Abstract
Medicinal innovation has led to the discovery and use of thousands of human and veterinary drugs. With this comes the potential for unintended effects on non-target organisms exposed to pharmaceuticals inevitably entering the environment. The impracticality of generating whole-organism chronic toxicity data representative of all species in the environment has necessitated prioritization of drugs for focused empirical testing as well as field monitoring. Current prioritization strategies typically emphasize likelihood for exposure (i.e. predicted/measured environmental concentrations), while incorporating only rather limited consideration of potential effects of the drug to non-target organisms. However, substantial mammalian pharmacokinetic and mechanism/mode of action (MOA) data are produced during drug development to understand drug target specificity and efficacy for intended consumers. An integrated prioritization strategy for assessing risks of human and veterinary drugs would leverage available pharmacokinetic and toxicokinetic data for evaluation of the potential for adverse effects to non-target organisms. In this reiview, we demonstrate the utility of read-across approaches to leverage mammalian absorption, distribution, metabolism and elimination data; analyse cross-species molecular target conservation and translate therapeutic MOA to an adverse outcome pathway(s) relevant to aquatic organisms as a means to inform prioritization of drugs for focused toxicity testing and environmental monitoring.
Keywords: pharmacokinetics, SeqAPASS, adverse outcome pathway, read-across, molecular target conservation
1. Introduction
Active pharmaceutical ingredients are increasingly detected in the environment due to several factors, including advances in human and veterinary medicinal practices, the ageing human population and improved sensitivity of analytical instrumentation. Sources such as wastewater treatment plant effluent and run-off associated with animal feeding operations have been implicated as important contributors of pharmaceuticals to aquatic environments [1]. Owing to the continuous introduction of some of these chemicals into waterbodies, they have been termed pseudo-persistent, a characteristic that increases the possibility of chronic exposures of non-target organisms. Unintended exposures of aquatic species to pharmaceuticals are inevitable and have been documented [2–4]. Unfortunately, only limited publically available ecotoxicity data exist for most drugs, making informed, transparent, assessments of their possible ecological risks problematic [5,6]. Further, much of the ecotoxicity data that do exist for pharmaceuticals focus on short-term exposure tests or acute lethality, which is not always suitable for predicting effects of pseudo-persistent chemicals specifically designed to produce sublethal biological effects [5,6]. That is, many pharmaceuticals are designed to target specific pathways, often at relatively low doses [7,8]. Certain of these pathways are critical to the long-term maintenance of physiological functions, and can be highly conserved across taxa, including non-target aquatic animal species. As such, it is reasonable to expect that some pharmaceuticals will elicit adverse sublethal responses in chronic exposures [5,6].
Generation of chronic ecotoxicity data for the large number of pharmaceuticals that may (or do) enter aquatic environments would be prohibitively costly, as well as requiring numerous test animals, which contradicts the growing international desire to decrease animal use. Given the number of drugs in use or development (5000+; [9]), whole-organism chronic toxicity studies for assessing all possible risks are impractical, so techniques for the prioritization of those chemicals most likely to be problematic are needed. In recognition of the challenges associated with testing pharmaceuticals for their effects to human and ecological health and the need to better focus research efforts, international experts recently gathered at a workshop to develop a list of the most critical questions to guide future studies, including questions pertaining to best practices for prioritization and effects characterization [10]. Consistent with these recommendations, development of several prioritization approaches that effectively and efficiently use available pharmaceutical knowledge is ongoing. Currently, the prominent focal point for this type of activity has been based on exposure (e.g. related to production volume, use patterns, potential for bioconcentration, etc.), occasionally with some consideration of potential for effects (e.g. predicted no effect concentration) [11,12].
The need for a more integrated exposure- and effects-based approach to pharmaceutical prioritization can be illustrated when considering the contraceptive ingredient ethinyl oestradiol (EE2), a synthetic oestrogen known to cause endocrine-disrupting effects in fish at low ng l−1 concentrations [13]. Predicted (based on production volume) or measured concentrations of EE2 in aquatic systems and demonstrated potential for acute lethality in a number of species would rank EE2 as a very low priority [5]. However, empirical data from chronic toxicity studies, as well as an understanding of cross-species pathway conservation would most certainly result in a high priority ranking of EE2 when considering potential for effects [12,14,15]. Caffeine, in contrast, is abundantly and consistently found in aquatic samples, but poses negligible potential for effects to exposed species [1], thus indicating a much lower priority ranking. These relatively simple examples emphasize the need for an integrated understanding of both the potential for exposure, and the plausible effects of the drug on non-target organisms for robust pharmaceutical prioritization.
Unlike most other classes of chemicals of possible ecological concern, insights as to possible environmental exposure and effects of both human and veterinary drugs can be gleaned from a priori knowledge. For example, for many pharmaceuticals, efficacy and safety data are available concerning adsorption, distribution, metabolism and elimination (ADME), and biological pathways affected in target species (i.e. humans, livestock). From this, it should be possible to employ systematic approaches to prioritize pharmaceuticals for monitoring and testing in two ways: (i) identification of chemicals with the most potential to elicit adverse effects and (ii) identification of which species/endpoints should be used for this testing or monitoring. In this review, we describe specific techniques we are applying to this challenge.
2. Focus on likelihood for adverse effects
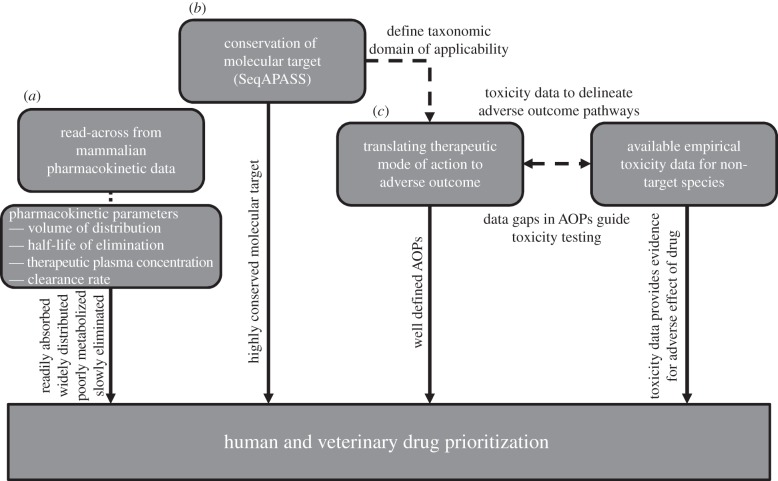
The fact that a drug must be present in the environment for it to cause adverse effects to non-target organisms is irrefutable. However, considerations of pharmacokinetic measures, biological pathway interactions (both with potential for specific and non-specific interactions) and knowledge of primary drug-metabolizing enzymes and conservation of molecular targets, can provide enhanced potential to rank and prioritize pharmaceuticals for their potential to cause unintended effects to wildlife (figure 1).
Figure 1.
Connections between key components of effects-based prioritization strategy for human and veterinary pharmaceuticals. Rectangular shapes describe the components considered for prioritization. Long dashed line represents instances where data or information derived from one of the components can be used to better understand or guide decisions within another component. Small dashed line represents parameters assessed within component (a). Solid arrows represent the information used to guide prioritization derived from each component. AOPs, adverse outcome pathways. (a–c) Correspond to §2a,b and c in the text.
(a). Utility of read-across from mammalian pharmacokinetic data
Approximately 40–60% of new drug candidates for humans fail owing to poor ADME profiles [16]. Therefore, accurately measuring or predicting mammalian pharmacokinetic parameters is fundamental to drug discovery and development, and typically, data for existing drugs are publically available. A number of online databases house this type of information, including Drugbank (6825 drug entries [9]), PK/DB database for pharmacokinetic properties (ca 1400 chemicals [17]) and the PharmacoKinetics Knowledge Base (PKKB; 1685 drugs [16]). However, due to the inconsistent nature of information in these databases relative to, for example, pharmacokinetic parameters measured (including method descriptions), reporting units, linkage to primary literature, etc., we deemed these sources unsuitable for transparent prioritization efforts. Therefore, we have developed a database of the most commonly prescribed drugs, over-the-counter drugs and veterinary medicines, which is representative of nearly all therapeutic classes. Our database is populated with information from selected review articles [18,19], the Physicians' Desk Reference [20], manufacturers/government agency monographs and the primary literature, and provides consistently referenced material with common units. This curated evaluation of the available literature includes data for 1200 drugs across 100 drug classes (defined by source description of mode of action (MOA)), and includes approximately 7000 data points related to ADME parameters. With this readily accessible mammalian pharmacokinetic data, read-across approaches can be employed to inform or hypothesize the potential pharmacodynamics of both specific and representative classes of drugs in non-target species.
Biological read-across as it relates to ecotoxicology has been described as the ability for a drug to have an effect on a non-target organism owing to molecular target conservation and similar pharmacology as the target species [21]. Pharmacokinetic parameters selected as a means to inform cross-species read-across include clearance rate, volume of distribution, therapeutic plasma concentration and half-life of elimination. Although a thorough understanding of pharmacokinetic nuances between species is lacking, currently available data can be used based on qualitative understandings of species similarities, particularly within vertebrates. Simply put, in the absence of evidence to the contrary, it is reasonable to assume that if a drug is readily absorbed, widely distributed, poorly metabolized and/or slowly eliminated in the mammalian target species, it has greater potential for hazard in non-target vertebrate species.
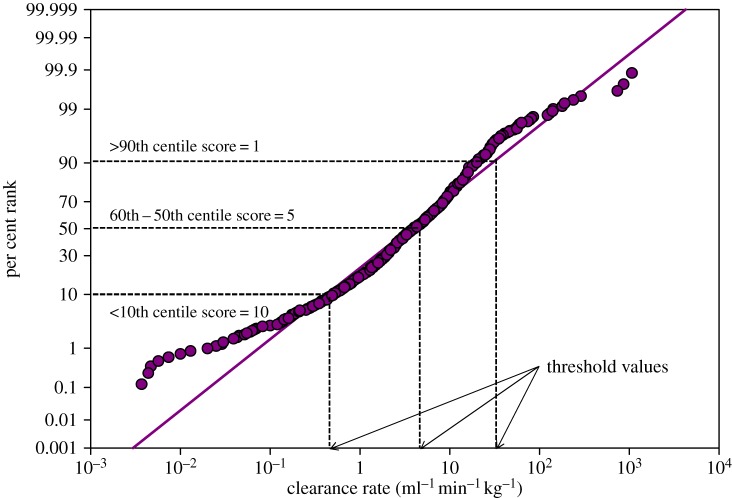
With this basic assumption in mind, our database serves as a component of a dynamic framework for prioritizing drugs. Specifically, each data point is ranked within the pharmacokinetic parameter using a probabilistic distribution [22]. The ranking provides a regression from which data can be scored, from 1 to 10, according to specific 10th centile thresholds, and translated into relative potential for hazard. For example, total body clearance (ml min−1 kg−1) data are considered important, with the assumption that the slower the clearance the greater the potential hazard (i.e. as a drug would have more time to initiate its biological action). The database includes 832 data points ranging between 0.0037 and 1070 ml min−1 kg−1 for total body clearance. Scores were established using the probabilistic distribution of the data, and subsequent regression threshold values for each 10th centile (figure 2). Drugs with lower clearance values are subsequently assigned higher scores based on potential hazard. When multiple pharmacokinetic parameters are assessed for each drug in this manner, scores can be summed to develop an overall prioritization metric for available ADME data as a component of a comprehensive prioritization strategy. A critical attribute of this approach is that relative prioritization rankings can be produced by grouping drugs by class or chemical structure allowing for predictions of probable ADME priorities for newly developed drugs or older pharmaceuticals falling within that class, for which significant pharmacokinetic information is lacking.
Figure 2.
Probabilistic distribution of total clearance values for 832 pharmaceuticals. Thresholds values and associated scores are identified for three of the 10th centile categories used to bin the data and identify prioritization scores. Per cent rank (y-axis) is based on Weibull distribution [22]. (Online version in colour.)
(b). Conservation of molecular target for cross-species extrapolation
Pharmaceuticals are generally designed to act on specific molecular targets to produce their desired therapeutic benefits and lessen the potential for undesirable off-target interactions. The biomolecular targets typically are involved in key metabolic or signal transduction pathways specific to a disease or medical condition. Conservation of these molecular targets at the protein level is likely when considering species with close phylogeny. However, some targets are well conserved across more diverse phyla. Another key component for focusing testing efforts on organisms with the greatest likelihood for susceptibility to a given pharmaceutical includes considering cross-species similarity of therapeutic molecular targets. Efforts have been made to explore the utility of protein sequence comparisons as a means to estimate sensitivity to pharmaceuticals, demonstrating cross-species conservation of many drug targets [11,23]. Recently, we developed a computational tool, Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS), that facilitates rapid and strategic examination of protein sequence similarity at the level of the primary amino acid sequence (including orthologue candidate identification), conserved functional domains and (when possible) individual amino acid residue position(s) across species, as a means to predict relative intrinsic susceptibility to chemicals with known MOA [14]. Output derived from the SeqAPASS analysis can be used to define the relevance (or lack thereof) of known protein targets across taxa (e.g. figure 3). A growing list of case studies, including examples with the human pharmaceutical EE2, the veterinary drug 17β-trenbolone (an anabolic steroid) and the pesticide permethrin, for which predictions of susceptibility compared favourably with empirical toxicity data [14,24], have demonstrated the relevance and utility of the SeqAPASS tool. Others have explored molecular docking of pharmaceuticals to drug targets in common ecotoxicological model species, further demonstrating the utility of protein structural information, when it exists or can be modelled, for predicting potential for effects [25,26]. These types of strategies for species extrapolation use existing (and rapidly expanding) protein sequence/structural information to explore species similarities and differences meaningful to the molecular initiating event (MIE; e.g. receptor/ligand interaction, enzyme inhibition, etc.) responsible for producing adverse effects [27,28]. Exploring commonalities among species with regard to structure of key protein targets such as receptors (or primary xenobiotic-metabolizing enzymes) is a key piece of information that can guide predictions of potential cross-species susceptibility to pharmaceuticals, particularly when quantitative data regarding receptor/ligand interaction, binding affinity and potency for most non-mammalian species are lacking. This approach, in conjunction with leveraging mammalian ADME data, can serve as the basis for prioritizing testing and monitoring of pharmaceuticals.
Figure 3.
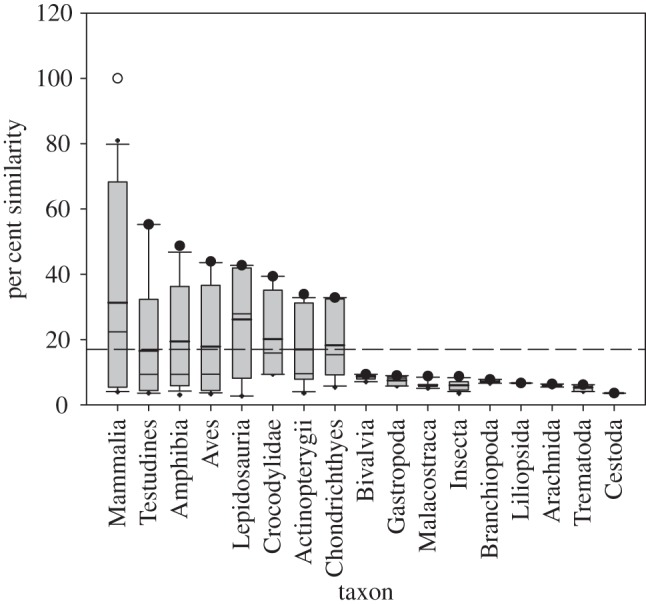
Protein sequence similarity across species compared with the bovine androgen receptor (AR) modified from LaLone et al. [14]. Open circle represents the Bos taurus AR and filled circle represents the species with the highest per cent similarity within a specified taxonomic group. Dashes indicates the cut-off for intrinsic susceptibility predictions, with those above the line predicted to be susceptible. Determination of the susceptibility cut-off using reciprocal best hit BLAST methods for orthologue candidate identification was described previously [14].
(c). Translating therapeutic mode of action into adverse outcome(s) relevant for risk assessment
Adverse outcome pathways (AOPs) have been proposed as a conceptual framework through which to link chemical–biological interactions at the molecular level (termed MIEs) with key events at multiple biological levels of organization, culminating in an adverse outcome of regulatory significance at the individual or population level [27]. The Organisation for Economic Cooperation and Development (OECD) is coordinating an internationally harmonized effort to develop a knowledgebase of AOPs relevant to both human health and ecological effects [29]. Examples of well-defined AOPs relevant to established molecular targets of pharmaceuticals include aromatase inhibition, androgen receptor (AR) activation, oestrogen receptor activation or antagonism, and steroidogenesis inhibition leading to impaired reproduction in fish [27]. Several existing AOPs, and/or studies from which putative AOPs relevant to the function(s) of fish orthologues to human drug targets are summarized in table 1. Based on ongoing efforts in the international scientific community to delineate and disseminate AOPs suitable for regulatory application (http://www.oecd.org/env/ehs/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm and www.aopwiki.org), it is expected that growing numbers of well-described AOPs associated with orthologues to human drug targets will become available. Notably, further development of AOPs relevant to ecological risk assessment can be accelerated through strategic application of the SeqAPASS tool to help define which pharmaceutical-relevant MIEs are applicable to different phyla of aquatic organisms.
Table 1.
Putative adverse outcome pathways for fish relevant to human and veterinary drugs. Single asterisk indicates established AOPs developed in accordance with OECD guidance and submitted to the AOP wiki (www.aopwiki.org). Double asterisk indicates hypothesized molecular initiating event or adverse outcome.
| drug class | molecular initiating event | example key events | adverse outcome | reference |
|---|---|---|---|---|
| anabolic steroids | androgen receptor activation* | — impaired vitellogenesis, ovulation and spawning | — reproductive impairment | [24,27,30,31] |
| anti-androgens | androgen receptor antagonism | — impaired ovulation and spawning — impaired sexual differentiation — decreased nest defence |
— reproductive impairment — reduced probability of young of year survival** |
[32–37] |
| hormonal contraceptive | oestrogen receptor activation | — impaired ovulation and spawning — impaired sexual development |
— reproductive impairment | [27] |
| hormonal contraceptive | oestrogen receptor antagonism* | — impaired vitellogenesis, ovulation and spawning — impaired sexual differentiation |
— reproductive impairment** | [38–42] |
| hormonal contraceptive | progesterone receptor activation | — reduced fecundity — reduced sperm motility |
— reproductive impairment | [43–46] |
| anti-inflammatory | glucocorticoid receptor activation | — reduced oestradiol synthesis and fecundity — morphological abnormalities — decreased immune response |
— reproductive impairment — reduced probability of young of year survival** |
[47–49] |
| anti-depressants | serotonin reuptake inhibition | — morphological abnormalities — reduced fecundity — decreased food intake — impaired predator avoidance |
— reproductive impairment — reduced probability of young of year survival** |
[50–55] |
| anti-convulsant | gamma-aminobutyric-acid receptor opening** sodium-channel inhibition |
— impaired predator avoidance | — reduced probability of young of year survival** | [56,57] |
| non-steroidal anti-inflammatory drugs | cyclooxygenase inhibition | — impaired growth — impaired hatching — decreased spawning behavioura — reduced fecunditya |
— increased probability of mortality** — reduced probability of young of year survival** — impaired reproductiona |
[58–60] |
| fibrates | peroxisome proliferator-activated receptor activation | — reduced fecundity | — impaired reproduction | [61] |
| beta-blockers | beta-adrenergic receptor antagonist | — reduced growth | — reduced probability of young of year survival** — increased probability of mortality** |
[62–64] |
| conazolesb | aromatase inhibition* | — impaired vitellogenesis, ovulation and spawning | — impaired reproduction | [65–69] |
| anti-psychotics | dopamine receptor antagonism | — increased male dominance | — adverse consequence unknown | [70,71] |
| goitrogens | thyroid peroxidase inhibition | — delayed development | — reduced probability of young of year survival** | [72–75] |
aD. Martinovic-Weigelt, University of St Thomas, St Paul, MN, personal communication.
bAromatase inhibition is an established molecular initiating event for propiconazole, however other conazoles have been shown to inhibit other cytochrome P450 enzymes [76].
3. Prioritization based on potential for effects reveals uncommonly identified drugs
To demonstrate the utility of our ADME database and SeqAPASS tool (in conjunction with AOP knowledge) for prioritizing pharmaceuticals for toxicity testing and environmental monitoring, we provide an example that focuses on drugs that interact with the AR in target species (humans, livestock). Owing to the critical role of the AR in endocrine function, it is an important drug target in humans for treating certain cancers, testosterone deficiencies, hypogonadism, dermatological conditions, hirsutism and delayed puberty in males, and in livestock, particularly beef cattle, for increasing muscle mass [24]. Using our ADME database, DrugBank and the Veterinary Substance Database [77], we first identified human and veterinary pharmaceuticals whose therapeutic MOA involved an interaction with the AR (table 2). The majority of these chemicals, which all have hormone-type structures, have neither been tested for toxicity to non-target species nor routinely monitored in the environment.
Table 2.
Prioritization of androgen receptor modulating drugs based on potential for adverse effects. Dashes represent drugs with limited pharmacokinetic data available for scoring individually. Tick indicates empirical evidence exists, represented by a select publication in the reference column.
| active ingredient | primary CAS no. | average ADME scorea | empirical evidence of adverse effect in fish | reference |
|---|---|---|---|---|
| bicalutamide | 90357-06-5 | 6.8 | √ | [78] |
| boldenone | 846-48-0 | 10.0 | ||
| calusterone | 17021-26-0 | — | ||
| cyproterone | 2098-66-0 | — | √ | [32] |
| danazol | 17230-88-5 | 7.0 | ||
| dihydrotestosterone | 521-18-6 | — | √ | [79] |
| drospirenone | 67392-87-4 | — | √ | [80] |
| drostanolone | 58-19-5 | — | ||
| enzalutamide | 915087-33-1 | 7.0 | ||
| fludrocortisone | 127-31-1 | — | ||
| fluoxymesterone | 76-43-7 | — | ||
| flutamide | 13311-84-7 | 5.0 | √ | [33] |
| levonorgestrel | 17489-40-6 | 7.3 | √ | [81] |
| methylnortestosterone, 7α-, 19- | 3764-87-2 | 2.7 | ||
| methyltestosterone | 58-18-4 | — | √ | [82] |
| methyltrienolone | 965-93-5 | — | ||
| nandrolone | 434-22-0 | 5.0 | ||
| nilutamide | 63612-50-0 | — | ||
| norgestimate | 35189-28-7 | — | ||
| oxandrolone | 53-39-4 | 7.3 | ||
| spironolactone | 52-01-7 | 5.3 | √ | [24] |
| testolactone | 968-93-4 | — | ||
| testosterone | 57-85-2 | — | √ | [83] |
| trenbolone | 10161-34-9 | — | √ | [30] |
aAverage ADME score: calculated by averaging hazard score across pharmacokinetic parameters (clearance rate, volume of distribution, therapeutic plasma concentration and half-life of elimination).
To determine the potential for effects in non-target species via interaction with the AR, we first assessed cross-species conservation of the human and bovine (target species) AR using SeqAPASS. Data from both analyses indicate a high degree of AR conservation across vertebrates, including fish species (figure 3; note that results are similar using either the bovine or human AR as queries, so only bovine is shown). The drugs in table 2 may target the AR as either agonists or antagonists. Importantly, both AR agonists and antagonists are linked to established or putative AOPs resulting in adverse reproductive effects in fish (table 1). Therefore, as either AR agonists or antagonists, these drugs would be considered high priority owing to conservation of their molecular target across vertebrate species, and the demonstration (through linkages established via AOPs) of the potential for adverse population-relevant effects.
To help assess AR modulators with incomplete mammalian pharmacokinetic information, we initially evaluated drugs classified as hormones in our ADME database (53 compounds) to derive a class-level priority ranking. Accordingly, probabilistic distributions were derived for each pharmacokinetic parameter individually to assess hormone-like drugs only. Within each parameter, we identified the value representing the 90th or 10th percentile (depending on hazard assumption) and used those values to represent hormones as a class. Subsequently, probabilistic distributions for all drugs in our database were derived for each pharmacokinetic parameter (e.g. figure 2). The hormone class assigned values were then scored based on the centiles from the distributions of all drugs. The final hazard score of 9.3 of 10 was then calculated by averaging hormone class scores across all parameters, ranking this drug class as high priority compared with other classes (e.g. narcotics score: 8.3; non-steroidal anti-inflammatory drugs score: 7; and antibiotics score: 6.8).
Relevant mammalian pharmacokinetic information (i.e. volume of distribution, clearance rate and/or half-life of elimination) was available in our ADME database for 10 of the 24 AR-active drugs identified. Based on this, individual AR-active drugs can be evaluated using distributions derived from all drugs for each pharmacokinetic parameter, scores assigned, and then averaged across each parameter (table 2). The resultant average ADME score allows for ranking an individual drug against all others evaluated, thus further helping assign priority based on the potential for hazard. For example, based on ADME properties, boldenone (an anabolic steroid illegally used to enhance athletic performance in canine, equine and human athletes, and improve food conversion in cattle [84]) would rank as a higher priority than 7α, 19-methylnortestosterone (under study in humans to treat hypogonadism in males and as a male contraceptive [85]) for testing or monitoring.
For some AR-active chemicals, empirical chronic toxicity data in non-target species (predominantly fish) are available, providing additional input for priority ranking and also illustrating where test data are needed (table 2). Significantly, a majority of the drugs listed in table 2 have not been identified by other prioritization strategies focused primarily on the potential for exposure in the environment. Analogous prioritizations have been and are being employed in our laboratory to guide our toxicity testing and field monitoring, which, as an example, recently led to the identification of spironolactone as a pharmaceutical of potential environmental concern to fish species [24]. In this case, the knowledge of a well-defined AOP for AR activation leading to reproductive impairment in small fish helped to focus toxicity testing with spironolactone on endpoints previously recognized as being impacted by perturbation of the AR. Additionally, SeqAPASS data directed the selection of test organisms likely to be susceptible to spironolactone, with predictions indicating fish would be more susceptible than invertebrates such as daphnids, which we subsequently confirmed experimentally [24]. In this manner, AOP knowledge in combination with SeqAPASS evaluation of cross-species susceptibility offers a powerful means to guide toxicity testing, with the potential to reduce the investment of time, resources and animal testing.
4. Summary and vision for prioritization
The read-across components of our effects-based prioritization strategy for human and veterinary drugs, combined with application of the AOP framework and approaches for defining taxonomic relevance of key events (e.g. MIE), and evaluation of available empirical toxicity data, provide insights that complement analyses based on exposure. Although potential for exposure is undeniably important, biological considerations are critical to a comprehensive approach for drug prioritization in terms of assessing potential for adverse effects in non-target organisms. Central to this strategy is evaluation of biological pathways and processes, and their conservation across taxa, a central theme to the broader issue of cross-species extrapolation of chemical effects in the discipline of toxicology [86].
A number of recent publications have presented various methods for drug prioritization. Briefly, these papers included consideration of conserved receptors and enzymes important as drug targets across species [11,14,23,24,87]; calculated effects ratios using human and fish plasma concentration data [87]; acute to therapeutic ratios [22,88]; identification of high production volume drugs, identification of those that are persistent, bioaccumulative and toxic [89]; maximum observed (environmental) concentrations, predicted effect concentrations and predicted no-effect concentrations [11,87]; prescription volume and sales data, evaluation of days of water consumption required to ingest equivalent of a single minimum daily therapeutic dose for a given pharmaceutical MOA [11]; and consideration of AOPs [24,88]. From these prioritization methods, it is clear that an integrated approach is on the horizon when the tools become available for rapidly and strategically disseminating available pharmaceutical data. Our strategy builds on some of the concepts described previously and specifically introduces a novel tool (i.e. SeqAPASS) and database (ADME database) being developed for use by the scientific community which will be publically released in the near future for others to use for drug prioritization.
Importantly, the prioritization strategy and tools described herein are intended to guide toxicological research and inform lists of chemicals to monitor in the environment. We recognize that the quality of the assumptions used for the read-across approaches described are based on current knowledge of the science and therefore must be applied with an understanding that uncertainties exist. Although research is ongoing, empirical studies are limited relative to the assumption that mammalian ADME data translates well to fish. For example, a recent study assessing metabolism of common human pharmaceuticals in fish provided evidence that, in rainbow trout liver S9 fractions, little substantial biotransformation occurred for several known substrates of human cytochrome P450 (CYP) 2D6, CYP2C9 or CYP3A4 [90]. These results suggest that further research is necessary to understand the challenges associated with the ADME read-across approach [91]. However, in the absence of fish-specific data, mammalian pharmacokinetic knowledge currently presents the most logical starting point for read-across approaches owing to its abundance and availability. Further, uncertainties related to cross-species extrapolation using protein sequence/structure comparisons have been identified and reviewed elsewhere [14,23]; for example, it is recognized that other factors play a role in determining susceptibility to a chemical beyond the presence of a molecular target (e.g. metabolism, life stage, life history) [92]. Off-target molecular effects can also impact predictions of susceptibility. Finally, fully developed AOPs, and even putative AOPs, for pharmaceuticals are sparse and though the AOP framework has strong support from the international scientific community, it will take time for new AOP constructs to be developed, accepted as having adequate supporting evidence, and be available for use in a prioritization strategy for the majority of pharmaceutical therapeutic classes. Nonetheless, important advances in hazard prediction relevant to drug prioritization for toxicity testing and monitoring can be obtained from making use of available pharmaceutical information with strategic and thoughtful approaches, such as those presented here. Specifically, the presence of a drug on a prioritized list indicates that sufficient predictive and/or empirical support ranks it as having an increased potential for adverse effects in non-target species, and therefore evidence to proceed with further exploration.
Acknowledgements
The authors thank A. Schroeder for providing thoughtful review comments on an earlier version of the paper. We also acknowledge J. Tietge and C. Russom for their insightful discussions and input regarding development of our methods for drug prioritization. This manuscript has been reviewed in accordance with the requirements of the US EPA Office of Research and Development; however, the recommendations made herein do not represent US EPA policy. Mention of products or trade names does not indicate endorsement by the US EPA.
References
- 1.Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol. 36, 1202–1211. ( 10.1021/es011055j) [DOI] [PubMed] [Google Scholar]
- 2.Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyer CR, Sumpter JP. 1994. Estrogenic effects of effluents from sewage treatment works. Chem. Ecol. 8, 275–285. ( 10.1080/02757549408038554) [DOI] [Google Scholar]
- 3.Oaks JL, et al. 2004. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427, 630–633. ( 10.1038/nature02317) [DOI] [PubMed] [Google Scholar]
- 4.Daughton CG, Brooks BW. 2011. Environmental contaminants in biota. New York, NY: CRC Press. [Google Scholar]
- 5.Ankley GT, Brooks BW, Huggett DB, Sumpter JP. 2007. Repeating history: pharmaceuticals in the environment. Environ. Sci. Technol. 41, 8211–8217. ( 10.1021/es072658j) [DOI] [PubMed] [Google Scholar]
- 6.Brain RA, Brooks BW. 2012. Considerations and criteria for the incorporation of mechanistic sublethal endpoints into environmental risk assessment for biologically active compounds. In Human pharmaceuticals in the environment, current and future perspectives, ch. 7, pp. 139–165. New York, N: Springer Science + Business Media, LLC. [Google Scholar]
- 7.Lin JH, Lu AY. 1997. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol. Rev. 49, 403–449. [PubMed] [Google Scholar]
- 8.Arnold KE, et al. 2013. Assessing the exposure risk and impacts of pharmaceuticals in the environment on individuals and ecosystems. Biol. Lett. 9, 20130492 ( 10.1098/rsbl.2013.0492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knox C, et al. 2011. DrugBank 3.0: a comprehensive resource for 'omics’ research on drugs. Nucleic Acids Res. 39, D1035–D1041. ( 10.1093/nar/gkq1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boxall ABA, et al. 2012. Pharmaceuticals and personal care products in the environment: what are the big questions? Environ. Health Perspect. 120, 1221–1229. ( 10.1289/ehp.1104477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostich MS, Lazorchak JM. 2008. Risks to aquatic organisms posed by human pharmaceutical use. Sci. Total Environ. 389, 329–339. ( 10.1016/j.scitotenv.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 12.Roos V, Gunnarsson L, Fick J, Larsson DG, Ruden C. 2012. Prioritising pharmaceuticals for environmental risk assessment: towards adequate and feasible first-tier selection. Sci. Total Environ. 421–422, 102–110. ( 10.1016/j.scitotenv.2012.01.039) [DOI] [PubMed] [Google Scholar]
- 13.Caldwell DJ, Mastrocco F, Hutchinson TH, Lange R, Heijerick D, Janssen C, Anderson PD, Sumpter JP. 2008. Derivation of an aquatic predicted no-effect concentration for the synthetic hormone, 17α-ethinyl estradiol. Environ. Sci. Technol. 42, 7046–7054. ( 10.1021/es800633q) [DOI] [PubMed] [Google Scholar]
- 14.LaLone CA, et al. 2013. Molecular target sequence similarity as a basis for species extrapolation to assess the ecological risk of chemicals with known modes of action. Aquat. Toxicol. 144–145, 141–154. ( 10.1016/j.aquatox.2013.09.004) [DOI] [PubMed] [Google Scholar]
- 15.Ankley GT, Gray LE. 2013. Cross-species conservation of endocrine pathways: a critical analysis of tier 1 fish and rat screening assays with 12 model chemicals. Environ. Toxicol. Chem. 32, 1084–1087. ( 10.1002/etc.2151) [DOI] [PubMed] [Google Scholar]
- 16.Cao D, Wang J, Zhou R, Li Y, Yu H, Hou T. 2012. ADMET evaluation in drug discovery. 11. Pharmacokinetics knowledge base (PKKB): a comprehensive database of pharmacokinetic and toxic properties for drugs. J. Chem. Inf. Model. 52, 1132–1137. ( 10.1021/ci300112j) [DOI] [PubMed] [Google Scholar]
- 17.Moda TL, Torres LG, Carrara AE, Andricopulo AD. 2008. PK/DB: database for pharmacokinetic properties and predictive in silico ADME models. Bioinformatics 24, 2270–2271. ( 10.1093/bioinformatics/btn415) [DOI] [PubMed] [Google Scholar]
- 18.Obach RS, Lombardo F, Waters NJ. 2008. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab. Dispos. 36, 1385–1405. ( 10.1124/dmd.108.020479) [DOI] [PubMed] [Google Scholar]
- 19.Schulz M, Iwersen-Bergmann S, Andresen H, Schmoldt A. 2012. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit. Care 16, R136 ( 10.1186/cc11441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anon 2013. Physicians desk reference, 67th edn Montvale, NJ: PDR Network. [Google Scholar]
- 21.Rand-Weaver M, Margiotta-Casaluci L, Patel A, Panter GH, Owen SF, Sumpter JP. 2013. The read-across hypothesis and environmental risk assessment of pharmaceuticals. Environ. Sci. Technol. 47, 11 384–11 395. ( 10.1021/es402065a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berninger JP, Brooks BW. 2010. Leveraging mammalian pharmaceutical toxicology and pharmacology data to predict chronic fish responses to pharmaceuticals. Toxicol. Lett. 193, 69–78. ( 10.1016/j.toxlet.2009.12.006) [DOI] [PubMed] [Google Scholar]
- 23.Gunnarsson L, Jauhiainen A, Kristiansson E, Nerman O, Larsson DG. 2008. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 42, 5807–5813. ( 10.1021/es8005173) [DOI] [PubMed] [Google Scholar]
- 24.LaLone CA, et al. 2013. Cross species sensitivity to a novel androgen receptor agonist of potential environmental concern, spironolactone. Environ. Toxicol. Chem. 32, 2528–2541. ( 10.1002/etc.2330) [DOI] [PubMed] [Google Scholar]
- 25.Walker SD, McEldowney S. 2013. Molecular docking: a potential tool to aid ecotoxicity testing in environmental risk assessment of pharmaceuticals. Chemosphere 93, 2568–2577. ( 10.1016/j.chemosphere.2013.09.074) [DOI] [PubMed] [Google Scholar]
- 26.McRobb FM, Sahagun V, Kufareva I, Abagyan R. 2014. In silico analysis of the conservation of human toxicity and endocrine disruption targets in aquatic species. Environ. Sci. Technol. 48, 1964–1972. ( 10.1021/es404568a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ankley GT, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741. ( 10.1002/etc.34) [DOI] [PubMed] [Google Scholar]
- 28.Miyagawa S, et al. 2014. Differing species responsiveness of estrogenic contaminants in fish is conferred by the ligand binding domain of the estrogen receptor. Environ. Sci. Technol. 48, 5254–5263. ( 10.1021/es5002659) [DOI] [PubMed] [Google Scholar]
- 29.Organisation of Economic Cooperation and Development. 2014 Adverse outcome pathways, molecular screening and toxicogenomics http://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm (accessed 31 March 2014).
- 30.Ankley GT, et al. 2003. Effects of the androgenic growth promoter 17-β-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ. Toxicol. Chem. 22, 1350–1360. ( 10.1002/etc.5620220623) [DOI] [PubMed] [Google Scholar]
- 31.Ekman DR, et al. 2011. Use of gene expression, biochemical and metabolite profiles to enhance exposure and effects assessment of the model androgen 17β-trenbolone in fish. Environ. Toxicol. Chem. 30, 319–329. ( 10.1002/etc.406) [DOI] [PubMed] [Google Scholar]
- 32.Kiparissis Y, Metcalfe TL, Balch GC, Metcalfe CD. 2003. Effects of the antiandrogens, vinclozolin and cyproterone acetate on gonadal development in the Japanese medaka (Oryzias latipes). Aquat. Toxicol. 63, 391–403. ( 10.1016/S0166-445X(02)00189-3) [DOI] [PubMed] [Google Scholar]
- 33.Jensen KM, Kahl MD, Makynen EA, Korte JJ, Leino RL, Butterworth BC, Ankley GT. 2004. Characterization of responses to the antiandrogen flutamide in a short-term reproduction assay with the fathead minnow. Aquat. Toxicol. 70, 99–110. ( 10.1016/j.aquatox.2004.06.012) [DOI] [PubMed] [Google Scholar]
- 34.Dey CJ, O'Connor CM, Gilmour KM, Van Der Kraak G, Cooke SJ. 2010. Behavioral and physiological responses of a wild teleost fish to cortisol and androgen manipulation during parental care. Horm. Behav. 58, 599–605. ( 10.1016/j.yhbeh.2010.06.016) [DOI] [PubMed] [Google Scholar]
- 35.Nakamura A, Takanobu H, Tamura I, Yamamuro M, Iguchi T, Tatarazako N. 2013. Verification of responses of Japanese medaka (Oryzias latipes) to anti-androgens, vinclozolin and flutamide, in short-term assays. J. Appl. Toxicol. 34, 545–553. ( 10.1002/jat.2934) [DOI] [PubMed] [Google Scholar]
- 36.Hatef A, Alavi SM, Milla S, Kristan J, Golshan M, Fontaine P, Linhart O. 2012. Anti-androgen vinclozolin impairs sperm quality and steroidogenesis in goldfish. Aquat. Toxicol. 122–123, 181–187. ( 10.1016/j.aquatox.2012.06.009) [DOI] [PubMed] [Google Scholar]
- 37.Martinovic D, Blake LS, Durhan EJ, Greene KJ, Kahl MD, Jensen KM, Makynen EA, Villeneuve DL, Ankley GT. 2008. Reproductive toxicity of vinclozolin in the fathead minnow: confirming an anti-androgenic mode of action. Environ. Toxicol. Chem. 27, 478–488. ( 10.1897/07-206R.1) [DOI] [PubMed] [Google Scholar]
- 38.Sun L, Zha J, Spear PA, Wang Z. 2007. Tamoxifen effects on the early life stages and reproduction of Japanese medaka (Oryzias latipes). Environ. Toxicol. Pharmacol. 24, 23–29. ( 10.1016/j.etap.2007.01.003) [DOI] [PubMed] [Google Scholar]
- 39.Sun L, Zha J, Wang Z. 2009. Effects of binary mixtures of estrogen and antiestrogens on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 93, 83–89. ( 10.1016/j.aquatox.2009.03.010) [DOI] [PubMed] [Google Scholar]
- 40.Singh R, Singh AK, Tripathi M. 2012. Effect of a non steroidal tamoxifen on the gonad and sex differentiation in Nile tilapia, Oreochromis niloticus. J. Environ. Biol. 33, 799–803. [PubMed] [Google Scholar]
- 41.Kitano T, Yoshinaga N, Shiraishi E, Koyanagi T, Abe S. 2007. Tamoxifen induces masculinization of genetic females and regulates P450 aromatase and Mullerian inhibiting substance mRNA expression in Japanese flounder (Paralichthys olivaceus). Mol. Reprod. Dev. 74, 1171–1177. ( 10.1002/mrd.20603) [DOI] [PubMed] [Google Scholar]
- 42.Williams TD, Caunter JE, Lillicrap AD, Hutchinson TH, Gillings EG, Duffell S. 2007. Evaluation of the reproductive effects of tamoxifen citrate in partial and full life-cycle studies using fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 26, 695–707. ( 10.1897/05-646R1.1) [DOI] [PubMed] [Google Scholar]
- 43.Zeilinger J, Steger-Hartmann T, Maser E, Goller S, Vonk R, Lange R. 2009. Effects of synthetic gestagens on fish reproduction. Environ. Toxicol. Chem. 28, 2663–2670. ( 10.1897/08-485.1) [DOI] [PubMed] [Google Scholar]
- 44.Paulos P, Runnalls TJ, Nallani G, La Point T, Scott AP, Sumpter JP, Huggett DB. 2010. Reproductive responses in fathead minnow and Japanese medaka following exposure to a synthetic progestin, Norethindrone. Aquat. Toxicol. 99, 256–262. ( 10.1016/j.aquatox.2010.05.001) [DOI] [PubMed] [Google Scholar]
- 45.Murack PJ, Parrish J, Barry TP. 2011. Effects of progesterone on sperm motility in fathead minnow (Pimephales promelas). Aquat. Toxicol. 104, 121–125. ( 10.1016/j.aquatox.2011.04.006) [DOI] [PubMed] [Google Scholar]
- 46.DeQuattro ZA, Peissig EJ, Antkiewicz DS, Lundgren EJ, Hedman CJ, Hemming JD, Barry TP. 2012. Effects of progesterone on reproduction and embryonic development in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 31, 851–856. ( 10.1002/etc.1754) [DOI] [PubMed] [Google Scholar]
- 47.LaLone CA, et al. 2012. Effects of a glucocorticoid receptor agonist, dexamethasone, on fathead minnow reproduction, growth, and development. Environ. Toxicol. Chem. 31, 611–622. ( 10.1002/etc.1729) [DOI] [PubMed] [Google Scholar]
- 48.Kugathas S, Sumpter JP. 2011. Synthetic glucocorticoids in the environment: first results on their potential impacts on fish. Environ. Sci. Technol. 45, 2377–2383. ( 10.1021/es104105e) [DOI] [PubMed] [Google Scholar]
- 49.Kugathas S, Runnalls TJ, Sumpter JP. 2013. Metabolic and reproductive effects of relatively low concentrations of beclomethasone dipropionate, a synthetic glucocorticoid, on fathead minnows. Environ. Sci. Technol. 47, 9487–9495. ( 10.1021/es4019332) [DOI] [PubMed] [Google Scholar]
- 50.Foran CM, Weston J, Slattery M, Brooks BW, Huggett DB. 2004. Reproductive assessment of Japanese medaka (Oryzias latipes) following a four-week fluoxetine (SSRI) exposure. Arch. Environ. Contam. Toxicol. 46, 511–517. ( 10.1007/s00244-003-3042-5) [DOI] [PubMed] [Google Scholar]
- 51.Mennigen JA, Stroud P, Zamora JM, Moon TW, Trudeau VL. 2011. Pharmaceuticals as neuroendocrine disruptors: lessons learned from fish on Prozac. J. Toxicol. Environ. Health B Crit. Rev. 14, 387–412. ( 10.1080/10937404.2011.578559) [DOI] [PubMed] [Google Scholar]
- 52.Lister A, Regan C, Van Zwol J, Van Der Kraak G. 2009. Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: a mechanistic evaluation. Aquat. Toxicol. 95, 320–329. ( 10.1016/j.aquatox.2009.04.011) [DOI] [PubMed] [Google Scholar]
- 53.Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL. 2009. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 28, 2677–2684. ( 10.1897/08-556.1) [DOI] [PubMed] [Google Scholar]
- 54.Schultz MM, Painter MM, Bartell SE, Logue A, Furlong ET, Werner SL, Schoenfuss HL. 2011. Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat. Toxicol. 104, 38–47. ( 10.1016/j.aquatox.2011.03.011) [DOI] [PubMed] [Google Scholar]
- 55.Valenti TW, Gould GG, Berninger JP, Connors KA, Keele NB, Prosser KN, Brooks BW. 2012. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ. Sci. Technol. 46, 2427–2435. ( 10.1021/es204164b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandao FP, Rodrigues S, Castro BB, Goncalves F, Antunes SC, Nunes B. 2013. Short-term effects of neuroactive pharmaceutical drugs on a fish species: biochemical and behavioural effects. Aquat. Toxicol. 144–145, 218–229. ( 10.1016/j.aquatox.2013.10.005) [DOI] [PubMed] [Google Scholar]
- 57.Thomas MA, Klaper RD. 2012. Psychoactive pharmaceuticals induce fish gene expression profiles associated with human idiopathic autism. PLoS ONE 7, e32917 ( 10.1371/journal.pone.0032917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stepanova S, Praskova E, Chromcova L, Plhalova L, Prokes M, Blahova J, Svobodova Z. 2013. The effects of diclofenac on early life stages of common carp (Cyprinus carpio). Environ. Toxicol. Pharmacol. 35, 454–460. ( 10.1016/j.etap.2012.09.011) [DOI] [PubMed] [Google Scholar]
- 59.van den Brandhof EJ, Montforts M. 2010. Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol. Environ. Saf. 73, 1862–1866. ( 10.1016/j.ecoenv.2010.08.031) [DOI] [PubMed] [Google Scholar]
- 60.Lee J, Ji K, Lim Kho Y, Kim P, Choi K. 2011. Chronic exposure to diclofenac on two freshwater cladocerans and Japanese medaka. Ecotoxicol. Environ. Saf. 74, 1216–1225. ( 10.1016/j.ecoenv.2011.03.014) [DOI] [PubMed] [Google Scholar]
- 61.Skolness SY, Durhan EJ, Jensen KM, Kahl MD, Makynen EA, Villeneuve DL, Ankley GT. 2012. Effects of gemfibrozil on lipid metabolism, steroidogenesis, and reproduction in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 31, 2615–2624. ( 10.1002/etc.1989) [DOI] [PubMed] [Google Scholar]
- 62.Winter MJ, et al. 2008. Defining the chronic impacts of atenolol on embryo-larval development and reproduction in the fathead minnow (Pimephales promelas). Aquat. Toxicol. 86, 361–369. ( 10.1016/j.aquatox.2007.11.017) [DOI] [PubMed] [Google Scholar]
- 63.Giltrow E, Eccles PD, Winter MJ, McCormack PJ, Rand-Weaver M, Hutchinson TH, Sumpter JP. 2009. Chronic effects assessment and plasma concentrations of the beta-blocker propranolol in fathead minnows (Pimephales promelas). Aquat. Toxicol. 95, 195–202. ( 10.1016/j.aquatox.2009.09.002) [DOI] [PubMed] [Google Scholar]
- 64.Lorenzi V, Mehinto AC, Denslow ND, Schlenk D. 2012. Effects of exposure to the beta-blocker propranolol on the reproductive behavior and gene expression of the fathead minnow, Pimephales promelas. Aquat. Toxicol. 116–117, 8–15. ( 10.1016/j.aquatox.2012.03.001) [DOI] [PubMed] [Google Scholar]
- 65.Ankley GT, Kahl MD, Jensen KM, Hornung MW, Korte JJ, Makynen EA, Leino RL. 2002. Evaluation of the aromatase inhibitor fadrozole in a short-term reproduction assay with the fathead minnow (Pimephales promelas). Toxicol. Sci. 67, 121–130. ( 10.1093/toxsci/67.1.121) [DOI] [PubMed] [Google Scholar]
- 66.Ankley GT, et al. 2005. Effects of two fungicides with multiple modes of action on reproductive endocrine function in the fathead minnow (Pimephales promelas). Toxicol. Sci. 86, 300–308. ( 10.1093/toxsci/kfi202) [DOI] [PubMed] [Google Scholar]
- 67.Villeneuve DL, et al. 2009. Direct effects, compensation, and recovery in female fathead minnows exposed to a model aromatase inhibitor. Environ. Health Perspect. 117, 624–631. ( 10.1289/ehp.11891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skolness SY, et al. 2013. Propiconazole inhibits steroidogenesis and reproduction in the fathead minnow (Pimephales promelas). Toxicol. Sci. 132, 284–297. ( 10.1093/toxsci/kft010) [DOI] [PubMed] [Google Scholar]
- 69.Ankley GT, Cavallin JE, Durhan EJ, Jensen KM, Kahl MD, Makynen EA, Thomas LM, Wehmas LC, Villeneuve DL. 2012. A time-course analysis of effects of the steroidogenesis inhibitor ketoconazole on components of the hypothalamic–pituitary–gonadal axis of fathead minnows. Aquat. Toxicol. 114–115, 88–95. ( 10.1016/j.aquatox.2012.02.012) [DOI] [PubMed] [Google Scholar]
- 70.Villeneuve DL, et al. 2010. I. Effects of a dopamine receptor antagonist on fathead minnow, Pimephales promelas, reproduction. Ecotoxicol. Environ. Saf. 73, 472–477. ( 10.1016/j.ecoenv.2009.09.007) [DOI] [PubMed] [Google Scholar]
- 71.Villeneuve DL, et al. 2010. II: Effects of a dopamine receptor antagonist on fathead minnow dominance behavior and ovarian gene expression in the fathead minnow and zebrafish. Ecotoxicol. Environ. Saf. 73, 478–485. ( 10.1016/j.ecoenv.2009.09.018) [DOI] [PubMed] [Google Scholar]
- 72.Sharma P, Patino R. 2013. Regulation of gonadal sex ratios and pubertal development by the thyroid endocrine system in zebrafish (Danio rerio). Gen. Comp. Endocrinol. 184, 111–119. ( 10.1016/j.ygcen.2012.12.018) [DOI] [PubMed] [Google Scholar]
- 73.Liu YW, Chan WK. 2002. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation 70, 36–45. ( 10.1046/j.1432-0436.2002.700104.x) [DOI] [PubMed] [Google Scholar]
- 74.Raldua D, Babin PJ. 2009. Simple, rapid zebrafish larva bioassay for assessing the potential of chemical pollutants and drugs to disrupt thyroid gland function. Environ. Sci. Technol. 43, 6844–6850. ( 10.1021/es9012454) [DOI] [PubMed] [Google Scholar]
- 75.Crane HM, Pickford DB, Hutchinson TH, Brown JA. 2006. The effects of methimazole on development of the fathead minnow, Pimephales promelas, from embryo to adult. Toxicol. Sci. 93, 278–285. ( 10.1093/toxsci/kfl063) [DOI] [PubMed] [Google Scholar]
- 76.Ankley GT, Jensen KM, Kahl MD, Makynen EA, Blake LS, Greene KJ, Johnson RD, Villeneuve DL. 2007. Ketoconazole in the fathead minnow (Pimephales promelas): reproductive toxicity and biological compensation. Environ. Toxicol. Chem. 26, 1214–1223. ( 10.1897/06-428R.1) [DOI] [PubMed] [Google Scholar]
- 77.University of Hertfordshire. 2013. The veterinary substance database (VSDB) developed by the Agriculture and Environment Research Unit (AERU). http://sitem.herts.ac.uk/aeru/vsdb/. [Google Scholar]
- 78.Panter GH, Glennon YC, Robinson J, Hargreaves A, Murray-Smith R. 2012. Effects of the anti-androgen, bicalutamide, in a reduced life-cycle study with the fathead minnow (Pimephales promelas). Aquat. Toxicol. 114–115, 31–38. ( 10.1016/j.aquatox.2012.02.002) [DOI] [PubMed] [Google Scholar]
- 79.Martyniuk CJ, Bissegger S, Langlois VS. 2013. Current perspectives on the androgen 5α-dihydrotestosterone (DHT) and 5α-reductases in teleost fishes and amphibians. Gen. Comp. Endocrinol. 194, 264–274. ( 10.1016/j.ygcen.2013.09.019) [DOI] [PubMed] [Google Scholar]
- 80.Runnalls TJ, Beresford N, Losty E, Scott AP, Sumpter JP. 2013. Several synthetic progestins with different potencies adversely affect reproduction of fish. Environ. Sci. Technol. 47, 2077–2084. ( 10.1021/es3048834) [DOI] [PubMed] [Google Scholar]
- 81.Svensson J, Fick J, Brandt I, Brunstrom B. 2013. The synthetic progestin levonorgestrel is a potent androgen in the three-spined stickleback (Gasterosteus aculeatus). Environ. Sci. Technol. 47, 2043–2051. ( 10.1021/es304305k) [DOI] [PubMed] [Google Scholar]
- 82.Rivero-Wendt CL, Miranda-Vilela AL, Ferreira MF, Borges AM, Grisolia CK. 2013. Cytogenetic toxicity and gonadal effects of 17α-methyltestosterone in Astyanax bimaculatus (Characidae) and Oreochromis niloticus (Cichlidae). Genet. Mol. Res. 12, 3862–3870. ( 10.4238/2013.September.23.4) [DOI] [PubMed] [Google Scholar]
- 83.Sanchez-Hernandez M, Chaves-Pozo E, Cabas I, Mulero V, Garcia-Ayala A, Garcia-Alcazar A. 2013. Testosterone implants modify the steroid hormone balance and the gonadal physiology of gilthead seabream (Sparus aurata L.) males. J. Steroid Biochem. Mol. Biol. 138, 183–194. ( 10.1016/j.jsbmb.2013.05.014) [DOI] [PubMed] [Google Scholar]
- 84.Tousson E, El-Moghazy M, Massoud A, El-Atrash E, Sweef O, Akel A. 2013. Physiological and biochemical changes after boldenone injection in adult rabbits. Toxicol. Ind. Health Epub ahead of print. ( 10.1177/0748233713501365) [DOI] [PubMed] [Google Scholar]
- 85.Nieschlag E, Kumar N, Sitruk-Ware R. 2013. 7α-Methyl-19-nortestosterone (MENTR): the population council's contribution to research on male contraception and treatment of hypogonadism. Contraception 87, 288–295. ( 10.1016/j.contraception.2012.08.036) [DOI] [PubMed] [Google Scholar]
- 86.Perkins EJ, Ankley GT, Crofton KM, Garcia-Reyero N, LaLone CA, Johnson MS, Tietge JE, Villeneuve DL. 2013. Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ. Health Perspect. 121, 1002–1010. ( 10.1289/ehp.1306638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huggett DB, Cook JC, Ericson JF, Williams RT. 2003. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk Assess. 9, 1789–1799. ( 10.1080/714044797) [DOI] [Google Scholar]
- 88.Caldwell Dj, Mastrocco F, Margiotta-Casaluci L, Brooks BW. In press. An integrated approach for prioritizing pharmaceuticals found in the environment for risk assessment, monitoring and advanced research. Chemosphere ( 10.1016/j.chemosphere.2014.01.021) [DOI] [PubMed] [Google Scholar]
- 89.Diamond JM, Latimer HA, Munkittrick KR, Thornton KW, Bartell SM, Kidd KA. 2011. Prioritizing contaminants of emerging concern for ecological screening assessments. Environ. Toxicol. Chem. 30, 2385–2394. ( 10.1002/etc.667) [DOI] [PubMed] [Google Scholar]
- 90.Connors KA, Du B, Fitzsimmons PN, Hoffman AD, Chambliss CK, Nichols JW, Brooks BW. 2013. Comparative pharmaceutical metabolism by rainbow trout (Oncorhynchus mykiss) liver S9 fractions. Environ. Toxicol. Chem. 32, 1810–1818. ( 10.1002/etc.2240) [DOI] [PubMed] [Google Scholar]
- 91.Hutchinson TH, Madden JC, Naidoo V, Walker CH. 2014. Comparative metabolism as a key driver of wildlife species sensitivity to human and veterinary pharmaceuticals. Phil. Trans. R. Soc. B 369, 20130583 ( 10.1098/rstb.2013.0583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown AR, Gunnarsson L, Kristiansson E, Tyler CR. 2014. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Phil. Trans. R. Soc. B 369, 20130576. ( 10.1098/rstb.2013.0576) [DOI] [PMC free article] [PubMed] [Google Scholar]