Abstract
Though pharmaceuticals are increasingly observed in a variety of organisms from coastal and inland aquatic systems, trophic transfer of pharmaceuticals in aquatic food webs have not been reported. In this study, bioaccumulation of select pharmaceuticals was investigated in a lower order effluent-dependent stream in central Texas, USA, using isotope dilution liquid chromatography–tandem mass spectrometry (MS). A fish plasma model, initially developed from laboratory studies, was tested to examine observed versus predicted internal dose of select pharmaceuticals. Pharmaceuticals accumulated to higher concentrations in invertebrates relative to fish; elevated concentrations of the antidepressant sertraline and its primary metabolite desmethylsertraline were observed in the Asian clam, Corbicula fluminea, and two unionid mussel species. Trophic positions were determined from stable isotopes (δ15N and δ13C) collected by isotope ratio-MS; a Bayesian mixing model was then used to estimate diet contributions towards top fish predators. Because diphenhydramine and carbamazepine were the only target compounds detected in all species examined, trophic magnification factors (TMFs) were derived to evaluate potential trophic transfer of both compounds. TMFs for diphenhydramine (0.38) and carbamazepine (1.17) indicated neither compound experienced trophic magnification, which suggests that inhalational and not dietary exposure represented the primary route of uptake by fish in this effluent-dependent stream.
Keywords: urban ecosystems, bioconcentration, trophic transfer, aquatic food webs, contaminants of emerging concern, instream flow
1. Introduction
Human pharmaceuticals are increasingly identified in effluent-dominated and -dependent urban ecosystems, which may represent worst-case scenarios for waterborne exposure to these pharmaceuticals and other contaminants of emerging concern (CECs) in developed countries [1]. Though pharmaceuticals are more water soluble than historical environmental contaminants (e.g. persistent organic pollutants), suggesting that these CECs may have lower propensities to bioconcentrate or biomagnify [2], instream flows of urban rivers and streams that are dominated by or dependent on effluent discharges result in increased effective exposure duration to aquatic life [3]. Understanding environmental exposure and ecological impacts of pharmaceuticals and other industrial chemicals is essential for sustainable management of environmental quality, particularly in urbanizing ecosystems [4]. For example, identifying regions where pharmaceuticals present high risk to terrestrial and aquatic wildlife was recently identified as a major research need during an expert horizon scanning workshop [5].
Perhaps the first report of a human pharmaceutical bioaccumulating in aquatic life was the contraceptive 17α-ethinyloestradiol in fish bile from Sweden [6]. Brooks et al. [7] then identified bioaccumulation of selective serotonin reuptake inhibiting (SSRI) antidepressants by fish from an effluent-dominated stream in north Texas, USA. These antidepressants were targeted for study because of high volume of distribution (VD) values, elevated lipophilicity (e.g. necessary to cross the blood–brain barrier to elicit therapeutic benefit), slow clearance rates, biologically important activities and human usage patterns [4]. Such observations were subsequently extended to other pharmaceuticals accumulating in fish from this urban stream [8] and to other urban rivers of the USA [9]. Biotransformation and clearance of many of these pharmaceuticals may be limited in fish [10], which may increase the potential for bioaccumulation of some pharmaceuticals, particularly when continuous exposure to effluents occurs in urban ecosystems.
Unlike many historical organic contaminants, partitioning dynamics of the majority of pharmaceuticals are not only due to hydrophobic interactions, but are also influenced by hydrogen bonding, cation exchange, cation bridging and surface complexation [11]. For example, uptake and elimination of ionizable pharmaceuticals (over 70% of drugs are ionizable) by fish [12,13] and invertebrates [14] are modified by surface water pH [13,15–17]. Unfortunately, environmental modelling approaches for predicting fate, transport, exposure and bioaccumulation, which were designed to address historical contaminants (e.g. persistent organic pollutants), are often inappropriate for assessing environmental risks of these chemicals [11]. In fact, Ramirez et al. [9] identified that pharmaceutical concentrations in fish were not explained by lipid normalization, an approach routinely performed when studying bioaccumulation and trophic transfer dynamics of historical environmental organic contaminants (e.g. polychlorinated biphenyls (PCBs), dioxins, furans). Understanding and predicting the uptake of ionizable pharmaceuticals in terrestrial and aquatic ecosystems was also recently identified as a major research need to better define environmental risks [5].
Trophic transfer of chemicals can result in elevated exposure to and effects on predators at higher trophic positions (TPs), including humans [18]. Studies investigating the trophic transfer of pharmaceuticals in aquatic ecosystems, which are critically necessary for understanding the environmental disposition and impacts of pharmaceuticals, are lacking. Herein, trophic magnification factors (TMFs) are useful, because TMFs are increasingly used to assess trophic transfer of environmental contaminants by relating chemical concentrations in tissues to relative TPs of organisms in a food web [19]. The objectives of this study were thus to examine the occurrence of select pharmaceuticals in surface water and various species from a stream with instream flows dependent on municipal effluent discharge, and to test whether a laboratory-derived partitioning model could predict uptake of pharmaceuticals by fish in the field. We further identified TP of the studied organisms using stable isotope analysis and determined whether trophic transfer of select pharmaceuticals occurred in an effluent-dependent stream.
2. Material and methods
(a). Study site and field sampling
The North Bosque River is located within the Brazos River watershed (www.brazos.org), which is the longest river basin in Texas, USA, stretching from Curry County, New Mexico, to the Gulf of Mexico. The North Bosque River was selected because instream flows are highly dependent on effluent discharge from six centralized wastewater treatment plants (WWTPs). We selected downstream of the Stephenville, Texas, WWTP as a study site for field sampling because it is the first and only major effluent discharger to this river (approx. 1 million US gallons (3.8 × 106 l) discharged per day). During the present study, no stream flow was observed upstream from this WWTP; thus, instream flows of the North Bosque River were dependent on a municipal effluent discharge at the study site.
Water and biological samples were extensively collected over a 3-day period, 17–19 June 2013. Water samples were collected for nutrient analysis in the laboratory (Lachat Quikchem 8500 Flow Injection Autoanalyzer), while in situ water chemistry measurements were taken with a calibrated YSI multi-parameter data sonde. Biological sample collection followed standard techniques for periphyton, six invertebrate species (Planorbis sp. (snail; Family: Planorbidae), Hyalella azteca (amphipod; Family: Hyalellidae), Ranatra elongata (water scorpion; Family: Nepidae), Corbicula fluminea (Asian clam; Family: Corbiculidae), Uniomerus tetralasmus (Pondhorn mussel; Family: Unionidae), Utterbackia imbecillis (paper pondshell mussel; Family: Unionidae)) and six fish species (Lepomis megalotis (longear sunfish; Family: Centrarchidae), Lepomis cyanellus (green sunfish; Family: Centrarchidae), Micropterus salmoides (largemouth bass; Family: Centrarchidae), Ameiurus natalis (yellow bullhead catfish; Family: Ictaluridae), Ictalurus punctatus (channel catfish; Family: Ictaluridae), Gambusia affinis (mosquitofish; Family: Poeciliidae)). Emergence of ephemeropterans and zygopterans, which are typically multivoltine in this region, was observed immediately prior to this study, which likely explains lack of mayfly and damselfly specimens collected during field sampling. Fish were collected via a back pack electrofisher (LR-24 Electrofisher, Smith-Root, Inc., Vancouver, WA, USA) with seines and dip nets following approved IACUC and Scientific Collection Permit methods. Fish were immediately anaesthetized using MS-222, and length and mass measured on site. Blood was collected from the caudal artery with heparinized micro-haematocrit capillary tubes, and subsequently centrifuged at 13 000g for 10 min. All samples were transported to the laboratory on ice and then stored at −80°C until further analyses. During the 3-day sampling event, triplicate water samples were collected daily in 4 l pre-rinsed amber glass bottles, transported on ice to the laboratory and stored for less than 48 h at 4°C prior to filtration and extraction.
(b). Chemicals, regents and sample analysis
Selection of 23 target analytes was based on previously reported occurrences of these pharmaceuticals in aquatic ecosystems [20,21]. All chemicals and their corresponding isotopically labelled analogues were obtained with various vendors [20,21] and used as received (see the electronic supplementary material, table S1). Sample extraction and analysis followed previous methods [21–23] in which isotope dilution was used to alleviate matrix interference with isotopically labelled internal standards for each target analyte. Plasma sample extraction followed a slightly modified methodology [24]. Similarly, this tissue sample extraction protocol generally followed previously developed methodologies [8,20]. Detailed information regarding mass, length and composite groupings is provided in the electronic supplementary material, table S2.
All samples were analysed using liquid chromatography–tandem mass spectrometry. Instrumentation parameters, separation strategy, detection of target analytes, calibration method and method detection limits (MDLs) generally followed previously reported methods [20]. In this study, MDLs represented the lowest concentrations of an analyte that were reported with 99% confidence that the concentration is different from zero in a given matrix. Less than MDL was defined as analytes being detected in the particular matrices, but below their corresponding MDLs. Half the MDLs were used for calculating bioaccumulation factors (BAFs) and blood water partition coefficient (PBW) if target analytes were not detected or detected below MDLs. One method blank sample and duplicate matrix spikes were included in each analytical sample batch.
(c). Therapeutic hazards of surface water quality
Predicted PBW values for each target analyte in fish plasma were generated using a previously reported physiological-based pharmacokinetic model, which derived an empirical relationship (equation (2.1)) to model branchial flux of organic chemicals across a wide range of log Kow values from 0 to 8 [25]. Additionally, we modified equation (2.1) and substituted octanol–water distribution coefficient (Dow) and liposome–water distribution coefficient (Dlipw) in equations (2.2) and (2.3), respectively
| 2.1 |
| 2.2 |
| 2.3 |
A comparative pharmacology approach proposed by Huggett et al. [26] was used to identify whether plasma levels of pharmaceuticals present potential hazards to fish by comparing observed fish plasma levels to human therapeutic plasma doses (Cmax). We then compared concentrations of pharmaceuticals observed in the North Bosque River to therapeutic hazard values (THVs; [4]) for each pharmaceutical. The THV describes the surface water concentration predicted to result in fish plasma concentrations equalling human therapeutic doses (Cmax) using equation (2.4) [27]
| 2.4 |
(d). Stable isotope analysis
Stable isotopes (δ15N and δ13C) were determined in the Stable Isotopes Laboratory of Baylor University using a dual-inlet gas-source Stable Isotope Mass Spectrometer (Thermo-Electron, Waltham, MA, USA) and an Elemental Analyzer (Costech, Valencia, CA, USA). Data were calibrated using international standards USGS-40 and USGS-41. Analytical precision was ±0.2%. Isotopic ratios in delta notation are calculated using the following equation:
| 2.5 |
where the heavier isotope is X, Rsample is the raw ratio of heavy to light isotope in the analysed sample and Rstandard is the ratio of heavy to light isotope in the internationally recognized standards [28,29]. TP was determined using the following equation [28]
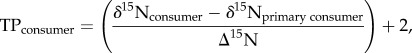 |
2.6 |
where the TPconsumer and δ15Nconsumer are the TP and stable isotope abundance, respectively, of the organism in question. δ15Nprimary consumer is the baseline of the trophic structure, in this case calculated from the small Asian clam (C. fluminea) (13.88 ± 0.21‰ (mean ± s.d.); n = 10), and is considered to be TP 2. Δ15N is the nitrogen-enrichment factor. An enrichment factor of 3.4‰ was chosen for this study based on a suite of previous studies [28,30]. Furthermore, the extent of basal resources and their specific contributions to the higher TPs can be examined by using isotopes of an element that do not fractionate appreciably with increasing TPs, but where the ratios can provide information on the source of energy. This can be achieved by using stable δ13C ratios [28]. If a species has a similar δ13C signature to a lower TP group, it is likely that the source of energy from the higher TP is derived from the group below with a similar δ13C signature. In order to appropriately calculate TMF throughout a food chain, similar δ13C values are important. In order to describe the food web of the North Bosque River, TP and trophic extent along the δ13C gradient were compared between all species/size classes using one-way ANOVA and Tukey HSD (honest significant difference) test. Significant differences in especially δ13C ratios can then help in choosing the right species for calculating TMF across TPs.
The Bayesian mixing model Stable Isotope Analysis in R (SIAR) [31] was used to estimate diet contributions towards the major top predators in the studied food web, i.e. longear sunfish separated by their three size classes and mosquitofish. SIAR is a library in the programming language R. SIAR uses a Bayesian approach to calculate likelihoods of dietary compositions using source and consumer-stable carbon and nitrogen isotope ratios. Sources for the model were defined as all lower TP species that could potentially be eaten by the fish. We eliminated all clams as sources and species that had a sample size of two or less. The sources for the model that remained were periphyton, amphipods, snails, water scorpions and mosquitofish. Their respective mean and standard deviation were used to predict diet likelihood contributions to the top predators using the SIAR model [32]. The model was run for each fish species/size class with a reiteration of 4 000 000 (400 000 burns). The model output data were summarized with standard summary statistics using the mode for each source as well as the 75th percentile and 97th percentile of the model output data. A graphical representation of diet choice likelihoods was plotted using the library ggplot2 in R [33].
(e). Trophic magnification factors
Trophic magnification of pharmaceuticals was quantified using the slope (b) of a linear equation (equation (2.7)), which was derived using organisms from the present study [34]
| 2.7 |
where a is the intercept of the line. The above equation is derived from an earlier form that used δ15N instead of TP [35], which is derived from δ15N and gives leeway for the use of unique enrichment factors for different species or animal groups [28,34]. TMFs were calculated from the slope b of the following equation [34]
| 2.8 |
The advantage of using TP instead of a δ15N-derived slope is that the TP corrects for the baseline and shows the biomagnifcation potential from one TP to the next averaged over the entire food web [28,36]. More detailed discussions on this approach can be found elsewhere [37].
(f). Statistical analyses
Regressions between log[contaminant] and TP were performed using SigmaPlot (Systat Software, San Jose, CA, USA). ANOVA was used to test variation of water exposure (SigmaPlot). Differences in TP and trophic extent were evaluated using one-way ANOVA between species, followed by Tukey HSD tests, performed using R (R core team, 2013); the libraries agricolae [38] for post hoc analysis and ggplot2 [33] were used for graphics.
3. Results and discussion
(a). Traditional water chemistry measures from the North Bosque River
Water chemistry remained typically uniform over the 3-day sampling period (electronic supplementary material, table S3); for example, water temperatures ranged from 26.2 to 30.0°C while dissolved oxygen and pH varied from 8.18 to 8.92 mg l−1 and 7.87 to 7.93, respectively. Elevated nutrient concentrations (e.g. mean total phosphorus = 1.07 ± 0.1 mg l−1; N = 3) suggest treatment performance of this WWTP was not optimal (total phosphorus screening level = 0.8 m l−1) [39] (electronic supplementary material, table S3).
(b). Human pharmaceuticals and wastewater tracers in surface water
Of 23 target analytes, none were detected in the method blank samples; however, various pharmaceuticals were detected in water, periphyton and aquatic organisms. Nine target compounds (electronic supplementary material, table S4) were detected in surface water samples. The wastewater tracer sucralose was detected at the highest level (up to 20 000 ng l−1) of any target analyte, while carbamazepine was the pharmaceutical detected at the highest concentrations (up to 370 ng l−1). Concentrations of each detected compound were consistent and not significantly (p > 0.05) different over the 3-day sampling period.
(c). Observed versus predicted fish plasma levels of human pharmaceuticals
Based on these surface water observations, we further examined fish plasma concentrations of targeted pharmaceuticals. Understanding internal doses of pharmaceuticals in fish tissues is more critical than traditional body burden approaches that have been applied for historical organic contaminants (e.g. PCBs, dioxins, furans) in whole organisms. This consideration is particularly important because plasma levels of pharmaceuticals can be associated with therapeutic dosage thresholds and resulting adverse outcomes of ecological importance [26]. In the present study, we evaluated therapeutic hazards of select pharmaceuticals to L. megalotis by comparing predicted surface water THV values of these pharmaceuticals to their concentration in surface waters and measured concentrations in fish plasma. Three pharmaceuticals, diphenhydramine, diltiazem and carbamazepine, were detected in L. megalotis plasma with a highest observed concentration of 4.1 µg l−1 (electronic supplementary material, table S4). If these plasma concentrations in fish are compared with human therapeutic doses (Cmax) [26], then L. megalotis plasma levels of diphenhydramine, diltiazem and carbamazepine were 17, 190 and 490 lower than human Cmax values, respectively (electronic supplementary material, table S5).
Huggett et al. [26] previously proposed that if pharmaceuticals occur in fish plasma within a factor of 1000 (based on three separate safety factors of 10 for mammalian to non-mammalian species–species differences and human to animal extrapolations) from the human therapeutic dose then these chemicals deserve additional study. Bioaccummulation of diltiazem and carbamazepine was previously reported from a similar study in Sweden [24]. Similarly, Fick et al. [24] identified diltiazem to accumulate in trout plasma within a factor of 1000 from the human Cmax value. Pharmacological effects or other adverse outcomes of these pharmaceuticals to L. megalotis are unknown and deserve further investigation.
Observations of plasma concentrations of the ionizable weak base pharmaceuticals diltiazem and diphenhydramine and the non-ionizable therapeutic carbamazepine in L. megalotis were considerably higher than plasma concentrations predicted using equations (2.1)–(2.3) (electronic supplementary material, table S5). In the case of ionizable contaminants, surface water pH and chemical pKa can influence bioaccumulation and toxicity because the non-polar neutral form is more lipophilic and bioavailable than the ionized form. In this study, pH of the North Bosque River, which was used to develop predictions of fish plasma levels presented in the electronic supplementary material, table S5, was collected on three consecutive days, but only at one point in time on each day. Variability of surface water pH can significantly shift how much of a compound is taken up by an organism [16]; thus, diel pH variability in the North Bosque River likely modified exposure and potential toxicity to aquatic organisms therein [16].
Such differences between observed and predicted fish plasma concentrations (electronic supplementary material, table S5) could have resulted from several other factors. Surface water samples collected from the North Bosque River were grab samples at one point in time; however, daily, weekly and seasonal variability of pharmaceuticals in wastewater effluents is inherent [21]. Furthermore, the fish plasma model tested here was based on laboratory studies with rainbow trout and non-ionizable contaminants [25]. An understanding of comparative pharmacokinetics is not available among fish species, though pharmaceuticals appear differentially metabolized by trout [10] and the fathead minnow model (KA Connors, B Du, PN Fitzsimmons, AD Hoffman, CK Chambliss, JW Nichols, BW Brooks 2014, unpublished data). It is also possible that dietary exposure contributed to under-predictions of field observations.
(d). Bioaccumulation of human pharmaceuticals by various aquatic organisms
Nine target compounds were detected in various species collected from the North Bosque River. Species-specific occurrence of these compounds in periphyton and whole organism tissues and their corresponding frequency of detection are given in table 1. Typically, both level and frequency of detection were higher in invertebrates relative to fish. This observation was particularly reflected by SSRI antidepressants and anti-inflammatory drugs (diclofenac and celecoxib). Among all pharmacetuicals, celecoxib was detected at the highest level (mean = 430 µg kg−1) in the water scorpion, R. elongata. Sertraline was also observed at elevated levels (greater than 130 µg kg−1) in the Asian clam, C. fluminea and the unionid mussels, U. tetralasmus and U. imbecillis.
Table 1.
Human pharmaceuticals in periphyton, invertebrates and fish (mean ± s.d.; µg kg−1) from the North Bosque River, TX, USA. DPH, diphenhydramine; CAR, carbamazepine; NOR, norfluoxetine; SER, sertraline; FLU, fluoxetine; DES, desmethylsertraline; CEL, celecoxib; DIC, diclofenac; DIL, diltiazem; freq., frequency; n.d., not detected; <MDL, below method detection limit.
| species name | analyte |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPH |
CAR |
NOR |
SER |
FLU |
DES |
CEL |
DIC |
DIL |
||||||||||
| mean ± s.d. | freq. | mean ± s.d. | freq. | mean ± s.d. | freq. | mean ± s.d. | freq. | mean ± s.d. | freq. | mean ± s.d. | freq. | mean ± s.d. | freq. | mean ± s.d. | freq. | mean ± s.d. | freq. | |
| Periphyton | 18 ± 12 | 10/10 | 1.3 ± 0.22 | 10/10 | n.d. | 0/4 | n.d. | 0/4 | n.d. | 0/4 | n.d. | 0/4 | 18 ± 13 | 7/10 | 3.6 ± 2.3 | 5/10 | <MDL | 0/10 |
| Invertebrates | ||||||||||||||||||
| H. azteca | 5.3 ± 0.96 | 4/4 | 1.6 ± 0.10 | 4/4 | n.d. | 0/4 | n.d. | 0/4 | n.d. | 0/4 | n.d. | 0/4 | 25 ± 19 | 4/4 | 20 ± 4.9 | 4/4 | 0.21 ± 0.11 | 2/4 |
| R. elongata | 6.8 ± 6.3 | 3/3 | <MDL | 1/3 | <MDL | 0/3 | n.d. | 0/3 | n.d. | 0/3 | 9.8 ± 4.7 | 3/3 | 430 ± 190 | 3/3 | <MDL | 0/3 | n.d. | 0/3 |
| Planorbis sp. | 2.3 ± 0.68 | 8/8 | 1.4 ± 0.59 | 7/8 | <MDL | 0/8 | 37 ± 11 | 8/8 | 6.9 ± 2.4 | 7/8 | 71 ± 16 | 8/8 | 21 ± 10 | 7/8 | 13 ± 5.6 | 7/8 | 0.37 ± 0.07 | 8/8 |
| C. fluminea (small) | 2.8 ± 0.87 | 10/10 | 1.2 ± 0.28 | 10/10 | <MDL | 0/10 | 140 ± 21 | 10/10 | 10 ± 3.6 | 9/10 | 86 ± 20 | 10/10 | 26 ± 14 | 9/10 | 19 ± 3.2 | 10/10 | <MDL | 0/10 |
| C. fluminea (medium) | 1.6 ± 0.40 | 9/9 | 1.9 ± 0.32 | 9/9 | <MDL | 0/9 | 130 ± 23 | 9/9 | 12 ± 1.3 | 9/9 | 97 ± 37 | 9/9 | 22 ± 7.9 | 8/9 | 33 ± 6.7 | 9/9 | <MDL | 0/9 |
| C. fluminea (large) | 2.7 ± 1.2 | 10/10 | 1.9 ± 0.77 | 10/10 | <MDL | 0/9 | 56 ± 19 | 10/10 | 10 ± 4.4 | 10/10 | 67 ± 19 | 10/10 | 24 ± 16 | 8/10 | 18 ± 9.3 | 10/10 | <MDL | 0/10 |
| U. tetralasmus | 1.2 | 1/1 | 1.4 | 1/1 | <MDL | 0/1 | 130 | 1/1 | 15 | 1/1 | 88 | 1/1 | <MDL | 0/1 | 11 | 1/1 | n.d. | 0/1 |
| U. imbecillis | 4.4 | 2/2 | 1.1 | 2/2 | <MDL | 0/2 | 370 | 2/2 | 22 | 2/2 | 78 | 2/2 | <MDL | 0/2 | 15 | 2/2 | 0.27 | 2/2 |
| Vertebrates | ||||||||||||||||||
| G. affinis | 1.4 ± 0.43 | 10/10 | 1.8 ± 0.27 | 10/10 | 13 ± 2.3 | 10/10 | 14 ± 4.6 | 10/10 | n.d. | 0/10 | <MDL | 3/10 | n.d. | 0/10 | <MDL | 4/10 | 0.14 ± 0.08 | 7/10 |
| L. megalotis (small) | 1.6 ± 0.99 | 10/10 | 1.4 ± 0.38 | 10/10 | 4.8 ± 3.3 | 5/10 | 6.9 ± 3.2 | 8/10 | n.d. | 0/10 | n.d. | 0/10 | n.d. | 0/10 | <MDL | 3/10 | 0.15 ± 0.18 | 3/10 |
| L. megalotis (medium) | 0.47 ± 0.33 | 9/10 | 1.3 ± 0.57 | 10/10 | <MDL | 4/10 | n.d. | 0/10 | n.d. | 0/10 | n.d. | 0/10 | n.d. | 0/10 | n.d. | 0/10 | <MDL | 4/10 |
| L. megalotis (large) | 0.45 ± 0.16 | 9/9 | 1.0 ± 0.28 | 9/9 | n.d. | 0/9 | 10 ± 6.8 | 7/9 | n.d. | 0/9 | 7.2 ± 6.1 | 3/9 | n.d. | 0/9 | n.d. | 0/9 | <MDL | 2/9 |
| M. salmoides | 1.0 | 2/2 | 1.3 | 2/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 |
| A. natalis | 0.64 | 1/1 | <MDL | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 |
| I. punctatus | 25 | 1/1 | <MDL | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 | n.d. | 0/1 |
| L. cyanellus | 0.59 | 2/2 | 1.1 | 2/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 | n.d. | 0/2 |
Diphenhydramine and carbamazepine were the only target compounds ubiquitously detected in all periphyton, invertebrate and fish samples. Ramirez et al. [9] reported fish tissue concentrations of pharmaceuticals from urban US rivers influenced by WWTP discharges; occurrence patterns of pharmaceuticals in fish in this study are typically consistent with this US Environmental Protection Agency (EPA) national pilot study [9]. Also similar to observations in this study, SSRIs were rarely observed in fish fillets of EPA's national pilot study (e.g. up to 11 µg kg−1 of sertraline in C. commersonii fillets [9] versus up to 14 µg kg−1 of sertraline in G. affinis whole tissues, table 1).
Previous observations of pharmaceutical accumulation in invertebrates are limited [2]. Meredith-Williams et al. [14] investigated the uptake of pharmaceuticals (5-fluorouracil, carbamazepine, carvedilol, diazepam, fluoxetine and moclobemide) by invertebrates Gammarus pulex (amphipod), Notonecta glauca (water boatman) and Planorbarius corneus (snail). When considering potential uptake routes in the three invertebrates, their results [14] suggested that aquatic species using a plastron (N. glauca) or pulmonary respiration (certain molluscs) could be exposed to less dissolved contaminants than those species using gill respiration. Other influences on uptake of pharmaceuticals by invertebrates may be locomotion and water column utilization (e.g. epibenthic, benthic, pelagic, surface swimmers).
Bringolf et al. [40] investigated the occurrence, distribution and bioaccumulation of fluoxetine in Elliptio complanata (mussel) in a stream near a WWTP effluent discharge. Fluoxetine BAFs for mussels at four stream sites ranged from 125 to 1347. In more recent laboratory studies, Hazelton et al. [41] observed fluoxetine BAFs for a unionid mussel to range between 229 and 1221. Elevated SSRI levels observed in bivalves (table 1) during this study may have resulted from partitioning to lysosomes [42]. Additionally, SSRIs and other pharmaceuticals, which are weakly basic and having an amino group, have been reported to be effectively sequestered by lysosomes of mammalian cells, resulting in drug accumulation [43]. In this study, SSRI concentrations may have also been influenced by filter feeding on suspended particles. Because we prefiltered surface water samples prior to extraction, sorbed SSRIs to suspended materials were not characterized. Freshwater mollusc morphological feeding and digestive anatomy varies depending on the taxa but C. fluminea can be broadly grouped together with typical unionids [44]. When considering bioaccumulation in molluscs by filter feeding, efficiencies of particle detainment, ingestion and assimilation need to be accounted for due to removal of particles from suspension indiscriminately, creating difficulties for exposure modelling [44]. An advanced understanding of mechanisms responsible for bivalve bioaccumulation of pharmaceuticals is needed.
Pharmaceutical exposures to aquatic organisms are of particular interest in effluent-dominated or -dependent streams [1], where effective exposure duration is increased due to limited dilution and constant introduction via effluent discharges [1]. In fact, such exposure scenarios challenge historical constructs defining ‘persistence’ of environmental contaminants, particularly in urban ecosystems [4]. Though sublethal effects data related to therapeutic mechanisms of action for pharmaceuticals in aquatic organisms are scarce [5], the anti-epileptic agent carbamazepine has been reported to elicit sublethal toxicity to cladocerans, albeit with concentrations that exceed relevant environmental scenarios for developed countries [45,46]. Similar to carbamazepine, diphenhydramine was also detected in all samples analysed. Diphenhydramine is a common over-the-counter medication with multiple modes of action, including histamine, serotonin and acetylcholine receptor antagonist functions. It has been demonstrated to suppress microbial respiration [47], modify bacterial community structure [48] and alter fish behaviour [27] at low microgram per litre concentrations. Similar to diphenhydramine, previous studies with sertraline, which also blocks serotonin reuptake transport, have also highlighted the effects on fish anxiety behaviour when plasma concentrations exceeded human Cmax values [17]. In the present study, sertraline was not observed in fish tissue but accumulated to relatively high levels in bivalves. An understanding of sertraline influences on unionid mussels deserves future study.
(e). Trophic transfer of select pharmaceuticals
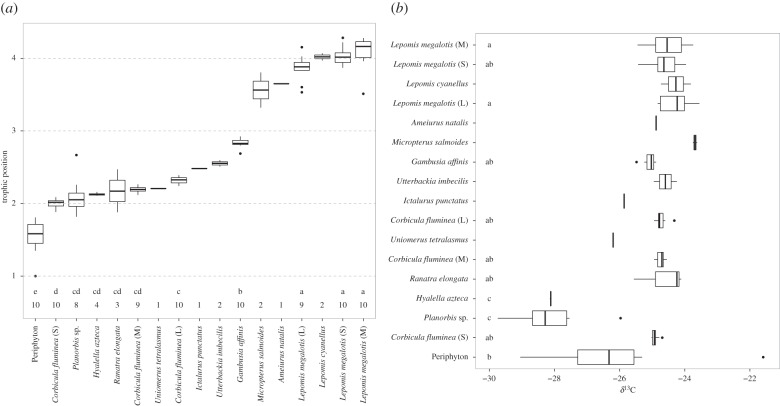
TPs of organisms collected from the North Bosque River were determined. δ15N and calculated TPs of study species are given in the electronic supplementary material, table S6. It was not surprising to see that lowest δ15N was found for periphyton, which was considered to be the bottom TP in the study food web (figure 1a). Invertebrates were assumed to occupy the next level (TP 2.00–2.55) with the δ15N in the range 13.88–15.76‰ (figure 1a). The range of δ15N for fish species was 15.52–20.96‰, resulting in the range of TPs as 2.48–4.08. δ15N of I. punctatus was slightly lower than of select invertebrate species, which may have resulted from the limited number of samples of this species.
Figure 1.
(a) TP and (b) trophic extent (δ13C) of species from the effluent-dependent North Bosque River, TX, USA. In both panels, boxplots represent median and 25% and 75% quartiles, respectively; whiskers are 95% and dots represent outliers. Grey numbers along the x-axis of panel (a) indicate sample size of the species. Letters along the x-axis of panel (a) or the y-axis of panel (b) represent significantly different groups.
Additionally, age may also affect TP and subsequently have an impact on characterization of trophic transfer [49]. We examined whether the size influenced TPs for L. megalotis and C. fluminea. Significant difference (p < 0.05) of TP was identified among C. fluminea with three different sizes (mass), with TP increasing slightly with an increase in size of the organism. By contrast, no significant differences were found between size (length, wet mass) of L. megalotis (p > 0.05). We also examined a potential relationship between fish TP and total length, but the relationship was not significant (p > 0.05).
When calculating a TMF, it is important to understand the flow of energy between organisms prior to generating a regression of contaminants versus the TP. One way to define a food web is to use isotopes that do not fractionate during transfer from prey to predator (δ13C). Such isotopic ratios are conserved and only slightly enriched (approx. 0.4 ± 1.3‰) from the diet to the consumer species [30]. Thus, isotopes can be used to define a relationship of consumers supported by the same primary producers and to define energy flows in the system [30]. Establishing energy flow can be achieved by examining a bi-plot of δ15N by δ13C (figure 1a,b). Any organisms that do not feed from the same base or rely on other organisms within the isotopic mixing space should be removed from analysis before generating a TMF [50]. In this study, defining the structure of the North Bosque River food web as precisely as possible was performed to reduce uncertainty associated with calculating TMFs for ionizable pharmaceuticals. Thus, the organisms Planorbis sp. and H. azteca were removed from the calculation and regression of TMF for both diphenhydramine and carbamazepine because the δ13C values were too depleted to consider these species as part of the same food web.
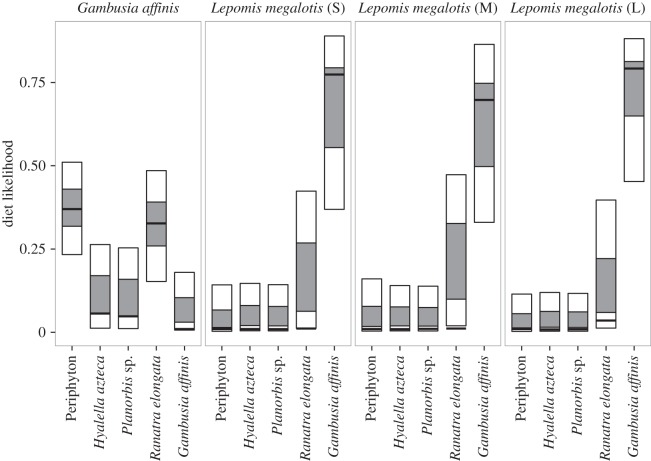
A diet likelihood model was used for higher TPs to describe with a measure of certainty the contributions of lower TPs to top predators. In the present study, this model was applied to L. megalotis and G. affinis. Confirming the results of TP estimates (figure 2), the model showed a linear transfer of energy up the food web in the North Bosque River. While mosquitofish had two major diet sources, longear sunfish received their energy mainly from one source (mosquitofish). Though the one-time sampling approach likely underestimates some possible diets, and the food web was influenced by emergence of other benthic macroinvertebrates (e.g. mayflies) prior to sampling, the results highlight a simplified food web in which possible routes for energy transfer to higher TPs are limited.
Figure 2.
Diet likelihood fraction estimates for top predators in the effluent-dependent North Bosque River, TX, USA. Boxes represent mode (black bar), 25–75% (dark grey area), 5–95% (light grey area) of the data.
TP adjusted biomagnification factors (BMFs) have previously been used to examine accumulation of chemicals from prey to predator with the presumption that dietary uptake is a critical route [47]. Chemicals with BMFs greater than unity are suggested to accumulate from prey to predator. However, if predators are able to metabolize a compound which is poorly biotransformed by organisms at lower TPs, it is difficult to evaluate the bioaccumulation between different predator–prey pairs without accurate biotransformation rates of chemicals in each species [37]. Clearly, comparative biotransformation of pharmaceuticals and other CECs are needed in fish [10].
Biomagnification occurs when concentrations of chemical residues within organisms increase with increasing TP [30,36] . When assessing the TMF for a chemical, it is presumed that the major exposure route for a species is through its diet. However, for aquatic species that take up chemicals through respiratory surfaces, the relative fractions of dietary uptake, active transport or passive diffusion for a specific chemical are less well studied and highly likely to affect the determination of TMF in an aquatic food web [51]. Additionally, such considerations are applicable to lower TP organisms with high surface area to body ratios. Such observations may be more pronounced for chemicals that are ionizable and experience speciation at environmentally relevant pHs.
Here, we present novel estimates of TMFs for the pharmaceuticals diphenhydramine and carbamazepine in the aquatic food web of the North Bosque River. As shown in figure 3, log[pharmaceutical] without additional treatment (e.g. lipid normalization) was regressed against TP, which resulted in a TMF for diphenhydramine (figure 3a) of 0.38 (b = −0.42, a = 1.47, r2 = 0.37, p < 0.05) and a TMF for carbamazepine (figure 3b) of 1.17 (b = 0.07, a = −0.21, r2 = 0.04, p > 0.05). Though a slightly positive slope resulted from the regression between log[carbamazepine] and TP, which resulted in a TMF for carbamazepine above unity, the relationship between log [carbamazepine] and TP was not significant (p > 0.05). Chemicals with Kow < 5, in general and without extensive metabolism, attain concentrations in organisms that represent thermodynamic equilibrium with the surrounding water. TMF > 1 (b > 0) indicates that a chemical is biomagnifying, but TMF < 1 (b < 0) indicates that a contaminant is being diluted with increased TPs. Such observations are known as trophic dilution. Thus, neither compound experienced trophic magnification across the studied aquatic food web.
Figure 3.
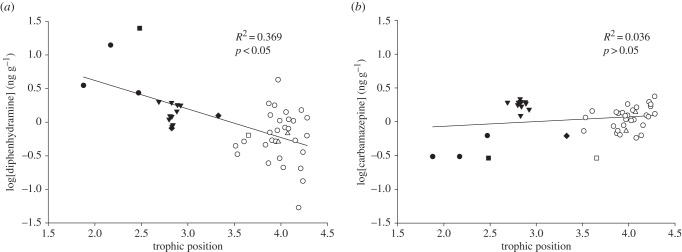
(a) Log[diphenhydramine] ng g−1 and (b) log[carbamazepine] ng g−1 as a function of TP for select species from the effluent-dependent North Bosque River, TX, USA. Filled circles represent R. elongata; open circles, L. megalotis; inverted triangles, G. affinis; triangles, L. cyanellus; filled squares, I. punctatus; open squares, A. natalis; diamonds, M. salmoides.
Trophic dilution of other organic compounds, such as polyaromatic hydrocarbons [52], non-polybrominated diphenyl ethers [53], polychlorinated dibenzo-dioxins, dibenzofurans and PCBs [54], has been previously reported elsewhere. These non-ionizable compounds are generally more hydrophobic than the pharmaceuticals examined in the present study. For more intrinsically persistent and hydrophobic compounds, dietary uptake critically contributes to elevated accumulation along with respiratory uptake [55]. Hydrophobic compounds, particularly those poorly metabolized and with elevated log Kow values, are more likely to present bioaccumulation and biomagnification hazards to aquatic organisms [56]. Tissue concentrations of these hydrophobic non-ionizable contaminants are normalized by lipid content, because hydrophobic partitioning is often described by fugacity models [56]. Unfortunately, such historical modelling approaches do not adequately address bioaccumulation of ionizable contaminants such as the pharmaceuticals studied here [2,4,5,11,14]. For example, as noted above, tissue samples were not lipid normalized in the present study because fish tissue levels of pharmaceuticals and lipid content were not significantly related [9].
When trophic dilution is observed for more hydrophobic compounds, it is likely due to substantial biotransformation rates and/or poor assimilation efficiencies in organisms at higher TPs [52]. Comparative biotransformation of pharmaceuticals by aquatic organisms at different TPs is not understood, though we recently reported that rainbow trout exhibited limited in vitro biotransformation for several pharmaceuticals, including diphenhydramine [10]. Future studies are needed to define pharmaceutical biotransformation by other fish and invertebrate species. But if biotransformation of diphenhydramine is also limited in species examined from the North Bosque River, assimilation efficiencies of diphenhydramine in organisms at higher TPs are expected to be low. Thus, it appears that uptake of ionizable pharmaceuticals by aquatic organisms may be more likely to occur from respiratory exchange (inhalation) than assimilation from diet, based on observations for diphenhydramine in the present study.
Supplementary Material
Acknowledgements
We thank Christopher Breed, Bruce Byars, Thomas Conry and Dr Jeffrey Arnold for useful discussions. We thank Rebekah Burket for laboratory assistance, Dr Jeffrey Back for assistance with nutrient analyses and Dr Ren Zhang for assistance with stable isotope measurements.
Funding statement
Support for this project was provided by grants from the Glasscock Fund for Excellence in Environmental Science (to B.D.), the City of Waco, Texas, Water Utilities (to B.W.B. and C.K.C.) and the United States Department of Agriculture (to B.W.B. and C.K.C.). B.W.B. received additional support from the Fulbright Visiting Research Chair in Water and the Environment at the University of Lethbridge.
References
- 1.Brooks BW, Riley TM, Taylor RD. 2006. Water quality of effluent-dominated ecosystems: ecotoxicological, hydrological, and management considerations. Hydrobiologia 556, 365–379. ( 10.1007/s10750-004-0189-7) [DOI] [Google Scholar]
- 2.Daughton C, Brooks B. 2011. Active pharmaceutical ingredients and aquatic organisms. In Environmental contaminants in biota (ed. Beyer JMWN.), pp. 286–347, 2nd edn Boca Raton, FL: CRC Press. [Google Scholar]
- 3.Ankley GT, Brooks BW, Huggett DB, Sumpter JP. 2007. Repeating history: pharmaceuticals in the environment. Environ. Sci. Technol. 41, 8211–8217. ( 10.1021/es072658j) [DOI] [PubMed] [Google Scholar]
- 4.Brooks BW. 2014. Fish on Prozac (and Zoloft): ten years later. Aquat. Toxicol. 151, 61–67. ( 10.1016/j.aquatox.2014.01.007) [DOI] [PubMed] [Google Scholar]
- 5.Boxall AB, et al. 2012. Pharmaceuticals and personal care products in the environment: what are the big questions? Environ. Health Perspect. 120, 1221–1229. ( 10.1289/ehp.1104477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson DGJ, Adolfsson-Erici M, Parkkonen J, Pettersson M, Berg AH, Olsson PE, Förlin L. 1999. Ethinyloestradiol: an undesired fish contraceptive? Aquat. Toxicol. 45, 91–97. ( 10.1016/S0166-445X(98)00112-X) [DOI] [Google Scholar]
- 7.Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ. 2005. Determination of select antidepressants in fish from an effluent-dominated stream. Environ. Toxicol. Chem. 24, 464–469. ( 10.1897/04-081R.1) [DOI] [PubMed] [Google Scholar]
- 8.Ramirez AJ, Mottaleb MA, Brooks BW, Chambliss CK. 2007. Analysis of pharmaceuticals in fish using liquid chromatography-tandem mass spectrometry. Anal. Chem. 79, 3155–3163. ( 10.1021/ac062215i) [DOI] [PubMed] [Google Scholar]
- 9.Ramirez AJ, et al. 2009. Occurrence of pharmaceuticals and personal care products in fish: results of a national pilot study in the United States. Environ. Toxicol. Chem. 28, 2587–2597. ( 10.1897/08-561.1) [DOI] [PubMed] [Google Scholar]
- 10.Connors KA, Du B, Fitzsimmons PN, Hoffman AD, Chambliss CK, Nichols JW, Brooks BW. 2013. Comparative pharmaceutical metabolism by rainbow trout (Oncorhynchus mykiss) liver S9 fractions. Environ. Toxicol. Chem. 32, 1810–1818. ( 10.1002/etc.2240) [DOI] [PubMed] [Google Scholar]
- 11.Brooks BW, Huggett DB, Boxall ABA. 2009. Pharmaceuticals and personal care products: research needs for the next decade. Environ. Toxicol. Chem. 28, 2469–2472. ( 10.1897/09-325.1) [DOI] [PubMed] [Google Scholar]
- 12.Paterson G, Metcalfe CD. 2008. Uptake and depuration of the anti-depressant fluoxetine by the Japanese medaka (Oryzias latipes). Chemosphere 74, 125–130. ( 10.1016/j.chemosphere.2008.08.022) [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Yamamoto H, Sekizawa J, Kondo T, Hirai N, Tatarazako N. 2008. The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere 70, 865–873. ( 10.1016/j.chemosphere.2007.06.089) [DOI] [PubMed] [Google Scholar]
- 14.Meredith-Williams M, Carter LJ, Fussell R, Raffaelli D, Ashauer R, Boxall AB. 2012. Uptake and depuration of pharmaceuticals in aquatic invertebrates. Environ. Pollut. 165, 250–258. ( 10.1016/j.envpol.2011.11.029) [DOI] [PubMed] [Google Scholar]
- 15.Valenti TW, Perez-Hurtado P, Chambliss CK, Brooks BW. 2009. Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH. Environ. Toxicol. Chem. 28, 2685–2694. ( 10.1897/08-546.1) [DOI] [PubMed] [Google Scholar]
- 16.Valenti TW, Taylor JM, Back JA, King RS, Brooks BW. 2011. Influence of drought and total phosphorus on diel pH in wadeable streams: implications for ecological risk assessment of ionizable contaminants. Integr. Environ. Assess. Manag. 7, 636–647. ( 10.1002/ieam.202) [DOI] [PubMed] [Google Scholar]
- 17.Valenti TW, Gould GG, Berninger JP, Connors KA, Keele NB, Prosser KN, Brooks BW. 2012. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ. Sci. Technol. 46, 2427–2435. ( 10.1021/es204164b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisk AT, et al. 2005. An assessment of the toxicological significance of anthropogenic contaminants in Canadian arctic wildlife. Sci. Total Environ. 351–352, 57–93. ( 10.1016/j.scitotenv.2005.01.051) [DOI] [PubMed] [Google Scholar]
- 19.Gobas FAPC, de Wolf W, Burkhard LP, Verbruggen E, Plotzke K. 2009. Revisiting bioaccumulation criteria for POPs and PBT assessments. Integr. Environ. Assess. Manag. 5, 624–637. ( 10.1897/IEAM_2008-089.1) [DOI] [PubMed] [Google Scholar]
- 20.Du B, Perez-Hurtado P, Brooks BW, Chambliss CK. 2012. Evaluation of an isotope dilution liquid chromatography tandem mass spectrometry method for pharmaceuticals in fish. J. Chromatogr. A 1253, 177–183. ( 10.1016/j.chroma.2012.07.026) [DOI] [PubMed] [Google Scholar]
- 21.Du B, Price AE, Scott WC, Kristofco LA, Ramirez AJ, Chambliss CK, Yelderman JC, Brooks BW. 2014. Comparison of contaminants of emerging concern removal, discharge, and water quality hazards among centralized and on-site wastewater treatment system effluents receiving common wastewater influent. Sci. Total Environ. 466–467, 976–984. ( 10.1016/j.scitotenv.2013.07.126) [DOI] [PubMed] [Google Scholar]
- 22.Lajeunesse A, Gagnon C, Sauvé S. 2008. Determination of basic antidepressants and their N-desmethyl metabolites in raw sewage and wastewater using solid-phase extraction and liquid chromatography−tandem mass spectrometry. Anal. Chem. 80, 5325–5333. ( 10.1021/ac800162q) [DOI] [PubMed] [Google Scholar]
- 23.Vanderford BJ, Snyder SA. 2006. Analysis of pharmaceuticals in water by isotope dilution liquid chromatography/tandem mass spectrometry. Environ. Sci. Technol. 40, 7312–7320. ( 10.1021/es0613198) [DOI] [PubMed] [Google Scholar]
- 24.Fick J, Lindberg RH, Parkkonen J, Arvidsson B, Tysklind M, Larsson DGJ. 2010. Therapeutic levels of levonorgestrel detected in blood plasma of fish: results from screening rainbow trout exposed to treated sewage effluents. Environ. Sci. Technol. 44, 2661–2666. ( 10.1021/es903440m) [DOI] [PubMed] [Google Scholar]
- 25.Fitzsimmons PN, Fernandez JD, Hoffman AD, Butterworth BC, Nichols JW. 2001. Branchial elimination of superhydrophobic organic compounds by rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 55, 23–34. ( 10.1016/S0166-445X(01)00174-6) [DOI] [PubMed] [Google Scholar]
- 26.Huggett DB, Cook JC, Ericson JF, Williams RT. 2003. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk Assess. 9, 1789–1799. ( 10.1080/714044797) [DOI] [Google Scholar]
- 27.Berninger JP, Du B, Connors KA, Eytcheson SA, Kolkmeier MA, Prosser KN, Valenti TW, Chambliss CK, Brooks BW. 2011. Effects of the antihistamine diphenhydramine on selected aquatic organisms. Environ. Toxicol. Chem. 30, 2065–2072. ( 10.1002/etc.590) [DOI] [PubMed] [Google Scholar]
- 28.Jardine TD, Kidd KA, Fisk AT. 2006. Applications, considerations, and sources of uncertainty when using stable isotope analysis in ecotoxicology. Environ. Sci. Technol. 40, 7501–7511. ( 10.1021/es061263h) [DOI] [PubMed] [Google Scholar]
- 29.Werner RA, Brand WA. 2001. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun. Mass Spectrom. 15, 501–519. ( 10.1002/rcm.258) [DOI] [PubMed] [Google Scholar]
- 30.Post DM. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718. ( 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2) [DOI] [Google Scholar]
- 31.Parnell AC, Jackson AL.2013. SIAR: stable isotope analysis in R. R package version 4.2. See http://CRAN.R-project.org/package=siar.
- 32.Parnell AC, Inger R, Bearhop S, Jackson AL. 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5, e9672 ( 10.1371/journal.pone.0009672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 34.Fisk AT, Hobson KA, Norstrom RJ. 2001. Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the northwater polynya marine food web. Environ. Sci. Technol. 35, 732–738. ( 10.1021/es001459w) [DOI] [PubMed] [Google Scholar]
- 35.Broman D, Rolff C, Näf C, Zebühr Y, Fry B, Hobbie J. 1992. Using ratios of stable nitrogen isotopes to estimate bioaccumulation and flux of polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in two food chains from the Northern Baltic. Environ. Toxicol. Chem. 11, 331–345. ( 10.1897/1552-8618(1992)11[331:UROSNI]2.0.CO;2) [DOI] [Google Scholar]
- 36.Lavoie RA, Hebert CE, Rail J-F, Braune BM, Yumvihoze E, Hill LG, Lean DRS. 2010. Trophic structure and mercury distribution in a Gulf of St Lawrence (Canada) food web using stable isotope analysis. Sci. Total Environ. 408, 5529–5539. ( 10.1016/j.scitotenv.2010.07.053) [DOI] [PubMed] [Google Scholar]
- 37.Borgå K, Kidd KA, Muir DCG, Berglund O, Conder JM, Gobas FAPC, Kucklick J, Malm O, Powell DE. 2011. Trophic magnification factors: considerations of ecology, ecosystems, and study design. Integr. Environ. Assess. Manag. 8, 64–84. ( 10.1002/ieam.244) [DOI] [PubMed] [Google Scholar]
- 38.de Mendiburu F. 2014. Agricolae: statistical procedures for agricultural research. R package version 1.2-0. See http://CRAN.R-project.org/package=agricolae. [Google Scholar]
- 39.TCEQ. 2003. Guidance for assessing Texas surface and finished drinking water quality data, 2004. Austin, TX: Surface Water Quality Monitoring Program, Texas Commission on Environmental Quality. [Google Scholar]
- 40.Bringolf RB, Heltsley RM, Newton TJ, Eads CB, Fraley SJ, Shea D, Cope WG. 2010. Environmental occurrence and reproductive effects of the pharmaceutical fluoxetine in native freshwater mussels. Environ. Toxicol. Chem. 29, 1311–1318. ( 10.1002/etc.157) [DOI] [PubMed] [Google Scholar]
- 41.Hazelton PD, Du B, Haddad SP, Fritts AK, Chambliss CK, Brooks BW, Bringolf RB. 2014. Chronic fluoxetine exposure alters movement and burrowing in adult freshwater mussels. Aquat. Toxicol. 151, 27–35. ( 10.1016/j.aquatox.2013.12.019) [DOI] [PubMed] [Google Scholar]
- 42.Franzellitti S, Buratti S, Capolupo M, Du B, Haddad SP, Chambliss CK, Brooks BW, Fabbri E. 2014. An exploratory investigation of various modes of action and potential adverse outcomes of fluoxetine in marine mussels. Aquat. Toxicol. 151, 14–26. ( 10.1016/j.aquatox.2013.11.016) [DOI] [PubMed] [Google Scholar]
- 43.Daniel WA, Wöjcikowski J. 1997. Contribution of lysosomal trapping to the total tissue uptake of psychotropic drugs. Pharmacol. Toxicol. 80, 62–68. ( 10.1111/j.1600-0773.1997.tb00285.x) [DOI] [PubMed] [Google Scholar]
- 44.Morton B. 1983. Feeding and digestion in Bivalvia. In The mollusca (eds Saleuddin ASM, Wilbur KM.), pp. 65–147. New York, NY: Academic Press. [Google Scholar]
- 45.Lamichhane K, Garcia S, Huggett D, DeAngelis D, La Point T. 2013. Chronic effects of carbamazepine on life-history strategies of Ceriodaphnia dubia in three successive generations. Arch. Environ. Contam. Toxicol. 64, 427–438. ( 10.1007/s00244-012-9845-5) [DOI] [PubMed] [Google Scholar]
- 46.De Lange HJ, Noordoven W, Murk AJ, Lürling M, Peeters ETHM. 2006. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 78, 209–216. ( 10.1016/j.aquatox.2006.03.002) [DOI] [PubMed] [Google Scholar]
- 47.Nfon E, Cousins IT, Broman D. 2008. Biomagnification of organic pollutants in benthic and pelagic marine food chains from the Baltic Sea. Sci. Total Environ. 397, 190–204. ( 10.1016/j.scitotenv.2008.02.029) [DOI] [PubMed] [Google Scholar]
- 48.Rosi-Marshall E, Royer T. 2012. Pharmaceutical compounds and ecosystem function: an emerging research challenge for aquatic ecologists. Ecosystems 15, 867–880. ( 10.1007/s10021-012-9553-z) [DOI] [Google Scholar]
- 49.Swanson HK, Kidd KA. 2010. Mercury concentrations in Arctic food fishes reflect the presence of anadromous Arctic charr (Salvelinus alpinus), species, and life history. Environ. Sci. Technol. 44, 3286–3292. ( 10.1021/es100439t) [DOI] [PubMed] [Google Scholar]
- 50.Wyn B, Kidd KA, Burgess NM, Curry RA. 2009. Mercury biomagnification in the food webs of acidic lakes in Kejimkujik National Park and National Historic Site, Nova Scotia. Can. J. Fish. Aquat. Sci. 66, 1532–1545. ( 10.1139/F09-097) [DOI] [Google Scholar]
- 51.Borgå K, Fisk AT, Hoekstra PF, Muir DCG. 2004. Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in Arctic marine food webs. Environ. Toxicol. Chem. 23, 2367–2385. ( 10.1897/03-518) [DOI] [PubMed] [Google Scholar]
- 52.Wan Y, Jin X, Hu J, Jin F. 2007. Trophic dilution of polycyclic aromatic hydrocarbons (PAHs) in a marine food web from Bohai Bay, North China. Environ. Sci. Technol. 41, 3109–3114. ( 10.1021/es062594x) [DOI] [PubMed] [Google Scholar]
- 53.Wu J-P, Guan Y-T, Zhang Y, Luo X-J, Zhi H, Chen S-J, Mai B-X. 2010. Trophodynamics of hexabromocyclododecanes and several other non-PBDE brominated flame retardants in a freshwater food web. Environ. Sci. Technol. 44, 5490–5495. ( 10.1021/es101300t) [DOI] [PubMed] [Google Scholar]
- 54.Wan Y, et al. 2010. Bioaccumulation of polychlorinated dibenzo-pS-dioxins, dibenzofurans, and dioxin-like polychlorinated biphenyls in fishes from the Tittabawassee and Saginaw Rivers, Michigan, USA. Sci. Total Environ. 408, 2394–2401. ( 10.1016/j.scitotenv.2010.02.003) [DOI] [PubMed] [Google Scholar]
- 55.Arnot JA, Gobas FAPC. 2004. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environ. Toxicol. Chem. 23, 2343–2355. ( 10.1897/03-438) [DOI] [PubMed] [Google Scholar]
- 56.Mackay D, Fraser A. 2000. Bioaccumulation of persistent organic chemicals: mechanisms and models. Environ. Pollut. 110, 375–391. ( 10.1016/S0269-7491(00)00162-7) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.