic supplementary material, table S1, for baseline), activity levels (table 1 and figure 2b, and electronic supplementary material, table S1) and boldness (table 1 and figure 2c, and electronic supplementary material, table S1) did not vary with treatment, day (1 or 2) or in the way that the birds of the different treatment groups habituated from day 1 to day 2, as shown by the non-significant interactions for treatment and day in table 1 (end) and the electronic supplementary material, table S1 (baseline).
Table 1.
Effects of treatment and day on behaviour, CORT and body mass measured after 16 weeks of treatment (end). Wald statistics from repeated measures GEE compared to a χ2-distribution with 1 d.f. The χ2-value along with p > χ are reported for each of the explanatory variables.
| endpoint | treatment |
day |
interaction |
|||
|---|---|---|---|---|---|---|
| χ2 | p-value | χ2 | p-value | χ2 | p-value | |
| exploration | 0.60 | 0.44 | 1.93 | 0.17 | 1.83 | 0.18 |
| activity | 2.48 | 0.12 | 0.30 | 0.58 | 0 | 1.00 |
| boldness | 0.26 | 0.61 | 0.020 | 0.89 | 0.033 | 0.86 |
| corticosterone metabolites | 0.016 | 0.90 | 4.47 | 0.035 | 1.38 | 0.24 |
| body mass | 3.02 | 0.082 | 7.11 | <0.01 | 0.026 | 0.87 |
Figure 2.
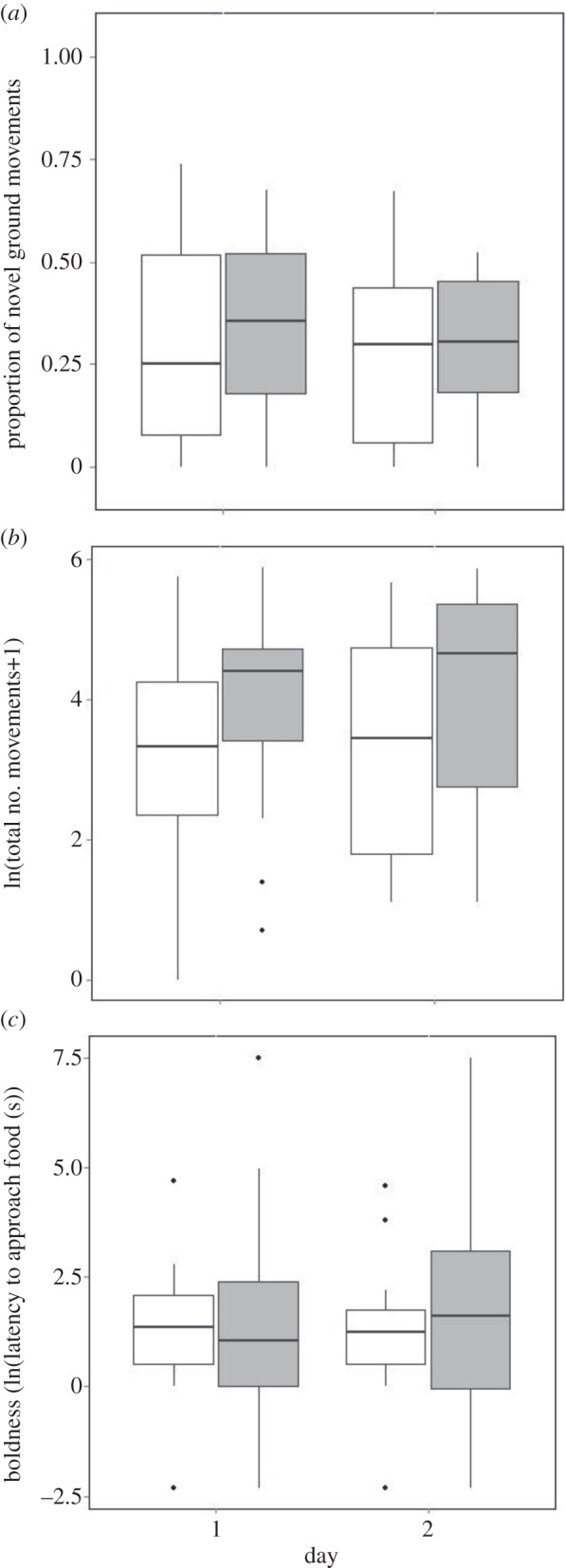
Median behaviour at the end trials (days 1 and 2) for control (white) and FLUOX birds (grey): (a) individual exploration (proportion of total movements that were novel ground movements), (b) individual activity (ln(total number of movements + 1)) and (c) boldness (ln(latency to approach food (s)). Boxes represent the upper and lower quartiles and points beyond the ends of the whiskers represent outliers as defined by Tukey [58].
(c). Corticosterone metabolites
The mean faecal CORT metabolite concentration was obtained from two replicates, for each sample the variability between replicates was significantly repeatable (single factor ANOVA) given the level of variation between birds (baseline day 1: F23,24 = 33.95, r = 0.94, p < 0.001; baseline day 2: F23,24 = 34.51, r = 0.94, p < 0.001; end day 1: F23,24 = 33.06, r = 0.94, p < 0.001 and end day 2: F23,24 = 7.78, r = 0.76, p < 0.001).
The level of faecal CORT metabolites in faeces did not differ significantly between treatments or change from day 1 to day 2 differently between the two treatments at baseline (electronic supplementary material, table S1). In the end trials, CORT metabolites were significantly lower for all birds on day 2 compared with day 1 (figure 3a and table 1). The variances in mean CORT metabolites for FLUOX birds were the same on days 1 and 2 (F1,22 = 0.979, p = 0.33), but they were significantly higher on day 1 than on day 2 for controls (F1,22 = 22.3, p < 0.001).
Figure 3.
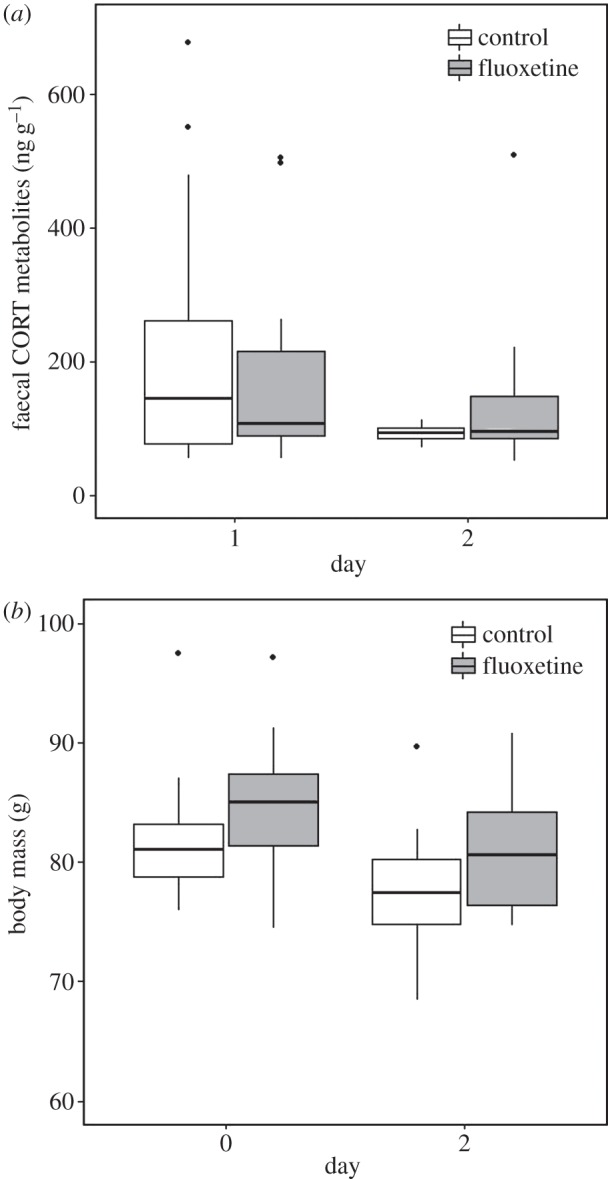
(a) Median faecal CORT metabolite concentration (ng/g of dry faeces) on days 1 and 2 for the end trials and (b) median body mass (g) on day 0 (the afternoon before the day 1 trials) and day 2 for end trials for control birds (white) and FLUOX birds (grey). Boxes represent the upper and lower quartiles and points beyond the ends of the whiskers represent outliers as defined by Tukey [58].
(d). Corticosterone metabolites and body mass change
In isolation, the birds in both treatment groups lost weight during the 2 days that they were held in individual test cages for the behaviour trials at baseline and end. There was no treatment effect on weight loss at baseline or end (table 1 and figure 3b, and electronic supplementary material, table S1). At baseline, there was no relationship between faecal CORT metabolites on the morning of day 1 (F = 1.35, p = 0.26), treatment (F = 0.17, p = 0.69) or their interaction (F = 1.37, p = 0.26) and body mass change over the 2 days in isolation. At the end, there was a significant interaction between CORT metabolites on day 1 and treatment (F = 5.67, p = 0.027): control birds that exhibited high CORT metabolites on the morning of day 1 lost more mass over the 2 days in individual cages than those that showed a lower physiological stress response at the start. In comparison, in FLUOX birds there was no relationship between CORT metabolites and mass change.
4. Discussion
Our fluoxetine treatment can be considered environmentally relevant based on our estimated and measured concentrations of fluoxetine in worms [18,45–47]. It is intriguing that there was no effect of fluoxetine on boldness, exploratory tendency or activity levels in individual isolation. Whether this was due to the environmentally relevant, but low, dose administered; the subtle, non-standard, endpoints investigated; or the small sample size, from a statistical but not an ecotoxicology perspective, is difficult to interpret. Even though our environmentally relevant exposure for birds corresponds to only 2.2–6.5% of the human therapeutic dose (when corrected for body mass differences) we found evidence that fluoxetine could potentially affect mass balance in starlings through both behavioural and physiological mechanisms. If starlings eliminate fluoxetine as slowly as humans [59], then it is possible that accumulation of fluoxetine and down regulation of the post-synaptic serotonin receptors in the brain could have occurred.
In terms of behavioural effects, we found the diurnal patterns in food tray visits of FLUOX-treated individuals did not show the normal peaks at times of highest nutritional need, that is immediately before and after the overnight fast [60]. Moreover, FLUOX birds overall made less visits to food trays than controls in the relatively low stress surroundings of their home aviaries within their familiar flock.
From a physiological perspective, we found no relationship between the levels of CORT metabolites in faeces and body mass loss during a stressful situation for FLUOX birds but there was the expected relationship with high CORT metabolites causing greater body mass loss in controls. However, the relationships between faecal CORT metabolites and circulating CORT [61] are notoriously difficult to interpret. Further experiments on the effects of fluoxetine on mass balance and circulating concentrations of basal and acute CORT would allow us to establish the proximate mechanisms and ultimate impacts of fluoxetine-induced changes in foraging and stress responsiveness.
To place these findings in context, maintenance of body mass plays a vital role in birds and they can change their body mass considerably in a short space of time [62]. Over the course of 24 h, birds must regulate their body mass precisely to ensure that they lay down enough fat reserves to provide sufficient insulation and energy to survive periods without food while not gaining so much mass that it inhibits their ability to avoid predators [62]. Under predation pressure, optimal foraging models suggest that birds' daily foraging patterns should exhibit bimodal peaks around the hours of sunrise, to stave off starvation risk and to build up energy reserves, and sunset, to build up fat reserves [63]. Controls showed the expected bimodal peaks in feeding around sunrise and sunset but for FLUOX birds the peaks occurred later in the morning and early in the afternoon. Additionally, the control birds had a higher overall feeding effort than FLUOX birds. Fluoxetine has been shown to alter appetite in humans [64], and it is believed that the serotonergic nerve terminals play a role in regulating feeding behaviour [65] with serotonin decreasing food intake and increasing energy expenditure [66]. It is possible that we have found evidence that fluoxetine, as in humans, caused individuals to be less sensitive to physiological signals stimulating foraging behaviour as displayed by their lower feeding effort and delayed foraging peaks. This has clear implications for mass balance and potentially for survival.
When individuals were moved into test cages for 2 days, as expected all birds showed relatively high levels of CORT metabolite in the faecal samples collected from their first night in isolation. Stress hormone metabolites in samples collected from the birds' second night in isolation were significantly lower than in the first sample. The evidence for the effects of fluoxetine on glucocorticoids in other species is inconsistent. For example, Debellis et al. [67] found evidence that fluoxetine decreased levels of corticotropin releasing hormone in humans, a precursor hormone to the release of cortisol. In fish, [68] acute activation of serotonin receptors reduced levels of glucocorticoid precursor hormones while chronic activation of the serotonin receptors elevated production of the glucocorticoid precursor hormones in a non-stressful situation. Thus, the effects of fluoxetine on CORT are likely to be complex and probably context dependent. Analysing CORT in blood samples might help differentiate between effects of fluoxetine on basal- and stress-induced CORT concentrations. Metabolites contained in faeces can be used as a non-invasive measure of CORT [69] which provides an integrated measure of CORT levels over a period of several hours which not only has a number of benefits over blood sampling but also has several limitations such as the effect of sex, diet and digestive efficiency [61].
Interestingly, control birds that had higher CORT levels on day 1 lost more body mass over 2 days than those with lower levels of CORT metabolites, as expected from other studies of CORT metabolites. For example, work by Dickens et al. [70] on chukars (Alectoris chukar) showed that on the first day of captivity, both baseline and stress-induced CORT concentrations were raised and birds lost weight. However, for FLUOX-treated starlings there was no relationship between CORT metabolites and mass change. CORT is intrinsically linked to energetic status through its role in glucose regulation [71] and has been linked to environmental conditions [34,35], diet [38,39] and body condition in birds [72,73]. Therefore, it is plausible that disruption to CORT production could also have influenced the way individuals responded to environmental stimuli associated with diurnal variation in starvation probability [71]. In order to establish whether the feedback pathways linking CORT and body mass have potentially been disrupted, we would need to repeat these experiments and collect plasma samples before and after isolation [74]. This was not done in this experiment as blood sampling in the middle of 2 days of behaviour trials would have been a significant stressor.
Exploratory tendency and boldness, but not activity, were repeatable across trials in both treatment groups, indicating that they were personality traits in our starlings that reflect stable individual differences in how individuals responded to environmental stressors. However, we found no evidence of a treatment effect on these behaviours. Previous studies have found contradictory evidence for the effects of fluoxetine on activity but have focused on aquatic species (Brodin et al. [19]). A reduction in activity was observed in mosquito fish (Gambusia affinis) by Henry & Black [75] but at much higher concentrations than De Lange et al. [76], who found no effect at environmental concentrations [77,78]. These studies support our findings which suggest that pharmaceuticals designed to treat anxiety-related conditions may not have the expected effects in wild animals based on therapeutic or side effects in humans.
Although effects on boldness, exploration and activity may not have been observed due to the low dose, the aim of this study was to be environmentally relevant. The null results could have been produced for a variety of reasons other than the low dose. These reasons include the small sample size, individual variation, simply looking at the wrong endpoints or the stress of the daily capture. One of the more challenging aspects of working with terrestrial species is administering the dose via a vehicle that closely mimics uptake in the environment without inducing capture and handling stress that outweighs the effects of the experimental treatment. It is known that birds are unlikely to habituate to repeated capture and handling stress and that the resultant repeated short-lived peaks in CORT can have lasting effects on physiology [53]. Therefore, it was important that the control group experienced the same stress as the FLUOX group. There is a realistic possibility that we were only measuring the effect of fluoxetine in stressed birds. However, the presence of significant variation in CORT levels (regardless of the treatment group) suggests that there was still variation in stress level. However, factors such as differences in digestive tract efficiency make differences in faecal CORT metabolites difficult to interpret.
5. Conclusion
This is one of few studies to have investigated the effects of pharmaceuticals in either wild or terrestrial vertebrates. To place our findings in a wider context, many terrestrial species forage on food sources contaminated with human sewage and, consequently, will uptake a range of pharmaceuticals [21,65]. However, the proportion of diet that is made up from prey items obtained from trickling filters is poorly defined and difficult to obtain. We have administered starlings with a predicted environmentally relevant dose. This dose was calculated based on values obtained from the literature and our laboratory and was within the same order of magnitude as the mean concentration found in Eisenia fetida in the environment, making our findings that fluoxetine may alter diurnal variation in food tray visits and disrupt the relationship between CORT metabolites and mass balance worthy of further investigation. Importantly, fluoxetine is not the only pharmaceutical, or indeed the only antidepressant, to be detected in the environment [20,21]; through additive or synergistic interactions, mixtures of pharmaceuticals could potentially be more potent than single compounds increasing the likelihood of adverse effects. Here we have demonstrated the potential for an antidepressant to alter behaviour and physiology in birds at environmentally relevant concentrations. We suggest that more research is required in both the field and the laboratory to determine the extent to which pharmaceuticals bioaccumulate in prey items, their uptake by wildlife via food and their potential to impact upon fitness-related traits.
Supplementary Material
Acknowledgements
We would like to thank the Wildlife Ecotoxicology team and the ASIST team at the Food and Environment Research Agency (FERA), M. Brash, M. Gomm, F. Bellamy, G. Bryning, G. Stubbings, L. Carter, S. Mason, E. Bergstrom, J. Thomas-Oates and R. Luck for their help and support. We would also like to thank Severn Trent Water who kindly provided access to wastewater treatment plants.
Ethics statement
All work was carried out under Home Office Licence (PPL 60/4213) and was approved by the ethics committees of FERA and the University of York. Birds were captured under licences from the British Trust for Ornithology and Natural England.
Funding statement
T.B. was funded by a PhD studentship from the Natural Environment Research Council and K.A. by a Royal Society University Research Fellowship.
References
- 1.Monteiro SC, Boxall ABA. 2010. Occurrence and fate of human pharmaceuticals in the environment. Rev. Environ. Contam. Toxicol. 202, 53–154. ( 10.1007/978-1-4419-1157-5_2) [DOI] [PubMed] [Google Scholar]
- 2.Cuthbert RJ, et al. 2014. Avian scavengers and the threat from veterinary pharmaceuticals. Phil. Trans. R. Soc. B 369, 20130574 ( 10.1098/rstb.2013.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markman S, Guschina IA, Barnsley S, Buchanan KL, Pascoe D, Muller CT. 2007. Endocrine disrupting chemicals accumulate in earthworms exposed to sewage effluent. Chemosphere 70, 119–125. ( 10.1016/j.chemosphere.2007.06.045) [DOI] [PubMed] [Google Scholar]
- 4.Fuller RJ, Glue DE. 1980. Sewage works as bird habitats in Britain. Biol. Conserv. 17, 165–181. ( 10.1016/0006-3207(80)90054-3) [DOI] [Google Scholar]
- 5.Park KJ, Cristinacce A. 2006. Use of sewage treatment works as foraging sites by insectivorous bats. Anim. Conserv. 9, 259–268. ( 10.1111/j.1469-1795.2006.00031.x) [DOI] [Google Scholar]
- 6.Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. 2010. Erratum to ‘The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters’ (vol. 43, p. 363, 2009). Water Res. 44, 2076 ( 10.1016/j.watres.2009.06.026) [DOI] [PubMed] [Google Scholar]
- 7.Ternes TA. 1998. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 32, 3245–3260. ( 10.1016/S0043-1354(98)00099-2) [DOI] [Google Scholar]
- 8.McClellan K, Halden RU. 2010. Pharmaceuticals and personal care products in archived US biosolids from the 2001 EPA national sewage sludge survey. Water Res. 44, 658–668. ( 10.1016/j.watres.2009.12.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topp E, et al. 2008. Runoff of pharmaceuticals and personal care products following application of biosolids to an agricultural field. Sci. Total Environ. 396, 52–59. ( 10.1016/j.scitotenv.2008.02.011) [DOI] [PubMed] [Google Scholar]
- 10.Fick J, Lindberg RH, Tysklind M, Joakim-Larsson DG. 2010. Predicted critical environmental concentrations for 500 pharmaceuticals. Regul. Toxicol. Pharmacol. 58, 516–523. ( 10.1016/j.yrtph.2010.08.025) [DOI] [PubMed] [Google Scholar]
- 11.Rhind SM. 2005. Are endocrine disrupting compounds a threat to farm animal health, welfare and productivity? Reprod. Domest. Anim. 40, 282–290. ( 10.1111/j.1439-0531.2005.00594.x) [DOI] [PubMed] [Google Scholar]
- 12.Gunnarson L, Jauhainen A, Kristiansson E, Nerman O, Larsson DGJ. 2008. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 42, 5807–5813. ( 10.1021/es8005173) [DOI] [PubMed] [Google Scholar]
- 13.Boxall A, et al. 2012. Pharmaceuticals and personal care products in the environment: what are the big questions? Environ. Health Perspect. 120, 1221–1229. ( 10.1289/ehp.1104477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shore RF, Taggart MA, Smits J, Mateo R, Richards NL, Fryday S. 2014. Detection and drivers of exposure and effects of pharmaceuticals in higher vertebrates. Phil. Trans. R. Soc. B 369, 20130570 ( 10.1098/rstb.2013.0570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oaks JL, et al. 2004. Diclofenac residues as the cause of vulture population decline in Pakistan. Lett. Nat. 427, 630–633. ( 10.1038/nature02317) [DOI] [PubMed] [Google Scholar]
- 16.Gross-Sorokin MY, Roast SD, Brighty GC. 2006. Assessment of feminization of male fish in English rivers by the environment agency of England and Wales. Environ. Health Perspect. 114, 147–151. ( 10.1289/ehp.8068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodin T, Fick J, Jonsson M, Klaminder J. 2013. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 339, 814–815. ( 10.1126/science.1226850) [DOI] [PubMed] [Google Scholar]
- 18.Markman S, Muller CT, Pascoe D, Dawson A, Buchanan KL. 2011. Pollutants affect development in nestling starlings Sturnus vulgaris. J. Appl. Ecol. 48, 391–397. ( 10.1111/j.1365-2664.2010.01931.x) [DOI] [Google Scholar]
- 19.Brodin T, Piovano S, Fick J, Klaminder J, Heynen M, Jonsson M. 2014. Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations. Phil. Trans. R. Soc. B 369, 20130580 ( 10.1098/rstb.2013.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calisto V, Esteves V. 2009. Psychiatric pharmaceuticals in the environment. Chemosphere 77 1257–1274. ( 10.1016/j.chemosphere.2009.09.021) [DOI] [PubMed] [Google Scholar]
- 21.Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ. 2005. Determination of select antidepressants in fish from an effluent-dominated stream. Environ. Toxicol. Chem. 24, 464–469. ( 10.1897/04-081R.1) [DOI] [PubMed] [Google Scholar]
- 22.Lesch K, Hoh A, Schulte H, Osterheider M, Muller T. 1991. Long-term fluoxetine treatment decreases 5-HT1a receptor responsivity in obsessive-compulsive disorder. Psychopharmacology 105, 415–420. ( 10.1007/BF02244438) [DOI] [PubMed] [Google Scholar]
- 23.Lilly E. 2009. Highlights of prescribing information: Prozac. Available at http://pi.lilly.com/us/prozac.pdf. [Google Scholar]
- 24.Tang TZ, DeRubeis RJ, Hollon SD, Amsterdam J, Shelton R, Schalet B. 2009. Personality change during depression treatment: a placebo-controlled trial. Arch. Gen. Psychiatry 66, 1322–1330. ( 10.1001/archgenpsychiatry.2009.166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du L, Bakish D, Ravindran A, Hrdina P. 2002. Does fluoxetine influence major depression by modifying five-factor personality traits? J. Affect. Disord. 71, 235–241. ( 10.1016/S0165-0327(01)00370-6) [DOI] [PubMed] [Google Scholar]
- 26.Gosling SD. 2001. From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86. ( 10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- 27.Sih A, Bell AM, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative review. Q. Rev. Biol. 79, 241–277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 28.Bell AM, Sih A. 2007. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834. ( 10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- 29.Clayton AH. 2002. Female sexual dysfunction related to depression and antidepressant medications. Curr. Women. Health Rep. 2, 182–187. [PubMed] [Google Scholar]
- 30.Fichtner C, Braun B. 1994. Hyperphagia and weight-loss during fluoxetine treatment. Ann. Pharmacother. 28, 1350–1352. [DOI] [PubMed] [Google Scholar]
- 31.Raap D, Van de Kar L. 1999. Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci. 65, 1217–1235. ( 10.1016/S0024-3205(99)00169-1) [DOI] [PubMed] [Google Scholar]
- 32.Caraco T, Martindale S, Whittam TS. 1980. An empirical demonstration of risk-sensitive foraging preferences. Anim. Behav. 28, 820–830. ( 10.1016/s0003-3472(80)80142-4) [DOI] [Google Scholar]
- 33.Kacelnik A, Bateson M. 1996. Risky theories—the effects of variance on foraging decisions. Am. Zool. 36, 402–434. ( 10.1093/icb/36.4.402) [DOI] [Google Scholar]
- 34.Kitaysky A, Piatt J, Wingfield J. 2007. Stress hormones link food availability and population processes in seabirds. Mar. Ecol. Prog. Ser. 352, 245–258. ( 10.3354/meps07074) [DOI] [Google Scholar]
- 35.Muller C, Jenni-Eiermann S, Blondel J, Perret P, Caro S, Lambrechts M, Jenni L. 2007. Circulating corticosterone levels in breeding blue tits Parus caeruleus differ between island and mainland populations and between habitats. Gen. Comp. Endocrinol. 154, 128–136. ( 10.1016/j.ygcen.2007.05.031) [DOI] [PubMed] [Google Scholar]
- 36.Romero LM, Reed JM, Wingfield JC. 2000. Effects of weather on corticosterone responses in wild free-living passerine birds. Gen. Comp. Endocrinol. 118, 113–122. ( 10.1006/gcen.1999.7446) [DOI] [PubMed] [Google Scholar]
- 37.Wingfield JC, Moore MC, Farner DS. 1983. Endocrine responses to inclement weather in naturally breeding populations of white-crowned sparrows (Zonotrichia leucophrys pugetensis). Auk 100, 56–62. [Google Scholar]
- 38.Kitaysky AS, Kitaiskaia EV, Piatt JF, Wingfield JC. 2006. A mechanistic link between chick diet and decline in seabirds? Proc. R. Soc. B 273, 445–450. ( 10.1098/rspb.2005.3351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitaysky AS, Kitaiskaia EV, Wingfield JC, Piatt JF. 2001. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J. Comp. Physiol. B 171, 701–709. ( 10.1007/s003600100230) [DOI] [PubMed] [Google Scholar]
- 40.Roberts ML, Buchanan KL, Hasselquist D, Bennett ATD, Evans MR. 2007. Physiological, morphological and behavioural effects of selecting zebra finches for divergent levels of corticosterone. J. Exp. Biol. 210, 4368–4378. ( 10.1242/jeb.007104) [DOI] [PubMed] [Google Scholar]
- 41.Roberts ML, Buchanan KL, Hasselquist D, Evans MR. 2007. Effects of testosterone and corticosterone on immunocompetence in the zebra finch. Horm. Behav. 51, 126–134. ( 10.1016/j.yhbeh.2006.09.004) [DOI] [PubMed] [Google Scholar]
- 42.Wingfield JC, Romero LM. 2001. Adrenocortical responses to stress and their modulation in free-living vertebrates. In Handbook of physiology, vol. 4 (eds McEwen BS, Goodman HM.), pp. 211–234. New York, NY: Oxford University Press. [Google Scholar]
- 43.Sperry TS, Thompson CK, Wingfield JC. 2003. Effects of acute treatment with 8-OH-DPAT and fluoxetine on aggressive behaviour in male song sparrows (Melospiza melodia morphna). J. Neuroendocrinol. 15, 150–160. ( 10.1046/j.1365-2826.2003.00968.x) [DOI] [PubMed] [Google Scholar]
- 44.Fuller RJ, Glue DE. 1978. Seasonal activity of birds at a sewage-works. Br. Birds 71, 235–244. [Google Scholar]
- 45.Markman S, Leitner S, Catchpole C, Barnsley S, Müller CT, Pascoe D, Buchanan KL. 2008. Pollutants increase song complexity and the volume of the brain area HVC in a songbird. PLoS ONE 3, e1674 ( 10.1371/journal.pone.0001674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feare CJ, McGinnity N. 1986. The relative importance of invertebrates and barley in the diet of starlings Sturnus vulgaris. Bird Study 33, 164–167. ( 10.1080/00063658609476915) [DOI] [Google Scholar]
- 47.Tinbergen JM. 1981. Foraging decisions in starlings (Sturnus vulgaris). Ardea 69, 1–67. [Google Scholar]
- 48.National Health Service. 2010. Prescription Cost Analysis—England 2009 Prescription: items dispensed in the community in England and listed alphabetically within chemical entity by therapeutic class http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescriptions/prescription-cost-analysis-england--2009 (accessed 14 May 2011).
- 49.Minderman J, Reid JM, Evans PGH, Whittingham MJ. 2009. Personality traits in wild starlings: exploration behavior and environmental sensitivity. Behav. Ecol. 20, 830–837. ( 10.1093/beheco/arp067) [DOI] [Google Scholar]
- 50.Feare CJ. 1984. The starling. Oxford, UK: Oxford University Press. [Google Scholar]
- 51.National Health Service. 2013. Prescription Cost Analysis—England 2012: health and social care information centre http://www.hscic.gov.uk/catalogue/PUB13887/pres-cost-anal-eng-2013-rep.pdf (accessed 12 October 2103). [Google Scholar]
- 52.Williams RT. 2005. Human health pharmaceuticals in the environment—an introduction. In Human pharmaceuticals: assessing the impacts an aquatic ecosystems (ed. Williams RT.), pp. 1–45. Pensacola, FL: Setac Press. [Google Scholar]
- 53.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 20133151 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herborn KA, Macleod R, Miles WTS, Schofield ANB, Alexander L, Arnold KE. 2010. Personality in captivity reflects personality in the wild. Anim. Behav. 79, 835–843. ( 10.1016/j.anbehav.2009.12.026) [DOI] [Google Scholar]
- 55.Minderman J, Reid JM, Hughes M, Denny MJH, Hogg S, Evans PGH, Whittingham MJ. 2010. Novel environment exploration and home range size in starlings Sturnus vulgaris. Behav. Ecol. 21, 1321–1329. ( 10.1093/beheco/arq151) [DOI] [Google Scholar]
- 56.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing. [Google Scholar]
- 57.Højsgaard S, Halekoh U, Yan J. 2006. The R package geepack for generalized estimating equations. J. Stat. Softw. 15, 1–11. [Google Scholar]
- 58.Tukey JW. 1977. Exploratory data analysis, p. 688 Reading, MA: Addison-Wesley. [Google Scholar]
- 59.McEvoy GK. 2003. American hospital formulary service—drug information, p. 2214 Bethesda, MD: American Society of Health-System Pharmacists, Inc. [Google Scholar]
- 60.Macleod R, Gosler A, Cresswell W. 2005. Diurnal mass gain strategies and perceived predation risk in the great tit Parus major. J. Anim. Ecol. 74, 956–964. ( 10.1111/j.1365-2656.2005.00993.x) [DOI] [Google Scholar]
- 61.Goymann W. 2005. Noninvasive monitoring of hormones in bird droppings—physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann. NY Acad. Sci. 1046, 35–53. ( 10.1196/annals.1343.005) [DOI] [PubMed] [Google Scholar]
- 62.Krebs JR, Davies NB. 1993. An introduction to behavioural ecology, 3rd edn, p. 420 Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 63.McNamara J, Houston A, Lima S. 1994. Foraging routines of small birds in winter—a theoretical investigation. J. Avian Biol. 25, 287–302. ( 10.2307/3677276) [DOI] [Google Scholar]
- 64.Visser M, Seidell J, Koppeschaar H, Smits P. 1993. The effect of fluoxetine on body-weight, body-composition and visceral fat accumulation. Int. J. Obes. 17, 247–253. [PubMed] [Google Scholar]
- 65.Broberger C, Hokfelt T. 2001. Hypothalamic and vagal neuropeptide circuitries regulating food intake. Physiol. Behav. 74, 669–682. ( 10.1016/S0031-9384(01)00611-4) [DOI] [PubMed] [Google Scholar]
- 66.Guimaraes R, Telles M, Coelho V, Mori R, Nascimento C, Ribeiro E. 2002. Adrenalectomy abolishes the food-induced hypothalamic serotonin release in both normal and monosodium glutamate-obese rats. Brain Res. Bull. 58, 363–369. ( 10.1016/S0361-9230(02)00799-2) [DOI] [PubMed] [Google Scholar]
- 67.Debellis MD, Gold PW, Geracioti TD, Listwak SJ, Kling MA. 1993. Association of fluoxetine treatment with reductions in concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am. J. Psychiatry 150, 656–657. [DOI] [PubMed] [Google Scholar]
- 68.Hoglund E, Balm P, Winberg S. 2002. Stimulatory and inhibitory effects of 5-HT1A receptors on adrenocorticotropic hormone and cortisol secretion in a teleost fish, the Arctic charr (Salvelinus alpinus). Neurosci. Lett. 324, 193–196. ( 10.1016/S0304-3940(02)00200-8) [DOI] [PubMed] [Google Scholar]
- 69.Millspaugh J, Washburn B. 2004. Use of fecal glucocorticold metabolite measures in conservation biology research: considerations for application and interpretation. Gen. Comp. Endocrinol. 138, 189–199. ( 10.1016/j.ygcen.2004.07.002) [DOI] [PubMed] [Google Scholar]
- 70.Dickens M, Delehanty D, Romero L. 2009. Stress and translocation: alterations in the stress physiology of translocated birds. Proc. R. Soc. B 276, 2051–2056. ( 10.1098/rspb.2008.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 72.Pike T, Petrie M. 2005. Maternal body condition and plasma hormones affect offspring sex ratio in peafowl. Anim. Behav. 70, 745–751. ( 10.1016/j.anbehav.2004.12.020) [DOI] [Google Scholar]
- 73.Love OP, Chin EH, Wynne-Edwards KE, Williams TD. 2005. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 166, 751–766. ( 10.1086/497440) [DOI] [PubMed] [Google Scholar]
- 74.Angelier F, Shaffer S, Weimerskirch H, Trouve C, Chastel O. 2007. Corticosterone and foraging behavior in a pelagic seabird. Physiol. Biochem. Zool. 80, 283–292. ( 10.1086/512585) [DOI] [PubMed] [Google Scholar]
- 75.Henry T, Black M. 2008. Acute and chronic toxicity of fluoxetine (selective serotonin reuptake inhibitor) in western mosquitofish. Arch. Environ. Contam. Toxicol. 54, 325–330. ( 10.1007/s00244-007-9018-0) [DOI] [PubMed] [Google Scholar]
- 76.De Lange H, Noordoven W, Murk A, Lurling M, Peeters E. 2006. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 78, 209–216. ( 10.1016/j.aquatox.2006.03.002) [DOI] [PubMed] [Google Scholar]
- 77.Mennigen JA, Sassine J, Trudeau VL, Moon TW. 2010. Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish Carassius auratus. Aquat. Toxicol. 100, 128–137. ( 10.1016/j.aquatox.2010.07.022) [DOI] [PubMed] [Google Scholar]
- 78.Brooks BW, et al. 2003. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett. 142, 169–183. ( 10.1016/S0378-4274(03)00066-3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


