Abstract
Endocrine-disrupting chemicals (EDCs) in municipal effluents directly affect the sexual development and reproductive success of fishes, but indirect effects on invertebrate prey or fish predators through reduced predation or prey availability, respectively, are unknown. At the Experimental Lakes Area in northwestern Ontario, Canada, a long-term, whole-lake experiment was conducted using a before-after-control-impact design to determine both direct and indirect effects of the synthetic oestrogen used in the birth control pill, 17α-ethynyloestradiol (EE2). Algal, microbial, zooplankton and benthic invertebrate communities showed no declines in abundance during three summers of EE2 additions (5–6 ng l−1), indicating no direct toxic effects. Recruitment of fathead minnow (Pimephales promelas) failed, leading to a near-extirpation of this species both 2 years during (young-of-year, YOY) and 2 years following (adults and YOY) EE2 additions. Body condition of male lake trout (Salvelinus namaycush) and male and female white sucker (Catostomus commersonii) declined before changes in prey abundance, suggesting direct effects of EE2 on this endpoint. Evidence of indirect effects of EE2 was also observed. Increases in zooplankton, Chaoborus, and emerging insects were observed after 2 or 3 years of EE2 additions, strongly suggesting indirect effects mediated through the reduced abundance of several small-bodied fishes. Biomass of top predator lake trout declined by 23–42% during and after EE2 additions, most probably an indirect effect from the loss of its prey species, the fathead minnow and slimy sculpin (Cottus cognatus). Our results demonstrate that small-scale studies focusing solely on direct effects are likely to underestimate the true environmental impacts of oestrogens in municipal wastewaters and provide further evidence of the value of whole-ecosystem experiments for understanding indirect effects of EDCs and other aquatic stressors.
Keywords: endocrine disrupters, ethynyloestradiol, indirect food-web effects, whole-ecosystem study, aquatic toxicology
1. Introduction
Reproduction and development in aquatic biota are adversely affected by exposure to endocrine-disrupting chemicals (EDCs), such as exogenous oestrogens found in municipal effluents [1]. Species responses to these environmental oestrogens vary considerably, with fishes having much higher sensitivities than invertebrates in laboratory toxicity studies [2,3]. Fishes exposed to EDCs exhibit an array of direct responses including depressed circulating sex steroids, reduced gonad size and fecundity, altered reproductive behaviours, and feminized males as evidenced by vitellogenin production and intersex [4,5]. Presently, the compounds of greatest concern are the natural (e.g. 17β-oestradiol; E2) and synthetic (17α-ethynyloestradiol; EE2) oestrogens [6]. Although some loss occurs during wastewater treatment [7,8], oestrogen concentrations considered to be protective [2] are sometimes exceeded in effluents and waters downstream [9,10], and could affect the sustainability of resident fish populations [11]. It is possible that declines in fish populations (such as in [11]) lead to indirect changes in other taxa through trophic cascades, and this is the main focus of this study.
A whole-lake experiment was conducted to understand whether continuous, summer additions of 5–6 ng l−1 of EE2 in 2001–2003 affected the development and abundance of fishes and other biota in Lake 260 at the Experimental Lakes Area (ELA) in northwestern Ontario, Canada. Elevated vitellogenin (induced up to 24 200-fold above reference fish) and delayed spermatocyte production in male fathead minnow (Pimephales promelas) preceded the species' reproductive failure, with losses of the smaller size classes and a rapid decline in abundance that continued for 2 years after EE2 additions stopped [11,12]. Males of another smaller bodied fish in the lake, the pearl dace (Margariscus margarita), had vitellogenin concentrations that were 2300- to 15 900-fold higher than reference or pre-treatment fishes. All exposed males showed delays in spermatocyte development, and intersex was found in about one-third of the males [12,13]. By contrast, while male white sucker (Catostomus commersonii) and lake trout (Salvelinus namaycush) in Lake 260 produced higher concentrations of plasma vitellogenin (inductions of 118- to 18 700-fold, respectively), no delays in sperm cell development were observed in either species in direct response to EE2 exposures [12].
Despite their importance in aquatic food webs, abundance and potential to act as sentinels, little is known about the impacts of EDCs on wild populations of freshwater invertebrates. The diversity of aquatic invertebrates is considerable and the details of their endocrine systems are well documented for only a few groups, such as insects and molluscs. Although vertebrate-like steroids have been reported in a wide range of invertebrate phyla, including insects (e.g. [14,15]), evidence for a role of steroid sex hormones in invertebrate reproduction is limited to the Mollusca, Annelida and Echinodermata [16,17], but findings remain controversial [18]. However, oestradiol may play a role in metabolic activities not directly related to reproduction in some invertebrate phyla (e.g. [19]). There is some evidence that aqueous exposure to EE2 and other oestrogens directly affect moulting, reproduction or enzyme activity. Oestrogens or their mimics (i.e. EE2, E2, nonylphenol) inhibit juvenile development, alter sex ratios and affect egg production of cladocerans, copepods and rotifers (e.g. [20–24]). Some effects such as earlier emergence, disturbed gonad development and delayed hatching have also been observed on benthic organisms such as amphipods, molluscs and chironomids (reviewed by Segner et al. [25]). Typically, responses in invertebrates are seen only at comparatively high concentrations (microgram per litre to milligram per litre levels), although decreased abundance of adult copepods and cyclopoids and enzyme activities can occur at concentrations of EE2 as low as 10 ng l−1 [24]. Few studies have examined whether invertebrate community structure is affected by EE2 or other oestrogens [26], and the long-term effects of oestrogen exposure on wild populations of zooplankton and benthic invertebrate populations and communities are unknown.
EDCs may affect aquatic invertebrates not only through direct toxic impacts but also by indirect changes in food-web interactions such as predation or competition [27]. The type and magnitude of indirect effect depends on whether the predator is more or less sensitive to the chemical than the prey [27]. Most studies of indirect food-web effects from contaminants (mainly pesticides) show top-down, rather than bottom-up, responses [27]. If EDCs such as EE2 affect the abundance of fishes like the fathead minnow [11] that feed mainly on invertebrates, it is likely that there will be increases in these taxa through reductions in predation pressure.
Herein, we examine the long-term responses of the Lake 260 food web to additions of EE2 by assessing the composition and abundance of plankton and littoral invertebrates and the abundance and biomass of fishes, including the top predator lake trout, before, during and after amendments to a whole lake. We sought evidence for both direct and indirect impacts of this potent oestrogen on taxa abundance and community composition in the Lake 260 food web. Species' abundances may decline if reproduction or survival is directly affected by EDCs (as in [11]). In turn, losses of species that are key components of the food web may indirectly affect the abundances of their predators or prey because of the tight trophic linkages found in aquatic systems [27,28]. Whereas direct effects are expected to appear during or shortly after EDC exposures, indirect effects may become manifest both during exposures and well after the chemicals are present in the system.
2. Material and methods
Lake 260 has a surface area of 34 ha and a maximum depth of 14.4 m. The lake is oligotrophic and had mean epilimnetic total phosphorous and chlorophyll a concentrations of 7.2 and 2.3 μg l−1, respectively, during the open water season and prior to manipulation. Other chemical characteristics are typical of boreal shield lakes in the ELA region [29,30]. Baseline data on plankton, macroinvertebrates and fish were collected over two summers (1999, 2000). Nearby lakes, Lakes 442, 224, 114, Roddy (468), 239 and 373, were used as reference systems. Sampling continued for 3 years during (2001–2003) and 2 years after (2004–2005; exceptions below) EE2 additions to Lake 260. When possible, results are categorized within ‘baseline’ (1999–2000), ‘treatment’ (2001–2003) and ‘post-treatment’ (2004–2005) periods.
After Lake 260 had stratified in the spring until shortly before turnover in the autumn of 2001–2003, EE2 was added to the epilimnion for 20–21 weeks. Details on the additions, water sampling and analyses to quantify EE2 are given in Palace et al. [13] and Park & Kidd [30]. Seasonal mean concentrations for the summer were 5.0, 6.1 and 4.8 ng l−1 for 2001 through 2003, respectively, and lower concentrations were also measured under the ice; these EE2 data are described in more detail in Palace et al. [13]. No water samples were collected after 2003 for EE2 analyses.
Phytoplankton and bacteria samples were obtained biweekly during the ice-free seasons from the epilimnion and over the deep station using an integrating sampler (2 l) [31] in Lake 260 and four unimpacted reference lakes (Lakes 224, 239, 373 and 442). The epilimnion was defined as the surface layer of water with uniform temperature (ignoring any shallow temporary stratification phenomena). Enumeration and biomass estimation of phytoplankton and bacteria followed methods described in Findlay et al. [32].
Zooplankton were collected biweekly during the day from Lake 260 and the four reference lakes throughout the ice-free season. In each lake, collection methods remained consistent throughout the period of study, although different approaches were used in Lakes 260, 373 and 442 and in Lakes 239 and 240. In Lakes 260, 373 and 442, zooplankton were collected from the entire water column at multiple stations using a PVC hose calibrated for volume, whereas Lakes 224 and 239 were sampled at the deep station using vertical hauls of a double-barrelled plankton net [33]. All samples were collected using a 53 µm mesh and preserved in 4% sugar-formalin. Counts and biomass estimation followed methods in Paterson et al. [33]. Rotifer data were only available for the full 7-year period from reference Lakes 224 and 239.
We used a before-after-control-impact (BACI) design to assess the effects of EE2 on bacteria, phytoplankton and zooplankton [34,35]. This analysis used the differences between monthly plankton biomasses in Lake 260 and the monthly means of comparable data from the unimpacted reference lakes. Using a one-way ANOVA, pre-impact (‘baseline’) differences (1999–2000) were compared with differences in two different ‘after’ periods: (i) the 3 years of EE2 additions (2001–2003; treatment) and (ii) the two post-treatment years (2004–2005). We separated the two post-impact periods to help distinguish direct toxic impacts of EE2 additions from indirect impacts mediated through the food web. Our rationale was that direct toxic impacts were most likely to occur during the years of EE2 addition, whereas indirect food-web effects may occur both in the years of EE2 addition and afterward. EE2 has a short half-life in the water column of this lake (12 days; K Kidd 1999, unpublished data) and was believed to be rapidly lost from the water column and from sediments of the lake after EE2 amendments stopped. All data were log-transformed prior to analysis.
Changes in monthly species composition of plankton were analysed using reciprocal averaging (RA; [36]). Density data (numbers per litre) were log-transformed and only taxa that represented greater than 5% of total abundance for Crustacea and rotifers, and greater than 1% for phytoplankton were included. Resulting scores on axes 1 and 2 of the RA were tested for differences using the BACI design described above. Ordinations were completed using PC-Ord 6, and all other statistical analyses were completed using SYSTAT 12.
Chaoborus is a dipteran zooplankton predator and an important food item for many fish species. It is typically benthic during the day and vertically migrates into the water column at night. Chaoborus were sampled in Lake 260 every four weeks from 1999 to 2004 during the ice-free season at least 1 h after sunset at a station located over the deepest part of the lake using vertical hauls from the entire water column of a 0.5 m diameter 150 μm net. Instars were separated using measures of head-capsule length [37]. Chaoborus were not sampled in 2005. We also provide data from unimpacted Lake 240, which was sampled from 2000 to 2004, as a reference for changes in Lake 260.
Duplicate pyramidal emergence traps [38], built following Schmude et al. [39], were set over approximately 70 cm of water at three sites in Lakes 260 and 442 and the emerging adult insects were collected weekly over the summers of 1999–2002. The three sites represented three different habitat types common in the littoral zone of the two lakes: small rock (mixture of cobble and gravel), cobble–boulder mix and boulder with woody debris. Emerged insects were initially preserved in a mixture of ethylene glycol and ethanol, and were then transferred to 70% ethanol upon weekly collection of the trap samples. Insects were identified to family using Merritt & Cummins [40]. No statistical analyses of these data were done because the emergence trap samples were not independent replicates. At the same sites, artificial substrates (0.01 m3 wire baskets containing local cobble) were used to monitor benthic invertebrate populations in Lakes 260 and 442 in 2000 and 2001 only (see the electronic supplementary material for details on methods). Molluscs were present in these two lakes, but were too small to use for anything other than abundance assessments.
The fish communities in Lakes 260 and 442, the two lakes used for fish assessments, consisted of fathead minnow, northern redbelly dace (Phoxinus eos), finescale dace (Phoxinus neogaeus), lake chub (Couesius plumbeus), pearl dace, slimy sculpin (Cottus cognatus), blacknose shiner (Notropis heterolepis), brook stickleback (Culaea inconstans), white sucker and the piscivorous lake trout. The main prey fish species for lake trout in Lakes 260 and 442 are fathead minnow, pearl dace and slimy sculpin (electronic supplementary material, figures S9 and S10).
Fishing abundance data were based on catch-and-release methods using trap nets (spring and autumn, all species) and short (30 min) evening gill net sets on spawning shoals for lake trout (autumn). The community composition of small-bodied fishes, based on relative abundance (i.e. catch-per-unit-effort, CPUE) is shown in the electronic supplementary material, figures S9 and S10 (derived from autumn trap nets). Abundance estimates for adult fathead minnow and pearl dace are from Guzzo et al. [41] and based on autumn sampling with minnow traps. Biomass of these minnow species was estimated as the product of abundance and mean size from minnow trap captures, standardized by lake area. Mark-and-recapture techniques were used to estimate the abundance of lake trout (autumn data) and white sucker (spring data) in Lakes 260 and 442. All lake trout were tagged, as were a subset of white sucker. Handling mortality for all fish encountered was minimal throughout the study (less than 1% of all netted fish). Abundance of lake trout and white sucker was estimated using the POPAN method in Program Mark (v. 7.1, [42]). A fully parametrized model was implemented to provide annual parameter estimates for survival, catchability and entry to the population, from which annual population estimates were derived. Population estimates were based on the complete dataset for Lakes 260 (1984–2006) and 442 (1990–2012), but the data presented here are limited to the period 1999–2005. A number of lake trout and white sucker were removed from the population to permit histopathological analyses during the experiment [12]. To account for known mortalities due to fish removal and to separate removal effects from an ecosystem response to EE2 in our population estimates, we adjusted the estimated abundance in both lakes to include the cumulative number of fish removed for histopathology. Significant changes in fish abundance were evaluated by comparison of 95% confidence intervals (CIs) around annual abundance estimates. Biomass was estimated as the product of abundance estimates and mean size, standardized by lake area.
Body condition of large fishes was evaluated in Lake 260, and compared with patterns in three reference lakes (Lakes 224, 373, 442). White sucker condition was estimated using Fulton's condition factor K [43]. To avoid length-based bias due to known size-dependence of this condition index [44], comparisons for this species were limited to fish that had achieved a size approaching asymptotic growth (greater than 400 mm total length in ELA lakes, M Rennie, K Mills, S Chalanchuk 2014, unpublished data). However, in Lakes 442 and 224 Fulton's K for males was estimated for fish between 300 and 400 mm total length to achieve sufficient sample sizes. Condition of lake trout was estimated for male and female fish separately using a published standard weight equation [45] and is expressed as a percentage of standard weight. Patterns in condition were compared with a BACI design in each lake for both species. Using the statistical package R [46], we used linear mixed effects modelling (lmer) in the lme4 package to compare patterns in condition within each lake. Three time periods were compared: baseline (1999–2000), treatment (2001–2003) and post-treatment (2004–2005) years, and year was modelled as a nested random effect within time period. Differences among time periods (fixed effects) were examined with a Tukey's test using glht() in the multcomp package. Although only Lake 260 received EE2 additions, these time periods were also compared among reference lakes.
3. Results
(a). Phytoplankton and bacteria
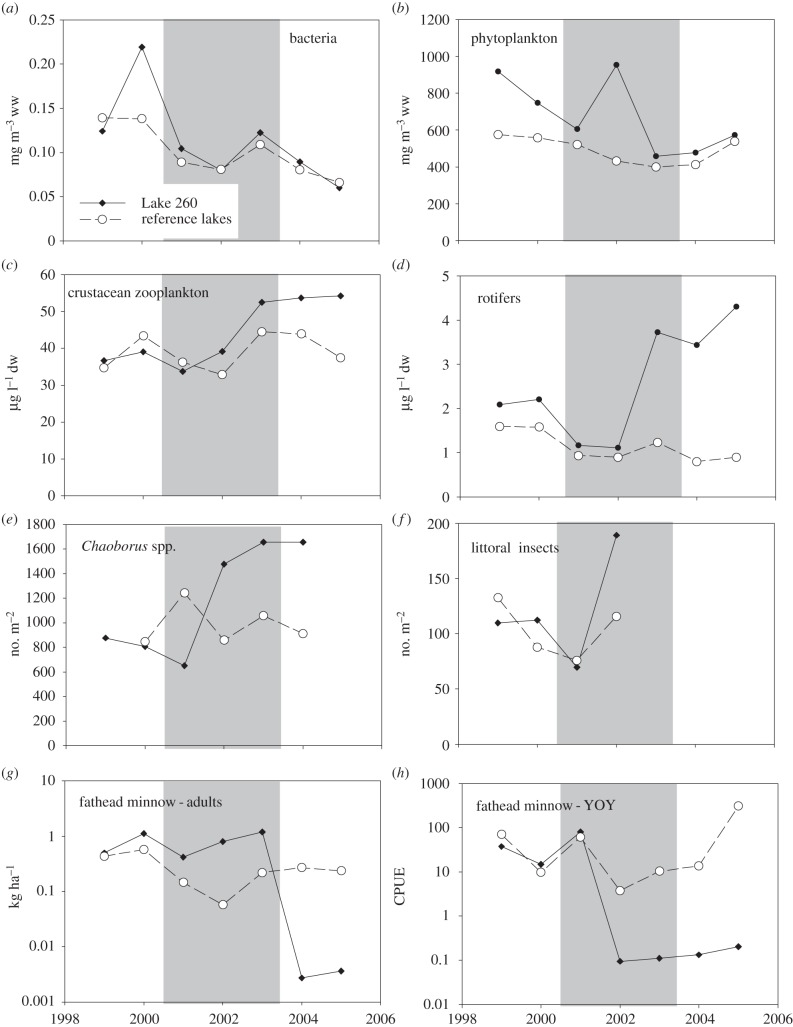
There was no apparent effect of EE2 additions on total phytoplankton biomass in Lake 260 (figure 1). Phytoplankton biomass in Lake 260 was variable in the baseline years, averaging 832 mg m−3 wet weight (ww) in 1999–2000. Annual algal biomass averaged 672 mg m−3 ww during treatment and then decreased to an average of 525 mg m−3 ww in the post-treatment period. A similar relative decline in total phytoplankton biomass from 566 to 474 mg m−3 ww was observed in the four reference lakes between 1990 and 2005 (figure 1) and none of the changes observed in Lake 260 were statistically significant when compared with baseline data and the reference lakes (one-way ANOVA; F2,37, p > 0.47; table 1).
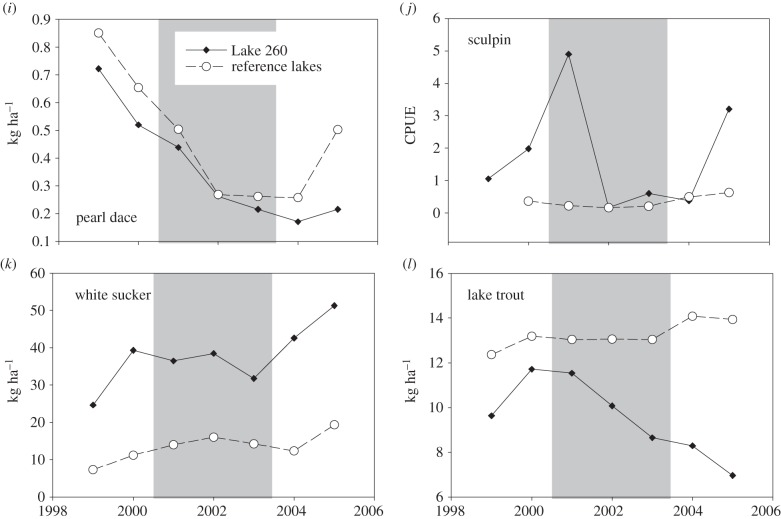
Figure 1.
Biomass or abundance of several taxa during baseline (1999–2000), treatment (2001–2003; shaded area) and post-treatment (2004–2005) periods in Lake 260, and comparisons with data from one or several reference lakes (see below) over the same time period. (a) Annual average biomass of epilimnetic bacteria (mg m−3) in Lake 260 and reference lakes (Lakes 224, 239, 373 and 442) standardized to the long-term average biomass in the suite of reference lakes. (b) Annual mean biomass of epilimnetic phytoplankton (mg m−3) in Lake 260 and reference lakes (Lakes 224, 239, 373 and 442). (c) Annual mean biomass of crustacean zooplankton (μg l−1) in Lake 260 and reference lakes (Lakes 224, 239, 373 and 442). (d) Annual mean biomass of rotifers (μg l−1) in Lake 260 and reference lakes (Lakes 224 and 239). (e) Annual aerial abundances of Chaoborus spp. (number m−2) in Lake 260 and reference Lake 240. (f) Annual average aerial abundances of emerging littoral invertebrates (number m−2) in Lake 260 and reference Lake 442. (g) Annual mean biomass of adult fathead minnow (kg ha−1) in Lake 260 and reference Lake 442 (autumn minnow traps). (h) Annual mean CPUE of YOY fathead minnow in Lake 260 and reference Lake 442 (autumn trap netting). (i) Annual mean biomass of adult pearl dace (kg ha−1) in Lake 260 and reference Lake 442 (autumn minnow traps). (j) Annual autumn CPUE of slimy sculpin in Lake 260 and reference Lake 442 (autumn trap netting). (k) Annual mean biomass of white sucker (kg ha−1) in Lake 260 and reference Lake 442 (spring trap netting). (l) Annual mean biomass of adult lake trout (kg ha−1) in Lake 260 and reference Lake 442 (autumn trap and gill netting).
Table 1.
Results of one-way BACI ANOVAs comparing differences between monthly mean data for bacteria, phytoplankton and zooplankton from Lake 260 and for unimpacted reference lakes before, during and after EE2 additions. No corrections were made for multiple comparisons.
| group | F ratio | d.f. | p-values |
|---|---|---|---|
| bacteria (mg m−3 ww) | 1.88 | 2, 34 | 0.17 |
| total phytoplankton (mg m−3 ww) | 0.78 | 2, 37 | 0.47 |
| Cyanophyta (mg m−3 ww) | 0.28 | 2, 37 | 0.76 |
| Chlorophyta (mg m−3 ww) | 0.19 | 2, 37 | 0.83 |
| Chrysophyta (mg m−3 ww) | 0.30 | 2, 37 | 0.74 |
| diatoms (mg m−3 ww) | 0.27 | 2, 37 | 0.49 |
| Cryptophyta (mg m−3 ww) | 0.98 | 2, 37 | 0.39 |
| total Crustacea (mg m−3 dw) | 3.01 | 2, 39 | 0.06 |
| Calanoida (mg m−3 dw) | 6.23 | 2, 39 | 0.004 |
| Cyclopoida (mg m−3 dw) | 1.91 | 2, 39 | 0.16 |
| Cladocera (mg m−3 dw) | 0.16 | 2, 39 | 0.86 |
| Rotifera (mg m−3 dw) | 10.03 | 2, 37 | <0.0001 |
| crustacean richness | 2.05 | 2, 39 | 0.14 |
| rotifer richness | 0.41 | 2, 37 | 0.67 |
Additions of EE2 to Lake 260 also did not affect the community composition or diversity of the epilimnetic phytoplankton community (electronic supplementary material, figure S1; RA ordination and BACI test on axes 1 and 2; ANOVA; F2,39, p > 0.43 and p > 0.19, respectively). The phytoplankton in Lake 260 were dominated by chrysophytes (71.4%) in 1999, similar to the reference lakes, and by chrysophytes (47.3%) and diatoms (mainly Cyclotella sp.; 30.6%) in 2000. During treatment years, phytoplankton in Lake 260 were dominated by colonial chrysophytes (56.2–68.5%), and there was an increase in cyanobacteria (Snowella sp.) that was also observed in the reference lakes. In 2004, the Lake 260 phytoplankton assemblage was co-dominated by colonial chrysophytes (47.8%) and diatoms (Cyclotella sp.; 25.2%), similar to that observed in 2000. Algal dominance shifted back to 66.8% chrysophytes in 2005, during the second year of recovery from EE2 additions. Within each algal group in Lake 260, no significant differences were found in epilimnetic biomass when comparing the baseline years (1999–2000) to years during and after EE2 treatment (table 1). Simpson's diversity index for algae decreased from an average of 0.81 in 1999 and 2000 to 0.74 and 0.67 in 2001 and 2002, respectively. This decrease did not continue in 2003–2005 and was not atypical when compared with reference lakes and to historical data from this lake (not shown).
EE2 also did not appear to affect the epilimnetic microbial community during or after treatment (figure 1). The Lake 260 bacterial community was consistently dominated by coccoidal-shaped cells ranging in size from 0.4 to 0.9 µm. Bacterial biomass averaged 0.16 µg l−1 in the baseline years, and 0.08 µg l−1 in 2001–2005 during the addition of EE2 and post-treatment years. However, bacterial biomass in reference Lake 373 averaged 0.12 µg l−1 in 1999–2000 and then also decreased from 2001 to 2005, averaging 0.07 µg l−1 over this time period. Changes in bacterial biomass were not statistically significant when compared with the changes in the unimpacted reference lakes (table 1; F2,34, p > 0.17).
(b). Zooplankton
The crustacean zooplankton community of Lake 260 was typical of ELA lakes and more than 90% of the biomass comprised seven taxa: Diaptomus minutus, Daphnia galeata mendotae, Diaptomus sicilis, Diacyclops thomasi, Mesocyclops edax and Tropocyclops extensus. Average crustacean zooplankton biomass in Lake 260 increased from a baseline average of 37.9 to 41.8 and 54 µg l−1 dry weight (dw) in the treatment and post-treatment periods, respectively (figure 1). When compared with the reference lakes and baseline data, this increase was not statistically significant (one-way ANOVA; F2,39, p > 0.06). The biomass of Cladocera and cyclopoid copepods did not change statistically post-treatment (electronic supplementary material, figure S2; table 1), but an increase in calanoid copepod biomass was statistically significant (table 1; ANOVA; F2,39, p < 0.005). This was primarily because calanoids declined in the reference lakes in 2005, but not in Lake 260 (electronic supplementary material, figure S2). In other years, calanoid biomass was similar in the reference lakes and in the study lake. Although the biomass of different crustacean groups did not change dramatically relative to the reference lakes, some variations in average densities of different taxa were observed (electronic supplementary material, figure S3a,b). Most notably, numbers of T. extensus declined in 2004 and this species was virtually absent from Lake 260 in 2005. Crustacean species richness was not statistically different during or after EE2 additions, when compared with the reference lakes (one-way ANOVA; F2,39, p > 0.14). Overall changes in crustacean species composition based on axes 1 and 2 of the RA ordination were not statistically different among treatment periods when compared with the reference lakes (electronic supplementary material, figure S4; BACI ANOVA axis 1; F2,39, p > 0.29; axis 2, p > 0.22). Where sufficient data were available, there was no evidence that EE2 additions affected sex ratios or egg ratios of dominant zooplankton taxa (data not shown).
The rotifer community of Lake 260 was also typical of ELA lakes, with the dominant taxa being Keratella cochlearis, Polyarthra vulgaris, Synchaeta sp., Collotheca sp. and Keratella taurocephala. Rotifer biomass averaged 2.6 μg l−1 dw in the baseline period and then decreased to an average of 1.1 μg l−1 in 2001 and 2002 (figure 1). From 2003 to 2005, rotifer biomass averaged 3.8 μg l−1. When compared with changes in the reference lakes, rotifer biomass was not statistically different in 2001 and 2002 from baseline years (Tukey's post hoc test; p = 0.38), but increased significantly in 2003–2005 (ANOVA; F2,37, p < 0.01; Tukey's post hoc test; p < 0.001). Not all rotifer taxa increased simultaneously in Lake 260. Most notably, P. vulgaris, Polyarthra remata and Synchaeta sp. increased in abundance, whereas densities of K. taurocephala decreased (electronic supplementary material, figure S5). Changes in rotifer richness were within expected levels of natural variation (electronic supplementary material, figure S2). Axis 1 of the RA ordination of rotifer data explained 25% of the variation in the overall dataset. From 1999 to 2005, rotifer RA scores shifted along axis 1, and overall species composition as measured by RA axis 1 was significantly different in the post-treatment years when compared with the two baseline years (ANOVA axis 1; F2,39, p < 0.01; axis 2, p < 0.1; electronic supplementary material, figure S6).
Both Chaoborus punctipennis and C. flavicans occurred in Lake 260. In 1999 and 2000, densities of instars 3 and 4 C. punctipennis and C. flavicans at centre-buoy were 212 and 378 animals per m2, respectively (electronic supplementary material, figure S7). After the initiation of EE2 additions in 2001, densities of C. punctipennis increased to an average of 438 and C. flavicans to an average of 563 animals per m2. Densities of instar 1 and 2 Chaoborus, which could not be identified to species, increased from an average of 252 animals per m2 in 1999–2000 to 377 animals per m2 in 2001–2004. Similar changes in total Chaoborus densities were not observed in unimpacted Lake 240 between 2000 and 2004 (figure 1). BACI analysis of the Chaoborus data was not possible because of the absence of pre-impact data from reference Lake 240 and the fact that Chaoborus have lifespans of 1–2 years in ELA lakes, making data points within years non-independent. Nonetheless, the data are consistent with an effect of EE2 additions.
(c). Adult and larval littoral insects
Total emergence of adult littoral insects was similar in Lakes 260 and 442 in all years but 2002; in the second year of EE2 additions, aerial abundances in emergence traps in Lake 260 increased about twofold when compared with previous years (electronic supplementary material, figures S1 and S8a). Chironomids were the dominant taxa emerging from Lakes 260 and 442 and their weekly emergence rates ranged from 16 to 99 individuals per m2 and from 19 to 122 individuals per m2 in Lakes 260 and 442, respectively, over the 4 years of sampling. Trichoptera and ceratopogonid midges made up smaller percentages of the adult emergence in both lakes over the period of the study (see the electronic supplementary material for details). Within major groups, results indicated no effects of EE2 on the emergence of adult Chironomidae, Trichoptera and Ceratopogonidae from Lake 260 (electronic supplementary material, figure S8b), nor any effects on taxa richness (not shown), but a suggestion of behavioural changes within the chironomids Tanytarsini (electronic supplementary material, figure S9). For the artificial substrates, there was a significant increase in the total number of invertebrates in Lake 260 during the first year of EE2 additions (p < 0.005; electronic supplementary material, figure S10), but no individual taxonomic group demonstrated a significant effect of EE2 (electronic supplementary material, table S1).
(d). Fish populations
In the baseline years, the small-bodied fish communities in Lakes 260 and 442 were dominated by fathead minnow (electronic supplementary material, figures S11 and S12). In the reference system (Lake 442), fathead minnow remained dominant for the duration of the study (electronic supplementary material, figure S12). After 2 years of EE2 additions, fathead minnow in Lake 260 declined dramatically and were replaced by pearl dace as the dominant forage fish species from 2002 through 2005 (electronic supplementary material, figure S11). Total small fish CPUE in Lake 260 was lower after EE2 additions in 2002 than in the first 3 years (1999–2001). Total CPUE of the small fish also declined in reference Lake 442 during the same period but was always more than an order of magnitude higher than the CPUE from EE2-treated Lake 260, and in 2005 the small fish CPUE estimates in Lake 442 were three orders of magnitude greater than in Lake 260.
Abundance and biomass of young-of-year (YOY) and adult fathead minnow, slimy sculpin and lake trout declined in Lake 260 during this experiment, in a fashion not observed in our reference lake (figure 1). Lake trout abundance during the post-treatment period was significantly lower than baseline years (non-overlapping 95% CIs, electronic supplementary material, figure S13). In 2003, lake trout biomass declined to 77% of the biomass that was measured at the start of the study, and 58% of the peak biomass observed in 2000, the year immediately prior to EE2 additions (figure 1). Fathead minnow YOY relative abundance declined sharply in 2002, and remained barely detectable and well below 1999–2001 estimates until after 2005. No similar degree of variation for either species was observed in Lake 442. The biomass of adult fathead minnow in Lake 260 declined by more than two orders of magnitude in the post-treatment years and abundance was statistically indistinguishable from zero (95% CIs included zero; electronic supplementary material, figure S13). Slimy sculpin relative abundance (CPUE) fell significantly below baseline levels in 2002 (CPUE was seven times lower during 2002–2004 compared with 1999–2001), and remained low until 2005. Although pearl dace also declined in Lake 260 during the oestrogen amendments, the trend was a continuation from the baseline period and was reflected in the pearl dace population in reference Lake 442. White sucker abundance showed no clear response to EE2 additions in Lake 260 and appeared to increase over the duration of the study in Lake 442. Although white sucker abundance in 2002 in Lake 260 was among the highest observed during the study (electronic supplementary material, figure S13), mean size of sucker was nearly half that observed in any other year, resulting in comparatively similar biomass estimates in 2002 compared with adjacent years (figure 1).
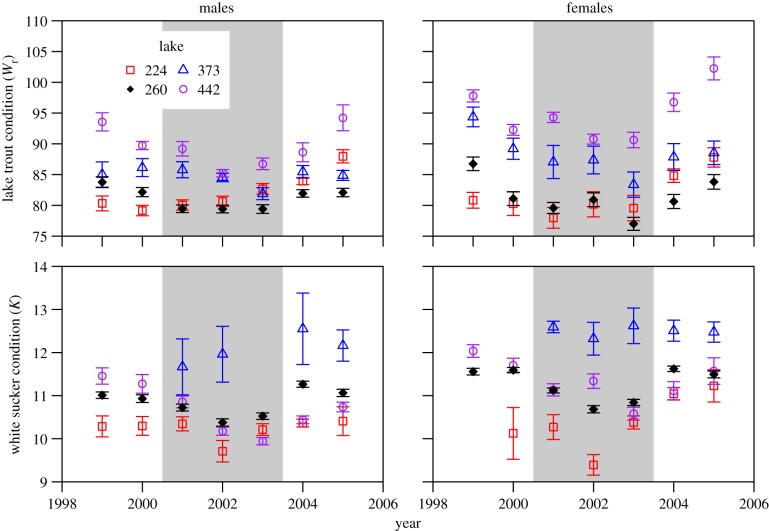
Condition declined significantly in male lake trout and both male and female white sucker during treatment years and increased in 2004, the first year post-treatment (figure 2; electronic supplementary material, table S2). No reference lakes demonstrated a similar pattern in condition; in most cases, there were no differences among time periods in reference lakes, and where differences occurred, they were not consistent with the pattern seen in Lake 260. Condition of female lake trout declined from the beginning of baseline observations to a minimum value in 2003 and increased thereafter; a similar pattern was observed in reference Lakes 373 and 442, suggesting this trend was unrelated to EE2 additions. There were no discernible changes in sex ratio for either lake trout or white sucker during the course of the experiment, nor any evidence of a change in size or age at maturity for either species (not shown).
Figure 2.
Condition of lake trout (expressed as relative weight (Wr), a percentage of standard weight) and white sucker (Fulton's K) for males and females. Lake 260 (filled diamonds) was amended with EE2 from 2001 to 2003; Lakes 224 (squares), 373 (triangles) and 442 (circles) are all reference lakes. Error bars are 1 s.e. (Online version in colour.)
4. Discussion
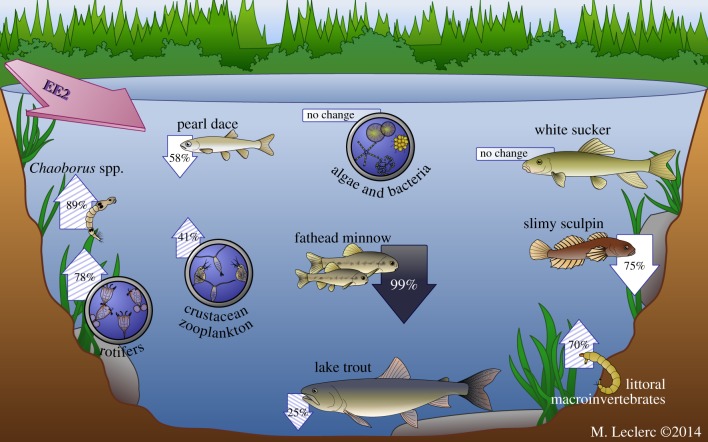
In this whole-ecosystem study, we found evidence of both direct and indirect effects of EE2 on the abundance of fishes but little evidence of direct effects of this synthetic oestrogen on lower-trophic-level organisms (figure 3). Both during and after treatment, the CPUE and biomass of YOY and adult fathead minnow, respectively, declined as a consequence of their reproductive failure beginning in year 2 of the EE2 treatments [11]. By contrast, the timing of abundance and biomass decline of the top predator fish, lake trout, is more consistent with a response to the decreases in its prey species, rather than from direct effects of EE2 on its reproduction [12]. No evidence of direct toxic effects of EE2 on abundance of bacteria, phytoplankton, zooplankton, Chaoborus and emerging or larval littoral invertebrates was found. However, increases in some taxa, such as Chaoborus, crustacean zooplankton, rotifers and total invertebrates on the artificial substrates and in the emergence traps, occurred during the experiment which could be explained as indirect effects based on a reduction in predation from fishes in the Lake 260 food web.
Figure 3.
Schematic of the population responses observed in the Lake 260 food web during and after three summers of EE2 additions. Solid arrows represent direct effects, hatched arrows represent likely indirect effects and white arrows represent potential direct effects. The relative magnitudes of the responses are indicated by percentage changes in abundance or biomass over the following periods: fathead minnow (combined; YOY 1999–2000 to 2002–2005; adults 1999–2000 to 2004–2005); pearl dace (1999–2000 to 2001–2005); slimy sculpin (1999–2000 to 2002–2004); lake trout (1999–2000 to 2003–2005); Chaoborus (1999–2000 to 2002–2004); littoral macroinvertebrates (emergence data 1999–2000 to 2002); crustacean zooplankton (1999–2000 to 2003–2005); rotifers (1999–2000 to 2003–2005). (Online version in colour.)
(a). Direct effects of EE2 on the Lake 260 food web
There was little evidence of direct negative effects of EE2 on the invertebrate community of Lake 260. Despite occasional studies showing increased reproduction of invertebrates following EE2 exposure [25,47] that may explain the increases in some Lake 260 taxa, it is generally expected that low nanogram per litre concentrations of EE2 would not reduce population abundance [48–50] and, in this study, no large or statistically detectable decreases in the abundances and biomass of any zooplankton or benthic invertebrate group in Lake 260 were observed in the first 2 years of EE2 additions. By 2003, several generations of zooplankton and of some littoral invertebrates had been exposed to EE2 without demonstrable negative effects. It is important to note that concentrations of EE2 in Lake 260 during our experiment were far lower than concentrations that are known to affect survival of zooplankton and macroinvertebrates [2,25].
We found strong evidence that EE2 additions to Lake 260 directly reduced populations of fathead minnow and possibly pearl dace and sculpin. By 2002, age-0 fathead minnow were virtually absent in the lake, and by 2004 the adult fathead minnow biomass had declined by two orders of magnitude, to less than 1% of the biomass in the previous 4 years. Fathead minnow showed elevated expression of vitellogenin, intersex and disruption of gonadal development, which ultimately led to reproductive failure and loss of the 2002–2005 year classes [11]. The delayed decline in adult biomass (2004) is probably because the lifespan of fathead minnow is typically 2 years [51]. Pearl dace from Lake 260 also displayed elevated vitellogenin and disruption of testicular and ovarian development, including intersex [12,13]. In association with these changes, pearl dace biomass declined steadily through to 2004, when the biomass was only 24% of that observed in 1999. However, interpretation of the cause of this decline is complicated by the fact that pearl dace biomass also declined in reference Lake 442. The relative abundance of slimy sculpin in Lake 260 also declined sharply in 2002, and remained low until 2004, a trend that was not observed in the reference system. While sculpin were not examined for vitellogenin or gonadal effects, the timing of the decline was coincident with that of YOY fathead minnow and suggests that it may also be a direct response to EE2 exposure.
There was also some evidence of direct effects of EE2 on individuals of the larger bodied fishes in Lake 260, but not on their populations. Condition of male lake trout and of male and female white sucker declined during EE2 additions, and the timing was concurrent with increases in plasma concentrations of vitellogenin in these two species [12], and before the major declines in prey species for lake trout (this study, [11]); in addition, condition of the fish improved once additions ceased and before recovery of the small-bodied fishes. In a whole-lake acidification experiment at the ELA, decreases in lake trout condition also occurred but only 2–3 years after prey species disappeared [52], lending strength to the idea that EE2 had a direct effect on this endpoint. Body condition is also subject to many other factors besides prey availability, including spawning state [44]. Changes in condition may, therefore, reflect effects of EE2 on the reproductive output of these two species, though gonadal somatic index was previously reported to be unaffected by EE2 exposure in these populations [12]. Lake trout abundance and biomass declined after 2002, the timing of which is more suggestive of indirect effects mediated through the food web (see below), rather than direct effects of EE2 given the lack of disruption in gonad development in both males and females and the occasional but low induction of vitellogenin in female lake trout [12]. Similarly, biomass and abundance of white sucker did not change in Lake 260 following EE2 additions, suggesting a lack of population-level response for this species.
(b). Indirect effects of EE2 on the Lake 260 food web
Because several small fishes declined in Lake 260 following EE2 additions, we sought evidence for a trophic cascade resulting from the associated reductions in planktivory and benthivory. In addition, both cyprinids and sculpin are important prey for lake trout [51] and, as such, we anticipated associated changes in this predator's biomass and abundance.
Some of the plankton in Lake 260 increased in abundance during and after treatments with EE2 and concurrently with declines in the abundances of small-bodied fishes, suggesting indirect effects of this EDC on planktonic invertebrates. We believe that increases in Chaoborus and crustacean zooplankton after 2002 were related to decreases in predation by small fishes, as increases in zooplankton biomass and changes in size structure can follow reductions of planktivorous fish (e.g. [28]). In addition, Chaoborus and zooplankton are important components of the diets of both fathead minnow and pearl dace in these and other systems [53,54]. While average rotifer biomass also increased starting in 2003, rotifers do not constitute a major part of the diet of the fish species that decreased in Lake 260. Intraguild competition and predation among omnivorous zooplankton predators including minnows, Chaoborus and cyclopoid copepods may lead to complex interactions. For example, the effect of decreased fish predation may have been partially offset by increases in Chaoborus predation on other invertebrates. Declines in Tropocyclops, which feed on rotifers [55,56], or changes in the behaviour of other invertebrate predators may also have affected rotifers and other small taxa. However, these explanations remain speculative at this time. Because zooplankton and nutrient availability did not change substantially during the experiment, the absence of responses in the phytoplankton and bacterial communities is not surprising. Manipulations of ELA lakes resulting in major changes in fish abundance and/or community composition have often not resulted in strong trophic cascades and responses of lower trophic levels were often delayed for several years (e.g. [33,57]).
Several of the fishes in Lake 260 are known to feed on littoral invertebrates, and declines in fish abundance may have contributed to observed increases in the abundance of benthos during this study. Pearl dace feed on a combination of Chironomidae, Cladocera, Ephemeroptera, Pelecypoda, detritus and occasionally fish, whereas fathead minnow feed mainly on Cladocera, Chironomidae and detritus [53]. Slimy sculpin and white sucker consume primarily benthic invertebrates and detritus [51]. Although the abundance of all invertebrates on the artificial substrates was higher in the first year of EE2 additions when compared with the reference year, the timing of this increase could not be linked to the declines in fathead minnow, pearl dace and slimy sculpin. However, total emergence did increase in the second year of additions and was concurrent with the declines in fathead minnow YOY and slimy sculpin and low abundance of pearl dace in the experimental lake, and is suggestive of indirect, food-web mediated effects of EE2 on littoral invertebrates.
Declines in lake trout biomass and abundance in Lake 260 following EE2 additions were probably an indirect response, due to major declines in prey fish species and the lack of direct effects on gonad development in lake trout [12] or on egg fertilization and hatching success of embryos from EE2-exposed adults [58]. Lake trout biomass declined continuously from 2003 to 2005, largely coincident with declines in their prey: slimy sculpin (declines in 2002–2004), YOY fathead minnow (major declines in 2002–2005) and adult fathead minnow (declines in 2004–2005). Sculpins and fathead minnow are key prey for lake trout ([51] and references therein). Lake trout abundance decreased the first year after EE2 treatment and occurred at the same time that the adult fathead minnow population declined by more than two orders of magnitude, 2 years after the start of the recruitment failure for this species. A similar indirect effect of environmental contamination was observed in a previous experiment at ELA, where dramatic losses of multiple prey species (slimy sculpin, pearl dace, fathead minnow and Mysis diluvania) due to acidification resulted in a decline in lake trout abundance and biomass that were generally consistent in timing with the loss of prey species [52,59,60]. Like the acidification experiment, declines in lake trout abundance and biomass in Lake 260 were coincident with or only slightly delayed when compared with declines in prey fish species.
Here, we report long-term and significant changes in a whole-lake ecosystem due to EDC exposure and provide a comprehensive evaluation of indirect effects of an EDC on an aquatic food web. Unlike the many studies that have shown direct effects of oestrogens and their mimics on aquatic species, especially fishes, our study advances the understanding of how oestrogen-induced changes in fish abundance can lead to indirect effects on aquatic food webs. We observed direct effects of the potent synthetic oestrogen EE2 on the abundance of forage fishes during a whole-lake experiment, and no evidence of direct toxic effects of EE2 on lower-trophic-level biota (bacteria, algae, zooplankton, Chaoborus, littoral invertebrates). Negative indirect effects on the food web were most notable as declines in the biomass of the top predator lake trout due to a decline in its food supply. While the increases in diverse invertebrate prey species were small, interpreted together they provide a strong indication of indirect effects on the lower food web due to observed declines in predation by small-bodied fishes. An ecosystem model developed to help understand the data from this experiment confirmed the indirect food-web effects of EE2 and, thus, highlights the importance of assessing ecosystem-level effects of endocrine disrupters through whole-system experiments [61]. The presence of indirect food-web effects of EE2 during this experiment suggests that similar kinds of responses may be occurring in systems receiving wastewater inputs of oestrogens and their mimics and that their environmental impacts may currently be underestimated.
Data accessibility
Data are publically available on ACCESS by contacting the International Institute of Sustainable Development—Experimental Lakes Area (mpaterson@iisd-ela.org).
Supplementary Material
Acknowledgements
The authors thank Sandy Chalanchuk, Ken Mills, Lori Tate and Doug Allan for conducting the mark–recapture fish study and collection of other fish data, Alex Salki and Willy Findlay for plankton identifications and counts, and Mark Lyng, Ken Sandilands and numerous field assistants for their help with other sampling. EE2 was supplied by Bayer Schering AG, Berlin, Germany.
Funding statement
Funding for this study was received from Fisheries and Oceans Canada's Environmental Sciences Strategic Research Fund, American Chemistry Council Long Range Initiative Program, the Canadian Federal Toxic Substances Research Initiative, the Canadian Network of Toxicology Centres and the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Tyler CR, Jobling S. 2008. Roach, sex and gender-bending chemicals: the feminization of wild fish in English rivers. Bioscience 58, 1051–1059. [Google Scholar]
- 2.Caldwell DJ, Mastrocco F, Anderson PD, Lange R, Sumpter JP. 2012. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol and 17α-ethinylestradiol. Environ. Toxicol. Chem. 31, 1396–1406. ( 10.1002/etc.1825) [DOI] [PubMed] [Google Scholar]
- 3.Brown AR, Gunnarsson L, Kristiansson E, Tyler CR. 2014. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Phil. Trans. R. Soc. B 369, 20130576 ( 10.1098/rstb.2013.0576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harries JE, Sheahan DA, Jobling S, Matthiessen P, Neall P, Sumpter JP, Tylor T, Zaman N. 1997. Estrogenic activity in five United Kingdom rivers detected by measurement of vitellogenesis in caged male trout. Environ. Toxicol. Chem. 16, 534–542. ( 10.1002/etc.5620160320) [DOI] [Google Scholar]
- 5.Jobling S, Beresford N, Nolan M, Rodgers-Gray T, Brighty GC, Sumpter JP, Tyler CR. 2002. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol. Reprod. 66, 272–281. ( 10.1095/biolreprod66.2.272) [DOI] [PubMed] [Google Scholar]
- 6.Routledge EJ, Sheahan D, Desbrow C, Brighty GC, Waldock M, Sumpter JP. 1998. Identification of estrogenic chemicals in STW effluent. 2. In vivo responses in trout and roach. Environ. Sci. Technol. 32, 1559–1565. ( 10.1021/es970796a) [DOI] [Google Scholar]
- 7.Layton AC, Gregory BW, Seward JR, Schultz TW, Sayler GS. 2000. Mineralization of steroidal hormones by biosolids in wastewater treatment systems in Tennessee U.S.A. Environ. Sci. Technol. 34, 3925–3931. ( 10.1021/es9914487) [DOI] [Google Scholar]
- 8.Ternes TA, Kreckel P, Mueller J. 1999. Behaviour and occurrence of estrogens in municipal sewage treatment plants—II. Aerobic batch experiments with activated sludge. Sci. Total Environ. 225, 91–99. ( 10.1016/S0048-9697(98)00335-0) [DOI] [PubMed] [Google Scholar]
- 9.Liu ZH, Kanjo Y, Mizutani S. 2009. Urinary excretion rates of natural estrogens and androgens from humans, and their occurrence and fate in the environment: a review. Sci. Total Environ. 407, 4975–4985. ( 10.1016/j.scitotenv.2009.06.001) [DOI] [PubMed] [Google Scholar]
- 10.Rojas MR, Leung C, Bonk F, Zhu Y, Edwards L, Arnold RG, Sáez AE, Klečka G. 2013. Assessment of the effectiveness of secondary wastewater treatment technologies to remove trace chemicals of emerging concern. Crit. Rev. Environ. Sci. Technol. 43, 1281–1314. ( 10.1080/10643389.2011.644221) [DOI] [Google Scholar]
- 11.Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick R. 2007. Collapse of a fish population following exposure to a synthetic estrogen. Proc. Natl Acad. Sci. USA 104, 8897–8901. ( 10.1073/pnas.0609568104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palace VP, Evans RE, Wautier KG, Mills KH, Blanchfield PJ, Park BJ, Baron CL, Kidd KA. 2009. Interspecies differences in biochemical, histopathological and population responses in four wild fish species exposed to ethynylestradiol added to a whole lake. Can. J. Fish. Aquat. Sci. 66, 1920–1935. ( 10.1139/F09-125) [DOI] [Google Scholar]
- 13.Palace VP, et al. 2006. Biochemical and histopathological effects of ethynylestradiol in pearl dace (Semotilus margarita) exposed to the synthetic estrogen in a whole lake experiment. Environ. Toxicol. Chem. 25, 1114–1125. ( 10.1897/04-557R1.1) [DOI] [PubMed] [Google Scholar]
- 14.Lafont R. 1991. Reverse endocrinology, or ‘hormones’ seeking functions. Insect Biochem. 21, 697–721. ( 10.1016/0020-1790(91)90112-R) [DOI] [Google Scholar]
- 15.Keshan B, Ray AK. 2001. The presence of estradiol-17β and its specific binding sites in posterior silk gland of Bombyx mori. Gen. Comp. Endocrinol. 123, 23–30. ( 10.1006/gcen.2001.7642) [DOI] [PubMed] [Google Scholar]
- 16.Lafont R, Mathieu M. 2007. Steroids in aquatic invertebrates. Ecotoxicology 16, 109–130. ( 10.1007/s10646-006-0113-1) [DOI] [PubMed] [Google Scholar]
- 17.Keay J, Thornton JW. 2009. Hormone-activated estrogen receptors in annelid invertebrates: implications for evolution and endocrine disruption. Endocrinology 150, 1731–1738. ( 10.1210/en.2008-1338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott AP. 2013. Do mollusks use vertebrate sex steroids as reproductive hormones? II. Critical review of the evidence that steroids have biological effects. Steroids 78, 268–281. ( 10.1016/j.steroids.2012.11.006) [DOI] [PubMed] [Google Scholar]
- 19.Canesi L, Borghi C, Fabbri R, Ciacci C, Lorusso LC, Gallo G, Vergani L. 2007. Effects of 17β-estradiol on mussel digestive gland. Gen. Comp. Endocrinol. 153, 40–46. ( 10.1016/j.ygcen.2007.02.005) [DOI] [PubMed] [Google Scholar]
- 20.Kashian DR, Dodson SI. 2004. Effects of vertebrate hormones on development and sex determination in Daphnia magna. Environ. Toxicol. Chem. 23, 1282–1288. ( 10.1897/03-372) [DOI] [PubMed] [Google Scholar]
- 21.Andersen HR, Wollenberger L, Halling-Sorensen B, Kusk KO. 2001. Development of copepod nauplii to copepodites: a parameter for chronic toxicity including endocrine disruption. Environ. Toxicol. Chem. 20, 2821–2829. [PubMed] [Google Scholar]
- 22.Zou E, Fingerman M. 1997. Effects of estrogenic xenobiotics on molting of the water flea, Daphnia magna. Ecotox. Environ. Saf. 38, 281–285. ( 10.1006/eesa.1997.1589) [DOI] [PubMed] [Google Scholar]
- 23.Preston BL, Snell TW, Robertson TL, Dingmann BJ. 2000. Use of freshwater rotifer Brachionus calcyflorus in screening assay for potential endocrine disruptors. Environ. Toxicol. Chem. 19, 2923–2929. () [DOI] [Google Scholar]
- 24.Souza MS, Hallgren P, Balseiro E, Hansson L-A. 2013. Low concentrations, potential ecological consequences: synthetic estrogens alter life-history and demographic structures of aquatic invertebrates. Environ. Pollut. 178, 237–243. ( 10.1016/j.envpol.2013.03.038) [DOI] [PubMed] [Google Scholar]
- 25.Segner H, Caroll K, Fenski M, Janssen CR, Maack G, Schafers C, Vandenbergh GF, Watts M, Wenzel A. 2004. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project. Ecotox. Environ. Saf. 54, 216–222. ( 10.1016/S0147-6513(02)00039-8) [DOI] [PubMed] [Google Scholar]
- 26.Hense BA, Severin GF, Pfister B, Welzl B, Jaser W, Schramm KW. 2005. Effects of anthropogenic estrogens nonylphenol and 17αethinylestradiol in aquatic model ecosystems. Acta Hydrochim. Hydrobiol. 33, 27–37. ( 10.1002/aheh.200300552) [DOI] [Google Scholar]
- 27.Fleeger JW, Carman KR, Nisbet RM. 2003. Indirect effects of contaminants in aquatic ecosystems. Sci. Total. Environ. 317, 207–233. ( 10.1016/S0048-9697(03)00141-4) [DOI] [PubMed] [Google Scholar]
- 28.Carpenter SR, Kitchell JF, Hodgson JR. 1985. Cascading trophic interactions and lake productivity. Biosciences 35 634–639. ( 10.2307/1309989) [DOI] [Google Scholar]
- 29.Cleugh TR, Hauser BW. 1971. Results of the initial survey of the Experimental Lakes Area, northwestern Ontario. J. Fish. Res. Board Can. 28, 129–137. ( 10.1139/f71-027) [DOI] [Google Scholar]
- 30.Park BJ, Kidd KA. 2005. Effects of the synthetic estrogen ethynylestradiol on early life states of mink frogs and green frogs in the wild and in situ. Environ. Toxicol. Chem. 24, 2027–2036. ( 10.1897/04-227R.1) [DOI] [PubMed] [Google Scholar]
- 31.Shearer JA. 1978. Two devices for obtaining water samples integrated over depth. Technical report no. 772, p. 9. Canada Fisheries and Marine Service.
- 32.Findlay DL, Podemski CL, Kasian SEM. 2009. Aquaculture impacts on the algal and bacterial communities in a small boreal forest lake. Can. J. Fish. Aquat. Sci. 66, 1936–1948. ( 10.1139/F09-121) [DOI] [Google Scholar]
- 33.Paterson MJ, Podemski CL, Findlay WJ, Findlay DL, Salki AG. 2010. The response of zooplankton in a whole-lake experiment on the effects of a cage aquaculture operation for rainbow trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 67, 1852–1861. ( 10.1139/F10-106) [DOI] [Google Scholar]
- 34.Stewart-Oaten A, Murdoch WW, Parker KR. 1986. Environmental impact assessment: ‘pseudoreplication’ in time? Ecology 67, 929–940. ( 10.2307/1939815) [DOI] [Google Scholar]
- 35.Stewart-Oaten A, Bence JR, Osenberg CW. 1992. Assessing effects of unreplicated perturbations: no simple solutions. Ecology 73, 1396–1404. ( 10.2307/1940685) [DOI] [Google Scholar]
- 36.Hill MO. 1974. Correspondence analysis: a neglected multivariate method. Appl. Stat. 23, 340–354. ( 10.2307/2347127) [DOI] [Google Scholar]
- 37.Carter JCH, Kwik JK. 1977. Instar succession, vertical distribution, and interspecific competition among four species of Chaoborus. J. Fish. Res. Board Can. 34, 113–118. ( 10.1139/f77-013) [DOI] [Google Scholar]
- 38.LeSage L, Harrison AD. 1979. Improved traps and techniques for the study of emerging aquatic insects. Entomol. News 90, 65–78. [Google Scholar]
- 39.Schmude KL, Liber K, Corry TD, Stay FS. 1999. Effects of 4-nonylphenol on benthic macroinvertebrates and insect emergence in littoral enclosures. Environ. Toxicol. Chem. 18, 386–393. ( 10.1002/etc.5620180304) [DOI] [PubMed] [Google Scholar]
- 40.Merritt RW, Cummins KW. 1996. An introduction to the aquatic insects of North America. Dubuque, IA: Kendall Hunt. [Google Scholar]
- 41.Guzzo MM, Rennie MD, Blanchfield PJ. 2014. Evaluating the relationship between mean catch per unit effort and abundance for littoral cyprinids in small boreal shield lakes. Fish. Res. 150, 100–108. ( 10.1016/j.fishres.2013.10.019) [DOI] [Google Scholar]
- 42.White GC, Burnham KP. 1999. Program MARK: survival estimation from populations of marked animals. Bird Study 46(Suppl.), 120–138. ( 10.1080/00063659909477239) [DOI] [Google Scholar]
- 43.Ricker WE. 1975. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. no. 191. Government of Canada. [Google Scholar]
- 44.Rennie MD, Verdon R. 2008. Development and evaluation of condition indices for the lake whitefish. N. Am. J. Fish. Manag. 28, 1270–1293. ( 10.1577/M06-258.1) [DOI] [Google Scholar]
- 45.Piccolo JJ, Hubert WA, Whaley RA. 1993. Standard weight equation for lake trout. N. Am. J. Fish. Manag. 13, 401–404. () [DOI] [Google Scholar]
- 46.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 47.Gallardo WG, Hagiwara A, Tomita Y, Soyano K, Snell TW. 1997. Effect of some vertebrate and invertebrate hormones on the population growth, mictic female production, and body size of the marine rotifer Brachionus plicatilis Muller. Hydrobiologia 358, 113–120. ( 10.1023/A:1003124205002) [DOI] [Google Scholar]
- 48.Hallgren P, Sorita Z, Berglund O, Persson A. 2012. Effects of 17α-ethinylestradiol on individual life-history parameters and estimated population growth rates of the freshwater gastropods Radix balthica and Bithynia tentaculata. Ecotoxicology 21, 803–810. ( 10.1007/s10646-011-0841-8) [DOI] [PubMed] [Google Scholar]
- 49.Dietrich S, Ploessl F, Bracher F, Laforsch C. 2010. Single and combined toxicity of pharmaceuticals at environmentally relevant concentrations in Daphnia magna: a multigenerational study. Chemosphere 79, 60–66. ( 10.1016/j.chemosphere.2009.12.069) [DOI] [PubMed] [Google Scholar]
- 50.Meregalli G, Ollevier F. 2001. Exposure of Chironomus riparius larvae to 17α-ethynylestradiol: effects on survival and mouthpart deformities. Sci. Total Environ. 269, 157–161. ( 10.1016/S0048-9697(00)00824-X) [DOI] [PubMed] [Google Scholar]
- 51.Scott WB, Crossman EJ. 1998. Freshwater fishes of Canada, p. 966. Oakville, Canada: Galt House Publications. [Google Scholar]
- 52.Mills KH, Chalanchuk SM, Mohr LC, Davies IJ. 1987. Response of fish populations in Lake 223 to 8 years of experimental acidification. Can. J. Fish. Aquat. Sci. 44, 114–125. ( 10.1139/f87-287) [DOI] [Google Scholar]
- 53.Tallman RF, Mills KH, Rotter RG. 1984. The comparative ecology of pearl dace (Semotilus margarita) and fathead minnow (Pimephales promelas) in Lake 114, the Experimental Lakes Area, northwestern Ontario, with an appended key to the cyprinids of the Experimental Lakes Area. Canadian Manuscript Report of Fisheries and Aquatic Science no. 1756.
- 54.Hambright KD. 1994. Morphological constraints in the piscivore-planktivore interaction: implications for the trophic cascade hypothesis. Limol. Oceanogr. 39, 897–912. ( 10.4319/lo.1994.39.4.0897) [DOI] [Google Scholar]
- 55.Adrian R, Frost TM. 1992. Comparative feeding ecology of Tropocyclops prasinus mexicanus (Copepoda, Cyclopoida). J. Plankton Res. 14, 1369–1382. ( 10.1093/plankt/14.10.1369) [DOI] [Google Scholar]
- 56.Peacock A, Smyly WJP. 1983. Experimental studies on the factors limiting Tropocyclops prasinus (Fischer) 1860 in an oligotrophic lake. Can. J. Zool. 61, 250–265. ( 10.1139/z83-031) [DOI] [Google Scholar]
- 57.Findlay DL, Vanni MJ, Paterson MJ, Mills KH, Kasian SEM, Findlay WJ, Salki AG. 2005. Dynamics of a boreal lake ecosystem during a long-term manipulation of top predators. Ecosystems 8, 603–618. ( 10.1007/s10021-005-1221-0) [DOI] [Google Scholar]
- 58.Werner J, Wautier K, Mills K, Chalanchuk S, Kidd K, Palace V. 2006. Reproductive fitness of lake trout (Salvelinus namaycush) exposed to environmentally relevant concentrations of the potent estrogen ethynylestradiol (EE2) in a whole lake exposure experiment. Sci. Mar. 70S2, 59–66. [Google Scholar]
- 59.Mills KH, Chalanchuk SM, Allan DJ. 2000. Recovery of fish populations in Lake 223 from experimental acidification. Can. J. Fish. Aquat. Sci. 57, 192–204. ( 10.1139/f99-186) [DOI] [Google Scholar]
- 60.Mills KH, Chalanchuk SM, Allan DJ. 2002. Biomass and production of lake charr during the acidification and recovery of a small Ontario lake. Environ. Biol. Fish. 64, 293–301. ( 10.1023/A:1016075001977) [DOI] [Google Scholar]
- 61.Clouzot L, Paterson M, Dupuis A, Blanchfield P, Rennie M, Kidd K, Vanrolleghem PA. 2014. A calibrated ecosystem model to assess the ecotoxicological risk of endocrine disrupters in aquatic environments Abstr. SETAC Europe 24th Annual Meeting , Basel, Switzerland, 11–15 May 2014, p. 140, abstr. 618. See http://c.ymcdn.com/sites/www.setac.org/resource/resmgr/Abstract_Books/SETAC-Basel-abstracts.pdf? [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are publically available on ACCESS by contacting the International Institute of Sustainable Development—Experimental Lakes Area (mpaterson@iisd-ela.org).