Abstract
Fish represent the planet's most diverse group of vertebrates and they can be exposed to a wide range of pharmaceuticals. For practical reasons, extrapolation of pharmaceutical effects from ‘model’ species to other fish species is adopted in risk assessment. Here, we critically assess this approach. First, we show that between 65% and 86% of human drug targets are evolutionarily conserved in 12 diverse fish species. Focusing on nuclear steroid hormone receptors, we further show that the sequence of the ligand binding domain that plays a key role in drug potency is highly conserved, but there is variation between species. This variation for the oestrogen receptor, however, does not obviously account for observed differences in receptor activation. Taking the synthetic oestrogen ethinyloestradiol as a test case, and using life-table-response experiments, we demonstrate significant reductions in population growth in fathead minnow and medaka, but not zebrafish, for environmentally relevant exposures. This finding contrasts with zebrafish being ranked as more ecologically susceptible, according to two independent life-history analyses. We conclude that while most drug targets are conserved in fish, evolutionary divergence in drug-target activation, physiology, behaviour and ecological life history make it difficult to predict population-level effects. This justifies the conventional use of at least a 10× assessment factor in pharmaceutical risk assessment, to account for differences in species susceptibility.
Keywords: drug target, orthologue, physiology, population ecology, species, susceptibility
1. Introduction
(a). Environmental risks associated with pharmaceuticals
Over 5000 human and veterinary pharmaceuticals are in use, or in development, and they target diverse physiological functions [1]. Many are highly potent, altering physiological processes at low therapeutic concentrations, between 0.05 and 100 μg ml−1 in blood plasma in human/mammalian systems [2]. Furthermore, many drug targets are highly conserved across diverse vertebrate phyla [3–6]. Following the widespread detection of pharmaceuticals in the environment [7–9], concern has been raised over their potential impact on vertebrate wildlife health. The most notable example of an adverse effect in wildlife is for exposure to the non-steroidal anti-inflammatory drug diclofenac. This has been shown to cause population-level declines, and even localized extinctions, in three Asian vulture species (Gyps sp.) scavenging on the carcasses of treated cattle [10,11]. In another case, the contraceptive oestrogen 17α-ethinyloestradiol (EE2) has been linked directly with population-level risks in wild fish, owing to feminization in males and reduced fertility in both sexes of several fish species [12–15]. Generally, however, wildlife populations are exposed to relatively low-level environmental concentrations of pharmaceuticals and data confirming adverse effects are extremely limited. It is widely recognized that better insight and understanding of environmental risks are required concerning both newly developed drugs and older pharmaceuticals, some of which have been present in the environment for decades [16]. Owing to the large number of compounds in use, several schemes have been proposed for prioritizing testing [4,17,18], which include ‘reading-across’ plasma concentrations and therapeutic effects of human and veterinary pharmaceuticals to non-target organisms [19]. However, quantifying both interindividual and interspecies variability in drug uptake and metabolism, and extrapolating between individual physiological responses and adverse population-level effects, represent major sources of uncertainty in pharmaceutical environmental risk assessment (ERA) [16]. Fish make up half of all vertebrate species, inhabiting virtually all aquatic environments [20] and exhibiting enormous diversity in morphology, physiology, behaviour, reproductive biology and population ecology [21]. ERAs concerning the susceptibility of fish to pharmaceuticals, however, are based on studies on only a few model fish species and rarely extend to the quantification of population-level effects.
(b). Assessing the potential susceptibility of fish to adverse effects from pharmaceuticals
The susceptibility of wildlife, including fish, to adverse population-level effects from chemicals and/or pharmaceuticals, depends on their exposure, physiological responsiveness and population resilience [22].
Exposure of fish to pharmaceuticals is generally assumed to be via water [23] (but also see [24]), because the majority of pharmaceuticals partition to the water phase in wastewater treatment and remain in solution following discharge to surface waters. In the cases where pharmaceuticals partition to solids and or lipids, this may trigger specific studies, simulating benthic sediment exposure and/or potential bioaccumulation in the food chain [25]. There are few data on pharmacokinetics (drug absorption, distribution, metabolism and excretion) in fish [26], but there is a wealth of human/mammalian data, which offers the potential for ‘read-across’ to fish [16]. More studies are needed to explore the extrapolation of external aqueous exposure concentrations to internal drug concentrations in blood plasma [19,27], to confirm whether expected ‘therapeutic’ effects occur in non-target organisms [28]. Drug uptake across fish gills may be controlled by a variety of membrane transporters, including multi-drug transporters [29] and/or more specific transporters such as sex hormone binding globulin (SHBG) which shows affinity across a wide dynamic range for both natural and synthetic steroids [30]. Predicting the metabolism and excretion of drugs in fish is even more challenging. While several fish species possess enzyme systems responsible for metabolism and excretion of most drugs in human/mammalian systems, limited comparative biotransformation data are available and they indicate that read-across is not straightforward. For example, in mammals, hepatic cytochrome P-450 enzymes (CYPs) are known to play a major role in xenobiotic metabolism and detoxification, with CYP1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1 and 3A4, in particular, mediating the metabolism of approximately 70% of pharmaceuticals [31]. However, no significant biotransformation could be measured for the known substrates of human CYP2D6, CYP2C9 or CYP3A4 for seven drugs in a study on rainbow trout (Oncorhynchus mykiss) [32]. Furthermore, the pregnane X receptor (PXR nuclear receptor subfamily 1, group I, member 2; NR1I2) which regulates many of the CYP enzymes, along with phase II metabolic enzymes and drug transporter proteins in human/mammalian systems, appears to play a role in metabolism in some fish species, but not others. PXR is active in common carp (Cyprinus carpio) [29], fathead minnow (Pimephales promelas) and zebrafish (Danio rerio) [33], but appears to be absent in cod (Gadus morhua), sea lamprey (Petromyzon marinus) and three-spined stickleback (Gasterosteus aculeatus); based on the latest gene builds for these species in Ensembl, version 74 [34]). At this time, therefore, it is difficult to predict drug metabolism and bioconcentration in fish based on the systems established for mammals, or to extrapolate between different fish species.
Physiological responsiveness of fish to pharmaceutical exposure will depend, in part, on the level of conservation of high-affinity interactions with designated drug targets (proteins) that cause the intended pharmacological action in human patients and veterinary animals. Thus, wildlife species that express proteins that are orthologous, i.e. proteins that share a common evolutionary origin to human and veterinary drug targets, may be more responsive than species lacking orthologues. Fish underwent evolutionary divergence from other vertebrates 450 Ma [21], but they nevertheless exhibit high evolutionary conservation of human and veterinary drug targets compared with other taxonomic groups used in aquatic ERA. For example, in zebrafish and three-spined stickleback, orthologues are predicted for 86% of human drug targets [3]. These (and other) fish species used in ecotoxicology belong to the last* of the following three superclasses: (i) Agnatha, jawless fish including lampreys and hagfish (ii) Chondrichthyes, cartilaginous fish, and (iii) Osteichthyes*, bony fish. The final class is the most diverse and comprises the Actinopterygii or ray-finned fishes, and Sarcopterygii, a paraphyletic class containing lobe-finned fishes, and all tetrapod vertebrates, including humans [21]. As Sarcopterygii are more directly related to tetrapods they may be expected to show greater conservation of human and veterinary drug targets compared with fish from other classes, particularly the more ancient superclasses Chondrichthyes and Agnatha. The influence of phylogeny on drug target conservation in fish has not yet been examined and existing orthologue predictions of drug targets have focused on only a few model ray-finned fish species [3], and/or employed simple best-match approaches [4,5]. Phylogenetically based predictions can better account for evolutionary events, including speciation and gene duplications [35], the latter being most prevalent in the ray-finned fish lineage [36]. The level of protein sequence similarity between the human drug target and the fish orthologue (especially for the ligand binding domain; LBD) may enable better predictions regarding the species responsiveness to pharmaceutical exposure [6]. However, currently, there are few experimental data comparing drug target responsiveness in fish, other than for the oestrogen receptor α (ESR1), and these indicate that LBD sequence similarity is not always predictive of receptor activation and therefore physiological response [37].
Population resilience (versus susceptibility) to environmental stressors is governed by life-history traits relating to reproductive strategy, longevity, dispersal, niche specificity, demographics and dynamic stock-recruitment [22,38,39]. A key challenge in ERA is determining whether or not physiological effects in individuals translate to adverse impacts on wild populations [16,40]. This extrapolation between effect-levels and between species has been attempted using population dynamics models, which project forward the life histories of wild populations, with and without superimposing chemical effects measured in surrogate, laboratory-exposed populations. Some models have indicated that short-lived, asynchronous spawning fish, such as the fathead minnow, may be more susceptible to population decline compared with longer-lived, seasonal spawning brook trout (Salvelinus fontinalis), following simulated multi-generation exposures to endocrine active chemicals [41]. These findings are consistent with studies on an experimental lake dosed for 3 years with the synthetic oestrogen EE2, which resulted in significant population decline in fathead minnows, but not the longer-lived, demersal white sucker (Catostomus commersonii) [15]. There is also some indication of increasing, heritable susceptibility to EE2 over generations, according to mesocosm studies on fathead minnows [42], and this is supported by laboratory life cycle studies on fathead minnows and zebrafish [43,44]. Other small fish species, such as the Chinese rare minnow (Gobiocypris rarus) may be even more susceptible to EE2 [45]. It is also possible that longer-term exposures to chemicals, including pharmaceuticals, may eventually become more problematic for longer-lived species with relatively low lifetime fecundity and inflexible life-history strategies [38,39] and/or fish species occurring higher in the food chain [15].
With a view to aiding the ERA of pharmaceuticals, we investigate a range of factors that may predict susceptibility in fish, spanning drug target conservation in model species to ecological life histories of these and other species. We assess the ability to extrapolate between species and biological effect-levels. This work encompasses assessments of exposure potential, physiological responsiveness and population resilience.
2. Methodological approach
We first examined variation in potential susceptibility in fish by describing the presence or absence of orthologues to 459 human drug targets in 12 diverse species with fully sequenced genomes and complete gene builds [34]. Then, prioritizing a subset of 45 active pharmaceutical ingredients (APIs) with potential to have direct effects on reproduction, we assessed the interspecies variation in target sequence similarity compared with the human target. The majority of the prioritized APIs mediate their pharmacological action via one or more steroid receptors, and the sequence similarity of the LBD of these receptors was assessed. Using experimental data, we then compared differences in ligand- and species-specificity of ESR1. In the next stage of our analysis, we evaluated fish life-history traits influencing environmental exposure to pharmaceuticals and population resilience (e.g. dispersal, reproductive strategy, generation time). Finally, a case study analysis was conducted for the highly potent steroidal oestrogen EE2, in order to investigate linkages between individual and population effect-levels, enabling an overall comparative assessment of risk for three model species commonly used in ERA. All fish species included in the study are listed in electronic supplementary material, table S1.
(a). Assessment of physiological responsiveness
(i). Orthologues in fish for human drug targets
Genomes were studied for 12 fully sequenced fish species with complete gene builds held in Ensembl Compara [46] (version 74, accessed January 2014): cod (G. morhua), coelacanth (Latimeria chalumnae), fugu (Taikfugu rubripes), medaka (Oryzias latipes), Mexican cavefish (Astyanax mexicanus), Nile tilapia (Oreochromis niloticus), sea lamprey (P. marinus), southern platyfish (Xiphophorus maculates), spotted gar (Lepisosteus oculatus), three-spined stickleback (G. aculeatus), tetraodon (Tetraodon nigroviridis) and zebrafish (D. rerio).
Information regarding human drugs and their targets were downloaded from DrugBank v. 3.0 [1]. Only drugs annotated in DrugBank as ‘small molecule’, ‘approved’ and with ‘humans and other mammals, as affected organisms’ were considered. Drug targets with ‘unknown pharmacological action’ were excluded from our analyses. In total, information on 978 APIs associated with 459 unique drug targets was downloaded. DrugBank previously listed over 1000 drug targets, including metabolizing enzymes and transporters, but now shows that only 459 have specific pharmacological action. These drug targets were mapped to the Ensembl database (version 74, accessed January 2014) [34] using protein sequences from Uniprot [47]. Drug target orthologues in the 12 fully sequenced fish species and in the tree frog (Xenopus tropicalis), included as a tetrapod outgroup, were then calculated (electronic supplementary material, table S2) based on the phylogenetic gene tree predictions in Ensembl Compara (version 74, accessed January 2014) [46]. A drug target was considered to be conserved in a species if it had at least one human orthologue. The associated taxonomic information was retrieved from the NCBI taxonomy database (accessed February 2014) [48].
(ii). Assessment of sequence similarities
Sequence similarities were calculated for drug targets (21) associated with APIs (45) with anatomical therapeutic chemical classification codes suggesting direct effects on reproduction (prostaglandins (A02BB), oxytocics/uterus-contracting agents (G02A), contraceptives for topical use (G02B), sex hormones and modulators of the genital system (G03) and endocrine agents used in the treatment of neoplastic diseases (L02)). The sequence similarity was estimated from the multiple alignments available in Ensembl (version 74, accessed January 2014) [34]. To reduce the effects of erroneously aligned gene regions, produced by the large evolutionary distance between the species, only aligned amino acids were considered in the estimates. All sequence similarities are presented in electronic supplementary material, table S3. LBDs were annotated using the position-specific scoring matrices from the Conserved Domain database [49]. The sequence similarities of LBDs were calculated in an analogous manner to the sequence similarities of complete drug targets.
(iii). Assessment of amino acid sequence alignments with the LDB for human ESR1
Multiple alignment of amino acid sequences of the LBD of ESR1 was assessed with the following sequences: human ENSP00000405330; tree frog ENSXETG00000012364; cavefish ENSAMXG00000006267; cod ENSGMOG00000014898; common carp BAF99812; fathead minnow AAV41373; fugu ENSTRUG00000018219; medaka ENSORLG00000014514; platyfish ENSXMAG00000003084; rainbow trout P16058; roach BAD91035; stickleback ENSGACG00000008711; tetraodon ENSTNIG00000012264; tilapia ENSONIG00000013354; sea lamprey ENSPMAG00000005727; zebrafish ENSDARG00000004111. The amino acid residues in the human ESR1 that have been shown to have direct contact with the co-crystallized ligands 17β-oestradiol (E2) and diethylstilboestrol (DES) according to pocketome.org [50] (accessed in April 2014) were highlighted, and the alignment is presented in electronic supplementary material, table S4. The ESR1 in the coelacanth was found to be erroneous and was therefore excluded from the alignment analysis.
(iv). Assessment of interactions of pharmaceutical oestrogens with ESR1
Interactions with ESR1 of oestrogenic pharmaceuticals E2, EE2, the equine oestrogen equilin (used in hormone replacement therapy) and DES were compared across six different fish species (common carp, fathead minnow, medaka, roach (Rutilus rutilus), three-spined stickleback and zebrafish). Full-coding regions for ESR1 in each species were cloned and transfected into separate HEK293 cell lines and ESR1 receptor trans-activation assays were conducted as described in [37].
(b). Assessment of exposure potential and population resilience
(i). Life-history trait analysis
Using the databases FishBase (version 12/2013) [51] and FishTraits (version 2) [52], and the available scientific literature, life-history data were obtained for a broad selection of fish species in which physiological responsiveness to pharmaceuticals had been assessed. Rainbow trout and the Chinese rare minnow were also included as an alternative model species used in ecotoxicology and ERA. Searches focused on the compilation of qualitative and quantitative trait data (electronic supplementary material, table S5) required to calculate susceptibility indices incorporating indicators of exposure potential (e.g. dispersal, niche specificity, lifespan) and population resilience (e.g. spawning frequency, parental care, recruitment). Two alternative indices were used, the population survivorship index [38] and the ecological vulnerability index [39].
(ii). Life-table-response experiments integrating life cycle assessments of individual and population-level effects of EE2
Published life cycle data (F1 embryo to F1 adult to F2 embryo) quantifying the effects of the oestrogen receptor (ESR1, ESR2) agonist EE2 in three model freshwater fish species, fathead minnow, medaka and zebrafish, under standard flow-through conditions and test temperatures (25–28°C), were compiled and scrutinized according to established quality/reliability criteria [53]. Those studies incorporating measurement of a range of effect levels (molecular, physiological, behavioural, population-relevant effects) and confirmation of exposure concentrations by chemical analysis were used to determine the lowest observed effect concentration (LOEC) for population-relevant endpoints in each species: zebrafish LOEC = 0.5–1 ng l−1 EE2 [54,55]; fathead minnow and medaka LOEC = 1 ng l−1 EE2 [43,56,57]. Endpoint values (vital rates: proportion of viable fertilized eggs; proportion of females; female fecundity (eggs per female per day); survival) were tabulated as mean values ± standard error of the mean (electronic supplementary material, table S6). A lower LOEC of 0.2 ng l−1 EE2 was reported for the Chinese rare minnow [45]; however, insufficient life-table data were available to permit life-table-response experiments (LTREs) for this species.
Species-specific vital rates for control (non-exposed) and EE2 (1 ng l−1) exposed fish were then input with specific life-table data for wild populations of fathead minnow, medaka, zebrafish (electronic supplementary material, table S7) to stage-based Leslie matrix population models (electronic supplementary material, figure S1), constructed for each species using PopTools (v. 3.2.5) [58]. Population projections were based on females, because female fecundity limits population numbers. Populations were assumed to be closed with no immigration or emigration, and spawning was assumed to be asynchronous and protracted in all three species [59–61]. Finite, geometric population growth rate (λ) was then projected from n = 100 replicate LTREs [62] for each species, by resampling vital rates from their means and standard deviations, assuming normal distributions for each vital rate [60]. The statistical significance of the effect of EE2 (1 ng l−1) exposure on projected population growth rate (λE) versus control population growth rate (λC) was assessed on ranked projections using the non-parametric Kruskal–Wallis test, owing to inequality of variances between exposed and control populations (according to Levene's test). These stochastic projections of treatment effects at the population-level enabled an integrated assessment of ecotoxicological and ecological population susceptibility. Determining the mean sensitivities (control + treatment/2) of each vital rate in each LTRE also enabled decomposition analysis to quantify the relative contribution and importance of these vital rates (performed according to Caswell [62]).
3. Results and discussion
(a). Assessment of physiological responsiveness
(i). Conservation of human drug targets
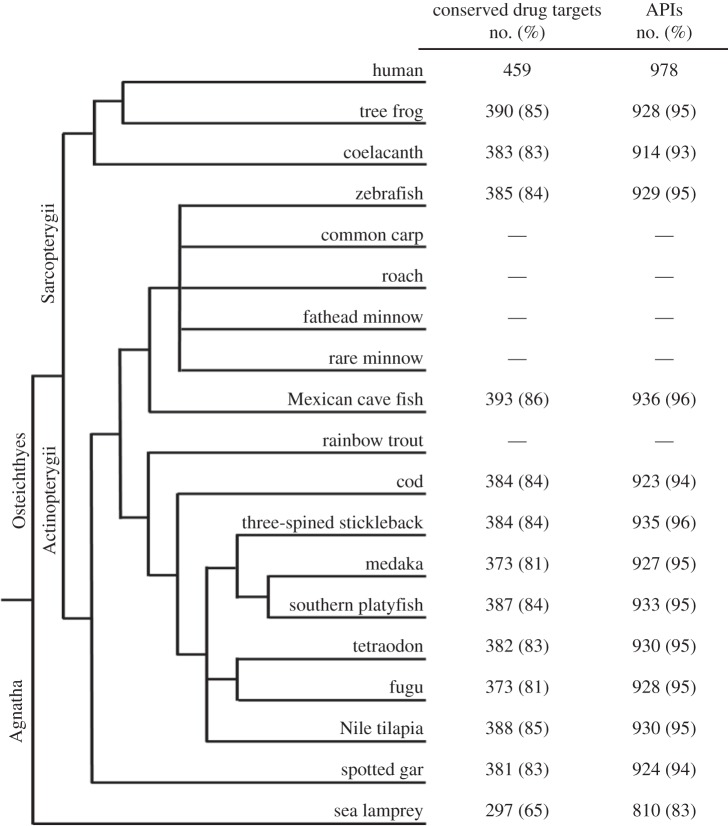
More than 80% of the 459 human drug targets had orthologues in all the investigated bony fish, while the sea lamprey (a jawless fish) had orthologues to 65% of the targets. These results suggest that responses in sea lamprey may differ frequently and substantially from those in bony fish (figure 1). Information regarding the APIs, the drug targets and their orthologues and co-orthologues in each species is presented in electronic supplementary material, table S2.
Figure 1.
Species tree illustrating the taxonomic relationships (based on NCBI taxonomy) between all species included in this study. The total number (no.) of conserved human drug targets for each of the 12 fish species with fully sequenced genomes and complete gene builds is presented, together with the number of active pharmaceutical ingredients (API) with at least one drug target orthologue.
Although the vast majority of the APIs had at least one orthologous drug target in fish, this does not necessarily mean all these APIs will have a functional drug interaction and invoke a physiological response in fish, or that this response will resemble that occurring in humans. Even between mammalian species (human–mouse), there are numerous examples of orthologues that have diverged functionally [63]. The general sequence similarity between orthologous proteins may potentially give more information, but the data should be interpreted with caution, because orthologous proteins with similar function can have significant domains that are missing or differ substantially. The LBD and especially the specific amino acids that interact with the ligands are, however, generally highly conserved and could provide additional information regarding potential drug interactions [6].
(ii). Variation in sequence similarity
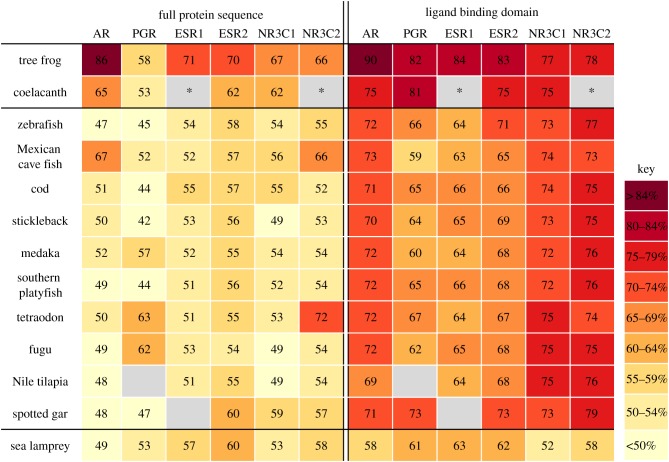
Alignments of the full protein sequence and LBD of the nuclear steroid hormone receptors, specifically, ESR1, oestrogen receptor β (ESR2), progesterone receptor (PGR), androgen receptor (AR), glucocorticoid receptor (NR3C1) and mineralocorticoid receptor (NR3C2), showed that the LBDs had higher sequence similarity to the corresponding human drug target than the full proteins (figure 2). Furthermore, in line with the established phylogenetics of fish evolution, the lobe-finned fish had the highest sequence similarities (75–81%) with the LBDs of the human steroid receptors, whereas the more primitive sea lamprey had the lowest LBD sequence similarities (52–63%; figure 2). This finding, however, does not necessarily mean that sea lamprey is less susceptible to drugs targeting these receptors, because it is also important to consider gene duplication and function. As an illustration of this, the human glucocorticoid- and mineralocorticoid-receptors are co-orthologous to a single corticoid receptor ‘CR’, and the human androgen and progesterone receptor are co-orthologous to a receptor annotated as ‘PGR’, in sea lamprey (electronic supplementary material, table S2). The CR in sea lamprey appears to be promiscuous for binding corticosteroids [64], but there are only limited experimental data assessing the responsiveness of this receptor to drugs designed to affect the human glucocorticoid- and mineralocorticoid-receptors. There are also very few data concerning the promiscuity, responsiveness and function of the PGR, ESR1 and ESR2 in sea lamprey. Nevertheless, plasma progesterone and E2 concentrations have been shown to vary between the sexes and reproductive stages, suggesting links with sexual function in this species [65]. Here, we show that sea lamprey has orthologues to both the human ESR1 and ESR2 and their LBDs are conserved to a similar degree in sea lamprey as in the ray-finned fishes (63–71%).
Figure 2.
Sequence similarities (%) between six human nuclear steroid hormone receptors and their corresponding orthologues in 13 species. Sequence similarities of the full sequences are presented on the left and similarities of the ligand binding domains are presented on the right. AR, androgen receptor; PGR, progesterone receptor; ESR1, oestrogen receptor 1 (α); ESR2, oestrogen receptor 2 (β); NR3C1, nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor); NR3C2, nuclear receptor subfamily 3, group C, member 2 (mineralocorticoid receptor). Percentage similarities are emphasized using a coloured heat map. Empty cells indicate that evidence for an orthologue is lacking in current genome versions and gene builds. Asterisk denotes missing value owing to errors in current gene build. (Online version in colour.)
(iii). Variation in target-ligand binding and activation of ESR1
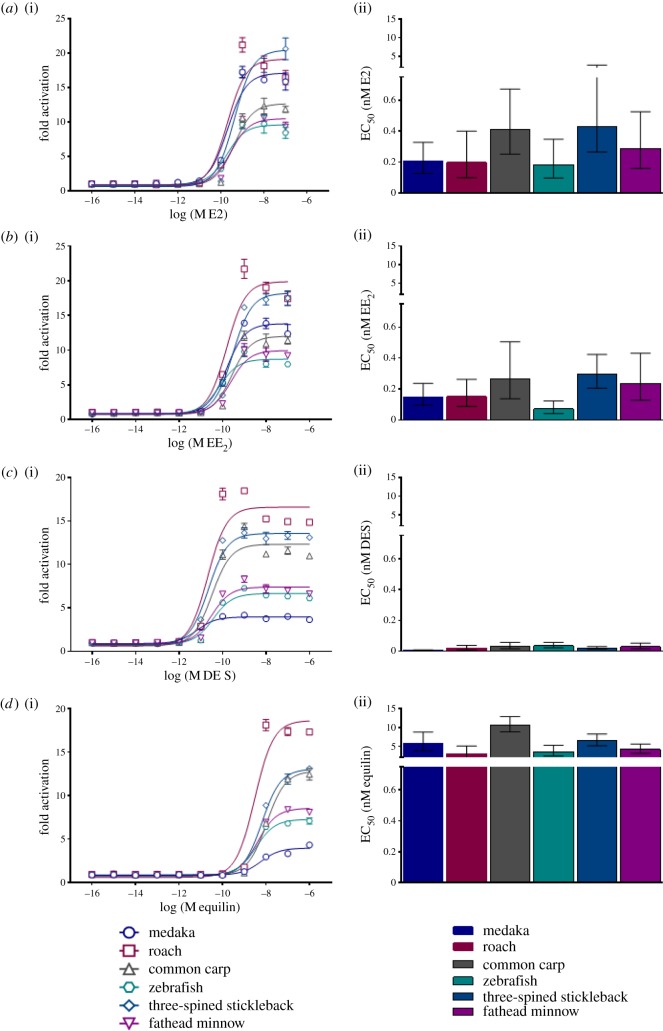
Assessment of pharmaceutical oestrogen interactions with fish ESR1 in trans-activation assays showed that DES was the most potent ligand and 10 times more potent than the natural oestrogen E2. EE2 was approximately twice the potency of E2, and equilin was around 10 times less potent (in all species) compared with E2.
The effective concentration corresponding with 50% of maximum ESR1 transactivation (EC50) showed little variation for E2 between the different fish species, ranging 2.4-fold between 0.18 and 0.43 nM (zebrafish and stickleback, respectively, figure 3). For the pharmaceutical oestrogens, however, there were somewhat larger differences between the different fish species. For EE2, the EC50 ranged 4.3-fold between 0.07 and 0.30 nM (zebrafish and stickleback, respectively) and for DES, the EC50 ranged 5.7-fold between 0.007 and 0.04 nM (medaka and zebrafish, respectively). For equilin, the EC50 varied 3.5-fold within the range 3.02–10.67 nM (roach and carp, respectively). There were also differences in the maximum fold-activation for ESR1 between the different fish species in these transactivation assays, for all the oestrogens analysed. For E2, this difference across the species was only around 2.5-fold, and similarly for EE2 around 2.2-fold, whereas for DES and equilin, these differences were around 4.0-fold (figure 3). Zebrafish and fathead minnow showed a tendency for lower levels of fold-activation by E2 and EE2 compared with roach, stickleback and medaka. Conversely, medaka showed lowest levels of activation by DES and equilin, followed by zebrafish and fathead minnow, whereas roach again showed highest fold-activation.
Figure 3.
Fish oestrogen receptor (ESR1) responses in transactivation assays induced by oestrogenic pharmaceuticals. (a) 17β-Oestradiol (E2), (b) 17α-ethinyloestradiol (EE2), (c) diethylstilboestrol (DES), (d) equilin. Receptor transactivation data are shown in terms of fold-activation (i) and EC50 (ii). Fold-activation represents the difference in receptor activity (non versus pharmaceutical exposure) of ESR1 from six selected fish species transfected into human HEK293 cells [32]. Receptor activity was quantified using a dual luciferase reporter assay. EC50 is the effective concentration (nM) corresponding with 50% of maximum ESR1 transactivation. Data are presented as mean ± s.e.m. from three independent assays, each consisting of three technical replicates per concentration tested. (Online version in colour.)
Although there were differences between the fish species studied for some of the interactions of pharmaceutical oestrogens with ESR1, far greater interspecies variation has been shown for other less potent environmental oestrogens. For example, ESR1 transactivation EC50s for 4-nonylphenol and 4-octylphenol are 30 times and 23 times lower, respectively, in common carp compared with medaka [37]. The much smaller 2.4-fold difference in the EC50 for E2 (and 4.3-fold for EE2) between the fish species studied, likely reflects the crucial roles of this natural oestrogen (and synthetic mimic) in a wide range of tissues, functions and processes in fish [66,67]. Curiously, 14 of the 15 amino acid residues of the human ESR1 known to have direct contact with E2 or DES were identical in all bony fish (electronic supplementary material, table S4). The greater interspecies variation in EC50s and fold-activation for DES (up to 5.7-fold variation) could therefore not easily be explained by the variations in sequence similarity in the LBD (electronic supplementary material, table S4). Instead, the influence of cofactors, differences in promoter sequences and amino acid residues in other domains, such as the DNA binding domain [37,68,69], may better account for the observed differences in ESR1 activation.
(b). Variation in exposure potential and population resilience
(i). Life-history trait analysis
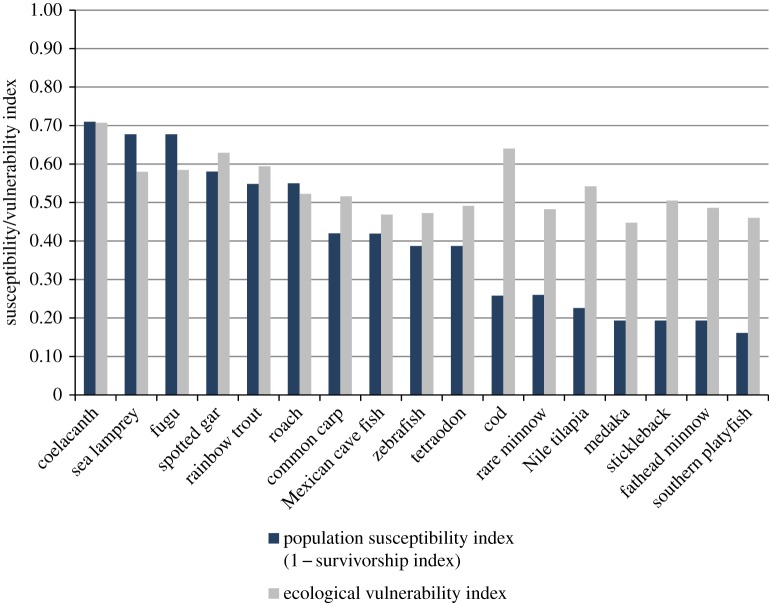
According to two alternative susceptibility indices, based on a range of life-history traits, species with longer generation times and lower spawning frequency, fecundity and recruitment (coelacanth, sea lamprey and fugu) were ranked as the most susceptible to environmental stressors (figure 4). The higher ranking of these species is consistent with their higher conservation status, compared with other species analysed (electronic supplementary material, table S1). The rapidly reproducing southern platyfish was ranked as the least susceptible to environmental stressors (figure 4). The ‘population survivorship index’, specifically tailored for fish and amphibians [38], provided greater differentiation between the most and least susceptible species, indicating a threefold difference, compared with a 1.6-fold difference indicated by the more general ‘ecological vulnerability index’ [39]. Both these ranges are within the 10× assessment factor traditionally used in pharmaceutical ERA to account for variation in species susceptibility [25]. Nevertheless, it is interesting to note that zebrafish were predicted to be more susceptible to population decline compared with the rare minnow, fathead minnow and medaka, which is due principally to broadcast spawning behaviour and lack of parental care in zebrafish. However, because the life-history strategies of many fish are highly plastic, enabling adaptation to their specific environments [38,70], our results should be used only as a general guide. Furthermore, the general life-history data analysis we adopted does not account for species-specific effects of pharmaceuticals. The inclusion of specific effects data in susceptibility indices requires some degree of weighting and/or expert judgement [39].
Figure 4.
Potential susceptibility of fish populations to environmental (chemical) stress. Population susceptibility index (1 − population survivorship index, Spromberg & Birge [38]) was calculated using a scoring system based on spawning frequency, parental care, lifespan, recruitment, niche specificity. Ecological vulnerability index (De Lange et al. [39]) was based on broader life-history data. Data were obtained mainly from www.fish.base.org/ and www.fishtraits.info/ (see electronic supplementary material, table S5). (Online version in colour.)
(ii). Life-table-response experiments
Available life-table data and full life-cycle studies for EE2 enabled integrated assessments of physiological susceptibility and population resilience for three model freshwater species commonly used in pharmaceutical ERA, specifically, fathead minnow, medaka and zebrafish.
Beginning with an analysis of life-cycle effects data, the LOECs for EE2 were: zebrafish LOEC = 0.5 ng l−1 [54]; fathead minnow and medaka LOEC = 1 ng l−1 [43,55–57]. While there were no effects on survival at these concentrations, there were significant effects on other population-relevant endpoints (vital rates), and their magnitudes of effect differed between species. There was a female-biased sex ratio in both zebrafish (57%) [55] and fathead minnow (65%) [43], but there was no female bias in medaka (50%) [56–57]. Plasticity in sexual differentiation is typical in some species, such as zebrafish, that are sometimes referred to as juvenile hermaphrodites. Alternatively, in gonochorists, such as fathead minnow and medaka, sex is determined around fertilization and feminization of males typically results in ovo-testes, as occurred in both species following exposure to 1 ng l−1 EE2 [43,56]. Female-bias appeared to be ‘compensated’ for by reductions in fecundity and fertilization success in fathead minnows. Greater reduction in fertilization success occurred in zebrafish, which may be linked to their broadcast spawning strategy, reduced likelihood of fertilization and oophagy, as opposed to substrate spawning and egg guarding displayed by fathead minnows. Fertilization success was affected least in medaka, whose females produced and brooded fewer eggs. EE2 exposure concentrations of between 0.5 and 2 ng l−1 resulted in no significant alteration in male courtship behaviour in zebrafish [54,71]. In contrast, male courtship was reduced in medaka [53,54]. Overall, there were reductions in fertilization/hatching success in all three species compared with controls: 25 ± 5% reduction in medaka [57]; 35 ± 30% in fathead minnows [43]; 54.5 ± 15% in zebrafish [44]. Female fecundity also showed declining (but non-significant) trends for all three species. Reciprocal pair-breeding of control (non-exposed) and EE2-exposed fish revealed that reproductive impairment occurred in both sexes, but was generally greater in males in both zebrafish [54] and medaka [57].
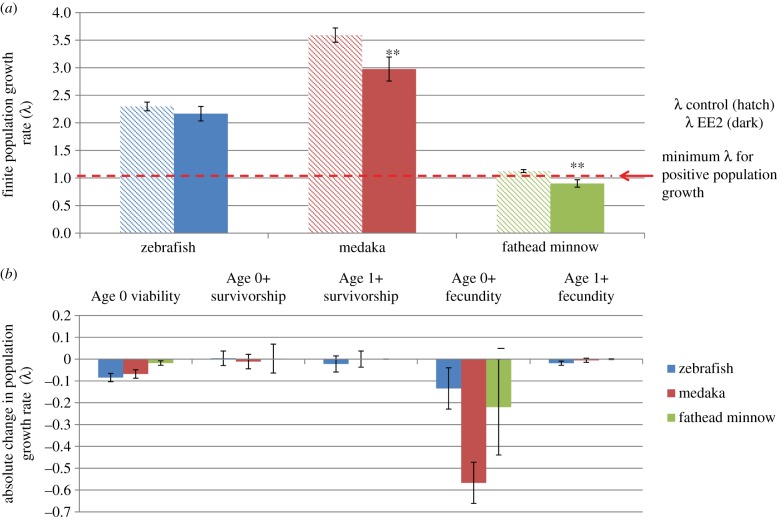
The integration of life-cycle effects data and ecological life-history data for fathead minnow, medaka and zebrafish, in separate LTREs, showed that finite population growth (λE) following exposure to 1 ng l−1 EE2 was more variable compared to controls (λC), but was reduced significantly in fathead minnow (λC = 1.12, λE = 0.90, −20%; Kruskal–Wallis H = 64.82, d.f. = 1, p < 0.001) and medaka (λC = 3.59, λE = 2.97, −17%; Kruskal–Wallis H = 43.55, d.f. = 1, p < 0.001), but not in zebrafish (λC = 2.30, λE = 2.17, −6%; Kruskal–Wallis H = 2.88, d.f. = 1, p = 0.089) (figure 5). Proportional reductions in population growth rate (fathead minnow > medaka > zebrafish) contrasted with the susceptibility indices derived from ecological life-history data for these species. Absolute population growth rate for fathead minnows was also projected to fall below λ = 1 under EE2 exposure, indicating population decline.
Figure 5.
(a) Mean (s.e.m.) projected finite population growth in model fish species following lifetime exposure (embryo to adult) to 1 ng l−1 ethinyloestradiol (EE2) compared with control populations with no chemical exposure. (b) Contribution of each vital rate to reductions in finite population growth rate. Mean of n = 100 stochastic matrix projections and standard error of the mean (SEM) shown. **p < 0.001 according to the Kruskal–Wallis test comparing ranked projections of finite population growth in EE2 exposed populations versus non-exposed (control) populations of model fish species. (Online version in colour.)
Decomposition analysis revealed that reduction in age 0+ fecundity (fecundity in the first year of life), although highly variable, was most influential on reducing population growth in all three model species under EE2 exposure. These results highlight potential drawbacks of traditional statistical evaluation of individual endpoints such as fecundity, which are often shown to be highly variable and ‘statistically insignificant’ [43,44,57]. Alternatively, integrative modelling approaches can use stochastic variation in multiple endpoints and, by extrapolating population-level effects, can indicate ‘ecological significance’. The second most influential parameter affecting population growth was age 0 viability (proportion of viable female eggs × proportion fertilized), in which the proportion of eggs fertilized is influenced by the effects of EE2 on reducing male fertility. Reduction in fertilization success, that can act directly to reduce population growth, was greatest in zebrafish, but this was compensated by female-biased sex ratios and high fecundity in this species. Other modelling approaches, including mechanistic, individual-based models [40,61], may be better able to discern effects of EE2 on male reproductive fitness, including those relating to effects on behaviour.
4. Conclusion
Here, we identify factors for consideration when extrapolating between fish species and effects endpoints in pharmaceutical ERA. This approach can help to identify which levels of biological organization, from the molecular to the population-level, are most likely to account for interspecies variation. Drug bioavailability and biotransformation in fish are likely to be further sources for interspecies variation in responsiveness to drugs, which we have not explored fully because data are currently sparse.
We illustrate that although fish generally show high conservation of human drug targets across diverse taxonomic groups, there is some interspecies variation, for example in the LBD sequences for nuclear hormone receptors. Greater variation (up to 5.7-fold) was identified in the physiological responsiveness of different fish to drugs targeting these receptors. Additional analysis of ecological life-history data indicated further interspecies variation (up to threefold) in potential susceptibility to population decline. Ultimately, we integrated ecological life-table data with life-cycle effects data for EE2 and showed distinct differences in proportional reductions in population growth between fathead minnow, medaka and zebrafish (around threefold). These results, however, were not entirely consistent with the physiological and ecological susceptibilities indicated by the preceding analyses. This work illustrates that extrapolating from individual-level effects in laboratory tests to population effects in wild fish is challenging. Furthermore, while small fish models offer considerable utility for pharmaceutical ERA, they are not necessarily protective of all fish owing to wide ranging evolutionary divergence of physiologies, behaviours and ecological life histories. Nevertheless, based on our analyses on the data available, the use of at least a 10× assessment factor to account for interspecies differences in ERA seems appropriate for application to pharmaceuticals.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Shinichi Miyagawa and Taisen Iguchi (Okazaki Institute for Integrative Bioscience, Okazaki, Japan) for allowing us to use the fish ESR transactivation data and Anke Länge (Biosciences, University of Exeter) for kindly preparing ESR data figures.
Funding statement
This research was supported financially by the Swedish Foundation for Strategic Environmental Research (Mistra), UK Natural Environment Research Council, and by AstraZeneca's Global SHE Research Programme.
References
- 1.Knox C, et al. 2011. DrugBank 3.0: a comprehensive resource for ‘Omics’ research on drugs. Nucleic Acids Res. 39(Suppl. 1), D1035–D1041. ( 10.1093/nar/gkq1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health 2014. Therapeutic drug levels: medline plus See http://www.nlm.nih.gov/medlineplus/ency/article/003430.htm (accessed January 2014).
- 3.Gunnarsson L, Jauhiainen A, Kristiansson E, Nerman O, Larsson DG. 2008. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 42, 5807–5813. ( 10.1021/es8005173) [DOI] [PubMed] [Google Scholar]
- 4.Kostich MS, Lazorchak JM. 2008. Risks to aquatic organisms posed by human pharmaceutical use. Sci. Total Environ. 389, 329–339. ( 10.1016/j.scitotenv.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 5.Lalone CA, et al. 2013. Molecular target sequence similarity as a basis for species extrapolation to assess the ecological risk of chemicals with known modes of action. Aquat. Toxicol. 144–145, 141–154. ( 10.1016/j.aquatox.2013.09.004) [DOI] [PubMed] [Google Scholar]
- 6.McRobb FM, Sahagún V, Kufareva I, Abagyan R. 2014. In silico analysis of the conservation of human toxicity and endocrine disruption targets in aquatic species. Environ. Sci. Technol. 48, 1964–1972. ( 10.1021/es404568a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daughton CG, Ternes TA. 1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 107(Suppl. 6), 907–938. ( 10.1289/ehp.99107s6907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder SA. 2008. Occurrence, treatment, and toxicological relevance of EDCs and pharmaceuticals in water. Ozone-Sci. Eng. 30, 65–69. ( 10.1080/01919510701799278) [DOI] [Google Scholar]
- 9.Barber LB, Keefe SH, Brown GK, Furlong ET, Gray JL, Kolpin DW, Meyer MT, Sandstrom MW, Zaugg SD. 2013. Persistence and potential effects of complex organic contaminant mixtures in wastewater-impacted streams. Environ. Sci. Technol. 7, 2177–2188. ( 10.1021/es303720g) [DOI] [PubMed] [Google Scholar]
- 10.Oaks JL. 2004. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427, 630–633. ( 10.1038/nature02317) [DOI] [PubMed] [Google Scholar]
- 11.Cuthbert RJ, et al. 2014. Avian scavengers and the threat from veterinary pharmaceuticals. Phil. Trans. R. Soc. B 369, 20130574 ( 10.1098/rstb.2013.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jobling S, et al. 2002. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol. Reprod. 67, 515–524. ( 10.1095/biolreprod67.2.515) [DOI] [PubMed] [Google Scholar]
- 13.Palace VP, Evans RE, Wautier KG, Mills KH, Blanchfield PJ, Park BJ, Baron CL, Kidd KA. 2009. Interspecies differences in biochemical, histopathological, and population responses in four wild fish species exposed to ethynylestradiol added to a whole lake. Can. J. Fish. Aquat. Sci. 66, 1920–1935. ( 10.1139/F09-125) [DOI] [Google Scholar]
- 14.Harris CA, et al. 2010. The consequences of feminization in breeding groups of wild fish. Environ. Health Perspect. 119, 306–311. ( 10.1289/ehp.1002555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd KA, Paterson MJ, Rennie MD, Podemski CL, Findlay DL, Blanchfield PJ, Liber K. 2014. Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen. Phil. Trans. R. Soc. B 369, 20130578 ( 10.1098/rstb.2013.0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell DJ, Mastrocco F, Margiotta-Casaluci L, Brooks BW. 2014. An integrated approach for prioritizing pharmaceuticals found in the environment for risk assessment, monitoring and advanced research. Chemosphere ( 10.1016/j.chemosphere.2014.01.021) [DOI] [PubMed] [Google Scholar]
- 17.Schreiber R, Gündel U, Franz S, Küster A, Rechenberg B, Altenburger R. 2011. Using the fish plasma model for comparative hazard identification for pharmaceuticals in the environment by extrapolation from human therapeutic data. Regul. Toxicol. Pharmacol. 61, 261–275. ( 10.1016/j.yrtph.2011.08.006) [DOI] [PubMed] [Google Scholar]
- 18.Roos V, Gunnarsson L, Fick J, Larsson DG, Rudén C. 2012. Prioritising pharmaceuticals for environmental risk assessment: towards adequate and feasible first-tier selection. Sci. Tot. Environ. 421–422, 102–110. ( 10.1016/j.scitotenv.2012.01.039) [DOI] [PubMed] [Google Scholar]
- 19.Huggett DB, Cook JC, Ericson JF, Williams RT. 2003. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk Assess. 9, 1789–1799. ( 10.1080/714044797) [DOI] [Google Scholar]
- 20.Nelson JS. 2006. Fishes of the world, 4th edn, p. 624 Hoboken, NJ: John Wiley and Sons. [Google Scholar]
- 21.Broughton RE, Betancur-R R, Li C, Arratia G, Ortí G. 2013. Multi-locus phylogenetic analysis reveals the pattern and tempo of bony fish evolution. PLoS Curr. 5 ( 10.1371/currents.tol.2ca8041495ffafd0c92756e75247483e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Straalen NM. 1994. Biodiversity of ecotoxicological responses in animals. Neth. J. Zool. 44, 112–129. ( 10.1163/156854294X00097) [DOI] [Google Scholar]
- 23.Du B, et al. 2014. Bioaccumulation and trophic dilution of human pharmaceuticals across trophic positions of an effluent-dependent wadeable stream. Phil. Trans. R. Soc. B 369, 20140058 ( 10.1098/rstb.2014.0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodin T, Piovano S, Fick J, Klaminder J, Heynen M, Jonsson M. 2014. Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations. Phil. Trans. R. Soc. B 369, 20130580 ( 10.1098/rstb.2013.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Medicines Agency 2006. Guideline on the environmental risk assessment of medicinal product for human use. London, UK: EMEA/CHMP/SWP4447/00. [Google Scholar]
- 26.Rand-Weaver M, Margiotta-Casaluci L, Patel A, Panter GH, Owen SF, Sumpter JP. 2013. The read-across hypothesis and environmental risk assessment of pharmaceuticals. Environ. Sci. Technol. 47, 11 384–11 395. ( 10.1021/es402065a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzsimmons PN, Fernandez JD, Hoffman AD, Butterworth BC, Nichols JW. 2001. Branchial elimination of superhydrophobic organic compounds by rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 55, 23–34. ( 10.1016/S0166-445X(01)00174-6) [DOI] [PubMed] [Google Scholar]
- 28.Hutchinson TH, Madden JC, Naidoo V, Walker CH. 2014. Comparative metabolism as a key driver of wildlife species sensitivity to human and veterinary pharmaceuticals. Phil. Trans. R. Soc. B 369, 20130583 ( 10.1098/rstb.2013.0583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corcoran J, Lange A, Cumming RI, Owen SF, Ball JS, Tyler CR, Winter MJ. 2014. Bioavailability of the imidazole antifungal agent clotrimazole and its effects on key PXR-regulated genes in the common carp (Cyprinus carpio). Aquat. Toxicol. 152C, 57–65. ( 10.1016/j.aquatox.2014.03.016) [DOI] [PubMed] [Google Scholar]
- 30.Miguel-Queralt S, Hammond GL. 2008. Sex hormone-binding globulin in fish gills is a portal for sex steroids breached by xenobiotics. Endocrinology 149, 4269–4275. ( 10.1210/en.2008-0384) [DOI] [PubMed] [Google Scholar]
- 31.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. 1994. Inter-individual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens, and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharm. Exp. Ther. 270, 414–423. [PubMed] [Google Scholar]
- 32.Connors KA, Du B, Fitzsimmons PN, Hoffman AD, Chambliss CK, Nichols JW, Brooks BW. 2013. Comparative pharmaceutical metabolism by rainbow trout (Oncorhynchus mykiss) liver S9 fractions. Environ. Toxicol. Chem. 32, 1810–1818. ( 10.1002/etc.2240) [DOI] [PubMed] [Google Scholar]
- 33.Milnes MR, et al. 2008. Activation of steroid and xenobiotic receptor (SXR, NR1I2) and its orthologs in laboratory, toxicologic, and genome model species. Environ. Health Perspect. 116, 880–885. ( 10.1289/ehp.10853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flicek P, et al. 2013. Ensembl. Nucleic Acids Res. 41, D48–D55. ( 10.1093/nar/gks1236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristensen DM, Wolf YI, Mushegian AR, Koonin EV. 2011. Computational methods for gene orthology inference. Brief Bioinform. 12, 379–391. ( 10.1093/bib/bbr030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer A, Van de Peer Y. 2005. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). Bioessays 27, 937–945. ( 10.1002/bies.20293) [DOI] [PubMed] [Google Scholar]
- 37.Miyagawa S, et al. 2014. Differing species responsiveness of estrogenic contaminants in fish is conferred by the ligand binding domain of the estrogen receptor. Environ. Sci. Technol. 48, 5254–5263. ( 10.1021/es5002659) [DOI] [PubMed] [Google Scholar]
- 38.Spromberg JA, Birge WJ. 2005. Population survivorship index for fish and amphibians: application to criterion development and risk assessment. Environ. Toxicol. Chem. 24, 1541–1547. ( 10.1897/04-159.1) [DOI] [PubMed] [Google Scholar]
- 39.De Lange HJ, Lahr J, Van der Pol JJ, Wessels Y, Faber JH. 2009. Ecological vulnerability in wildlife: an expert judgment and multicriteria analysis tool using ecological traits to assess relative impact of pollutants. Environ. Toxicol. Chem. 28, 2233–2240. ( 10.1897/08-626.1) [DOI] [PubMed] [Google Scholar]
- 40.Van den Brink PJ, Baird DJ, Baveco HJM, Focks A. 2013. The use of traits-based approaches and eco(toxico)logical models to advance the ecological risk assessment framework for chemicals. Integr. Environ. Assess. Manag. 9, e47–e57. ( 10.1002/ieam.1443) [DOI] [PubMed] [Google Scholar]
- 41.Brown AR, Riddle AM, Cunningham NL, Kedwards TJ, Shillabeer N, Hutchinson TH. 2003. Predicting the effects of endocrine disruptors on fish populations. Hum. Ecol. Risk Assess. 9, 761–788. ( 10.1080/713609966) [DOI] [Google Scholar]
- 42.Schwindt AR, Winkelman DL, Keteles K, Murphy M, Vajda AM. 2014. An environmental oestrogen disrupts fish population dynamics through direct and transgenerational effects on survival and fecundity. J. Appl. Ecol. ( 10.1111/1365-2664.12237) [DOI] [Google Scholar]
- 43.Länge R, Hutchinson TH, Croudace CP, Siegmund F, Schweinfurth H, Hampe P, Panter GH, Sumpter JP. 2001. Effects of the synthetic estrogen 17 α-ethinyloestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 20, 1216–1227. ( 10.1002/etc.5620200610) [DOI] [PubMed] [Google Scholar]
- 44.Schäfers C, Teigeler M, Wenzel A, Maack G, Fenske M, Segner H. 2007. Concentration- and time-dependent effects of the synthetic estrogen, 17 α-ethinyloestradiol, on reproductive capabilities of the zebrafish, Danio rerio. J. Toxicol. Environ. Health A. 70, 768–779. ( 10.1080/15287390701236470) [DOI] [PubMed] [Google Scholar]
- 45.Zha J, Sun L, Zhou Y, Spear PA, Ma M, Wang Z. 2008. Assessment of 17 α-ethinylestradiol effects and underlying mechanisms in a continuous, multigeneration exposure of the Chinese rare minnow (Gobiocypris rarus). Toxicol. Appl. Pharmacol. 226, 298–308. ( 10.1016/j.taap.2007.10.006) [DOI] [PubMed] [Google Scholar]
- 46.Vilella AJ, Severin J, Ureta-Vidal A, Durbin R, Heng L, Bimey E. 2009. EnsemblCompara GeneTrees: complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 19, 327–335. ( 10.1101/gr.073585.107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UniProt 2013. Consortium, Update on activities at the Universal Protein Research (UniProt) in 2013. Nucleic Acids Res. 41, D43–D47. ( 10.1093/nar/gks1068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Centre for Biotechnology Information. 2014. Taxonomy database. See www.ncbi.nln.nih.gov/taxonomy (accessed February 2014). [Google Scholar]
- 49.Marchler-Bauer A, et al. 2013. CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res. 41, D348–D352. ( 10.1093/nar/gks1243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kufareva I, Ilatovskiy AV, Abagyan R. 2012. Pocketome: an encyclopedia of small-molecule binding sites in 4D. Nucleic Acids Res. 40, D535–D540. ( 10.1093/nar/gkr825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Froese R, Pauly D. (eds). 2013. FishBase. See http://www.fishbase.org (accessed December 2013).
- 52.Xie Z, Frimpong EA, Lee S. 2013. FishTraits version 2: integrating ecological, biogeographic and bibliographic information. In Proc. 13th ACM/IEEE-CS joint Conference on Digital Libraries, pp. 447–448. New York, NY: ACM; See http://www.fishtraits.info/search/attr/ (accessed December 2013). [Google Scholar]
- 53.Ågerstrand M, Breitholtz M, Rudén C. 2011. Comparison of four different methods for reliability evaluation of ecotoxicity data: a case study of non-standard test data used in environmental risk assessments of pharmaceutical substances. Environ. Sci. Eur. 23, 17 ( 10.1186/2190-4715-23-17) [DOI] [Google Scholar]
- 54.Nash JP, Kime DE, Van der Ven LT, Wester PW, Brion F, Maack G, Stahlschmidt-Allner P, Tyler CR. 2004. Long-term exposure to environmental concentrations of the pharmaceutical ethynyloestradiol causes reproductive failure in fish. Environ. Health Perspect. 112, 1725–1733. ( 10.1289/ehp.7209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soares J, et al. 2009. Disruption of zebrafish (Danio rerio) embryonic development after full life-cycle parental exposure to low levels of ethinyloestradiol. Aquat. Toxicol. 95, 330 ( 10.1016/j.aquatox.2009.07.021) [DOI] [PubMed] [Google Scholar]
- 56.Scholz S, Gutzeit HO. 2000. 17 α-Ethinyloestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquat. Toxicol. 50, 363–373. ( 10.1016/S0166-445X(00)00090-4) [DOI] [PubMed] [Google Scholar]
- 57.Balch GC, Mackenzie CA, Metcalf CD. 2004. Alterations to gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17α-ethinyloestradiol. Environ. Toxicol. Chem. 23, 782–791. ( 10.1897/02-539) [DOI] [PubMed] [Google Scholar]
- 58.Hood GM. 2010. PopTools version 3.2.5. See http://www.poptools.org.
- 59.Miller DH, Ankley GT. 2004. Modeling impacts on populations: fathead minnow (Pimephales promelas) exposure to the endocrine disruptor 17 β-trenbolone as a case study. Ecotoxicol. Environ. Saf. 59, 1–9. ( 10.1016/j.ecoenv.2004.05.005) [DOI] [PubMed] [Google Scholar]
- 60.Meng Y, Lin B-L, Tominga M, Nakanishi J. 2006. Simulations of the population-level effects of 4 nonyl-phenol on wild Japanese medaka (Oryzias latipes) . Ecol. Model. 197, 350–360. ( 10.1016/j.ecolmodel.2006.03.022) [DOI] [Google Scholar]
- 61.Hazlerigg CRE, Tyler CR, Lorenzen K, Wheeler JR, Thorbek P. 2014. Population relevance of toxicant mediated changes in sex ratio in fish: an assessment using an individual-based zebrafish (Danio rerio) model. Ecol. Model. ( 10.1016/j.ecolmodel.2013.12.016) [DOI] [Google Scholar]
- 62.Caswell H. 1996. Analysis of life table response experiments II. Alternative parameterizations for size- and stage-structured models. Ecol. Model. 88, 73–82. ( 10.1016/0304-3800(95)00070-4) [DOI] [Google Scholar]
- 63.Gharib WH, Robinson-Rechavi M. 2011. When orthologs diverge between human and mouse. Brief Bioinform. 12, 436–441. ( 10.1093/bib/bbr031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bridgham JT, Carroll SM, Thornton JW. 2006. Evolution of hormone-receptor complexity by molecular exploitation. Science 312, 97–101. ( 10.1126/science.1123348) [DOI] [PubMed] [Google Scholar]
- 65.Bryan MB, Scott AP, Li W. 2008. Sex steroids and their receptors in lampreys. Steroids 73, 1–12. ( 10.1016/j.steroids.2007.08.011) [DOI] [PubMed] [Google Scholar]
- 66.Lee O, Takesono A, Tada M, Tyler CR, Kudoh T. 2012. Biosensor zebrafish provide new insights into potential health effects of environmental estrogens. Environ. Health Perspect. 120, 990–996. ( 10.1289/ehp.1104433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segner H, Casanova-Nakayama A, Kase R, Tyler CR. 2013. Impact of environmental estrogens on fish considering the diversity of estrogen signaling. Gen. Comp. Endocrinol. 191, 190–201. ( 10.1016/j.ygcen.2013.05.015) [DOI] [PubMed] [Google Scholar]
- 68.Matthews JB, Clemons JH, Zacharewski TR. 2001. Reciprocal mutagenesis between human (L349, M528) and rainbow trout (M317, I496) estrogen receptor residues demonstrates their importance in ligand binding and gene expression at different temperatures. Mol. Cell. Endocrinol. 183, 127–139. ( 10.1016/S0303-7207(01)00586-X) [DOI] [PubMed] [Google Scholar]
- 69.Schreurs RHMM, Quaedackers ME, Seinen W, van der Burg B. 2002. Transcriptional activation of estrogen receptor ERα and ERβ by polycyclic musks is cell type dependent. Toxicol. Appl. Pharmacol. 15, 183–192. ( 10.1006/taap.2002.9458) [DOI] [PubMed] [Google Scholar]
- 70.Mims MC, Olden JD, Shattuck ZR, Poff NL. 2010. Life history trait diversity of native freshwater fishes in North America. Ecol. Freshw. Fish 19, 390–400. ( 10.1111/j.1600-0633.2010.00422.x) [DOI] [Google Scholar]
- 71.Larsen MG, Hansen KB, Henriksen PG, Baatrup E. 2008. Male zebrafish (Danio rerio) courtship behaviour resists the feminising effects of 17 α-ethinyloestradiol, morphological sexual characteristics do not. Aquat. Toxicol. 87, 234–244. ( 10.1016/j.aquatox.2008.02.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.