Abstract
During the past two decades scientists, regulatory agencies and the European Commission have acknowledged pharmaceuticals to be an emerging environmental problem. In parallel, a regulatory framework for environmental risk assessment (ERA) of pharmaceutical products has been developed. Since the regulatory guidelines came into force the German Federal Agency (UBA) has been evaluating ERAs for human and veterinary pharmaceutical products before they are marketed. The results show that approximately 10% of pharmaceutical products are of note regarding their potential environmental risk. For human medicinal products, hormones, antibiotics, analgesics, antidepressants and antineoplastics indicated an environmental risk. For veterinary products, hormones, antibiotics and parasiticides were most often discussed as being environmentally relevant. These results are in good correlation with the results within the open scientific literature of prioritization approaches for pharmaceuticals in the environment. UBA results revealed that prospective approaches, such as ERA of pharmaceuticals, play an important role in minimizing problems caused by pharmaceuticals in the environment. However, the regulatory ERA framework could be improved by (i) inclusion of the environment in the risk–benefit analysis for human pharmaceuticals, (ii) improvement of risk management options, (iii) generation of data on existing pharmaceuticals, and (iv) improving the availability of ERA data. In addition, more general and integrative steps of regulation, legislation and research have been developed and are presented in this article. In order to minimize the quantity of pharmaceuticals in the environment these should aim to (i) improve the existing legislation for pharmaceuticals, (ii) prioritize pharmaceuticals in the environment and (iii) improve the availability and collection of pharmaceutical data.
Keywords: pharmaceuticals, regulation, environmental risk assessment, emerging substances, monitoring, prioritization
1. Introduction
During the past three decades, the presence of pharmaceuticals in the environment has gained increasing attention. The open scientific literature demonstrates that pharmaceuticals enter the environment and can produce adverse effects. A recent study commissioned by the German Federal Environment Agency (UBA) reviewed the literature reporting data from environmental monitoring in Germany [1]. The study confirms that a total of 156 pharmaceuticals have been detected in Germany in environmental media such as surface water, groundwater and drinking water. In this report, most of the pharmaceuticals (n = 131) were found in surface waters, mainly in the concentration range 0.1–10.0 μg l−1. On a global scale, several review papers have highlighted aquatic monitoring of pharmaceuticals in the European Union [2], the USA [3] and China [4], and demonstrated that pharmaceutical substances are frequently detected. A project currently being carried out by the UBA collects published worldwide data on measured concentrations of pharmaceuticals. The results illustrate that detected aquatic concentrations on a global scale are comparable with those found in Europe. More than 600 pharmaceutical substances have been shown to be present in the environment worldwide. Despite different usage patterns and specific local exposure scenarios, to date 17 pharmaceutical substances have been detected in each of the United Nations (UN) regions (http://www.pharmaceuticals-in-the-environment.org).
Of particular concern is the exposure of non-target organisms to pharmaceuticals, as these compounds are designed to be biologically active and low exposure can elicit physiological change. Over the past two decades, investigations on how exposure to pharmaceuticals may affect organisms have revealed that ethinyloestradiol (EE2) contributed to feminization of male fish in effluent-dominated rivers [5]. The review by Brausch et al. [6] gives a comprehensive overview on toxic effects in aquatic organisms, endpoints, investigated organisms, and mode of action (MOA) and toxicity of pharmaceutical classes. Acute toxicities of 150 pharmaceutical substances, comprising 35 pharmaceutical classes, are presented. In the same study, chronic toxicities are given for 65 pharmaceutical substances from 20 pharmaceutical classes. The review describes standard and advanced ecotoxicological test systems that have been applied to study MOA in aquatic organisms. Toxic effects are proved from molecular (e.g. inhibition of cyclooxygenase) to population levels (e.g. behavioural changes, effects on reproduction) [6]. By contrast, studies that have investigated the presence of pharmaceuticals in biota and/or wildlife and their subsequent effects are still scarce. Huerta et al. [7] reviewed the occurrence of pharmaceuticals in aquatic biota and reported that until now studies have mainly focused on hormones, antibiotics, analgesics and antidepressants. In this review, the most commonly used organisms for biota monitoring were fishes, followed by crustaceans and molluscs. Reported average concentrations were in the range 0.1–100 ng g−1 dry mass.
More recently, research focusing on the issue of pharmaceuticals in the environment has shifted from either effect- or exposure-directed studies to linking biological effects to exposure [8]. Brodin et al. [9], for example, state that the antidepressant oxazepam alters the behaviour and feeding rate of the wild fish Perca fluviatilis at environmentally relevant concentrations. The authors conclude that antidepressants in surface water alter animal behaviours that are known to have ecological and evolutionary consequences. As exposure may occur along a concentration gradient, some studies have been expanded to monitor receiving waters at given distances from wastewater treatment plants. Using this approach, Metcalfe et al. [10] showed a correlation between the distribution of antidepressants in a water body and bioaccumulation in the fish Pimephales promelas.
During the past decade, the European Commission (EC) has funded approximately 15 projects on the topic of pharmaceuticals in the environment. The results of these projects have created an increasing amount of open scientific literature and increased public awareness and the European Environment Agency (EEA) finally acknowledged pharmaceuticals to be an emerging environmental problem [11]. One of their key findings was that ‘there is definitely a need to look at impacts across the whole life cycle of a pharmaceutical’. Subsequently, the EC recognized that environmental pollution via pharmaceuticals was an emerging problem. In 2010, the EC ‘was called upon by the European Parliament and the European Council to prepare a report on the scale of the problem of environmental effects of pharmaceuticals, following recognition that residues from these substances in water and soil posed environmental problems’ [12]. In this context, the EC had to evaluate whether amendments to existing EU legislation on medicinal products or other relevant EU legislation were required. The report has just been published (http://ec.europa.eu/health/human-use/environment-medicines/index_en.htm) and will help in providing measures across the whole life cycle of pharmaceuticals to decrease their potential environmental risks.
This article is intended to present information on the development and existing regulatory framework of the environmental risk assessment (ERA) of pharmaceuticals. Results obtained by the UBA during evaluation of ERAs are given and discussed. In addition, we have developed some important challenges for different stakeholders which should be considered in order to inform and/or adapt regulations where necessary.
2. Environmental risk assessments: the regulatory framework
Initially, ERAs that compared environmental concentrations with toxic effects in aquatic organisms were carried out in parallel with the first scientific findings concerning pharmaceuticals in the environment. The necessity for and the development of formal pharmaceutical ERAs led from the 1990s onwards to legal guidelines as part of the registration dossier for pharmaceutical products. Effects in the environment posed by the use of a single product are difficult to detect and can easily be overlooked. Often, it is difficult to relate a visible effect to the release of a certain pharmaceutical substance contained in a product. Thus, a prospective ERA as part of the authorization process for human and veterinary products was established in European legislation. The ERA was strengthened by consolidating some older EU Directives to give the EC Directive [13,14]. As a consequence, for all new marketing applications for human and veterinary medicinal products, an assessment of potential risks to the environment is required in the pre-approval phase. The legislation includes products with new active substances as well as existing substances that are used in generic applications (herein the term ‘existing substances’ is used for pharmaceutical substances in products that entered the market before ERA guidelines came into force). Assessment of impacts is carried out according to guidelines on ERA for human (since 2006) as well as for veterinary (in 2001, 2005 and 2007, respectively) medicinal products [15–18] (all guidelines and other ERA relevant papers are accessible on the EMA homepage http://www.ema.europa.eu/ema/; see ‘human’ or ‘veterinary regulatory’, then ‘scientific guidelines’). The prospective ERA aims to protect aquatic and terrestrial ecosystems (including top predators), groundwater, and microorganisms in sewage treatment plants. In 2004, environmental risk was included in the risk–benefit analysis [14] for veterinary medicinal products. Thus, veterinary medicinal products may not be authorized in the EU if they pose environmental risks. By contrast, the authorization of a human medicinal product cannot be denied if an environmental risk is identified (because the environmental risk is not included in the risk–benefit analysis).
The ERA for pharmaceutical products prior to their authorization follows two different approaches: risk- and hazard-based. The risk assessment is a two-phase approach. Phase I identifies those products which require more detailed, experimental assessment. In phase II, a base dataset on fate and effects in the environment is generated according to OECD test guidelines. An exposure threshold value or ‘action limit’ separates the exposure estimation in phase I from the test requirements in the subsequent phase II. Substances that might have an effect on the environment even at very low concentrations such as hormones and parasiticides are exempted from the threshold value. The hazard approach focuses on the intrinsic properties of a substance defining its persistence, bioaccumulation potential and toxicity (PBT). Because of their hazardous properties these substances should not enter the environment [19]. Whereas exposure is a key parameter for the risk identification of a pharmaceutical substance, it cannot be considered for the hazard assessment. In consequence, a ‘harmless’ concentration regarding the environment for a potentially hazardous substance cannot be defined. Because identified hazardous substances should not enter the environment, no mitigation measures are possible. By contrast, for substances with an identified environmental risk, mitigation measures can be proposed that generally aim to minimize the quantity discharged into the environment, and these are communicated in the pharmaceutical product information [20].
3. Environmental risk assessments: results
The UBA has been involved in developing the guidelines for ERAs of pharmaceuticals for more than 15 years. Since the respective veterinary and human guidelines came into force, the UBA has been reviewing the ERAs that are included in dossiers for new medicinal products. Our own results and experience gained during this time are presented in this section. Particularly during the first few years after implementation of the guidelines, ERAs were submitted incompletely, e.g. without some of the required test results. In consequence, the conclusions regarding the potential environmental risks for many medicinal products could not be finalized. Recently, the quality and completeness of ERAs being reviewed by the UBA has improved. However, some of the ERAs still omit relevant studies that are requested according to the guidelines. To date, the UBA has evaluated ERAs for approximately 650 human and 120 veterinary pharmaceutical products, respectively. Complete (phase I and phase II) and valid ERAs are available for 120 human medicinal products. The evaluation of these substances resulted in the conclusion that approximately 10% are notable regarding their potential environmental risk. These medicinal products comprised the following pharmaceutical classes: hormones, antibiotics, analgesics, antidepressants and antineoplastics. The same trend of approximately 10% of products with an environmental risk is revealed for veterinary products of the following classes: hormones, antibiotics and parasiticides. In general, the following additional effects have been identified within the UBA during evaluation of ERAs: parasiticides showed harmful effects on non-target organisms, e.g. for dung insects, aquatic invertebrates, protozoa, worms in soil and surface water. Some antibiotics were assessed to have harmful effects on algae and plants. Antibiotics have also been discussed because of their potential accumulation in soil, tendency to reach groundwater and their contribution to microbial antibiotic resistance. Hormones have been assessed to have effects on the hormonal system of fish, molluscs, invertebrates and birds. Toxicological endpoints such as impaired reproduction, changed behaviour and intersex were additionally determined in the effects assessment of hormones. In addition, hazardous properties (PBT) have been identified for some parasiticides and several antineoplastic compounds.
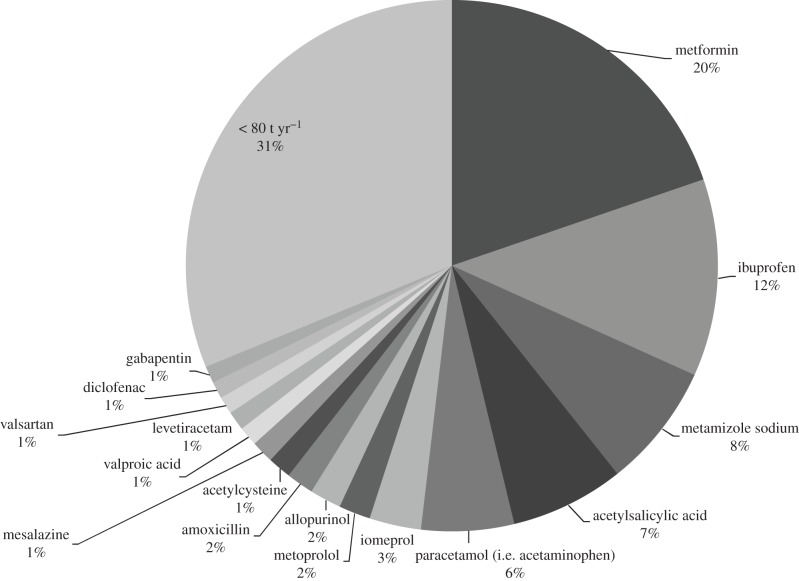
In addition, the experiences of the UBA highlight the lack of data for pharmaceutical products which entered the market before the respective guidelines came into force. Consequently, the environmental risk of these substances cannot and has not been properly assessed. For several of these existing substances insufficient or no ERA data are available but may have environmental relevance. We should like to point out that according to German consumption data in 2012 (IMS Health, DE) many existing substances are still produced in high amounts, such as metformin (1.200 t), ibuprofen (975 t), metamizole (615 t), acetaminophen (458 t), iomeprol (255 t) and metoprolol (157 t) (figure 1).
Figure 1.
Best selling environmentally relevant human pharmaceuticals in Germany in 2012. Pharmaceutical substances with an annual consumption of more than 80 t are indicated by name, and the percentage total consumption is presented. (Data from IMS Health, DE.)
German consumption data of the past 10 years additionally show that the volume of these compounds may still heavily increase. As an example, between 2002 and 2012 the consumption of metformin and ibuprofen in Germany increased from 390 to 1200 t and from 250 to 975 t, respectively. Many of the existing substances have been detected in the environment [1–3]. Thus, there is an urgent need for proper ERAs for these substances together with the respective data. A review programme of existing pharmaceuticals (similar to existing pesticide and biocide substances) would be an appropriate measure to compile missing data. Comprehensive and verified data on the fate and effects of drug substances—as demanded for the ERAs—are the essential basis for any kind of risk management. They are required not only for the authorization of pharmaceuticals but also for several other areas of environmental risk management, e.g. water pollution control. Clear rules on the collection and sharing of data, the assurance of property rights, etc., are thus needed. Hence, European legislation should be amended as necessary to meet the requirements of the EC regarding product stewardship and identification and management of risks for the environment.
Regarding possible risk management options during the ERA, a critical review of the existing mitigation measures has been published by the EMA [20]. For human pharmaceuticals, comments have been included in the product information for substances with an identified environmental risk to raise awareness and minimize waste, such as ‘Remaining hormonal active ingredients of the transdermal patch may have harmful effects if reaching the aquatic environment. Therefore, the used transdermal patch should be discarded carefully’. By contrast, risk mitigation measures for veterinary pharmaceuticals have been discussed and implemented more often. Implemented comments include the following: ‘Treated animals (cattle, horses and sheep) should not have access to surface water for <x> days after treatment to avoid adverse effects on aquatic organisms’; ‘Animals must remain stabled for <x> days after treatment, until the concentration of <active substance> in excreta is low enough to avoid adverse effects on dung fauna and their predators' or '<Active substance> is toxic for aquatic organisms. Remove the collar before allowing the dog to swim and before bathing the dog to avoid adverse effects on aquatic organisms’ [20]. However, in order to decrease the environmental exposure these risk mitigation measures have to be harmonized throughout the labelling of products authorized in the EU containing the same active substance for the same indication. In addition, due to the lack of appropriate risk mitigation measures for identified PBT substances, the substitution of these substances by less hazardous substances needs to be discussed and implemented.
Within the authorization process, an increasing number of so-called ‘referral procedures’ have been started for veterinary pharmaceutical products due to their assessed potential risks. In a referral procedure, the EMA is requested to conduct a scientific assessment of a particular medicine or class of medicines on behalf of the EC. This procedure is used to resolve issues such as concerns over the safety of a medicine or a class of medicines and/or to resolve divergent opinions between Member States (for details, see http://www.ema.europa.eu/ema/index.jsp?curl=pages/home/Home_Page.jsp). Since 2008, 11 of 77 referrals for veterinary medicinal products have been performed at the EMA owing to unresolved issues between EU Member States. All referral procedures that were related to the assessment of environmental risk are listed in table 1.
Table 1.
EU referral procedures related to potential environment risks (2008–2013) according to Article 33(4) and Article 35. For reference, see http://ec.europa.eu/health/documents/community-register/html/refv_others.htm.
| name of product | active substance | year of referral | divergent opinions on | decision on overall (environmental) risk benefit profile |
|---|---|---|---|---|
| Article 33(4) referralsa | ||||
| Ecomectin oral paste | ivermectin | 2008 | ERA incomplete | positive |
| Enro-K, Unisol 10% oral solution | enrofloxacin | 2009 | risk for aquatic and terrestrial compartment | positive |
| Fenflor, Shotaflor 300 mg ml−1 | florfenicol | 2009 | different opinions regarding exposure assessment | positive |
| Cevazuril 50 mg ml−1 oral suspension | toltrazuril | 2010 | ERA incomplete | positive |
| Pharmasin 100% w/w water soluble granules | tylosin | 2010 | risk for aquatic and terrestrial compartment, ERA incomplete | negative owing to lack of data |
| Prontax | doramectin | 2012 | ERA incomplete | positive |
| Deltanil | deltamethrin | 2013 | ERA incomplete | positive |
| Strenzen 500/125 mg g−1 powder | amoxicillin + clavularic acid | 2013 | ERA incomplete | positive |
| Suifertil 4 mg ml−1 oral solution | altrenogest | 2013 | ERA incomplete | positive |
| Article 35 referralsb | ||||
| toltrazuril | 2008 | risk for terrestrial compartment and for groundwater | positive | |
| doramectin | 2013 | risk for aquatic compartment, dung fauna, PBT assessment incomplete | positive | |
aArticle 33(4) of Directive 2001/82/EC, as amended (‘referral following disagreement at the coordination group on a mutual recognition and decentralized procedure’).
bArticle 35 of Directive 2001/82/EC, as amended (‘referral of issues raised with regard to a product or a class of products which is of community interest’).
However, it is important to note that no veterinary pharmaceutical has so far been refused based on a negative (environmental) risk—benefit analysis. Because the environment is exempted from the risk–benefit analysis for human pharmaceuticals, referral procedures owing to environmental risk have not been performed for human drugs to date.
4. Challenges for regulation, policy development and research
The prospective ERA implemented in the EU legislation has been shown to be a suitable instrument to assess the environmental risks of pharmaceuticals. Potential environmental risks of human as well as veterinary pharmaceuticals have already been identified for substances of different pharmaceutical classes such as parasiticides, analgesics, antidepressants, antibiotics, hormones and antineoplastics. The ERA procedure has increasingly been accepted by the involved parties, i.e. regulatory authorities, industry and EU agencies. However, based on our experiences, the following approaches and measures should be implemented and additional legislation gaps closed.
(a). Improve existing legislation
With regard to risk management options during ERAs, the following approaches have already been highlighted. First, the environmental risk should be included in the risk–benefit analysis of human medicinal products as in the regulation of veterinary medicinal products. This measure is not intended to block marketing of new products but aims to confirm environmental risks and include them within the authorization procedure. This step could be implemented within the next revision of the current EC Directive [13]. Second, risk mitigation measures for human pharmaceuticals in the ERA process are currently limited and should therefore be developed and controlled. To improve risk management options outside the ERA procedures, pharmaceutical substances should be incorporated into existing legislation. Because pharmaceuticals in the environment are a topic of emerging concern, they were not considered when drafting existing chemical regulations. Environmental quality norms for the entire EU, for example, have been defined for biocides and pesticides in the Groundwater Directive (Annex I) [21] but not for pharmaceuticals. In addition, a list of pollutants with considered threshold values (Annex II) does not yet include pharmaceuticals. It has to be noted that in order to ensure higher stability and higher bioavailability in patients, increasing numbers of pharmaceutical substances are being halogenated [22]. Halogenated substances are listed in Annex VII (point 1) of the Water Framework Directive (WFD) and thus Member States (according to the Groundwater Directive) should establish all measures necessary to prevent the input of halogenated compounds into groundwater. To date this has not been addressed, and therefore halogenated pharmaceutical substances do need special attention when addressing the groundwater legislation and environmental risks.
Post marketing control mechanisms for pharmaceuticals are still missing. Up to now, these mechanisms have not been included in regular monitoring programmes and possible risk management approaches do not exist. Monitoring data for pharmaceuticals most often result from specific targeted studies and not comprehensive monitoring. In 2012, the European Parliament, in agreement with EU Member States, added three pharmaceuticals to the so-called ‘watch list’ of emerging pollutants that could one day be added to the EU priority list of the WFD. The WFD [23] is the EU's main policy instrument for setting water anti-pollution strategies, including measures to progressively reduce emissions of chemicals listed as priority substances. Three substances (diclofenac, 17α-ethinyloestradiol and 17ß-oestradiol) are now foreseen for water monitoring to determine if they should be listed as priority substances. At present, it is uncertain whether and when (i) environmental quality standards (EQS) will apply to these pharmaceutical substances and (ii) appropriate water protection measures will be implemented for more pharmaceutical substances. However, the inclusion of pharmaceuticals in the priority list is an important next step in order to define and improve possible risk management options.
(b). Prioritization of pharmaceuticals
There is a definite need for prioritization of environmentally relevant pharmaceutical substances. Existing pharmaceutical substances with missing environmental data as well as those substances that are considered for monitoring campaigns have to be prioritized in order to define and minimize their environmental risk. Approximately 5000 active substances are currently on the market, including a large number that entered the market before the ERA guidelines came into force. According to the World Health Organization, increasing concentrations of pharmaceuticals in aquatic systems are to be expected, because pharmaceutical use is projected to increase as they become more available for the increasing global population. In order to be proactive, the substances which are most environmentally relevant have to be identified and prioritized, and this has been an issue within the past few years. A variety of approaches have been suggested depending on the chemical properties of the substances. Most often, a combination of exposure and effect data has been used to prioritize environmentally relevant chemicals. In several approaches, the use of toxicological data to predict adverse effects in aquatic organisms has been proposed (a comparison of several but not all approaches is given in [24]). The majority of published prioritization approaches indicated high potential environmental relevance for various pharmaceutical classes. Within the past few years, the UBA has funded different projects on prioritization approaches for human and veterinary pharmaceuticals. Those projects investigated prioritizing pharmaceuticals via general risk assessment together with MOA approaches and the use and extrapolation of toxicological data (for details and results, see [25–27]). In addition, publicly available prioritization data and results have been screened. The preliminary findings are that, for human pharmaceuticals, those most often prioritized have been hormones, antibiotic, psychotropic, anti-inflammatory and cytostatic substances and ß-blockers. For veterinary pharmaceuticals, antibiotics and parasiticides together with hormones have been shown to be environmentally relevant. These findings for prioritization approaches correlate well with the prospective findings of ERAs for pharmaceutical products assessed by the UBA and, for veterinary pharmaceuticals, the referral discussions at the EMA (table 1). In consequence, the preliminary results of these prioritization approaches could be used to identify candidates for complete ERA testing of existing substances as well as for retrospective water regulation.
(c). Data availability and collection
Comprehensive and verified data on the fate and effects of pharmaceutical substances in the environment are the essential basis for assessing their environmental risk and identifying possible risk management measures. These data are required not only for the authorization of pharmaceuticals but also for several other areas of environmental risk management (e.g. water pollution control). Better quality data on the fate and effects of pharmaceutical substances have been collected for both human and veterinary pharmaceuticals since the ERA guidelines came into force; however, these datasets are, in general, not publicly available. Thus, ERA data submitted in a dossier can only be used by the EU agencies during the review of the ERAs for the specific pharmaceutical product. This fact, among others, leads to a repetitive testing of pharmaceutical substances for different pharmaceutical products. This approach is expensive and very time consuming, and is not environmentally friendly. During the past few years, an increasing number of authorities (including the UBA) and scientists have requested that the ERA data be made publicly available. The EMA has started to publish the fate and effects endpoints of human and veterinary pharmaceuticals in Environmental Public Assessment Reports (EPARs/PARs) of products, but the quality and quantity of environmental data within the EPARs still need to be improved. Because long-term ERA data are not yet accessible to scientists or water boards, publicly available acute datasets on pharmaceutical substances have been used to assess the environmental risk of pharmaceuticals, e.g. for water regulation; such results may underestimate the environmental risk for pharmaceuticals and contradict ERA results. To ensure a more harmonized approach for the management of environmental risks associated with pharmaceuticals, it is important that the assessed data and conclusions of ERAs should be published as soon as possible, for example by the EMA.
Recent studies have shown that pharmaceuticals, according to their envisaged action in humans or animals (e.g. antidepressants that inhibit the serotonin uptake), are likely to exert side effects based on their MOA, such as a change in growth and feeding behaviour in fish [6]. These observed effects may not be the only action, due to the variable conservation status of molecular targets in aquatic organisms. Schreiber et al. [27] reported that the conservation of molecular targets in fish varied from 50 to 70% for diclofenac. In addition, researchers have recently started to study potential non-standard effects with improved test designs. Investigations on, for example, behavioural changes in laboratory and wild fish have already indicated potential endocrine effects for several antidepressants [28,29]. In addition, the importance of the chemical structure of pharmaceuticals has already been shown to influence the uptake by and thus possible adverse effects on biota in the aquatic environment. Many pharmaceuticals are designed as ionizable compounds to ensure that active components of administered doses reach a specific target location within the body. Therefore, exposure and ultimately toxic effects of these pharmaceuticals may depend on their dissociation constant (pKa) and environmental conditions (temperature, pH) [30]. Studies have been published that show pH-dependent effects of pharmaceuticals such as antidepressants and antihistamines on wildlife [31]; these authors suggest that considering how site-specific pH may affect the ionization state of drugs may reduce uncertainty during ERA. In conclusion, more and better information on pharmaceutical substances that (i) act on more than the main molecular target, (ii) exert unwanted adverse side effects, (iii) might be detected with more appropriate test designs and (iv) have varying structural characteristics (e.g. pKa), are considered to be very helpful for improved ERAs of pharmaceuticals.
5. Conclusion and outlook
The occurrence in and potential effects on aquatic and terrestrial organisms of human and veterinary pharmaceuticals are relatively new issues. Nevertheless, quite a number of studies have been published during the past few decades that indicate numerous effects on organisms and the presence of pharmaceuticals in several environmental compartments on a global scale. It has now been recognized that pharmaceuticals in the environment is a topic of global relevance, and not just an issue for industrialized countries. The majority of people, regardless of their background, i.e. general public, industrial, research or regulatory, do not want to have biologically active pharmaceuticals in the environment and thus potentially in their drinking water. Therefore the quantity of pharmaceuticals in the environment has to be minimized by all strategies available. Prospective approaches such as ERAs play an important role in minimizing problems before pharmaceuticals enter the environment. These strategies should be strengthened and adjusted to minimize the quantity of pharmaceuticals entering the environment.
With regard to ERAs, (i) inclusion of the environment in the risk–benefit analysis for human pharmaceuticals, (ii) improvement of risk management options, (iii) generation of data on existing pharmaceuticals, and (iv) improved availability of ERA data, represent some major next steps. It must be mentioned that valuable studies have already been published identifying potential environmental risks. New approaches that link biological effects to environmental exposure promise interesting results, although to date studies on wildlife or caged organisms that have been performed in the environment or in environmentally relevant conditions, for example, are very limited. This might be due to missing protocols for analytical methods and also to the variety of pharmaceutical structure characteristics that are not easy to handle but have to be considered. Possible side effects owing to MOAs of pharmaceuticals do represent additional valuable research fields. All these research approaches are definitely needed to verify potential risks in different environmental compartments. Results can be used for both prospective and retrospective legislation to minimize the environmental risks of pharmaceuticals.
Disclaimer
The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the German Federal Environment Agency.
Acknowledgements
Arne Hein did valuable work on data evaluation that is very much acknowledged. We very much appreciate the comments of John Kucklick and three anonymous reviewers.
References
- 1.Bergmann A, Fohrmann R, Weber FA. 2011. Zusammenstellung von Monitoringdaten zu Umweltkonzentrationen von Arzneimitteln. Texte Umweltbundesamt, p. 66 See http://www.umweltbundesamt.de/sites/default/files/medien/461/publikationen/4188.pdf.
- 2.Loos R, et al. 2013. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 47, 6475–6487. ( 10.1016/j.watres.2013.08.024) [DOI] [PubMed] [Google Scholar]
- 3.Kostich MS, Batt AL, Lazorchak JM. 2014. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ. Pollut. 184, 354–359. ( 10.1016/j.envpol.2013.09.013) [DOI] [PubMed] [Google Scholar]
- 4.Liu JL, Wong MH. 2013. Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Environ. Int. 59, 208–224. ( 10.1016/j.envint.2013.06.012) [DOI] [PubMed] [Google Scholar]
- 5.Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. 1998. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 32, 2498–2506. ( 10.1021/es9710870) [DOI] [Google Scholar]
- 6.Brausch JM, Connors KA, Brooks BW, Rand GM. 2012. Human pharmaceuticals in the aquatic environment: a review of recent toxicological studies and considerations for toxicity testing. Rev. Environ. Contam. Toxicol. 218, 1–99. ( 10.1007/978-1-4614-3137-4_1) [DOI] [PubMed] [Google Scholar]
- 7.Huerta B, Rodriguez-Mazaz S, Barcelo D. 2012. Pharmaceuticals in biota in the aquatic environment: analytical methods and environmental implications. Anal. Bioanal. Chem. 404, 2611–2624. ( 10.1007/s00216-012-6144-y) [DOI] [PubMed] [Google Scholar]
- 8.Metcalfe CD. 2013. Pharmaceutical contaminants of emerging concern in the environment. Environ. Toxicol. Chem. 32, 1683–1684. ( 10.1002/etc.2293) [DOI] [PubMed] [Google Scholar]
- 9.Brodin T, Fick J, Johnsson M, Klaminder J. 2013. Dilute concentrations of a psychiatric drug alter behaviour of fish from natural populations. Science 339, 814–815. ( 10.1126/science.1226850) [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe CD, Chu S, Judt C, Li H, Oakes KD, Servos MR, Andres DM. 2010. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 29, 79–89. ( 10.1002/etc.27) [DOI] [PubMed] [Google Scholar]
- 11.European Environment Agency 2010. Pharmaceuticals in the environment—results of an EEA workshop. EEA technical report no 1/2010. Luxembourg: Office for Official Publications of the European Communities.
- 12.European Commission 2010. Directive 2010/84/EU of the European Parliament and of the Council amending, as regards pharmacovigilance, Directive 2001/83/EC on the community code relating to medicinal products for human use.
- 13.European Commission 2004. Directive 2004/27/EC of the European Parliament and of the Council amending Directive 2001/83/EC on the community code relating to medicinal products for human use.
- 14.European Commission 2004. Directive 2004/28/EC of the European Parliament and of the Council amending Directive 2001/82/EC on the community code relating to veterinary medicinal products.
- 15.European Medicines Agency 2000. Committee for Medicinal Products for Veterinary use (CVMP): guideline on environmental impact assessment (EIAS) for veterinary medicinal products phase I. CVMP/VICH/592/98.
- 16.European Medicines Agency 2003. Committee for Medicinal Products for Veterinary use (CVMP): guideline on environmental impact assessment for veterinary medicinal products phase II. CVMP/VICH/790/03.
- 17.European Medicines Agency 2008. Committee for Medicinal Products for Veterinary use (CVMP): revised guideline on environmental impact assessment for veterinary medicinal products in support of the VICH guidelines GL6 and GL38. EMEA/CVMP/ERA/418282/2005-Rev.1.
- 18.European Medicines Agency 2006. Committee for Medicinal Products for Human Use (CHMP): guideline on the environmental risk assessment of medicinal products for human use. EMEA/CHMP/SWP/4447/00.
- 19.Moermond CTA, Janssen MPM, de Knecht JA, Montforts MHMM, Peijnenburg WJGM, Zweers PGPC, Sijmy DTHM. 2011. PBT Assessment using the revised Annex XIII of REACH: a comparison with other regulatory frameworks. Integr. Environ. Assess. Manag. 8, 359–371. ( 10.1002/ieam.1248) [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency 2010. Reflection paper on risk mitigation measures related to the environmental risk assessment of veterinary medicinal products. EMA/CVMP/ERAWP/409328/2010.
- 21.European Commission 2006. Directive 2006/118/EC of the European Parliament and of the Council on the protection of groundwater against pollution and deterioration. 2006/118/EC.
- 22.Sumpter JP. 2010. Pharmaceuticals in the environment: moving from a problem to a solution. In Green and sustainable pharmacy (eds Kümmerer K, Hempel M.), pp. 11–22. Berlin, Germany: Springer. [Google Scholar]
- 23.European Commission 2000. Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for community action in the field of water policy.
- 24.Roos V, Gunnarsson L, Fick J, Larsson DGJ, Ruden C. 2012. Prioritising pharmaceuticals for environmental risk assessment: towards adequate and feasibile first-tier selection. Sci. Total Environ. 421–422, 102–110. ( 10.1016/j.scitotenv.2012.01.039) [DOI] [PubMed] [Google Scholar]
- 25.Kools SAE, Boxall ABA, Moltmann JF, Bryning G, Koschorreck J, Knacker T. 2008. A ranking of European veterinary medicines based on environmental risks. Integr. Environ. Assess. Manag. 4, 399–408. ( 10.1897/IEAM_2008-002.1) [DOI] [PubMed] [Google Scholar]
- 26.Christen V, Hickmann S, Rechenberg B, Fent K. 2010. Highly active human pharmaceuticals in aquatic systems: a concept for their identification based on their mode of action. Aquat. Toxicol. 96, 167–181. ( 10.1016/j.aquatox.2009.11.021) [DOI] [PubMed] [Google Scholar]
- 27.Schreiber R, Gündel U, Franz S, Küster A, Rechenberg B, Altenburger R. 2011. Using the fish plasma model for comparative hazard identification for pharmaceuticals in the environment by extrapolation from human therapeutic data. Regul. Toxicol. Pharmacol. 61, 261–275. ( 10.1016/j.yrtph.2011.08.006) [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Yamamoto H, Sekizawa J, Kondo T, Hirai N, Tatrazako N. 2008. The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere 70, 865–873. ( 10.1016/j.chemosphere.2007.06.089) [DOI] [PubMed] [Google Scholar]
- 29.Valenti TW, Perez-Hurtado P, Chambliss CK, Brooks BW. 2009. Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH. Environ. Toxicol. Chem. 28, 2685–2694. ( 10.1897/08-546.1) [DOI] [PubMed] [Google Scholar]
- 30.Babic S, Horvat AJM, Mutavdzic Pavlovic M, Katelan-Macan M. 2007. Determination of pKa values of active pharmaceutical ingredients. Trends Anal. Chem. 26 ( 10.1016/j.trac.2007.09.004) [DOI] [Google Scholar]
- 31.Berninger JP, Du B, Connors KA, Eycheson SA, Kolkmeier MA, Prosser KN, Valenti TW, Chambliss K, Brooks BW. 2011. Effects of the antihistamine diphenhydramine on selected aquatic organisms. Environ. Toxicol. Chem. 30, 2065–2072. ( 10.1002/etc.590) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Comprehensive and verified data on the fate and effects of pharmaceutical substances in the environment are the essential basis for assessing their environmental risk and identifying possible risk management measures. These data are required not only for the authorization of pharmaceuticals but also for several other areas of environmental risk management (e.g. water pollution control). Better quality data on the fate and effects of pharmaceutical substances have been collected for both human and veterinary pharmaceuticals since the ERA guidelines came into force; however, these datasets are, in general, not publicly available. Thus, ERA data submitted in a dossier can only be used by the EU agencies during the review of the ERAs for the specific pharmaceutical product. This fact, among others, leads to a repetitive testing of pharmaceutical substances for different pharmaceutical products. This approach is expensive and very time consuming, and is not environmentally friendly. During the past few years, an increasing number of authorities (including the UBA) and scientists have requested that the ERA data be made publicly available. The EMA has started to publish the fate and effects endpoints of human and veterinary pharmaceuticals in Environmental Public Assessment Reports (EPARs/PARs) of products, but the quality and quantity of environmental data within the EPARs still need to be improved. Because long-term ERA data are not yet accessible to scientists or water boards, publicly available acute datasets on pharmaceutical substances have been used to assess the environmental risk of pharmaceuticals, e.g. for water regulation; such results may underestimate the environmental risk for pharmaceuticals and contradict ERA results. To ensure a more harmonized approach for the management of environmental risks associated with pharmaceuticals, it is important that the assessed data and conclusions of ERAs should be published as soon as possible, for example by the EMA.
Recent studies have shown that pharmaceuticals, according to their envisaged action in humans or animals (e.g. antidepressants that inhibit the serotonin uptake), are likely to exert side effects based on their MOA, such as a change in growth and feeding behaviour in fish [6]. These observed effects may not be the only action, due to the variable conservation status of molecular targets in aquatic organisms. Schreiber et al. [27] reported that the conservation of molecular targets in fish varied from 50 to 70% for diclofenac. In addition, researchers have recently started to study potential non-standard effects with improved test designs. Investigations on, for example, behavioural changes in laboratory and wild fish have already indicated potential endocrine effects for several antidepressants [28,29]. In addition, the importance of the chemical structure of pharmaceuticals has already been shown to influence the uptake by and thus possible adverse effects on biota in the aquatic environment. Many pharmaceuticals are designed as ionizable compounds to ensure that active components of administered doses reach a specific target location within the body. Therefore, exposure and ultimately toxic effects of these pharmaceuticals may depend on their dissociation constant (pKa) and environmental conditions (temperature, pH) [30]. Studies have been published that show pH-dependent effects of pharmaceuticals such as antidepressants and antihistamines on wildlife [31]; these authors suggest that considering how site-specific pH may affect the ionization state of drugs may reduce uncertainty during ERA. In conclusion, more and better information on pharmaceutical substances that (i) act on more than the main molecular target, (ii) exert unwanted adverse side effects, (iii) might be detected with more appropriate test designs and (iv) have varying structural characteristics (e.g. pKa), are considered to be very helpful for improved ERAs of pharmaceuticals.